Abstract
Objectives
Home diagnostics are essential to assist members of the general population become active agents of case detection. In Indonesia, a country with an over‐burdened healthcare system, individuals could use rapid SARS‐CoV‐2 antigen tests to self‐detect COVID‐19. To assess the general population's values and attitudes towards SARS‐CoV‐2 self‐testing, a survey was conducted in mid‐2021 in Jakarta and the provinces of Banten and North Sulawesi.
Methods
This was a quantitative survey that approached respondents in >600 randomly selected street‐points in the three study geographies in July–August 2021. A 35‐item questionnaire was used to collect data on key variables, such as likelihood to use a SARS‐CoV‐2 self‐test, willingness to pay for a self‐test device, and likely actions following a positive self‐test result. Bivariate and multivariate regression analyses were performed.
Results
Of 630 respondents (318 were female), 15.53% knew about COVID‐19 self‐testing, while 62.70% agreed with the idea of people being able to self‐test at home, unassisted, for COVID‐19. If self‐tests were available in Indonesia, >60% of respondents would use them if they felt it necessary and would undertake regular self‐testing for example weekly if recommended. Upon receiving a positive self‐test result, most respondents would communicate it (86.03%), request post‐test counselling (80.79%), self‐isolate (97.46%), and/or warn their close contacts (90.48%).
Conclusions
The use of rapid SARS‐CoV‐2 antigen detection tests for self‐testing appears acceptable to a majority of the Indonesian public, to learn whether they have COVID‐19. Self‐testing should be prioritised to complement to an over‐burdened healthcare system by helping the public, asymptomatic individuals included, become agents of change in epidemiological surveillance of SARS‐CoV‐2 in their communities.
Keywords: COVID‐19, home diagnostics, Indonesia, SARS‐CoV‐2 testing, self‐testing, survey
INTRODUCTION
In December 2019, the first case of a person infected by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was reported in Wuhan, China [1]. WHO declared this disease to be a pandemic in March 2020 [2], by which time Indonesia was among the most affected countries [3]. As of 13 February 2022, this nation had dealt with over 4,708,043 confirmed cases, with close to 145,000 deaths attributable to coronavirus disease 2019 (COVID‐19) [4].
In an effort to control the pandemic in Indonesia, country‐wide, free‐of‐charge screening interventions to detect new cases of COVID‐19 were implemented by the Jaminan Kesehatan Nasional (national health insurance) [5]. Testing for SARS‐CoV‐2 infection in laboratories and clinical settings was scaled up to include individuals with COVID‐19‐compatible symptoms and close contacts of confirmed cases [5, 6]. However, systematic screening for COVID‐19 cases requires a significant investment in human resources and diagnostic technology that Indonesia cannot afford. Additionally, even if provided free of charge by the national health authorities, some people cannot still access testing due to long distances to health facilities, transport costs, or conflicting work schedules.
Those with impediments to access health provider‐initiated testing have the possibility to purchase saliva‐based rapid SARS‐CoV‐2 antigen detection tests (RADT) in some private pharmacies [7, 8]. The concept of self‐testing is not novel; diagnostics for the home detection of infectious diseases such as human immunodeficiency virus (HIV) have been available since the mid‐1990s [9].
Self‐testing devices for private, home‐based detection of SARS‐CoV‐2 have been promoted to increase case detection while allowing the healthcare system to prioritise testing persons with COVID‐19‐compatible symptoms and their contacts [10]. With the aim to have SARS‐CoV‐2 self‐testing as a complement to health provider‐initiated testing to break the chain of transmission, countries such as India [11] or the United States [12] approved their use during 2021. At the start of 2022, following the surge of the Omicron variant of concern, middle‐income countries such as Peru [13], or Brazil [14] have decided to approve their use. In Indonesia, despite their availability in private retailers, self‐testing is not yet part of the national testing strategy.
To ensure that SARS‐CoV‐2 self‐testing in Indonesia has an impact on case detection, reduction of clinic workloads, and reduction of COVID‐19 morbimortality, society‐grounded strategies are necessary to introduce this innovative approach to the public in such a way that isolation, contact tracing, and effective requests for confirmatory testing and further clinical care can occur following any positive result. Therefore, information about the Indonesian public's values around SARS‐CoV‐2 self‐testing is required. To address this, a survey was conducted to assess general population's values and attitudes towards self‐testing. Other objectives of this survey were to understand the population's likelihood of using self‐testing, willingness to pay for self‐testing, and likely actions to be taken upon a reactive result.
METHODS
Design, population, and sites
This was a cross‐sectional, population‐based survey conducted in mid‐2021. At the time the survey was conducted, the incidence curve was flattening, with 37,284 confirmed cases the day the survey started (31st July), and 17,384 confirmed cases the day the survey ended (16th August) [4]. During the survey period, there were 29,622, 13,504, and 5965 confirmed cases in Jakarta, Banten, and North Sulawesi, respectively [15, 16].
The survey population was the general population of three geographies in Indonesia: the capital city of Jakarta, and the provinces of Banten (Java) and North Sulawesi (Celebes). These geographies were selected in consideration of the regions where the implementing organisation (Peduli Hati Bangsa) was authorised to operate. Among the catchment areas of this non‐profit organisation, Jakarta was selected to represent urban dwellers' views, while Banten and North Sulawesi were selected to represent rural dwellers' views.
The eligibility criteria were that participants were ≥18 years old, willing to provide informed consent, and free of symptoms compatible with COVID‐19 disease. To understand whether differences based on geographical location influenced the acceptability of SARS‐CoV‐2 self‐testing, sample size calculations were performed separately for each site. It was estimated that 196 or more respondents at each of the three sites, for a total of 588, would be necessary to have a confidence level of 95%, so that the real value (acceptability of COVID‐19 self‐testing) was within ±7% of the measured value.
Sampling and recruitment
A multi‐staged sampling process was applied. First, the boundary of each study geography was defined using Google MyMaps®. Once defined, each site map was divided into 40 areas of similar width, which were numbered from 1 to 40. Second, using a random number generator (RANDOM.ORG®), the three lists of 40 areas were randomly reordered and the first 14 areas in the newly arranged lists were selected as recruitment areas. Third, in each of the 14 areas, 21 randomly selected street‐points were manually marked. These street‐points were where the study staff would be stationed to recruit respondents. Subsequently, using RANDOM.ORG®, the three lists of 14 areas were reordered again to determine the sequence that the surveyors would follow when visiting each area. The areas were assigned, in exactly the same order in which they appeared after this new randomisation, to a survey calendar (one per geography), which included 7‐morning shifts and 7‐afternoon shifts.
When conducting the survey, pairs of surveyors arrived at the area assigned in their respective schedules and used ViewRanger® to guide them to each street‐point. The surveyors attempted to recruit just one respondent at each street‐point, by stopping the first passer‐by they saw and inviting them to participate. If the person declined to participate, the surveyors had to wait 3 min before stopping a new passer‐by. If a person agreed to participate, they were asked to provide informed consent before data collection.
Data collection and analysis
Informed consent was obtained and data were collected either on‐the‐spot where privacy could be guaranteed or, if necessary, in a nearby site of the respondent's choice.
A 35‐item structured questionnaire was used; the questionnaire was informed by a previous assessment of communities' values and preferences for hepatitis C virus self‐testing carried out by FIND, the global alliance for diagnostics [15, 16]. The questionnaire included items on respondents' socio‐demographics; perception of risk of COVID‐19; experiences with conventional COVID‐19 testing; knowledge of other self‐test kits; likelihood‐to‐use a SARS‐CoV‐2 self‐test; willingness‐to‐pay for a SARS‐CoV‐2 self‐test; and likely actions after self‐testing for SARS‐CoV‐2 [17]. This questionnaire was designed in English, translated into Indonesian, and pre‐piloted in Jakarta in the premises of Peduli Hati Bangsa. The finalised questionnaire was developed using the web‐based data‐collection form builder KoBoToolbox®, re‐tested, and deployed in the KoBoCollect® app.
Statistical analyses were conducted using STATA® v.14. As proxies for the acceptability of self‐testing, the primary outcomes of the analyses were: likelihood to use self‐testing, willingness to pay for a self‐testing device (i.e., maximum amount respondents would pay, in local currency), and intention to comply with expected COVID‐19 prevention behaviours following a reactive self‐test result. Associations between respondents' sociodemographic characteristics, risk perception, previous testing experience, and primary outcomes of interest were explored. Descriptive statistics were run. Frequencies and percentages are used to report findings for analyses of categorical variables. Mean (standard deviation) or median (interquartile range) are used to report measures of central tendency or dispersion. Univariate analyses were run using t‐tests or ANOVA, where appropriate. Self‐isolate, warn close contacts, report the result, and request post‐test counselling.
Bivariate and multivariate regression analyses were performed for each of the three outcomes of interest. The variables found by the bivariate analyses as significantly associated with the outcomes at a p‐value <0.05 were considered for the multivariate analyses. An ordinal logistic regression model (Odd's ratio for bivariate analysis, adjusted Odd's ratio for multivariate analysis) was used to identify associations between binary responses to outcomes on likelihood to self‐test (likely/unlikely), willingness to pay (any amount/no amount), and potential predictors. An ordinary least squares (OLS) regression was used to identify potential predictors of compliance with four expected COVID‐19 prevention behaviours following a reactive self‐test.
Ethics considerations
All respondents provided written informed consent to participate. Respondents received no incentive, other than a small bag containing face masks and hand sanitiser. The survey protocol received ethical clearance from the Universitas Katolik Indonesia Atma Jaya (Ref.: 0674A/III/LPPM‐PM.10.05/06/2021). To safeguard respondents' and surveyors' safety, the survey conduct complied with national policies and health authorities' recommendations (e.g., maintain 1.5–2 metres distance) to prevent the spread and acquisition of SARS‐CoV‐2 in the course of social interactions.
RESULTS
Respondents' characteristics
There was a total of 630 respondents (210 in each study setting) (Table 1). No respondent opted out of the study. Of the total, 50.47% were female. Their median age was 36 (standard deviation (SD) = 12.542) years, with just 10.63% of them aged ≥56 years. Java (16.35%), Minahasa (23.02%), and Sunda (33.02%) were the ethnicities most represented in the sample.
TABLE 1.
Respondents' age, education, and employment status, by sex and location
| Rural (Banten, North Sulawesi) | Urban (Jakarta) | Sub‐total (Rural and urban) | Total (N = 630) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Banten (13,504 confirmed cases) a | North Sulawesi (5965 confirmed cases) a | Jakarta (29,633 confirmed cases) a | Rural & Urban | ||||||
| Female (n = 106) | Male (n = 104) | Female (n = 109) | Male (n = 101) | Female (n = 103) | Male (n = 107) | Female (n = 318) | Male (n = 312) | ||
| Mean age in years (standard deviation) | 38.53 (12.37) | 35.66 (11.90) | 41.14 (13.89) | 42.71 (14.65) | 38.19 (11.73) | 37.69 (10.99) | 39.31 (12.75) | 38.64 (12.87) | 37.41 (12.54) |
| Age range | |||||||||
| 18–35 | 44 (41.51) | 61 (58.65) | 41 (37.61) | 34 (33.66) | 44 (42.72) | 45 (42.06) | 129 (40.57) | 140 (44.87) | 269 (42.70) |
| 36–55 | 53 (50.00) | 35 (33.65) | 55 (50.46) | 45 (44.55) | 53 (51.46) | 53 (49.53) | 161 (50.63) | 133 (42.63) | 294 (46.67) |
| ≥56 | 9 (8.49) | 8 (7.69) | 13 (11.93) | 22 (21.78) | 6 (5.83) | 9 (8.41) | 28 (8.81) | 39 (12.50) | 67 (10.63) |
| Education | |||||||||
| None | 2 (1.89) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (2.91) | 0 (0.00) | 5 (1.57) | 0 (0.00) | 5 (0.79) |
| Primary | 38 (35.85) | 23 (22.12) | 12 (11.01) | 7 (6.93) | 21 (20.39) | 13 (12.15) | 71 (22.33) | 43 (13.78) | 114 (18.10) |
| Secondary | 60 (56.85) | 66 (63.46) | 81 (74.31) | 79 (78.22) | 56 (54.37) | 66 (61.68) | 197 (61.95) | 211 (67.63) | 408 (64.76) |
| College/vocational | 4 (3.77) | 8 (7.69) | 3 (2.75) | 3 (2.97) | 8 (7.77) | 8 (7.48) | 15 (4.72) | 19 (6.09) | 34 (5.40) |
| Degree | 2 (1.89) | 5 (4.81) | 12 (11.01) | 11 (10.89) | 12 (11.65) | 18 (16.82) | 26 (8.18) | 34 (10.90) | 60 (9.52) |
| Post‐graduate | 0 (0.00) | 1 (0.96) | 1 (0.92) | 1 (0.99) | 0 (0.00) | 2 (1.87) | 1 (0.31) | 4 (1.28) | 5 (0.79) |
| Quranic education | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.97) | 0 (0.00) | 1 (0.31) | 1 (0.32) | 2 (0.32) |
| Other | 0 (0.00) | 1 (0.96) | 0 (0.00) | 0 (0.00) | 2 (1.94) | 0 (0.00) | 2 (0.63) | 0 (0.00) | 2 (0.32) |
| Employment status | |||||||||
| Unemployed | 10 (9.43) | 18 (17.31) | 8 (7.34) | 6 (7.23) | 5 (4.85) | 5 (4.67) | 23 (7.23) | 29 (9.29) | 52 (8.25) |
| Student | 47 (44.34) | 0 (0.00) | 59 (54.13) | 0 (39.62) | 20 (19.42) | 0 (0.00) | 126 (39.62) | 0 (0.00) | 126 (20.00) |
| Employed, part‐time | 3 (2.83) | 27 (25.96) | 9 (8.26) | 46 (9.75) | 19 (18.45) | 31 (28.97) | 31 (9.75) | 104 (33.33) | 135 (21.43) |
| Employed, full‐time | 12 (11.32) | 29 (27.88) | 6 (5.50) | 14 (11.95) | 20 (19.42) | 52 (48.60) | 38 (11.95) | 95 (30.45) | 133 (21.11) |
| Self‐employed, part‐time | 9 (8.49) | 8 (7.69) | 13 (11.93) | 15 (11.64) | 15 (14.56) | 9 (8.41) | 37 (11.64) | 32 (10.26) | 69 (10.95) |
| Self‐employed, full‐time | 25 (23.58) | 22 (21.15) | 14 (12.84) | 15 (19.50) | 23 (22.33) | 10 (9.35) | 62 (19.50) | 47 (15.06) | 109 (17.30) |
| Retired, on a pension | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (0.31) | 1 (0.97) | 0 (0.00) | 1 (0.31) | 5 (1.60) | 6 (0.95) |
Number of confirmed cases during the survey period (31st July–16th August) in the setting, as per [Ref [15]].
Most respondents (64.76%) had completed secondary school. Completion of university studies varied from as high as 16.82% (n = 18/107) among males in Jakarta, to 6.51% (n = 14/215) among females in the provinces. More than two thirds of the sample were employed full‐time (38.41%) or part‐time (32.38%). The largest proportion of unemployment was found among male respondents in the rural geographies (n = 24/205, 11.71%), with the lowest among male respondents in Jakarta (n = 5/107, 4.67%).
Experience with COVID‐19 testing
More urban respondents reported that they felt at high and moderate risk of COVID‐19 than rural respondents (n = 113/210, 53.80% vs. n = 98/420, 23.33%) (Table 2). Thirty‐four respondents (26 of them from Jakarta) reported that they had COVID‐19 (Table 2). There were 10.79%, 12.53%, and 21.11% of respondents, respectively, who perceived that they were living with people with chronic disease, children, or elders at increased risk of COVID‐19.
TABLE 2.
Respondents' perceived access to and utilisation of COVID‐19 testing
| Rural (Banten, North Sulawesi) | Urban (Jakarta) | Sub‐total (Rural and urban) | Total (N = 630) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Banten (13,504 confirmed cases) a | North Sulawesi (5965 confirmed cases) a | Jakarta (29,633 confirmed cases) a | Rural & Urban | ||||||
| Female (n = 106) | Male (n = 104) | Female (n = 109) | Male (n = 101) | Female (n = 103) | Male (n = 107) | Female (n = 318) | Male (n = 312) | ||
| Feeling at risk | |||||||||
| No risk | 10 (9.43) | 13 (12.50) | 7 (6.42) | 2 (1.98) | 7 (6.80) | 9 (8.41) | 24 (7.55) | 24 (7.69) | 48 (7.62) |
| Low risk | 46 (43.40) | 31 (29.81) | 24 (22.02) | 20 (19.80) | 14 (13.59) | 10 (9.35) | 84 (26.42) | 61 (19.55) | 145 (23.02) |
| Mild risk | 28 (26.42) | 36 (34.62) | 51 (46.79) | 54 (53.47) | 29 (28.16) | 28 (26.17) | 108 (33.96) | 118 (37.82) | 226 (35.87) |
| Moderate risk | 17 (16.04) | 17 (16.35) | 12 (11.01) | 14 (13.86) | 21 (20.39) | 20 (18.69) | 50 (15.72) | 51 (16.35) | 101 (16.03) |
| High risk | 5 (4.72) | 7 (6.73) | 15 (13.76) | 11 (10.89) | 32 (31.07) | 40 (37.38) | 52 (16.35) | 58 (18.59) | 110 (17.46) |
| Household members | |||||||||
| Children only | 13 (12.26) | 10 (9.62) | 14 (12.84) | 9 (8.91) | 2 (1.90) | 1 (0.90) | 29 (9.12) | 20 (6.41) | 49 (7.78) |
| Children and elders | 13 (12.26) | 2 (1.92) | 0 (0.00) | 4 (3.96) | 0 (0.00) | 0 (0.00) | 13 (4.09) | 6 (1.92) | 19 (3.02) |
| Children, elders and chronic disease (CD) | 5 (4.72) | 5 (4.81) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (1.57) | 5 (1.60) | 10 (1.59) |
| Elders only | 14 (13.21) | 11 (10.58) | 17 (15.60) | 13 (12.87) | 8 (7.80) | 6 (5.60) | 39 (12.26) | 30 (9.62) | 69 (10.95) |
| Elders and CD | 20 (18.87) | 11 (10.58) | 2 (1.83) | 1 (0.99) | 0 (0.00) | 1 (0.90) | 22 (6.92) | 13 (4.17) | 35 (5.56) |
| CD only | 2 (1.89) | 4 (3.85) | 4 (3.67) | 6 (5.94) | 4 (3.90) | 2 (1.90) | 10 (3.14) | 12 (3.85) | 22 (3.49) |
| Children and CD | 0 (0.00) | 0 (0.00) | 1 (0.92) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.31) | 0 (0.00) | 1 (0.16) |
| Previously had COVID‐19 | |||||||||
| Yes, confirmed by test | 3 (2.83) | 2 (1.92) | 2 (1.83) | 0 (0.00) | 4 (3.88) | 9 (8.41) | 9 (2.83) | 11 (3.53) | 20 (3.17) |
| Yes, confirmed by a healthcare worker | 0 (0.00) | 1 (0.96) | 0 (0.00) | 0 (0.00) | 6 (5.83) | 7 (6.54) | 6 (1.89) | 8 (2.56) | 14 (2.22) |
| Feeling they cannot access testing when needed | |||||||||
| Never | 102 (96.23) | 100 (96.15) | 105 (96.33) | 90 (89.11) | 97 (94.17) | 103 (96.26) | 304 (95.60) | 293 (93.91) | 597 (94.76) |
| At least once | 3 (3.77) | 3 (3.84) | 4 (3.64) | 9 (10.99) | 6 (5.83) | 4 (3.74) | 13 (4.09) | 16 (5.13) | 29 (4.60) |
| Previously tested for COVID‐19 | |||||||||
| Never | 76 (71.70) | 64 (61.54) | 88 (80.73) | 78 (77.23) | 54 (52.43) | 49 (45.79) | 218 (68.55) | 191 (61.22) | 409 (64.92) |
| At least once | 30 (28.30) | 40 (38.46) | 21 (19.27) | 23 (22.77) | 49 (47.57) | 58 (54.21) | 99 (31.13) | 121 (38.78) | 220 (34.92) |
| For the respondents who had ever tested for COVID‐19 (n=) | 29 | 40 | 21 | 23 | 49 | 58 | 99 | 121 | 220 |
| Months ago (mean, SD) | 3.241 (0.87) | 3.19 (0.92) | 3.19 (0.87) | 3.30 (0.86) | 3.1 (3.57) | 3.1 (3.37) | 2.8 (3.21) | 2.5 (2.78) | 2.6 (2.98) |
| – | |||||||||
| Very convenient | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.04) | 2 (3.45) | 1 (1.01) | 2 (1.65) | 3 (1.36) |
| Convenient | 15 (51.72) | 25 (62.50) | 10 (47.62) | 13 (56.52) | 11 (22.45) | 26 (44.83) | 36 (36.36) | 64 (52.89) | 100 (45.45) |
| Neutral | 6 (20.69) | 5 (12.50) | 5 (23.81) | 4 (17.39) | 7 (14.29) | 17 (29.31) | 18 (18.18) | 26 (21.49) | 44 (20.00) |
| Inconvenient | 8 (27.59) | 9 (22.50) | 6 (28.57) | 6 (26.09) | 26 (53.06) | 11 (18.97) | 40 (40.40) | 26 (21.49) | 66 (30.00) |
| Very inconvenient | 0 (0.00) | 1 (2.50) | 0 (0.00) | 0 (0.00) | 4 (8.16) | 2 (3.45) | 4 (4.04) | 3 (2.48) | 7 (3.18) |
| – | |||||||||
| Result in less than 1 h | 5 (17.24) | 11 (27.50) | 13 (61.90) | 15 (65.22) | 8 (16.33) | 12 (20.69) | 26 (26.26) | 38 (31.40) | 64 (29.09) |
| Result the same day | 20 (68.97) | 21 (52.50) | 2 (9.52) | 5 (21.74) | 27 (55.10) | 25 (43.10) | 49 (49.49) | 51 (42.15) | 100 (45.45) |
| The following day | 1 (3.45) | 1 (2.50) | 1 (4.76) | 0 (0.00) | 7 (14.29) | 13 (22.41) | 9 (9.09) | 14 (11.57) | 23 (10.45) |
| Two days later | 0 (0.00) | 1 (2.50) | 1 (4.76) | 0 (0.00) | 3 (6.12) | 2 (3.45) | 4 (4.04) | 3 (2.48) | 7 (3.18) |
| 3–7 days later | 3 (10.34) | 3 (7.50) | 1 (4.76) | 1 (4.35) | 4 (8.16) | 5 (8.62) | 8 (8.08) | 9 (7.44) | 17 (7.73) |
| More than 1 week later | 0 (0.00) | 3 (7.50) | 3 (14.29) | 0 (0.00) | 0 (0.00) | 1 (1.72) | 3 (3.03) | 4 (3.31) | 7 (3.18) |
| Never received the result | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (8.70) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.65) | 2 (0.91) |
| – | |||||||||
| Paid for the test | 13 (44.83) | 17 (42.50) | 7 (33.33) | 4 (18.18) | 29 (59.18) | 36 (62.07) | 49 (49.49) | 57 (47.50) | 106 (48.40) |
| Payment, USD (median, IQR) | 12.25 (2.1) | 10.5 (1.75) | 17.5 (3.5) | 15.75 (3.5) | 11.2 (3.5) | 10.5 (4.55) | 12.25 (7) | 10.5 (2.1) | 10.5 (3.5) |
Number of confirmed cases during the survey period (31st July–16th August) in the setting, as per [15]. The First ‘–’ is “Perceived convenience of last test”, The Second ‘–’ is “Turnaround time of last test”, and The Third ‘–’ is “Payment done for last lest”.
74.82% (n=306/420) of rural respondents and 49.05% (n = 103/210) of urban respondents had never been tested for COVID‐19. Among those who had ever been tested (n = 219/630, 34.76%), the most recent test was an average of 2.64 (SD = 2.98) months ago. Of these, almost half (n = 103/219, 46.81%) rated their experience as convenient or very convenient; 164 (74.54%) received their result the same day; 113 (51.60%) received their test free of charge, and the remainder paid a median of 10.5 USD.
Regarding perceived ease of access to testing, 95.60% of female and 93.91% of male respondents stated they never felt as if they needed a test but could not access it. Of these, 34.17% reported having ever received a test.
Acceptability of COVID‐19 self‐testing
The respondents were asked to list the self‐test devices they knew. This was an open question. Pregnancy tests were mentioned by 81.65% of females and 61.05% of males. Knowledge of self‐testing devices for other infectious diseases was scarce, with devices for HIV (0.86%), malaria (0.15%), and syphilis (0.15%) being the most mentioned. 15.53% of the sample mentioned COVID‐19 self‐testing (Table 3).
TABLE 3.
Acceptability of self‐testing for COVID‐19 disease
| Rural (Banten, North Sulawesi) | Urban (Jakarta) | Sub‐total (Rural and urban) | Total (N = 630) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Banten (13,504 confirmed cases) a | North Sulawesi (5965 confirmed cases) a | Jakarta (29,633 confirmed cases) a | Rural & Urban | ||||||
| Female (n = 106) | Male (n = 104) | Female (n = 109) | Male (n = 101) | Female (n = 103) | Male (n = 107) | Female (n = 318) | Male (n = 312) | ||
| Previous knowledge of COVID‐19 self‐testing | 9 (4.24) | 14 | 0 (0.00) | 0 (0.00) | 32 (31.10) | 33 (30.80) | 41 (12.99) | 55 (18.17) | 96 (15.53) |
| Agreement with the concept of COVID‐19 self‐testing | 49 (46.23) | 61 (58.65) | 94 (86.24) | 81 (80.20) | 48 (46.60) | 62 (57.94) | 191 (60.06) | 204 (65.38) | 395 (62.70) |
| Likelihood of using self‐testing | |||||||||
| Very unlikely | 3 (2.83) | 3 (2.88) | 2 (1.83) | 0 (0.00) | 4 (3.88) | 3 (2.80) | 10 (3.14) | 6 (1.92) | 16 (2.54) |
| Unlikely | 40 (37.74) | 31 (29.81) | 6 (5.50) | 3 (2.97) | 28 (27.18) | 21 (19.63) | 74 (23.27) | 55 (17.63) | 129 (20.48) |
| Neutral | 13 (12.26) | 8 (7.69) | 12 (11.01) | 18 (17.82) | 27 (26.21) | 25 (23.36) | 52 (16.35) | 51 (16.35) | 103 (16.35) |
| Likely | 45 (42.45) | 56 (53.85) | 73 (66.97) | 63 (62.38) | 30 (29.13) | 39 (36.45) | 148 (46.54) | 158 (50.64) | 306 (48.57) |
| Very likely | 5 (4.72) | 6 (5.77) | 16 (14.68) | 17 (16.83) | 14 (13.59) | 19 (17.76) | 35 (11.01) | 42 (13.46) | 77 (12.22) |
| Average Likelihood (mean, SD) | 3.084 (1.05) | 3.29 (1.05) | 3.87 (0.79) | 3.93 (0.68) | 3.21 (1.108) | 3.47 (1.0843) | 3.39 (1.047) | 3.56 (.9932) | 3.48 (1.023) |
| Willing to serially test | |||||||||
| Yes | 43 (40.57) | 47 (45.19) | 77 (70.64) | 79 (78.22) | 65 (63.10) | 72 (67.30) | 185 (58.20) | 198 (63.50) | 383 (60.82) |
| No | 44 (41.51) | 44 (42.13) | 24 (22.02) | 11 (10.89) | 33 (32.04) | 28 (26.17) | 101 (31.76) | 83 (26.60) | 184 (29.21) |
| For the respondents willing to pay for a self‐test (n=) | 79 (74.53) | 75 (72.12) | 31 (28.44) | 29 (28.71) | 86 (83.49) | 91 (85.04) | 196 (61.63) | 195 (62.50) | 391 (62.06) |
| Maximum acceptable payment in USD (median, IQR) | 1.05 (1.05) | 1.4 (0.7) | 1.75 (2.45) | 2.45 (2.1) | 3.5 (3.5) | 2.1 (2.1) | 1.4 (2.55) | 1.75 (2.45) | 1.4 (2.45) |
Number of confirmed cases during the survey period (31st July–16th August) in the setting, as per [15].
Almost two in three respondents (62.70%, 95% Confidence Interval [CI]: 0.58–0.66) agreed with the idea or concept of people being able to self‐test at home for COVID‐19. Agreement with the concept of self‐testing was slightly lower in Jakarta than in the rural geographies for both females (57.94%, CI: 0.48–0.66 vs. 69.27%, CI: 0.62–0.75, respectively) and males (46.60%, CI: 0.37–0.56 vs. 66.51%, CI: 0.59–0.72, respectively).
If freely available and recommended by health authorities, 60.82% (CI: 0.56–0.64) of respondents were willing to test weekly. The likelihood to use a self‐test when needed rated highly, with an average rating of 3.48/5 (SD = 1.023) in total, and 3.39/5 (SD = 1.047) for females and 3.56/5 (SD = 0.9932) for males. Overall, if self‐testing were available, 12.22% (CI: 0.09–0.15) and 48.57% (CI: 0.44–0.52) of respondents would be very likely or likely, respectively, to use them if they felt it necessary; of these, the majority (n = 281/383, 73.36%, CI: 0.68–0.77) were from the rural geographies. While only 42.72% (n = 44/103, CI: 0.33–0.52) of females from Jakarta answered ‘very likely’ or ‘likely’ to use self‐testing, 69.27% (n = 142/205, CI: 0.62–0.75) of males in rural areas gave these responses.
As per the bivariate analyses (Figure 1a), potential predictive factors of likelihood to use self‐testing included living in a rural area, knowledge of pregnancy self‐tests, feeling at mild risk of COVID‐19, agreement with the concept of COVID‐19 self‐testing, being employed part‐time, and having completed any education above primary school. The multivariate model showed that rural respondents (adjusted odds ratio [AOR]: 3.33, CI: 2.18–5.08, p < 0.001), those having secondary education (AOR: 1.87, CI: 1.19–2.92, p < 0.006) or a college degree (AOR: 3.90, CI: 2.08–7.33, p < 0.001), and those working part‐time (AOR: 1.73, CI: 1.09–2.72, p < 0.018) had comparatively higher odds of using self‐testing (Figure 1b). People cohabiting with persons perceived to be at‐increased risk of COVID‐19 (AOR: 0.45, CI: 0.30–068, p < 0.001), and people who knew pregnancy kits (AOR 0.61, CI: 0.40–0.92, p < 0.020) had comparatively lower odds of using self‐testing.
FIGURE 1.
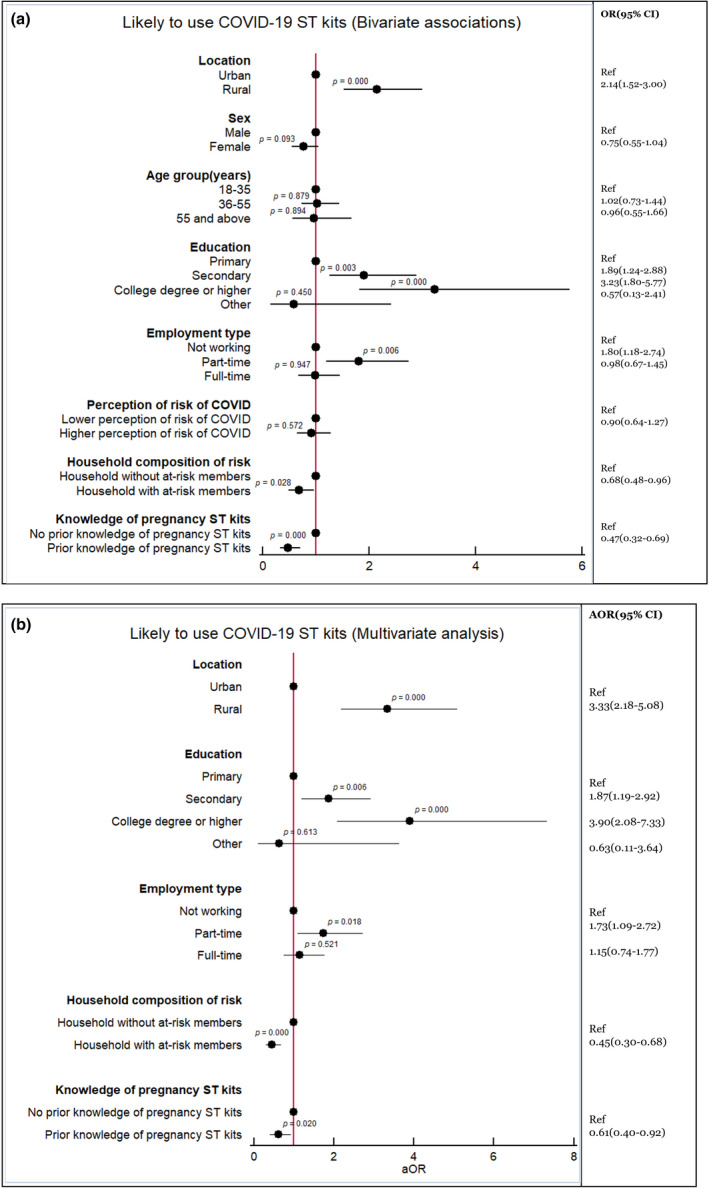
(a, b) Associations with likelihood to use self‐testing (Bivariate and Multivariate analyses)
If COVID‐19 self‐tests are not made available free‐of‐charge by health authorities, 62.06% (CI: 0.58–0.65) of respondents would be willing to pay for a self‐test if they needed it (i.e., a median of 1.4 USD (Interquartile range 2.45)) (Table 3). The bivariate associations showed that respondent characteristics such as urban location, age <35 years, having secondary education or a college degree, full‐time employment, and a higher perception of risk of COVID‐19 were potential predictors of willingness to pay for self‐testing devices (Figure 2a). The multivariate model confirmed that individuals aged 36–55 years (AOR: 0.58, CI: 0.39–0.86, p < 0.007) and >55 years (AOR: 0.032, CI: 0.16–0.061, p < 0.001) were less likely to pay for a self‐test compared with individuals aged <36 years. Rural residents were less likely to pay for self‐testing kits than urban residents (AOR: 0.26. CI: 0.16–0.41, p < 0.001). The respondents with a moderate to high perception of COVID‐19 risk were more likely to pay for a self‐test (AOR: 1.85, CI: 1.19–2.87, p < 0.006) (Figure 2b).
FIGURE 2.
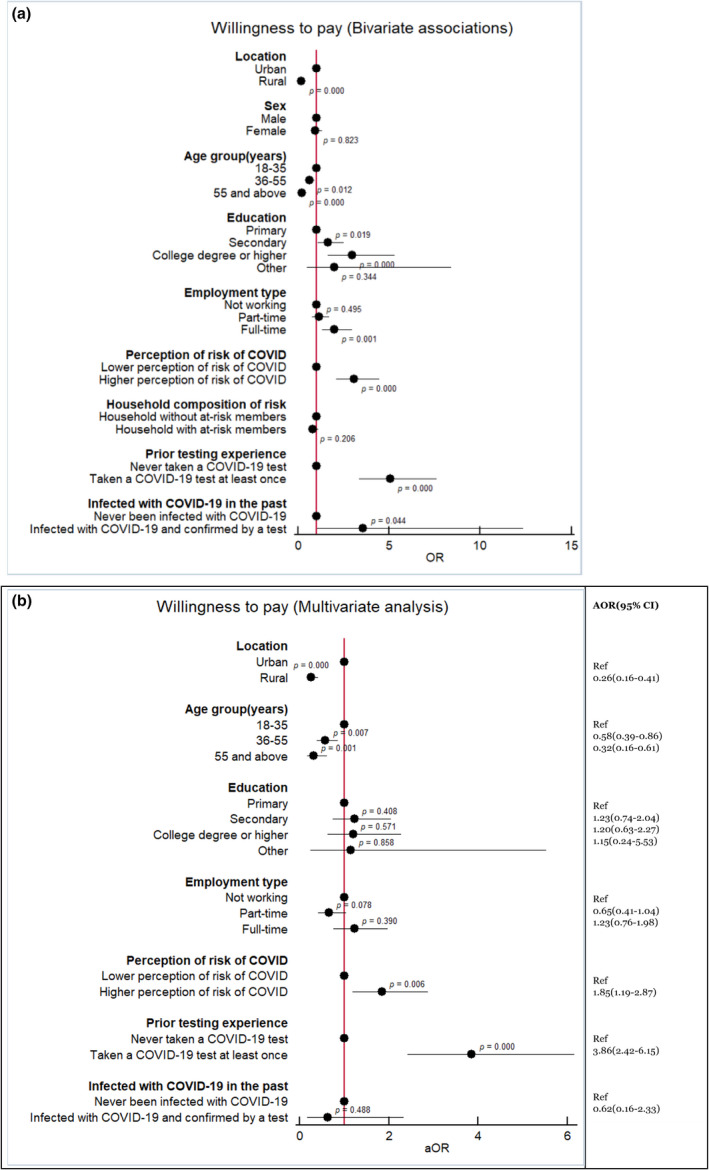
(a, b) Associations with willingness to pay for a self‐test device (Bivariate and Multivariate analyses)
Actions upon self‐testing for SARS‐CoV‐2
Respondents' preferred channels for reporting positive results were attending a clinic in person (81.05%) and use of community healthcare workers (60.99%). Just 6.53% of respondents stated that they would not report a positive result.
In the event that they had symptoms and knew they had been exposed to a COVID‐19 patient but their self‐test result was negative, the majority would not stop social distancing (only 13.81% would) or stop wearing masks (only 5.08% would). However, in this scenario, 74.60% would stop self‐isolating.
Most respondents stated that if they performed a self‐test and its result was positive, they would communicate the result (86.03%, CI: 0.83–0.88), visit a clinic to request post‐test counselling (80.79%, CI: 0.77–0.83), self‐isolate (97.46%, CI: 0.95–0.98), and warn their contacts (90.48%, CI: 0.87–0.92) (Table 4). The bivariate associations showed that respondents' characteristics such as urban location, being male, having a college degree or higher education, part‐time or full‐time employment, or higher perception of COVID‐19 risk were potential predictors of compliance with recommended measures following a positive self‐test (Figure 3a). The multivariate analysis confirmed that respondents from rural areas were 0.28 SD less likely to comply with expected actions than those from urban areas (Coefficient −0.29, CI: −0.44 to −0.14, p < 0.001). Similarly, part‐time employed individuals had higher odds to comply with expected actions after a positive self‐test in comparison to those where were unemployed (Coefficient 0.19, CI: −0.01 to 0.41, p < 0.10) (Figure 3b).
TABLE 4.
Actions following a SARS‐CoV‐2 self‐test
| Rural (Banten, North Sulawesi) | Urban (Jakarta) | Sub‐total (Rural and urban) | Total (N = 630) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Banten (13,504 confirmed cases) a | North Sulawesi (5965 confirmed cases) a | Jakarta (29,633 confirmed cases) a | Rural & Urban | ||||||
| Female (n = 106) | Male (n = 104) | Female (n = 109) | Male (n = 101) | Female (n = 103) | Male (n = 107) | Female (n = 318) | Male (n = 312) | ||
| Practices following receipt of a positive self‐test result | |||||||||
| Communicate the result to a clinic, hospital, and/or COVID hotline | 87 (82.08) | 88 (84.62) | 86 (78.90) | 84 (83.17) | 97 (94.17) | 100 (93.46) | 270 (84.91) | 272 (87.18) | 542 (86.03) |
| Go in person to a clinic or hospital to get post‐test counselling from a healthcare worker | 76 (71.70) | 73 (70.19) | 86 (78.90) | 91 (90.10) | 90 (87.38) | 93 (86.92) | 252 (79.25) | 257 (82.37) | 509 (80.79) |
| Self‐isolate | 105 (99.06) | 99 (95.19) | 103 (94.50) | 99 (98.02) | 102 (99.03) | 106 (99.07) | 310 (97.48) | 304 (97.44) | 614 (97.46) |
| Identify and warn close contacts | 88 (83.02) | 92 (88.46) | 90 (82.57) | 95 (94.06) | 102 (99.03) | 103 (96.26) | 280 (88.05) | 290 (92.95) | 570 (90.48) |
| Inform their employer (n = respondents employed) |
n = 49 28 (26.42) |
n = 86 63 (60.58) |
n = 42 34 (31.19) |
n = 90 80 (79.21) |
n = 77 52 (67.53) |
n = 102 79 (77.45) |
n = 168 114 (67.86) |
n = 278 222 (79.86) |
n = 446 336 (75.34) |
| Practices following receipt of a negative self‐test for a person with symptoms and exposed to a COVID‐19 case | |||||||||
| Stop self‐isolation | 82 (77.36) | 77 (74.04) | 76 (69.72) | 75 (74.26) | 76 (73.79) | 84 (78.50) | 234 (73.58) | 236 (75.64) | 470 (74.60) |
| Stop wearing a face‐mask | 11 (10.38) | 8 (7.69) | 3 (2.75) | 2 (1.98) | 2 (1.94) | 6 (5.61) | 16 (5.03) | 16 (5.13) | 32 (5.08) |
| Stop social distancing | 31 (29.25) | 25 (24.04) | 10 (9.17) | 8 (7.92) | 4 (3.88) | 9 (8.41) | 45 (14.15) | 42 (13.46) | 87 (13.81) |
Number of confirmed cases during the survey period (31st July–16th August) in the setting, as per [15].
FIGURE 3.
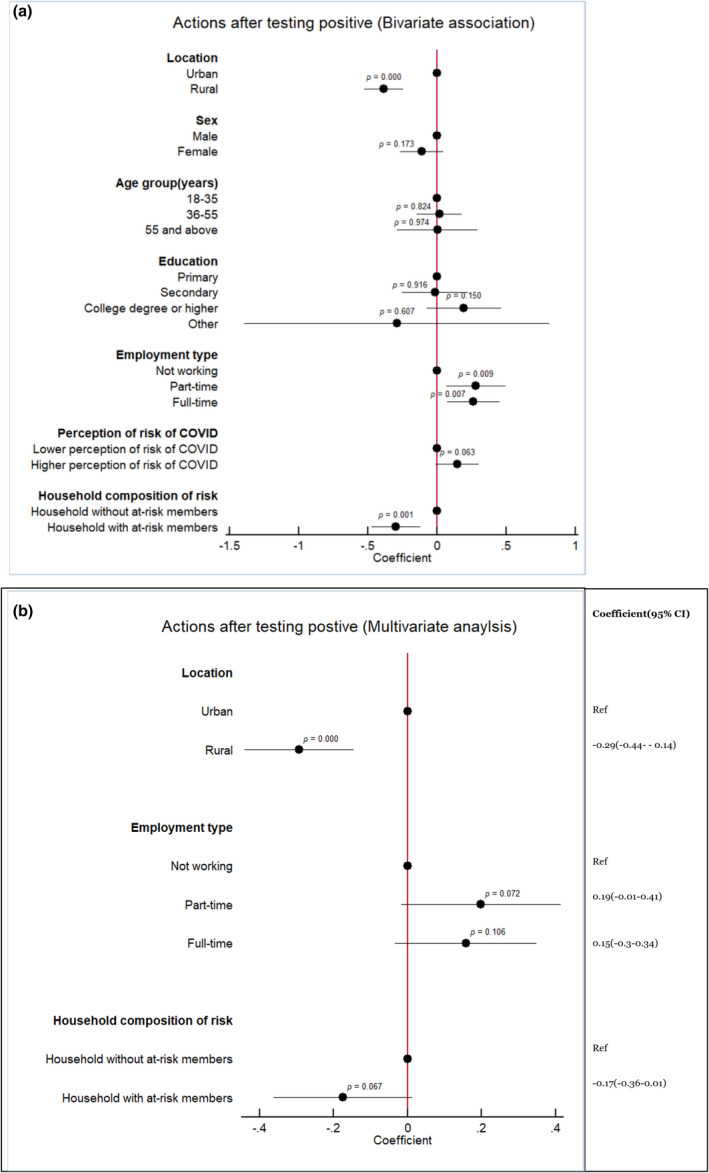
(a, b) Associations with actions upon self‐testing for SARS‐CoV‐2 (Bivariate and Multivariate analyses)
DISCUSSION
The use of RADTs for SARS‐CoV‐2 self‐testing represents an innovative approach that could positively impact case detection in Indonesia. This survey, which involved 630 individuals from a variety of urban and rural geographies, suggested that the Indonesian public appears to be willing to use SARS‐CoV‐2 self‐testing, and would react positively if they received a reactive result. Two in three respondents expressed agreement with the concept of home self‐testing for COVID‐19 and stated that they would use them if they felt they needed to test. The majority also expressed that, in accordance with health authorities' recommendations [18, 19], they would report a reactive result, request counselling, self‐isolate, and notify their contacts.
These findings must be interpreted with caution. The fact that one‐third of respondents did not express interest in using a self‐test might not be related to them disagreeing with the approach per se, but rather to them having convenient or free of charge access to conventional provider‐initiated COVID‐19 testing or to their self‐perception of being at low risk of experiencing severe COVID‐19 disease. It is also worth reflecting on the fact that, in both urban and rural geographies, females were less likely than males to state that they would use self‐testing. In Indonesia, women exhibit an increased likelihood compared with men to attend health facilities [20, 21], and this health‐seeking behaviour may extend to women feeling more comfortable than men in visiting health facilities to request COVID‐19 testing. Hence, we could hypothesise that females may not place as much value as males on the option of confidential home self‐testing, without the assistance of a healthcare worker. Further qualitative research may help to clarify the reasons for these differences once self‐testing devices become widely available in Indonesia.
There are concerns that people self‐testing for infectious diseases may not behave in the optimal manner to maximise public health benefits of the self‐test upon receiving their result. These concerns have been assessed in HIV and hepatitis C self‐testing acceptability studies [22, 23]. In the present survey, the majority of respondents expressed that, if they self‐tested positive for SARS‐CoV‐2, they would continue adhering to health authorities recommendations such as wearing masks and/or reporting the result. Almost all respondents (97.46%) would self‐isolate following a positive self‐test result. This finding is aligned with the results of other SARS‐CoV‐2 self‐testing studies carried out in the United Kingdom [24] and Germany [10]. However, in our survey, 74.60% of respondents said they would not isolate following a negative self‐test even if they had COVID‐19 symptoms and had been in contact with a COVID‐19 patient. This raises the following concern: would mildly symptomatic people who suspect they might be SARS‐CoV‐2 carriers and who fail to access a self‐test device (and, thus, to receive a negative result) manifest the preventative behaviour to self‐isolate to increase the possibility to avoid onward transmission? This aspect would deserve further exploration in future studies.
While the need for self‐test users to ensure they can generate income to provide for their offspring and themselves is a reason to not isolate, this survey did not assess the reasons behind participants' responses. It might be possible that they would not isolate but they would still report a negative result and request a confirmatory test at their nearest facility. As there is potential for social harm arising from the risk that self‐test users with COVID‐19‐compatible symptoms do not isolate, it is recommended that the distribution of self‐tests in Indonesia be performed in conjunction with clear sensitisation on what actions to take following a positive or a negative result. As suggested by a clinical evaluation of two RADTs for self‐testing in the Netherlands [25], as sensitivity of self‐test devices might not be optimal, the Indonesian public should better be advised not to use self‐testing as a diagnostic approach for severely ill individuals. Based on the findings of a review of evidence on RADT [26], it is important to emphasise that clear messages should be conveyed to self‐testers to understand that it remains possible that they are infectious even if the result of the self‐test is negative.
Respondents in our survey would prefer to report self‐test results directly to a healthcare worker in a clinic or hospital or through community health workers. Despite the public's distrust of the government for its management of the COVID‐19 pandemic [21, 27], this finding suggests the public do trust healthcare workers. While it is important to develop phone‐ and web‐based reporting mechanisms for self‐test users living in remote areas and in the islands, it is also important to capitalise on the good relationships that many Indonesians have established with their healthcare workers.
The favourable attitudes towards self‐testing found in our survey might be related to the awareness of the local availability of saliva‐based SARS‐CoV‐2 tests [28, 29]. Saliva‐based RADTs were identified as diagnostic tools that provide promise for self/home testing by the responsible of a clinical evaluation of the SD Biosensor SARS‐CoV‐2 saliva rapid test in the Netherlands [30]. In Indonesia, major news channels and websites as well as information from telemedicine platforms may have increased awareness of the accessibility of these kits [7, 8]. However, despite the public's awareness of home diagnostics, Indonesia is a middle‐income country where many households lack the resources to afford new technologies for health. In this regard, it is significant that 62.06% of the sample were willing to pay (a median of 1.4 USD). This finding has implications for the delivery of SARS‐CoV‐2 self‐testing. For self‐testing to have an impact in terms of case detection in Indonesia, quality, affordable SARS‐CoV‐2 self‐tests will need to be either brought to market or made available free of charge or at a low cost by employers, municipalities, or health insurance companies.
The importance of self‐testing in resource‐constrained countries where diagnostic testing capacity is scarce has been highlighted previously [31]. Ours is, to our knowledge, the only survey of the Indonesian public's values in relation to SARS‐CoV‐2 self‐testing. However, previous studies of HIV self‐testing in Indonesia suggest that many people may appreciate SARS‐CoV‐2 self‐testing, for myriad reasons: fear of shame, embarrassment, and social exclusion; issues around breach of confidentiality; fear of invasive testing methods; and concerns around privacy and inconvenience [32, 33]. Outside Indonesia, other similar cross‐sectional SARS‐CoV‐2 self‐testing acceptability studies carried out in Cyprus and Greece [34], Germany [10], the United States [35] or France [36] have also reported good public's acceptability of the approach. Of these studies, the study by Goggolidou and colleagues [34] is the most similar to our study in terms of its design (population‐based survey), its large study sample (n = 248 respondents from Cyprus and Greece), and its main finding (79% of their sample being willing to self‐test). The study of Bien‐Gund and colleagues [35], an online survey in the United States (n = 586), reported high motivation to order self‐test kits online (82.2% of the sample) or to use a self‐test if given to them from a potentially infected contact (86.1%).
A number of limitations must be considered. The survey was not designed as a household‐based survey due to concerns that approached potential respondents would not welcome the surveyors in their homes (especially, in Jakarta). Had we conducted a household‐based survey instead of a street‐based survey, the results might have been different as, perhaps, more people in the >55 years old group or more unemployed people could have participated. Also, it must be noted that during the implementation of this survey in rural geographies, the surveyors found a significant number of neighbourhoods whose perimeters could not be crossed by order of local authorities. The surveyors had to select new, nearby recruitment street‐points, which may have introduced recruitment bias. In Jakarta, the surveyors worked on some very crowded streets. Many individuals who were approached (n = 235) refused to even let the surveyors explain the purpose of the survey; it is impossible to know whether these individuals' characteristics differed from consenting respondents' characteristics. Another limitation relates to the country's cultural and socio‐economic diversity. Despite the choice of Banten and North Sulawesi as geographies with very different social strata, it is possible that the survey findings would have been different if other regions had been sampled. Also, it needs to be considered that awareness of the increasing COVID‐19 incidence rates in the different geographies and at the time the survey was conducted could have influenced the respondents' acceptance of SARS‐CoV‐2 self‐testing. Finally, it must be noted that the cross‐sectional design limited our capacity to statistically establish causal relationships between likelihood to use self‐testing, willingness to pay, and associated factors.
In conclusion, the Indonesian public appreciates self‐testing diagnostics for detection of infectious diseases and would use SARS‐CoV‐2 self‐tests if widely available. As recommended by health authorities, it is highly probable that self‐test users would report positive results and would self‐isolate, warn their contacts, and continue wearing face masks. In Indonesia, self‐testing kits should be introduced in a way that would encourage users to access confirmatory testing and COVID‐19 treatment following a positive self‐test result, as well as continue adhering to preventive behaviours, irrespective of the test result.
ACKNOWLEDGEMENT
The authors of this article are indebted to all survey respondents and surveyors in Jakarta, Banten, and North Sulawesi, who made this research possible. We thank Adam Bodley for editing the manuscript.
Thomas C, Shilton S, Thomas C, Batheja D, Goel S, Mone Iye C, et al. Values and preferences of the general population in Indonesia in relation to rapid COVID‐19 antigen self‐tests: A cross‐sectional survey. Trop Med Int Health. 2022;27:522–536. 10.1111/tmi.13748
Sustainable Development Goal: Good Health and Wellbeing.
REFERENCES
- 1. Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy. 2020;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cucinotta D, Vanelli M. WHO Declares COVID‐19 a Pandemic. Acta Biomed. 2020;91(1):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Setiati S, Azwar MK. COVID‐19 and Indonesia. Acta Med Indones. 2020;52(1):84–9. [PubMed] [Google Scholar]
- 4. Johns Hopkins Coronavirus Resource Center . Indonesia: COVID‐19 Overview 2021. Available from: https://coronavirus.jhu.edu/region/indonesia
- 5. Hendarwan H, Syachroni S, Aryastami NK, Sa'udi A, Susilawati MD, Despitasari M, et al. Assessing the COVID‐119 diagnostic laboratory capacity in Indonesia in the early phase of the pandemic. WHO South East Asia. J Public Health. 2020;9(2):134–40. [DOI] [PubMed] [Google Scholar]
- 6. Aisyah DN, Mayadewi CA, Igusti G, Manikam L, Adisasmito W, Kozlakidis Z. Laboratory Readiness and Response for SARS‐CoV‐2 in Indonesia. Front Public Health. 2021;9:705031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. QuickSpit AR. Tes Covid‐19 Pakai Air Liur Dinilai Akurat Deteksi Virus Corona: Suara.com. 2021. Available from: https://www.suara.com/health/2021/07/26/204615/quickspit‐tes‐COVID‐19‐pakai‐air‐liur‐donilai‐akurat‐deteksi‐virus‐corona?page=all
- 8. Anastasia T. Fatka‐fatka tentang Tes COVID‐19 Menggunakan Air Liur: klikdokter; 2021. Available from: https://www.klikdokter.com/info‐sehat/read/3648725/fakta‐fakta‐tentang‐tes‐covid‐19‐menggunakan‐air‐liur
- 9. Ganguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource‐limited settings. Curr HIV/AIDS Rep. 2009;6(4):217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wachinger J, Schirmer M, Täuber N, McMahon SA, Denkinger CM. Experiences with opt‐in, at‐home screening for SARS‐CoV‐2 at a primary school in Germany: an implementation study. BMJ Paediatr Open. 2021;5(1):e001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indian Council of Medical Research (ICMR) . Advisory for COVID‐19 Home Testing using Rapid Antigen Tests (RATs): Icmr.gov.in; 2021. Available from: https://www.icmr.gov.in/pdf/covid/kits/archive/Advisory_Home_Test_kit_02062021.pdf
- 12. U.S. Food & Drug Administration (FDA) . Coronavirus (COVID‐19) update: FDA authorizes antigen test as first over‐the‐counter fully at‐home diagnostic test for COVID‐19: fda.gov; 2021. Available from: https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐authorizes‐antigen‐test‐first‐over‐counter‐fully‐home‐diagnostic
- 13. da Saúde M. Nota técnica Nº 3/2022‐SECOVID/GAB/SECOVID/MS. 13/01/2022: gov.br; 2022. Available from: https://www.gov.br/saude/pt‐br/coronavirus/vacinas/plano‐nacional‐de‐operacionalizacao‐da‐vacina‐contra‐a‐covid‐19/notas‐tecnicas/2022/nota‐tecnica‐no‐3‐2022‐secovid‐gab‐secovid‐ms/
- 14. Gob.Pe Plataforma Digital Única del Estado Peruano . El Indecopi Brinda recomendaciones para adquirir “autotest COVID‐19” y medicamentos genéricos: gob.pe; 2022. Available from: https://www.gob.pe/institucion/indecopi/noticias/576959‐el‐indecopi‐brinda‐recomendaciones‐para‐adquirir‐autotest‐covid‐19‐y‐medicamentos‐genericos
- 15. Government of Indonesia . Peta Sebaran Kasus Per Provinsi. Available from: https://data.covid19.go.id/public/index.html
- 16. WHO . Recommendations and guidance on hepatitis C virus self‐testing: web annex D: values and preferences on hepatitis C virus self‐testing. Geneva: World Health Organization; 2021. [Google Scholar]
- 17. Shilton S, Ivanova Reipold E, Roca Álvarez A, Martínez‐Pérez GZ. Assessing values and preferences towards SARS‐CoV‐2 self‐testing among the general population and their representatives, health care personnel, and decision‐makers: protocol for a multicountry mixed methods study. JMIR Res Protoc. 2021;10(11):e33088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO . Advice for the public: Coronavirus disease (COVID‐19). Geneva: WHO; 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/advice‐for‐public [Google Scholar]
- 19. Better Work Indonesia . Compilation of guidelines on Covid‐19 transmission, prevention and management and the best practices in the workplace 2020. Available from: https://betterwork.org/wp‐content/uploads/2020/04/BWI_covid_guidance_eng_web.pdf
- 20. Handayani PW, Dartanto T, Moeis FR, Pinem AA, Azzahro F, Hidayanto AN, et al. The regional and referral compliance of online healthcare systems by Indonesia National Health Insurance agency and health‐seeking behavior in Indonesia. Heliyon. 2021;7(9):e08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad RA, Richardus JH, de Vlas SJ. Care‐seeking behaviour among individuals with TB symptoms in Jogjakarta Province, Indonesia: a community‐based study. Int Health. 2013;5(1):51–7. [DOI] [PubMed] [Google Scholar]
- 22. Jamil MS, Eshun‐Wilson I, Witzel TC, Siegfried N, Figueroa C, Chitembo L, et al. Examining the effects of HIV self‐testing compared to standard HIV testing services in the general population: a systematic review and meta‐analysis. eClinicalMedicine. 2021;38:100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martínez‐Pérez GZ, Nikitin DS, Bessonova A, Fajardo E, Bessonov S, Shilton S. Values and preferences for hepatitis C self‐testing among people who inject drugs in Kyrgyzstan. BMC Infect Dis. 2021;21(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wanat M, Logan M, Hirst JA, Vicary C, Lee JJ, Perera R, et al. Perceptions on undertaking regular asymptomatic self‐testing for COVID‐19 using lateral flow tests: a qualitative study of university students and staff. BMJ Open. 2021;11(9):e053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stohr JJJM, Zwart VF, Goderski G, Meijer A, Nagel‐Imming CRS, Kluytmans‐van der Bergh MFQ, et al. Self‐testing for the detection of SARS‐CoV‐2 infection with rapid antigen tests for people with suspected COVID‐19 in the community. Clin Microbiol Infect. 2021. 10.1016/j.cmi.2021.07.039. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barjaková M, Lunn P. Perception, behaviour and communication in relation to rapid antigen Economic & Social Research Institute detection tests – a narrative review of evidence: Economic & Social Research Institute; 2021. Available from: https://www.esri.ie/publications/perception‐behaviour‐and‐communication‐in‐relation‐to‐rapid‐antigen‐detection‐tests‐a
- 27. Riefky, Hutasoit Ina R, Nopiyanto Ananda M D, Nugrahani Henny S D, Zulkarnain Rizky A. Growing public distrust towards the Indonesian Government for lack of response to COVID‐19 outbreak. IOP Conference Series: Earth and Environmental Science. 2021;716:12072. [Google Scholar]
- 28. Kalbe . Kalbe launches the First Ever Saliva‐Based COVID‐19 Test Kit which is the Brainchild of Indonesia’s Own Researchers 2021.
- 29. Bio Farma . Bio Farma X Nusantics Launches Limited Bio Saliva, Covid‐19 PCR Detection Test With Gargle Method 2021. Available from: https://www.biofarma.co.id/en/latest‐news/detail/bio‐farma‐x‐nusantics‐launches‐limited‐bio‐saliva‐covid19‐pcr‐detection‐testwith‐gargle‐method
- 30. Igloi Z, Velzing J, Huisman R, Geurtsvankkessel C, Comvalius A, IJpelaar J, et al. Clinical evaluation of the SD Biosensor SARS‐CoV‐2 saliva antigen test with symptomatic and asymptomatic, non‐hospitalized patients. PLoS One. 2021;16(12):e0260894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boum Y, Eyangoh S, Okomo MC. Beyond COVID‐19‐will self‐sampling and testing become the norm? Lancet Infect Dis. 2021;21(9):1194–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wulandari LPL, Ruddick A, Guy R, Kaldor J. “Self‐testing sounds more private, rather than going to the clinic and everybody will find out”: facilitators and barriers regarding HIV testing among men who purchase sex in Bali, Indonesia. PLoS One. 2019;14(4):e0214987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wulandari LPL, Kaldor J, Guy R. Uptake and acceptability of assisted and unassisted HIV self‐testing among men who purchase sex in brothels in Indonesia: a pilot intervention study. BMC Public Health. 2020;20(1):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goggolidou P, Hodges‐Mameletzis I, Purewal S, Karakoula A, Warr T. Self‐testing as an invaluable tool in fighting the COVID‐19 pandemic. J Prim Care Community Health. 2021;12:21501327211047782. 10.1177/21501327211047782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bien‐Gund C, Dugosh K, Acri T, Brady K, Thirumurthy H, Fishman J, et al. Factors associated with US public motivation to use and distribute COVID‐19 self‐test. JAMA Netw Open. 2021;4(1):e2034001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tonen‐Wolyec S, Dupont R, Batina‐Agasa S, Hayette MP, Bélec L. Capillary whole‐blood IgG‐IgM COVID‐19 self‐test as a serological screening tool for SARS‐CoV‐2 infection adapted to the general public. PLoS One. 2020;15(10):e0240779. [DOI] [PMC free article] [PubMed] [Google Scholar]


