Abstract
To combat the coronavirus disease 2019 (COVID‐19) pandemic, many countries have started population vaccination programs using messenger ribonucleic acid (mRNA) vaccines. With the widespread use of such vaccines, reports are emerging worldwide, of the vaccine's association with the development of myocarditis. Younger men are more likely to develop postvaccine myocarditis, which usually presents as self‐limiting chest pain within a week after the second dose. We present a case of myocarditis following vaccination with tozinameran (BNT162b2, Pfizer‐BioNTech), which presented late, with ventricular tachycardia (VT) reduced left ventricular ejection fraction (LVEF).
Keywords: acute myocarditis, COVID‐19, mRNA vaccine, ventricular tachycardiac
Abbreviations
- CMR
cardiovascular magnetic resonance imaging
- COVID‐19
coronavirus disease 2019
- ECG
electrocardiogram
- LGE
late gadolinium enhancement
- LVEF
left ventricular ejection fraction
- mRNA
messenger ribonucleic acid
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- PCR
polymerase chain reaction
- TTE
transthoracic echocardiogram
- VT
ventricular tachycardia.
1. INTRODUCTION
The Coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has been a worldwide pandemic since end‐2019, with more than 200 million cases reported by early August 2021, accounting for more than 4 million deaths to date. 1 Population vaccination has been the strategy being employed by most countries to combat the pandemic. More than 4 billion doses of COVID‐19 vaccines have been administered at the time of writing, with the messenger ribonucleic acid (mRNA) vaccine tozinameran (BNT162b2, Pfizer‐BioNTech, Mainz, Germany) being one of the most used vaccines worldwide due to its proven efficacy. 2 , 3 However, the use of mRNA COVID‐19 vaccines has been reported to be associated with the development of myocarditis. 4 , 5 , 6 , 7 We report a case of sustained ventricular tachycardia (VT) secondary to acute myocarditis following COVID‐19 vaccination in a young adult male.
2. CASE REPORT
A 26‐year‐old Chinese male with no significant past medical history presented to his primary care physician, with a 1‐day history of epigastric discomfort and general malaise. Prior to this, he had completed his COVID‐19 vaccination with the tozinameran mRNA vaccine, receiving his first and second doses 22 days apart. He had refrained from exercise for a week after his second dose, only returning to his usual running after that. He was able to complete his usual 8 km jogging route on 14 days after his second dose but was only able to manage 4 km on 2 days after that, limited by fatigue. He saw his primary care physician 18 days after his second dose for nonspecific symptoms. At his primary care clinic, he was found to be tachycardic at 170 bpm and was promptly referred to the emergency department.
At the emergency department, patient was noted to be tachycardiac with heart rate of about 170 bpm. Blood pressure was 98/73 mmHg. Patient was still alert and physical examination was unremarkable apart from tachycardia. Initial electrocardiogram (ECG) showed a regular, wide complex tachycardia at a rate of 173 bpm, with atrioventricular dissociation. QRS duration was 150 ms, with a right bundle branch block (RBBB) pattern in lead V1. Right axis deviation was noted (Figure 1).
FIGURE 1.
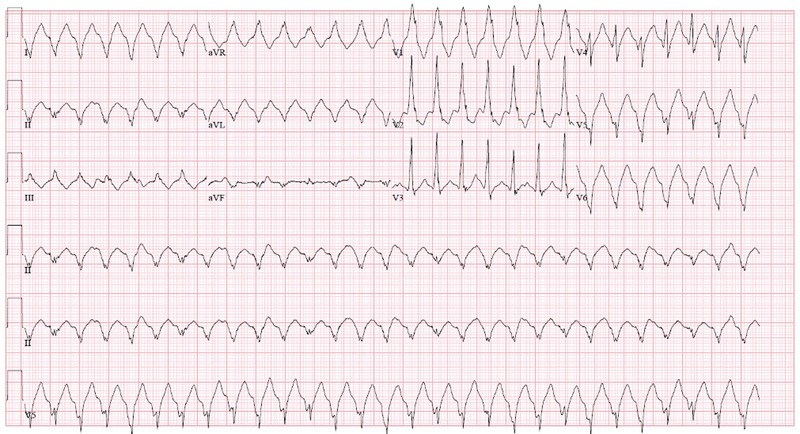
ECG at presentation, showing VT with ventricular rate of 173 beats per minute (bpm) with RBBB pattern (QRS duration 150 ms). The QRS axis was right superior. Atrioventricular dissociation was noted. ECG, electrocardiogram, RBBB, right bundle branch block, VT, ventricular tachycardia [Colour figure can be viewed at wileyonlinelibrary.com]
He was initially treated for presumed fascicular VT by the ED, receiving a total of 20 mg intravenous (IV) verapamil in four divided doses without successful cardioversion. Subsequently, a bolus dose of IV amiodarone 300 mg was successful in terminating the VT, achieving sinus rhythm (Figure 2). This ECG done in sinus rhythm demonstrated incomplete RBBB (QRS duration 118 ms) with right axis deviation. There were wide‐spread T wave inversions, likely as a result of recent tachyarrhythmia. His troponin‐I level was elevated at 2475 ng/dL (upper limit normal 17.4 ng/L) and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was also increased at 1884 pg/mL (upper limit of normal 63 pg/mL). His other initial laboratory investigations including complete blood count and renal panel were unremarkable. Transthoracic echocardiogram (TTE) on day of admission showed normal cardiac chamber sizes. However, left ventricular ejection fraction (LVEF) was reduced at 37% and moderate right ventricular dysfunction was seen. A cardiovascular magnetic resonance imaging (CMR) scan was performed 2 days after his VT episode. This documented subepicardial late gadolinium enhancement (LGE) at the LV apex extending to the inferior and lateral left ventricular wall (Figure 3). There was also focal limited LGE of the mid RV inferior wall with associated hypokinesia and myocardial edema. This CMR, which was done 2 days after his initial TTE, showed significant recovery of LVEF to 66%, with right ventricular ejection fraction quantified at 47% (mildly reduced) (Figure 3).
FIGURE 2.
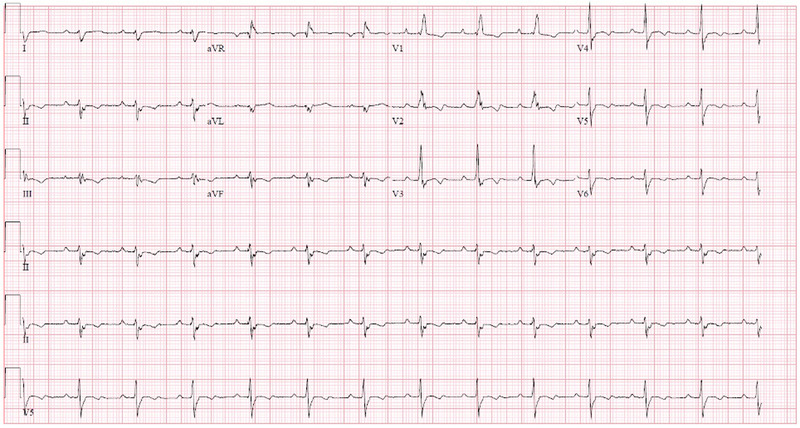
Electrocardiogram (ECG) following administration of intravenous amiodarone. Patient was in sinus rhythm, with incomplete right bundle branch block seen (QRS duration 118 ms). Wide‐spread T‐wave inversions seen [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
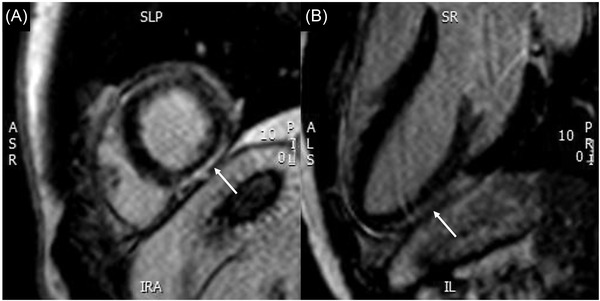
CMR with LGE, showing epicardial LGE of inferolateral wall (white arrows) in the short axis view (panel A) and in the left ventricular outflow tract view (panel B). CMR, cardiovascular magnetic resonance imaging; LGE, late gadolinium enhancement
Extensive viral serology tests, including the COVID‐19 polymerase chain reaction (PCR) test, as well as autoimmune screen were all returned negative. He was given a final diagnosis of myocarditis complicated by VT, secondary to COVID‐19 vaccination with tozinameran. During his 5‐day index admission, he remained hemodynamically stable with no further recurrence of VT. He was discharged with low‐dose beta blocker bisoprolol at 1.25 mg daily.
However, he presented again 8 days after his discharge, due to recurrent palpitations. He was found to have frequent nonsustained ventricular VT on telemetry monitoring and received an implantable cardiac defibrillator. During the defibrillation implantation procedure, endomyocardial biopsy was performed for the patient at the same setting. Histology of the myocardial tissue revealed focal hypertrophy of cardiomyocytes, with interstitial fibrosis. Immunohistochemistry with CD3 showed isolated T lymphoid cells while CD163 highlighted scattered histiocytes. This was consistent with recent/resolved myocarditis.
To our knowledge, this is the first case of myocarditis related to COVID‐19 mRNA vaccination that has presented with VT.
3. DISCUSSION
There have been reports of an association between COVID‐19 mRNA vaccines and myocarditis, primarily among young males within a few days after the second vaccination dose. 4 , 5 , 6 , 7 , 8 Based on these reports, myocarditis in this group tends to present with chest pain and were mostly mild in terms of clinical severity, with most of the patients having preserved LVEF on echocardiogram. They also tended to present within a week of the second vaccine dose. According to the United States Centers for Disease Control and Prevention (CDC), the estimated incidence of myocarditis or pericarditis following mRNA COVID‐19 vaccine administration was around 12.6 per million doses of second‐dose mRNA vaccine, among individuals between 12 and 39 years of age. 8 They most commonly present 2–3 days following their second dose of vaccine.
Our patient presented with VT 18 days after receiving his second dose of COVID‐19 vaccine, which was later than most reported cases of postvaccine myocarditis. Moreover, his clinical presentation was atypical. His early phase of myocarditis was likely asymptomatic, with none of the usual chest pain complaints. However, both myocardial inflammation and subsequent myocardial scarring can be milieus for ventricular arrhythmias. At the time of our patient's presentation, the inflammatory phase of myocarditis would have passed. This initial inflammation, however, resulted in residual myocardial scarring. This scarring process was detected as LGE on the CMR, fulfilling the revised Lake Louise diagnostic criteria for myocarditis. 9 His positive CMR, together with the initial symptoms, elevated cardiac troponin levels and lack of other identifiable cause for these findings, fulfilled the CDC criteria for confirmed myocarditis following COVID‐19 vaccination. 10 The right superior axis, QS pattern in lateral leads with an atypical RBBB morphology in V1 suggests that the VT was originating from the inferolateral left ventricular wall. This correlated well with the site of delayed gadolinium enhancement on CMR. The mechanism of his monomorphic VT is likely to be scar related re‐entry.
4. CONCLUSION
Acute myocarditis is increasingly being recognized as a side effect of mRCA COVID‐19 vaccination, especially in younger males. While usually presenting early with chest pain, we report a late presentation of potentially life‐threatening VT. This may be due to the myocardial inflammation and edema early in the course of myocarditis, or scar related later on in the disease process, in the case of our patient. With more younger individuals expected to receive their mRNA vaccines in the coming months, heightened vigilance for this serious complication would be prudent.
DISCLOSURES
None for all authors.
Lin W, Yip ACL, Evangelista LKM, et al. Ventricular tachycardia from myocarditis following COVID‐19 vaccination with tozinameran (BNT162b2, Pfizer‐BioNTech). Pacing Clin Electrophysiol. 2022;00 1–4. 10.1111/pace.14486
Toon Wei Lim and Devinder Singh cosupervised this manuscript.
REFERENCES
- 1. World Health Organization (WHO) . COVID‐19 Weekly Epidemiological Update Edition 52, published 10 August 2021. Accessed August 16, 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐10‐august‐2021.
- 2. World Health Organization (WHO) . WHO Coronavirus (COVID‐19) Dashboard. Accessed August 16, 2021. https://covid19.who.int.
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA‐1273 vaccination. Circulation. 2021;144:506–508. 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol. 2021:e212833. 10.1001/jamacardio.2021.2833. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickey JB, Albert E, Badr M, et al. A series of patients with myocarditis following SARS‐CoV‐2 vaccination with mRNA‐1279 and BNT162b2. JACC Cardiovasc Imaging. 2021;14:1862–1863. 10.1016/j.jcmg.2021.06.003. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID‐19 vaccination. Circulation. 2021;144:502–505. 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID‐19 mRNA vaccines. Circulation. 2021;144:471–484. 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira VM, Schulz‐Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC) . Use of mRNA COVID‐19 Vaccine after Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices – United States, June 2021. Accessed August 16, 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7027e2.htm. [DOI] [PMC free article] [PubMed]


