Abstract
Background
Diabetes has been recognized as a major comorbidity for COVID‐19 severity in adults. This study aimed to characterize the clinical outcomes and risk factors for COVID‐19‐related death in a large cohort of hospitalized pediatric patients with diabetes.
Methods
We performed an analysis of all pediatric patients with diabetes and COVID‐19 registered in SIVEP‐Gripe, a Brazilian nationwide surveillance database, between February 2020 and May 2021. The primary outcome was time to death, which was evaluated considering discharge as a competitive risk by using cumulative incidence function.
Results
Among 21,591 hospitalized pediatric patients with COVID‐19, 379 (1.8%) had diabetes. Overall, children and adolescents with diabetes had a higher prevalence of ICU admission (46.6% vs. 26%), invasive ventilation (16.9% vs. 10.3%), and death (15% vs. 7.6%) (all P < 0.0001). Children with diabetes had twice the hazard of death compared with pediatric patients without diabetes (Hazard ratio [HR] = 2.0, 95% CI, 1.58–2.66). Among children with diabetes, four covariates were independently associated with the primary outcome, living in the poorest regions of the country (Northeast, HR, 2.17, 95% CI 1.18–4.01, and North, (HR 4.0, 95% CI 1.79–8.94), oxygen saturation < 95% at admission (HR 2.97, 95% CI 1.64–5.36), presence of kidney disorders (HR 3.39, 95% CI 1.42–8.09), and presence of obesity (HR 3.77, 95% CI 1.83–7.76).
Conclusion
Children and adolescents with diabetes had a higher risk of death compared with patients without diabetes. The higher risk of death was associated with clinical and socioeconomic factors.
Keywords: children, COVID‐19, diabetes, outcome, risk factors, SARS‐CoV‐2
1. INTRODUCTION
The SARS‐CoV‐2 infection has been devastating worldwide particularly for older adults and those from vulnerable groups, including racial and ethnic minority populations. 1 , 2 Additionally, individuals with certain underlying medical conditions are also at increased risk of severe COVID‐19. 3 Recently, clinical and epidemiological studies have consistently identified diabetes as one of the major comorbidities associated with COVID‐19 severity and mortality among adults. 4 , 5 , 6 , 7 , 8
As most research on COVID‐19 and diabetes has been done in adults, it is unclear whether this increased risk of a more severe course of COVID‐19 is also present in the pediatric population. There is a paucity of data on the risk of death related to COVID‐19 in pediatric patients with diabetes. The few studies reported are from high‐ or upper‐middle income countries, often with a small sample size, which may limit the generalizability of findings. For example, in the first months of the pandemic, Cardona‐Hernandez et al. 9 collected data from large pediatric diabetes centers around the world and concluded that young individuals with diabetes (<25 years) were not at increased risk of hospitalization for COVID‐19. On the other hand, in 2021, Elbarbay et al. 10 conducted an international survey on the management of children with diabetes and COVID‐19. In this survey, 44% of respondents reported increased episodes of diabetic ketoacidosis in newly diagnosed cases and 30% in established cases. However, there is still limited data on many aspects of how COVID‐19 has affected children and adolescents with diabetes, especially in developing countries. 11
We recently described clinical outcomes and risk factors for death in children and adolescents hospitalized during the first and second waves of COVID‐19 in Brazil. 12 , 13 In both waves, we found an important and additive effect related to the presence of comorbidities in general. Based on data from the adult population, we hypothesized that COVID‐19 in pediatric patients with diabetes would have a poorer outcome compared to COVID‐19 in pediatric patients without diabetes. The aim of this study was to compare the risk of mortality in children with and without diabetes and to identify risk factors for mortality in a subsample of pediatric patients with diabetes, using the SIVEP‐Gripe (Surveillance Information System for Influenza) dataset, a Brazilian nationwide registry of hospitalized COVID‐19 cases.
2. METHODS
2.1. Study design
We performed a retrospective cohort study including all hospitalized pediatric cases recorded in the SIVEP‐Gripe. Detailed information regarding this database, including reporting form and data dictionary, codes, and all de‐identified data, are publicly available at https://opendatasus.saude.gov.br/dataset/srag-2020 for data from 2020, and at https://opendatasus.saude.gov.br/dataset/srag-2021-e-2022 for data from 2021–2022.
2.2. Participants and case‐defining
We included all consecutively registered patients, aged less than 20 years, with a positive quantitative RT‐PCR (RT‐qPCR) test result for SARS‐CoV‐2 who had been admitted to the hospital. The RT‐qPCR tests for SARS‐CoV‐2 were completed after hospital admission. For the present study, we integrated two datasets. We downloaded the first database on January 10, 2021 and the second database on May 29, 2021. For the purpose of analysis, we merged both datasets into a unique database and the cases were divided into two groups, (1) Wave 1 (44 epidemiological weeks from February 16, 2020 to December 31, 2020) and (2) Wave 2 (19 epidemiological weeks from January 1, 2021 to May 29, 2021). In addition, we updated on May 29, 2021, the outcomes of interest for pediatric patients admitted at Wave 1. The rationality in separate the sample into two waves was due to the emergence of the Gamma variant identified in January 2021 in the city Manaus, Brazil. 14 This lineage became predominant in Brazil around February 2021 and was characterized by an increased transmissibility and with more severe spectrum of the disease both in adults and children. 13 , 15
We identified diabetes cases in the SIVEP‐Gripe database by retrieving data from specific fields for comorbidities. In the database, information about comorbidities is provided in closed fields (yes/no) without any detailed information about the underlying condition. In addition to diabetes, the dataset provides data on the following comorbidities: asthma, obesity, and cardiovascular, lung, kidney, liver, autoimmune, neurologic, and hematologic diseases. The complete information about included and excluded cases are displayed in the flowchart (Figure 1).
FIGURE 1.
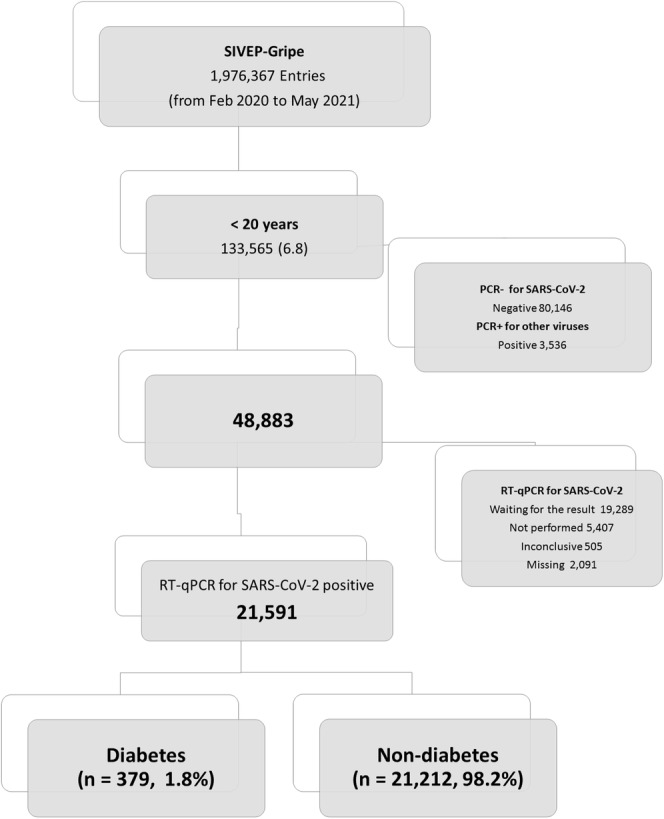
Flow diagram of cohort selection
2.3. Covariates and definitions
Clinical, demographic, and epidemiological data recorded in SIVEP‐Gripe are described elsewhere. 12 Briefly, demographic patient data included age, sex, ethnicity, and geographic regions of Brazil (North, Northeast, Central‐West, Southeast, and South). The Brazilian Institute of Geography and Statistics (IBGE) classifies ethnicity in five categories: Branco (White), Preto (Black), Pardo (Brown), Amarelo (Asian), or Indígeno (Indigenous). 16 Clinical data included date of signs and/or symptoms onset, date of hospital admission, and findings at disease presentation (fever, cough, respiratory distress, gastrointestinal symptoms). Oxygen saturation obtained at admission was classified as a dichotomous variable in the SIVEP‐Gripe database (cut‐off point of <95%). In addition, the presence of comorbidities was assessed as risk factors for the event of the interest. For this analysis, we included separately three comorbidities: cardiovascular disease, kidneys disorders, and obesity. The remaining associated conditions were analyzed together. For this purpose, we created a categorical covariate “other_comorbities.” This variable considered the sum of the other comorbidities included in the SIVEP‐gripe. The other comorbidities included were asthma, pulmonary, hepatic, autoimmune disease, neurologic, and hematologic diseases. The variable “other_comorbities” was categorized into three groups (none, 1, and 2 or more comorbidities).
The clinical course of the disease was reported in terms of respiratory support (none, noninvasive oxygen support, and invasive ventilation), admission to intensive care unit (ICU), hospital discharge, COVID‐19‐related death, and ongoing clinical situation. Severe spectrum of COVID‐19 was defined by the admission to the intensive care unit (ICU), the need of mechanical ventilation, and disease‐related death.
2.4. Missing data management
Although several variables were mandatory in the SIVEP‐Gripe registration form, others had the option “Ignored.” These variables presented a considerable amount of missing information. The following covariates had missing information: gender (0.08%), ethnicity (19.6%), oxygen saturation at admission (24.7%), ICU admission (8%), ventilatory support (5.5%), and primary outcome (0.8%). We use various strategies to partially overcome this problem. We describe these strategies in detail elsewhere. 13 Briefly, in the first step, patients with missing information about the primary outcome were removed from the survival analyses. For those cases with missing data on a particular symptom or comorbidity, we assumed that the clinical condition was absent. Finally, we performed a multiple imputation using all predictors plus the cumulative incidence function for the primary outcome. Ten imputed data sets were generated using the multiple imputation chain equations (MICE) package from the R software (R Foundation for Statistical Computing, Vienna, Austria. Available on https://cran.r-project.org/web/packages/mice/index.html). We combined the results from analyses on each of the imputed values using Rubin's rules to produce estimates and confidence intervals that incorporate the uncertainty of imputed values. 17 , 18
2.5. Outcomes
The primary outcome was time until COVID‐19‐related death (in‐hospital‐mortality). The survival time was defined from the day of admission until the event (death or discharge). We also analyzed the need of health‐care resources (ICU admission and respiratory support, defined as none, noninvasive, or invasive).
2.6. Statistical analysis
The analysis was performed in three steps. First, we compared clinical, demographic and outcomes data in children and adolescents with and without diabetes. We used medians and interquartile ranges (IQRs) or means and standard deviations (SDs) to summarize continuous variables and calculated frequencies and proportions for categorical variables. We compared means and proportions using respectively the F‐test and the chi‐square test. Second, to assess the impact of diabetes on the survival of pediatric patients with COVID‐19, we performed a survival analysis, using the cumulative incidence function (CIF) 19 and the Fine‐Gray subdistribution risk model 20 to estimate the incidence of outcomes over time in the presence of competing risks. A competing risk is an event whose occurrence precludes observation of the primary event of interest. In our study, as COVID‐19‐related death is the primary outcome, hospital discharge was analyzed as a concurrent event in the analysis. We performed both univariate and multivariate analyses of competitive risk survival, including age, sex, ethnicity, geographic macroregion, signs and symptoms at admission, oxygen saturation, obesity, and other comorbidities as covariates. Finally, the third step was performed on the subsample of pediatric patients with diabetes in order to identify risk factors for mortality in this group. For this step, we also performed a competitive risk survival analysis using the same methodology described in the second step. All statistical tests were two tailed, and statistical significance was defined as P < 0.05.
Ethical aspects.
We accessed data in SIVEP‐Gripe, which are already deidentified and publicly available. Following ethically agreed principles on open data, this analysis did not require ethical approval in Brazil. We reported our findings following the guideline STROBE for observational cohort studies. 21
3. RESULTS
3.1. Baseline demographic, clinical characteristics, and outcomes
The cohort comprised 21,591 cases, including 379 cases (1.8%) of diabetes. The demographic and clinical characteristics of the cohort according to presence of diabetes are shown in Table 1. As expected, pediatric patients with diabetes were significantly older at presentation. Proportionally, children and adolescents with diabetes were more frequently of female gender, from the richest regions (South and Southeast) of the country and of White ethnicity compared with non‐diabetic children. These patients also presented at baseline greater proportion of respiratory distress, dyspnea, and oxygen saturation < 95%. Cardiovascular disease, kidney disorders, and obesity were significantly more prevalent in children and adolescents with diabetes compared with those without. Pediatric patients with diabetes also had more associated comorbidities than those nondiabetics.
TABLE 1.
Demographic, clinical characteristics, and outcomes of children and adolescents with laboratory‐confirmed COVID‐19 according to presence of diabetes (n = 21,591)
| Overall (%) | Nondiabetes (%) | Diabetes (%) | P | |
|---|---|---|---|---|
| 21,591 (100) | 21,212 (98.2) | 379 (1.8) | ||
| Age (years) | ||||
| Median (interquartile range) | 4.7 (0.8–14.6) | 4.5 (0.8–14.3) | 14.7 (8.2–18.3) | <0.0001 |
| Mean (SD) | 7.4 (7.0) | 7.3 (7.0) | 12.5 (6.7) | <0.0001 |
| Age group (years) | ||||
| 0.0–9.9 | 13,856 (64.2) | 13,750 (64.8) | 106 (28.0) | <0.0001 |
| 10–19.9 | 7735 (35.8) | 7462 (35.2) | 273 (72.0) | |
| Gender (n = 21,573) | ||||
| Female | 10,411 (48.3) | 10,178 (48.0) | 233 (61.5) | <0.0001 |
| Male | 11,162 (51.7) | 11,016 (52.0) | 146 (38.5) | |
| Wave | ||||
| First | 11,574 (53.6) | 11,366 (53.6) | 208 (54.9) | 0.64 |
| Second | 10,017 (46.4) | 9846 (46.4) | 171 (45.1) | |
| Region | ||||
| Southeast | 8075 (37.4) | 7892 (37.2) | 183 (48.3) | <0.0001 |
| South | 2204 (10.2) | 2160 (10.2) | 44 (11.6) | |
| Central‐West | 2293 (10.6) | 2259 (10.6) | 34 (8.9) | |
| Northeast | 5748 (26.6) | 5651 (26.6) | 97 (25.6) | |
| North | 3271 (15.1) | 3250 (15.3) | 21 (5.5) | |
| Ethinicity | ||||
| White | 8047 (37.2) | 7866 (37.0) | 181 (47.7) | <0.0001 |
| Black/Brown | 13,107 (60.7) | 12,913 (60.9) | 194 (51.2) | |
| Asian | 181 (0.84) | 179 (0.84) | 2 (0.5) | |
| Indigenous | 256 (1.2) | 254 (1.2) | 2 (0.5) | |
| Signs and symptoms at admission | ||||
| Fever | 14,140 (65.5) | 13,938 (65.7) | 202 (53.3) | <0.001 |
| Cough | 12,971 (60.1) | 12,773 (60.2) | 198 (58.2) | 0.002 |
| Respiratory distress | 9733 (45.1) | 9540 (45.0) | 193 (50.9) | 0.022 |
| Oxygen saturation < 95% | 9580 (44.4) | 9399 (44.3) | 181 (47.8) | 0.19 |
| Dyspnea | 10,470 (48.5) | 10,227 (48.2) | 243 (64.1) | <0.001 |
| Odynophagia | 3476 (16.5) | 3512 (16.6) | 78 (20.6) | 0.041 |
| Diarrhea | 3094 (14.7) | 3144 (14.8) | 43 (11.3) | 0.06 |
| Vomit | 3629 (16.8) | 3537 (16.7) | 92 (24.3) | <0.001 |
| Abdominal pain | 1454 (6.7) | 1412 (6.7) | 42 (11.1) | <0.001 |
| Kidney disorders | ||||
| Yes | 290 (1.3) | 277 (1.3) | 13 (3.4) | 0.002 |
| No | 21,301 (98.7) | 20,935 (98.7) | 366 (96.6) | |
| Cardiovascular disorders | ||||
| Yes | 698 (3.2) | 647 (3.1) | 51 (13.5) | <0.0001 |
| No | 20,893 (96.8) | 20,565 (96.9) | 328 (86.5) | |
| Obesity | ||||
| Yes | 477 (2.2) | 446 (2.1) | 31 (8.2) | <0.0001 |
| No | 21,114 (97.8) | 20,776 (97.9) | 348 (91.8) | |
| Other omorbidities | ||||
| None | 17,243 (79.9) | 16,920 (79.8) | 323 (85.2) | 0.002 |
| 1 | 3850 (17.8) | 3807 (17.9) | 43 (11.3) | |
| ≥2 | 498 (2.3) | 485 (2.3) | 13 (3.4) | |
| ICU admission (n = 19,867) | ||||
| Yes | 5243 (26.4) | 5076 (26.0) | 167 (46.6) | <0.0001 |
| No | 14,624 (73.6) | 14,433 (74.0) | 191 (53.4) | |
| Ventilatory support (n = 20.396) | ||||
| Invasive | 11,272 (55.3) | 2069 (10.3) | 61 (16.9) | <0.0001 |
| Noninvasive | 6994 (34.3) | 6849 (34.2) | 145 (40.3) | |
| None | 2130 (10;4) | 11,118 (55.5) | 154 (42.8) | |
| Death | ||||
| Yes | 1661 (7.7) | 1604 (7.6) | 57 (15.0) | <0.0001 |
| No | 19,930 (92.3) | 19,608 (92.4) | 322 (85.0) |
The clinical outcomes are also shown in Table 1. Overall, children and adolescents with diabetes had higher prevalence of the severe spectrum of COVID‐19 compared with nondiabetic cohort, considering ICU admission (46.6% vs. 26%), use of invasive ventilation (16.9% vs. 10.3%), and death (15% vs. 7.6%) (all P < 0.001).
The CIF of death for patients with and without diabetes is shown in Figure 2. The estimated probability of fatal outcome for the first 10, 30, and 60 days of hospitalization for pediatric patients without diabetes was, respectively, 4.5%, 7.4%, 8%. For patients with diabetes, the respective figures were about 8%, 15%, and 18%. According to the Fine‐Gray model, in the univariate analysis, children and adolescents with diabetes had twice hazard of death compared with pediatric patients without diabetes (HR = 2.0, 95% CI, 1.58–2.66. P < 0.0001). In the competing‐risk multivariate survival analysis, adjusted by age, gender, region, ethnicity, respiratory symptoms, cardiovascular diseases, kidney disorders and obesity, children and adolescents with diabetes remained at higher risk of death compared with pediatric patients without diabetes (HR = 1.53, 95% CI, 1.17–2.03, P = 0.002).
FIGURE 2.
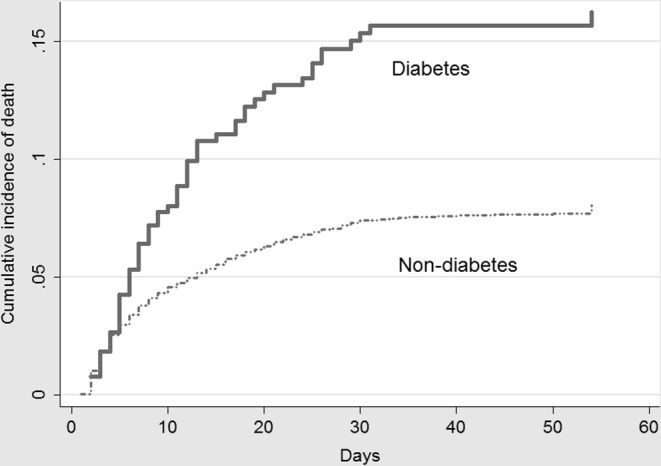
Cumulative incidence function of death according to the presence of diabetes (n = 21,591)
3.2. Risk factors of fatal outcome
Table 2 shows the competing‐risk univariate analysis for the risk of death regarding the demographic characteristics and clinical features among the 379 children and adolescents with diabetes. Using the Fine and Gray model for the main outcome, geographic region, presence of respiratory symptoms at admission, oxygen saturation < 95% at admission, associated kidney disorders, and obesity were significantly associated with higher hazard of death. (Table 2).
TABLE 2.
Univariate competing‐risk survival analysis of children and adolescents with diabetes and COVID‐19 (n = 376)
| Discharged (%)—283 (75.3) | Death (%)—57 (15.2) | Censored (%)—36 (9.6) | HR (95% CI) | P‐value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 0.0 ‐ 9.9 | 74 (70.5) | 21 (20.0) | 10 (9.5) | 1.0 | |
| 10–19.9 | 209 (77.1) | 36 (13.3) | 26 (9.6) | 0.62 (0.36; 1.06) | 0.086 |
| Gender | |||||
| Male | 110 (75.9) | 21 (14.5) | 14 (9.7) | 1.0 | |
| Female | 173 (74.9) | 36 (15.6) | 22 (9.5) | 1.06 (0.62; 1.81) | 0.22 |
| Wave | |||||
| First | 162 (78.6) | 33 (16.0) | 11 (5.3) | 1.0 | |
| Second | 121 (71.2) | 24 (14.1) | 25 (14.7) | 0.88 (0.52; 1.49) | 0.64 |
| Region | |||||
| Southeast | 144 (79.5) | 21 (11.6) | 16 (8.8) | 1.0 | |
| South | 33 (75.0) | 6 (13.6) | 5 (11.4) | 1.17 (0.47; 2.88) | 0.34 |
| Central‐West | 29 (85.3) | 3 (8.8) | 2 (5.9) | 0.77 (0.22; 2.64) | 0.68 |
| Northeast | 64 (66.7) | 20 (20.8) | 12 (12.5) | 1.94 (1.05; 3.57) | 0.033 |
| North | 13 (61.9) | 7 (33.3) | 1 (4.8) | 3.15 (1.38; 7.14) | 0.006 |
| Ethinicity | |||||
| White | 137 (76.1) | 24 (13.3) | 19 (10.6) | 1.0 | |
| Non‐White | 146 (74.5) | 33 (16.8) | 17 (8.7) | 1.26 (0.75;2.14) | 0.37 |
| Signs and symptoms at admisson | |||||
| Fever | 156 (77.6) | 30 (14.9) | 15 (7.5) | 0.95 (0.56, 1.59) | 0.85 |
| Cough | 153 (77.3) | 48 (13.1) | 28 (9.6) | 0.72 (0.43, 1.21) | 0.22 |
| Odynophagia | 62 (80.5) | 10 (13.0) | 5 (6.5) | 0.80 (0.41, 1.59) | 0.54 |
| Respiratory distress | 132 (69.1) | 38 (19.9) | 21 (11.0) | 2.07 (1.20, 3.58) | 0.009 |
| Dyspnea | 170 (70.2) | 46 (19.0) | 26 (10.7) | 2.44 (1.23, 4.73) | 0.008 |
| O2 saturation < 95% | 120 (66.6) | 41 (22.8) | 19 (10.6) | 2.99 (1.67, 5.32) | <0.0001 |
| Diarrhea | 36 (85.7) | 5 (11.9) | 1 (2.4) | 0.72 (0.29, 1.79) | 0.48 |
| Vomit | 75 (82.4) | 9 (9.9) | 7 (7.7) | 0.55 (0.27, 1.11) | 0.098 |
| Abdominal pain | 33 (80.5) | 1 (2.4) | 7 (17.1) | 0.13 (0.01, 0.98) | 0.05 |
| Kidney disorders | |||||
| No | 277 (97.9) | 52 (91.2) | 34 (94.5) | 1.0 | |
| Yes | 6 (2.1) | 5 (8.8) | 2 (5.5) | 3.05 (1.26, 7.39) | 0.013 |
| Cardiovascular disorders | |||||
| No | 253 (89.4) | 47 (82.4) | 26 (72.2) | 1.0 | |
| Yes | 30 (10.6) | 10 (17.6) | 10 (27.8) | 1.56 (0.78, 3.11) | 0.27 |
| Obesity | |||||
| No | 270 (78.3) | 46 (13.3) | 29 (8.4) | 1.0 | |
| Yes | 13 (41.9) | 11 (35.5) | 7 (22.6) | 3.41 (1.76, 6.61) | <0.0001 |
| Other comorbidities | |||||
| 0 | 242 (85.5) | 45 (78.9) | 33 (91.7) | 1.0 | |
| 1 | 32 (11.3) | 8 (14.0) | 3 (8.3) | 1.31 (0.63, 2.76) | 0.11 |
| ≥2 | 9 (3.2) | 4 (7.0) | 0 (0.0) | 2.46 (0.85, 7.06) | 0.094 |
Note: Three missing information regarding outcome.
After adjustment by the competing‐risk multivariate regression analysis, four covariates remained significantly associated with the main outcome, living in the poorest regions of the country (Northeast, Hazard ratio [HR] 2.17, 95% CI 1.18–4.01, P = 0.013, and North, (HR 4.0, 95% CI 1.79–8.94, P = 0.001), oxygen saturation < 95% at admission (HR 2.97, 95% CI 1.64–5.36, P < 0.0001), presence of kidney disorders (HR 3.39, 95% CI 1.42–8.09, P = 0.006), and presence of obesity (HR 3.77, 95% CI 1.83–7.76, P < 0.0001).
4. DISCUSSION
In this retrospective cohort study, we describe the clinical presentation and outcomes of SARS‐CoV‐2 infection in 379 pediatric patients with diabetes included in a cohort of hospitalized children and adolescents with proven SARS‐CoV‐2 infection in Brazil. First, our findings have shown that, overall, children and adolescents with diabetes had a higher risk of severe COVID‐19 and about twice the hazard of death compared with the nondiabetic cohort. Second, among diabetic pediatric patients, after adjustment by the competing risk survival analysis, four covariates were independently associated with fatal outcome: living in the poorest regions of Brazil (North and Northeast), an oxygen saturation < 95% at entry, and presence of associated obesity and kidney disorders.
The main outcome of interest in our study was COVID‐19‐related death. In our analysis, children with diabetes had an approximately 50% higher risk of fatal outcome compared to the general pediatric population, even after adjusting by the clinical and demographic characteristics. Few studies have addressed the association of diabetes with the severity of COVID‐19 in children and adolescents. Of note, Demeterco‐Berggren et al. 22 reported a multicenter cross‐sectional study of patients with type 1 diabetes and COVID‐19 from 56 clinical centers in the United States. A total of 767 patients were analyzed, of which 415 (54%) were younger than 19 years. The analysis showed that older patients (>40 years) were at higher risk for COVID‐19 severity, while children and younger adults had milder illness and no deaths were reported for this age group. Instead, our analysis revealed that hospitalized children and adolescents with diabetes from a developing country had a higher need for mechanical ventilation, ICU admission, and mortality rate compared to those without diabetes.
Our findings were similar to those previously reported for adult patients with diabetes. For example, several studies of adults with diabetes infected with SARS‐CoV‐2 have shown an increased prevalence of more severe COVID‐19 outcomes, including death. 23 , 24 , 25 , 26 Huang et al. 25 reported a meta‐analysis of a total of 6452 patients from 30 studies in which adult patients with diabetes had an unfavorable outcome compared with the general population. The meta‐analysis showed that diabetes was associated with a poor outcome, including disease progression (RR 3.31, 95%CI, 1.08, 10.14) and mortality (RR 2.12. 95%CI, 1.44–3.11). Another meta‐analysis further demonstrated that adult patients with diabetes had more than twice the risk of ICU admission and more than triple the risk of death. 27 The population‐based study by Barron et al. 28 showed that Type 1 and Type 2 diabetes were independently associated with a significantly increased odds of in‐hospital death from COVID‐19. Compared with adults without diabetes, the odds ratios of in‐hospital death related to COVID‐19 were 3.51 (95% CI 3.16–3.90) in individuals with Type 1 diabetes and 2.03 (1.97–3.90). 2.09) in those with Type 2 diabetes. It is noteworthy that these findings are quite similar to those found in our study. We emphasize that we did not find cohort studies with large samples of pediatric patients hospitalized with diabetes infected with SARS‐CoV‐2, highlighting the novelty and relevance of the present study.
Several mechanisms have been suggested as an underlying explanation for the more severe course of COVID 19 in patients with diabetes. While diabetes itself appears to be an independent risk factor, other important factors may contribute to an increased risk of COVID‐19 severity and mortality in this group of patients, including older age, hypertension, cardiovascular disease, chronic kidney disease, obesity, a pro‐inflammatory and hypercoagulable state, and glucose dysregulation. 7 , 29 However, it is important to point out that most mechanisms responsible for the higher mortality in adult patients with diabetes clearly are absent in pediatric population. Furthermore, it is unknown whether potential changes in immune response and increased cytokine storm propensity demonstrated in adults may also occur in pediatric patients with diabetes. Thus, further clinical studies in children with diabetes are clearly needed to assess the mechanisms involved in the poor outcome in pediatric age.
In the second step of our analysis, we evaluate risk factors of COVID‐related death among children and adolescents with diabetes. After adjustment by the competing‐risk survival analysis, patients from the poorest Brazilian geographic macro‐regions (North and Northeast), those with low oxygen saturation at admission, and those with associated kidney diseases and obesity exhibited a higher hazard of death. The finding of an increased risk of in‐hospital death in patients from the poorest Brazilian macroregions (North and Northeast) is similar to our previous studies about the entire cohort of hospitalized children and adolescents. 12 , 13 In these studies, we provided evidence that the social factors were inextricably associated with clinical factors to determine the outcomes of COVID‐19 in Brazil, especially for the most socioeconomically disadvantaged population. 12 , 13 Brazil is a country of continental dimensions and is geopolitically divided into five macroregions: North, Northeast, Central‐West, Southeast, and South. These macroregions have historical differences in social, economic, and health system infrastructure. In Brazil, despite the existence of an equitable universal public health system (SUS), there are disparities in the provision of health services, in health needs across different segments of the population, and in the access to healthcare. 30 , 31 For example, Rocha et al. 32 reported that among other social vulnerabilities, the figures of public ICU beds were significantly smaller in North and Northeast regions. In an analysis of the first 250,000 adults hospitalized with COVID‐19, Ranzani et al. 33 clearly showed the regional inequality in the risk of dying during hospitalization. They reported an in‐hospital mortality rate in nonelderly patients of 31% in the Northeast region compared to 15% in the South of the country. Another independent predictive factor of mortality in our cohort was an oxygen saturation lower than 95% at hospital admission. This finding is expected since the lung is one of the main target organs for SARS‐CoV‐2 infection and its damage results in greater risk of death for patients with COVID‐19. 34 Therefore, early signs of lung involvement, such as reduced oxygen saturation on admission, likely indicate greater disease severity. It is noteworthy that two relevant comorbidities in the context of diabetes, kidney disorders and obesity, were independent risk factors for fatal outcome. Pre‐existing chronic medical conditions were strongly related to the prognosis of COVID‐19 in the general population, including children and adolescents. 12 , 35 , 36 In adult cohorts, chronic kidney disease has been shown to be an independent risk factor for in‐hospital death related to COVID‐19 in the general population 35 , 36 and recent studies have shown that this association is also present in patients with diabetes. 5 , 37 , 38 Obesity has been widely associated with the severity and mortality of COVID‐19. 36 , 39 , 40 Since diabetes and obesity lead to pro‐inflammatory, hypercoagulable and immune altered conditions, it would be expected an additional effect of obesity upon COVID‐19 severity in diabetes patients. However, we must emphasize again that data on the role of obesity in the prognosis of children with diabetes and COVID‐19 are limited in the pediatric population. 29 In addition, unfortunately the lack of detailed information on the clinical characteristics of the cohort in the SIVEP‐Gripe database prevented us from further evaluating the relationship between these comorbidities and outcomes in children with diabetes.
The strength of this study relies on the size of the cohort, allowing the analysis of clinical characteristics, risk factors, and outcomes of hospitalized diabetic children and adolescents with proven SARS‐CoV‐2 infection. On the other hand, several limitations must be acknowledged in the current study. First, we should point out that our sample is comprised only for hospitalized patients with certainly a more severe spectrum of the disease. The major limitation of our study is the absence of fundamental information regarding both the patients' clinical characteristics prior to admission and data about in‐hospital management in the SIVEP‐Gripe database. Therefore, we were unable to include some important covariates in the analysis, such as data on diabetes type, treatment, and glycated hemoglobin A levels. 5 The lack of information about the type of diabetes in our dataset precluded the assessment of whether this increased risk of a severe course of COVID‐19 differs between types of diabetes in the pediatric population. In adults, for example, large cohort studies have shown an increased risk of COVID‐19‐related mortality, ICU admission, and hospitalization for Type 1 diabetes compared with Type 2 diabetes. 28 , 41 , 42 It can be speculated, however, that our sample is possibly composed of the majority of Type I diabetes, since it was constituted by a young age group and with a relative low prevalence of other clinical characteristics of interest, such as obesity, hypertension, and kidney disorders.
Another relevant limitation is the lack of information on the level of care and clinical evolution during hospitalization. For example, in COVID‐19, glycemic control also plays an important role in the severity of outcomes. 29 For example, Holman et al 37 demonstrated that adult patients with preadmission HbA1c ≥ 86 mmol/mol (10.0%) compared with patients with HbA1c of 48–53 mmol/mol (6.5–7.0%) increased COVID‐19‐related mortality in both types of diabetes. Furthermore, glycemic control during hospital stay is an important prognostic factor for worse outcomes in hospitalized patients with COVID‐19. 26 , 43 Unfortunately, the SIVEP‐Gripe database did not provide information on glycated hemoglobin A levels. Finally, missing data is another inherent issue due to the nature of a registry based on point‐of‐care case report forms. We used various strategies to try to partially overcome this limitation. For example, to clarify the fundamental point of the relationship between diabetes and its complications with the outcome, we have analyzed separately the relationship between fundamental covariates such as obesity and kidney disorders. Regarding, missing variables, we used the multiple imputation technique for relevant predictors.
In conclusion, in this analysis of a large nationwide database of hospitalized patients with proven COVID‐19, we found that children and adolescents with diabetes had a more severe spectrum of the disease and a higher risk of death than patients without diabetes. Among pediatric patients with diabetes and COVID‐19, the higher hazard of death was associated with living in the poorest geographic regions of Brazil, low oxygen saturation at admission, and presence of obesity and kidney disease. Our findings suggest that the high mortality rates in children and adolescents with diabetes and COVID‐19 in Brazil may be partially related to social and economic conditions and with the lack of appropriate support for these pediatric patients. Further prospective studies with large samples of pediatric patients with diabetes are necessary to investigate COVID‐19 outcome, risk factors and potential mechanisms related to disease severity.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Eduardo A. Oliveira, Robert H. Mak; Methodology: Eduardo A. Oliveira, Maria Christina L. Oliveira, Enrico A. Colosimo, Investigation: Eduardo A. Oliveira, Maria Christina L. Oliveira, Ana Cristina Simõese Silva, Ana Carmen Q. Mendonça, Hercílio Martelli‐Júnior, Ludmila R. Silva, Mariana A. Vasconcelos, Clara C. Pinhati; Formal analysis: Eduardo A. Oliveira, Maria Christina L. Oliveira, Ana Cristina Simõese Silva, Ana Carmen Q. Mendonça, Hercílio Martelli‐Júnior, Ludmila R. Silva, Robert H. Mak, Enrico A. Colosimo, Mariana A. Vasconcelos; Writing—original draft preparation: Eduardo A. Oliveira, Enrico A. Colosimo, Robert H. Mak, Ana Cristina Simõese Silva; Writing—review and editing: Eduardo A. Oliveira, Maria Christina L. Oliveira, Ana Cristina Simõese Silva, Robert H. Mak, Mariana A. Vasconcelos, Clara C. Pinhati; Data Curation: Eduardo A. Oliveira, Maria Christina L. Oliveira; Data access and verification: Eduardo A. Oliveira, Maria Christina L. Oliveira, Enrico A. Colosimo, Supervision: Eduardo A. Oliveira. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Eduardo A. Oliveira takes responsibility regarding the fact that this study has been reported honestly, accurately, and transparently.
FUNDING
This study was partially supported by the CNPq (National Council for Scientific and Technological Development) and FAPEMIG (Research Support Foundation of Minas Gerais).
ACKNOWLEDGMENTS
The authors are profoundly grateful and in debt to all frontline health‐care workers for their impressive efforts to tackle the COVID‐19 pandemic in Brazil. All data from the SIVEP‐Gripe (Influenza Epidemiological Surveillance Information System) were systematically collected in challenging circumstances by these frontline healthcare workers.
Oliveira EA, Mak RH, Colosimo EA, et al. Risk factors for COVID‐19‐related mortality in hospitalized children and adolescents with diabetes mellitus: An observational retrospective cohort study. Pediatr Diabetes. 2022;1‐10. doi: 10.1111/pedi.13335
Funding information Conselho Nacional de Desenvolvimento Cientifico e Tecnologico; Fundao de Amparo Pesquisa do Estado de Minas Gerais; National Council for Scientific and Technological Development; CNPq
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Ministerio da Saude at https://opendatasus.saude.gov.br/dataset
REFERENCES
- 1. Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397(10286):1711‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nafilyan V, Islam N, Mathur R, et al. Ethnic differences in COVID‐19 mortality during the first two waves of the coronavirus pandemic: a nationwide cohort study of 29 million adults in England. Eur J Epidemiol. 2021;36(6):605‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID‐19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2022. [online ahead of print]. doi: 10.1007/s12016-022-08921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. J Diabetes. 2020;12(11):851‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta‐analysis. Diabetes Metab Syndr. 2020;14(4):535‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muniyappa R, Gubbi S. COVID‐19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736‐E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muniyappa R, Wilkins KJ. Diabetes, obesity, and risk prediction of severe COVID‐19. J Clin Endocrinol Metab. 2020;105(10):e3812‐e3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cardona‐Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID‐19. Pediatr Diabetes. 2021;22(2):202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elbarbary NS, Dos Santos TJ, de Beaufort C, Wiltshire E, Pulungan A, Scaramuzza AE. The challenges of managing pediatric diabetes and other endocrine disorders during the COVID‐19 pandemic: results from an international cross‐sectional electronic survey. Front Endocrinol (Lausanne). 2021;12:735554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiMeglio LA, Albanese‐O'Neill A, Munoz CE, Maahs DM. COVID‐19 and children with diabetes‐updates, unknowns, and next steps: first, do no extrapolation. Diabetes Care. 2020;43(11):2631‐2634. [DOI] [PubMed] [Google Scholar]
- 12. Oliveira EA, Colosimo EA, Simoes ESAC, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID‐19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5(8):559‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliveira EA, Simoes ESAC, Oliveira MCL, et al. Comparison of the first and second waves of the COVID‐19 pandemic in children and adolescents in a middle‐income country: clinical impact associated with SARS‐CoV‐2 gamma lineage. J Pediatr. 2022. [online ahead of print]. doi: 10.1016/j.jpeds.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS‐CoV‐2 lineage in Manaus, Brazil. Science. 2021;372(6544):815‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bastos LS, Ranzani OT, Souza TML, Hamacher S, Bozza FA. COVID‐19 hospital admissions: Brazil's first and second waves compared. Lancet Respir Med. 2021;9(8):e82‐e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Health. 2020;8(8):e1018‐e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janssen KJ, Vergouwe Y, Donders AR, et al. Dealing with missing predictor values when applying clinical prediction models. Clin Chem. 2009;55(5):994‐1001. [DOI] [PubMed] [Google Scholar]
- 18. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3‐15. [DOI] [PubMed] [Google Scholar]
- 19. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi‐state models. Stat Med. 2007;26(11):2389‐2430. [DOI] [PubMed] [Google Scholar]
- 20. Fine JP, Gray RJ. A proportional hazards model for the sub‐distribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. [DOI] [PubMed] [Google Scholar]
- 22. Demeterco‐Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID‐19: data from the T1D exchange surveillance study. J Clin Endocrinol Metab. 2021;107(2):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 24. Chudasama YV, Zaccardi F, Gillies CL, et al. Patterns of multimorbidity and risk of severe SARS‐CoV‐2 infection: an observational study in the U.K. BMC Infect Dis. 2021;21(1):908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14(4):395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu L, She ZG, Cheng X, et al. Association of Blood Glucose Control and Outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077 e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID‐19 infection are at higher risk of ICU admission and poor short‐term outcome. J Clin Virol. 2020;127:104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol. 2020;8(10):813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landstra CP, de Koning EJP. COVID‐19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol (Lausanne). 2021;12:649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011;377(9779):1778‐1797. [DOI] [PubMed] [Google Scholar]
- 31. Pereira CCA, Martins M, Lima SML, de Andrade CLT, Soares FRG, Portela MC. Geographical variation in demand, utilization, and outcomes of hospital services for COVID‐19 in Brazil: a descriptive serial cross‐sectional study. PLoS One. 2021;16(9):e0257643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rocha R, Atun R, Massuda A, et al. Effect of socioeconomic inequalities and vulnerabilities on health‐system preparedness and response to COVID‐19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9(6):e782‐e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250 000 hospital admissions for COVID‐19 in Brazil: a retrospective analysis of nationwide data. Lancet . Respir Med. 2021;9:407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID‐19 lung injury. Crit Care Clin. 2021;37(4):749‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holman RR, Clark A, Rorsman P. Beta‐cell secretory dysfunction: a key cause of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8(5):370. [DOI] [PubMed] [Google Scholar]
- 38. Maddaloni E, D'Onofrio L, Alessandri F, et al. Clinical features of patients with type 2 diabetes with and without Covid‐19: a case control study (CoViDiab I). Diabetes Res Clin Pract. 2020;169:108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID‐19. Obesity (Silver Spring). 2020;28(7):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregory JM, Slaughter JC, Duffus SH, et al. COVID‐19 severity is tripled in the diabetes community: a prospective analysis of the Pandemic's impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID‐19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Ministerio da Saude at https://opendatasus.saude.gov.br/dataset


