Key Points
Question
What are the effects of oral glucocorticoids in patients with IgA nephropathy and proteinuria of 1 g per day or greater receiving optimal supportive therapy?
Findings
In this randomized clinical trial that included 503 participants, a 6- to 9-month course of oral methylprednisolone, compared with placebo, significantly reduced the risk of the composite outcome of kidney function decline, kidney failure, or death due to kidney disease (hazard ratio, 0.53), although the risk of serious adverse events was increased.
Meaning
Among patients with IgA nephropathy and proteinuria greater than or equal to 1 g per day, oral methylprednisolone significantly reduced the risk of the composite outcome of kidney function decline, kidney failure, or death due to kidney disease, with an increased incidence of serious adverse events.
Abstract
Importance
The effect of glucocorticoids on major kidney outcomes and adverse events in IgA nephropathy has been uncertain.
Objective
To evaluate the efficacy and adverse effects of methylprednisolone in patients with IgA nephropathy at high risk of kidney function decline.
Design, Setting, and Participants
An international, multicenter, double-blind, randomized clinical trial that enrolled 503 participants with IgA nephropathy, proteinuria greater than or equal to 1 g per day, and estimated glomerular filtration rate (eGFR) of 20 to 120 mL/min/1.73 m2 after at least 3 months of optimized background care from 67 centers in Australia, Canada, China, India, and Malaysia between May 2012 and November 2019, with follow-up until June 2021.
Interventions
Participants were randomized in a 1:1 ratio to receive oral methylprednisolone (initially 0.6-0.8 mg/kg/d, maximum 48 mg/d, weaning by 8 mg/d/mo; n = 136) or placebo (n = 126). After 262 participants were randomized, an excess of serious infections was identified, leading to dose reduction (0.4 mg/kg/d, maximum 32 mg/d, weaning by 4 mg/d/mo) and addition of antibiotic prophylaxis for pneumocystis pneumonia for subsequent participants (121 in the oral methylprednisolone group and 120 in the placebo group).
Main Outcomes And Measures
The primary end point was a composite of 40% decline in eGFR, kidney failure (dialysis, transplant), or death due to kidney disease. There were 11 secondary outcomes, including kidney failure.
Results
Among 503 randomized patients (mean age, 38 years; 198 [39%] women; mean eGFR, 61.5 mL/min/1.73 m2; mean proteinuria, 2.46 g/d), 493 (98%) completed the trial. Over a mean of 4.2 years of follow-up, the primary outcome occurred in 74 participants (28.8%) in the methylprednisolone group compared with 106 (43.1%) in the placebo group (hazard ratio [HR], 0.53 [95% CI, 0.39-0.72]; P < .001; absolute annual event rate difference, −4.8% per year [95% CI, −8.0% to −1.6%]). The effect on the primary outcome was seen across each dose compared with the relevant participants in the placebo group recruited to each regimen (P for heterogeneity = .11): full-dose HR, 0.58 (95% CI, 0.41-0.81); reduced-dose HR, 0.27 (95% CI, 0.11-0.65). Of the 11 prespecified secondary end points, 9 showed significant differences in favor of the intervention, including kidney failure (50 [19.5%] vs 67 [27.2%]; HR, 0.59 [95% CI, 0.40-0.87]; P = .008; annual event rate difference, −2.9% per year [95% CI, −5.4% to −0.3%]). Serious adverse events were more frequent with methylprednisolone vs placebo (28 [10.9%] vs 7 [2.8%] patients with serious adverse events), primarily with full-dose therapy compared with its matching placebo (22 [16.2%] vs 4 [3.2%]).
Conclusions and Relevance
Among patients with IgA nephropathy at high risk of progression, treatment with oral methylprednisolone for 6 to 9 months, compared with placebo, significantly reduced the risk of the composite outcome of kidney function decline, kidney failure, or death due to kidney disease. However, the incidence of serious adverse events was increased with oral methylprednisolone, mainly with high-dose therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT01560052
This randomized clinical trial examines the effect of oral methylprednisolone on the risk of major kidney outcomes, including 40% decline in eGFR, kidney failure (dialysis, transplant), or death due to kidney disease, and adverse effects in individuals with IgA nephropathy and proteinuria.
Introduction
IgA nephropathy is the most common primary glomerular disease. A systematic review published in 2011 estimated an incidence of 2.5 cases per 100 000 individuals per year.1 The incidence is likely underestimated due to variability in kidney biopsy practice in different regions.2 Several long-term studies have reported rates of kidney failure of up to 25% at 10-year follow-up and up to 50% at 30-year follow-up.3,4
Although there have been advances in understanding the pathogenesis of IgA nephropathy, highlighting its immunological basis, no disease-specific therapy has been proven to prevent kidney failure. Recent discovery of specific autoimmunity against galactose-deficient IgA1, immune complex deposition, and activation of the complement have strengthened the rationale for immunomodulatory therapy.5,6
Although glucocorticoid therapy has been variably used in IgA nephropathy, its role remains controversial based on persistent uncertainty about the benefits and risks.7 Although previous clinical trials collectively suggested potential benefit for corticosteroids,8,9 the largest previous individual clinical trial found that the addition of immunosuppression to supportive care resulted in more adverse effects, but had no effect on kidney function over the trial period or during long-term follow-up.3,10 The latest Kidney Disease: Improving Global Outcomes guidelines in glomerulonephritis therefore suggest consideration of glucocorticoid therapy (evidence level 2B) in individuals with IgA nephropathy, but recommend warning about glucocorticoid-related toxicity.11
The Therapeutic Effects of Steroids in IgA Nephropathy Global (TESTING) study was designed to determine the effects of oral methylprednisolone on the risk of major kidney outcomes, as well as adverse effects, in people with IgA nephropathy and proteinuria.
Methods
Study Design and Oversight
The detailed design of this randomized, double-blind, placebo-controlled, multicenter clinical trial has been described previously.12 The study was approved by the ethics committees of each of the participating sites, and all participants provided written informed consent. The study was overseen by a management committee, coordinated by The George Institute for Global Health, and was designed to be conducted in centers in China, Australia, India, Canada, and Malaysia. The study protocol and the statistical analysis plan are available in Supplement 1.13
The study started recruitment in May 2012 but was halted on November 13, 2015, when 262 patients had been randomized, based on an excess of serious infections, 2 of which were fatal, in the corticosteroid group. All of the participants and investigators were informed; 23 participants who were still receiving methylprednisolone or placebo had treatment discontinued, while scheduled follow-up was continued, and were included in an interim analysis.14
The steering committee subsequently made the decision to revise the protocol to recruit 240 additional participants to receive a reduced methylprednisolone dose regimen or matching placebo (see below; summary of and timelines for the main changes of the protocol are available in eTable 1 and eFigure 1 in Supplement 2), with ongoing follow-up of all participants (including the original full-dose cohort) to a revised target total sample size of 500 participants, reduced from the 750 participants planned at the time this change was made. Recruitment to the reduced-dose protocol was recommenced on March 21, 2017.
Participants
Eligibility required a diagnosis of primary IgA nephropathy proven on kidney biopsy (without time limits), an estimated glomerular filtration rate (eGFR) between 20 and 120 mL/min/1.73 m2 (calculated using the Chronic Kidney Disease Epidemiology Collaboration formula; modified to 30 to 120 mL/min/1.73 m2 for the reduced-dose cohort), and 24-hour urinary protein excretion greater than or equal to 1 g per day. Exclusion criteria included a strong indication for, or contraindication to, glucocorticoid therapy based on the judgement of the treating physician, or the use of systemic immunosuppressive therapy in the past year. Full inclusion and exclusion criteria are described in eTable 2 in Supplement 2. Because there are data suggesting differential disease prevalence and progression of IgA nephropathy in various regions being a more common cause of kidney failure in East Asian countries than in European and North American countries, we collected participant self-reported race and ethnicity based on questionnaires with fixed categories.
Randomization
Potentially eligible participants entered a run-in period of up to 12 weeks, during which background therapy was optimized, including ensuring that maximal renin-angiotensin system inhibitor therapy was used for a minimum of 3 months prior to randomization. Those who were adherent to treatment, with persistent proteinuria greater than 1 g per day during the run-in period, were centrally randomized in a 1:1 ratio to receive oral methylprednisolone or matching placebo, stratified by site, proteinuria (<3 g/d or ≥3 g/d), eGFR (<50 mL/min/1.73 m2 or ≥50 mL/min/1.73 m2), and kidney biopsy findings (endocapillary proliferation status according to the Oxford classification definitions at the time the trial was designed: E1 or E0).15 Randomization was performed using a minimization algorithm based on the 4 stratification variables; the algorithm was centrally generated and used by all centers to minimize any imbalances in key variables. Patients, investigators, site staff, and sponsors were masked to treatment assignment.
Intervention
Participants were originally randomized to receive oral methylprednisolone, 0.6 to 0.8 mg/kg per day, for 2 months (maximum, 48 mg/d), tapering by 8 mg per day each month for a total treatment period of 6 to 8 months or matching placebo. For participants randomized from 2017 onward (the reduced-dose cohort), a lower dose of methylprednisolone was used: 0.4 mg/kg per day (maximum, 32 mg/d) for 2 months followed by dose tapering by 4 mg per day each month, or matching placebo, for a total of 6 to 9 months. Antibiotic prophylaxis for pneumocystis pneumonia (a single-strength tablet daily or half of a double-strength tablet daily) was also added during the first 12 weeks of the treatment period for all participants randomized to the reduced-dose cohort.
Study visits occurred monthly for 3 months, then every 3 months until month 12, and then annually until the end of the study. Additional study visits (either telephone or face-to-face at the choice of the investigator) were conducted every 3 months after the first year to ascertain kidney end points and adverse events.
Outcomes
The primary end point was the composite of the first occurrence of a sustained 40% decrease in eGFR, the development of kidney failure (defined as a need for maintenance dialysis or kidney transplant and adjudicated by a blinded, independent committee), or death due to kidney disease (defined as death due to kidney failure that required dialysis and that could be avoided by timely dialysis). Occurrences of 40% eGFR reduction were included as end points if they were sustained on a repeated serum creatinine test at least 30 days after the first occurrence or if they occurred at the final available measurement.
Secondary end points were sustained decreases in eGFR (of 30%, 40%, and 50%) combined with kidney failure and all-cause death; the separate individual components of the composite primary and secondary outcomes; proteinuria reduction evaluated by time-averaged mean proteinuria; and eGFR slope, using all eGFR values or those excluding eGFR at the time of high-dose treatment exposure.
Predefined adverse event outcomes were total serious adverse events, serious infections needing hospitalization, new-onset diabetes, gastrointestinal hemorrhage, fracture or osteonecrosis, and cardiovascular events. Serious adverse events were defined according to the International Conference on Harmonization Guideline for Clinical Safety Data Management.
Sample Size
The trial was event-driven, requiring at least 160 primary outcome events to provide 90% power (α = .05) to detect a 40% overall reduction in the hazard for the methylprednisolone group. This, in turn, required the enrollment of at least 500 patients across the trial (pooling the original and reduced-dose cohorts). The trial had been designed with 90% power to detect a 30% risk reduction (sample size of 750) based on a balance between what was clinically important to detect and what was feasible, but was modified for pragmatic and funding-related reasons after the serious infection–related pause. A meta-analysis of previous trials8 suggested that glucocorticoids might have a relative risk for the primary end point of 0.32 (95% CI, 0.15-0.67), but had been considered likely to be an overestimate. When recruitment of the full-dose cohort was stopped, feasibility was affected, so the trial was powered to detect a still-conservative risk reduction of 40%.
Statistical Analysis
All analyses were conducted by analyzing all patients according to the group they were randomized to, regardless of treatment adherence or protocol violations. The effect of treatment on the primary composite end point was estimated using an adjusted Cox model, with site as a random effect and the 3 other stratification variables (baseline proteinuria, baseline eGFR, and kidney biopsy findings) as fixed effects. Data for patients were censored at the date they died (for causes other than kidney disease), were lost to follow-up, or withdrew from the study or at the end of the trial assessment, whichever occurred first. Survival curves were generated with the Kaplan-Meier method. The proportional hazards assumption was assessed graphically and checked using the Kolmogorov-type supremum test, and a post hoc sensitivity analysis using flexible parametric survival models with either constant or time-varying hazard ratios (HRs) was performed.
All secondary outcomes of a time-to-event nature were analyzed using the same approach as the primary outcome (using an adjusted Cox model). Decline in eGFR was analyzed as the mean rate of decline and compared between groups using a t test. Time-averaged mean proteinuria was calculated for each participant and was also compared using a t test. In addition, overall trajectories of eGFR and proteinuria over time were analyzed using longitudinal linear mixed models that included all postrandomization measurements. For this analysis, proteinuria values were log-transformed using the natural logarithmic function to remove skewness.
Adjusted annual event rates and absolute risk differences with 95% CIs were estimated using a Poisson model adjusted by the stratification factors. Site was not included as a random effect due to convergence issues. CIs around adjusted risk differences were obtained using the normal approximation and the standard error was obtained from the model. Secondary outcomes were considered supportive and were not adjusted for multiplicity. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Almost all participants were followed up until the end of the trial, thus resulting in negligible missing data. All analyses were conducted by including all available data points. For time-to-event analyses, participants were censored when they were last known to be free of the event.
The homogeneity of the treatment effects on the primary outcome across specified subgroups was tested by adding interaction terms to the Cox models, with predefined subgroups including methylprednisolone dosage regimen (full or reduced dose, each compared with the participants randomized to receive placebo during that trial period), baseline proteinuria (<3.0 g/d vs ≥3.0 g/d), baseline estimated GFR (<50 mL/min/1.73 m2 vs ≥50 mL/min/1.73 m2), endocapillary hypercellularity on histological scoring (yes [E1] vs no [E0]), self-reported ethnicity (Chinese vs other), and time between biopsy and randomization. All P values were 2-sided, and P values less than .05 were considered to indicate statistical significance. All analyses were performed using SAS software, version 9.4 (SAS Institute). Further details are included in the statistical analysis plan (Supplement 1).13
Results
Enrollment and Follow-up
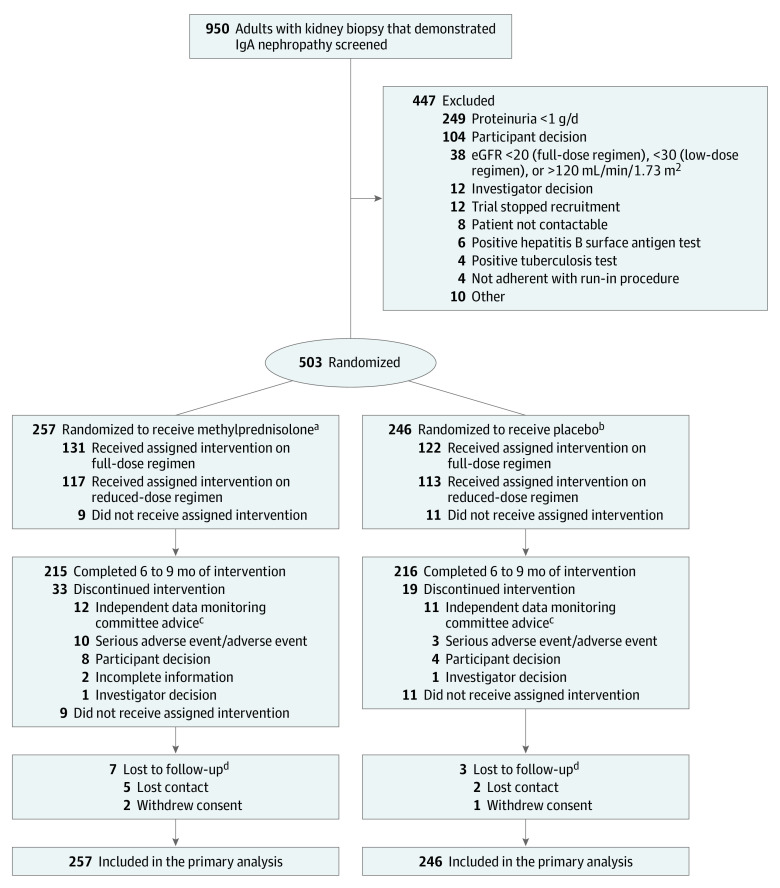
Between May 2012 and November 2019, a total of 950 potentially eligible patients were screened at 67 sites from Australia (6 sites), Canada (8 sites), China (41 sites), India (6 sites), and Malaysia (6 sites). Overall, 503 participants were randomized (257 to the methylprednisolone group and 246 to the placebo group), including 262 based on the original full-dose protocol and 241 based on the reduced-dose protocol (Figure 1). Recruitment began earlier in China and Australia and continued throughout the trial in these countries, whereas in Canada, India, and Malaysia, recruitment commenced only after recruitment to the full-dose protocol had been stopped.
Figure 1. Flow of Participants in a Study of the Effect of Oral Methylprednisolone on Kidney Function Decline in Patients With IgA Nephropathy.
eGFR indicates estimated glomerular filtration rate.
aDosing of methylprednisolone starting at 0.6-0.8 mg/kg per day (maximum, 48 mg/d) was reduced to a starting dose of 0.4 mg/kg per day (maximum, 32 mg/d) after an interim analysis demonstrated a high rate of adverse events in the intervention group.
bA total of 126 participants were randomized to receive placebo during the period when full-dose methylprednisolone was being used for the intervention group and 120 were randomized to receive placebo during the period when reduced-dose methylprednisolone was being used.
cA total of 23 participants were still receiving full-dose methylprednisolone or placebo in 2015 when the excess serious adverse events were identified. Based on the advice from the independent data safety monitoring committee, treatment was discontinued for these 23 individuals, but scheduled follow-up was continued and they were included in the primary analysis.
dPatients lost to follow-up may have received, completed, or discontinued the assigned intervention.
The baseline characteristics of participants were similar across randomized groups (Table 1). The mean age of participants was 38 years, 198 (39%) were women, the mean baseline eGFR was 61.5 mL/min/1.73 m2, and the mean 24-hour urinary protein was 2.46 g per day. Compared with those enrolled to the full-dose cohort, participants in the reduced-dose cohort were less likely to be from China (95% vs 51%) and had a higher body mass index , higher eGFR, a longer mean time since diagnostic kidney biopsy, and less tubular atrophy and fibrosis on that biopsy (eTable 3 in Supplement 2).
Table 1. Baseline Characteristics of Participants in a Study of the Effect of Oral Methylprednisolone on Kidney Function Decline in Patients With IgA Nephropathy.
| Characteristics | No. (%)a | |
|---|---|---|
| Methylprednisolone (n = 257) | Placebo (n = 246) | |
| Age, median (IQR), y | 35.6 (29.4-46.3) | 36.6 (29.0-45.9) |
| Sex | ||
| Men | 155 (60) | 150 (61) |
| Women | 102 (40) | 96 (39) |
| Race/ethnicityb | ||
| Chinese | 195 (76) | 184 (75) |
| Japanese | 0 | 1 (0.4) |
| Mixed | 0 | 1 (0.4) |
| Other Eastern Asian | 1 (0.4) | 0 |
| South Asian | 30 (12) | 33 (13) |
| Southeast Asian | 18 (7) | 15 (6) |
| White/European | 13 (5) | 12 (5) |
| BMI, median (IQR) | 24.2 (21.6-26.7) | 24.7 (22.0-28.0) |
| Smoking history | ||
| Previous | 39 (15) | 27 (11) |
| Current | 19 (7) | 23 (9) |
| Medical historyc | ||
| Hypertension | 128 (50) | 113 (46) |
| Macrohematuria | 42 (16) | 38 (15) |
| Previous corticosteroids | 18 (7) | 10 (4) |
| Previous other immunosuppressant | 17 (7) | 12 (5) |
| Diabetes | 7 (3) | 10 (4) |
| Family history of IgA nephropathy | 3 (1) | 9 (4) |
| Tonsillectomy | 2 (0.8) | 1 (0.4) |
| Blood pressure, median (IQR), mm Hg | ||
| Systolic | 123.8 (115.0-132.5) | 125.0 (115.5-131.0) |
| Diastolic | 80.0 (73.5-85.0) | 80.0 (74.0-86.0) |
| Laboratory findings, median (IQR) | ||
| eGFR, mL/min per 1.73 m2d | 56.1 (43.2-75.0) | 59.0 (42.0-77.6) |
| Urine protein, g/de | 1.99 (1.36-3.09) | 1.93 (1.38-2.88) |
| Time since kidney biopsy, median (IQR), mo | 5 (4-11) | 5 (3-14) |
| Histology on kidney biopsy, No./total No. (%)f | ||
| Mesangial hypercellularity (M1) | 147/252 (58) | 148/241 (61) |
| Segmental glomerulosclerosis (S1) | 172/252 (68) | 164/241 (68) |
| Endocapillary hypercellularity (E1) | 72 (28) | 55 (22) |
| Tubular atrophy/interstitial fibrosis (T) | ||
| T0: 0%-25% | 123/252 (48.8) | 118/241 (49.0) |
| T1: 26%-50% | 92/252 (36.5) | 95/241 (39.4) |
| T2: >50% | 37/252 (14.7) | 28/241 (11.6) |
| Medication | ||
| ACE inhibitors | 140 (54.5) | 128 (52.0) |
| ARBs | 119 (46.3) | 120 (48.8) |
| Dose of ACE inhibitor or ARB | ||
| No ACE or ARB received | 0 | 1 (0.4) |
| <50% of maximum labeled doseg | 30 (11.7) | 35 (14.2) |
| ≥50% of maximum labeled doseg | 222 (86.4) | 201 (81.7) |
| Received but dose unknown | 5 (1.9) | 9 (3.7) |
| Calcium channel blocker | ||
| Dihydropyridine | 44 (17.1) | 41 (16.7) |
| Nondihydropyridine | 13 (5.1) | 17 (6.9) |
| β and α blocker | 21 (8.2) | 20 (8.1) |
| Diuretic | 20 (7.8) | 21 (8.5) |
| Mineralocorticoid receptor antagonist | 4 (1.6) | 11 (4.5) |
| Nitrates | 2 (0.8) | 0 |
| Centrally acting | 5 (1.9) | 2 (0.8) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Percentages may not total 100 because of rounding.
Participant self-reported ethnicity was listed as 13 categories in the study case report form. There were no participants of African, Australian (Aboriginal), Arabic, Maori/Polynesian, Japanese, or North American Indian ancestry. South Asian refers to participants from India; Southeast Asian refers to participants from Malaysia. One participant reported as mixed race and 1 participant reported as Other Eastern Asian.
Medical history was reported by the patients.
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula; normal values were 90 mL/min/1.73 m2 or greater.
Normal values of urine protein excretion were less than 0.2 g/d.
Histological findings were scored by Oxford Classification (MEST) and reviewed by each site. The median (IQR) time between biopsy and randomization was 5 (4-13) months. T score relates to the proportion of the kidney biopsy affected by tubular atrophy/interstitial fibrosis and is divided into 3 categories, as listed.
Maximum labeled dose is based on individual country local practice. All participants received stable maximum tolerated dose of ACE inhibitors or ARBs for at least 12 weeks.
Participants were followed up for a mean of 4.2 years (5.7 y for the full-dose vs 2.6 y for the reduced-dose cohort) and a median of 3.5 years (6.1 y for the full-dose vs 2.5 y for the reduced-dose cohort). Among those randomized, 431 patients (85.7%) completed the full treatment course, 20 (4.0%) did not receive the randomized therapy, and 52 (10.3%) discontinued prematurely (including 23 randomized to the original full-dose protocol who had randomized therapy discontinued when the excess of serious infections was identified; Figure 1). A total of 493 patients (98%) had vital status available at completion of the trial, with 3 (0.6%) who withdrew consent and 7 (1.4%) who were lost to follow-up.
Efficacy
Primary Outcome
The primary outcome occurred significantly less frequently in participants randomized to the methylprednisolone group than the placebo group (74 [28.8%] vs 106 [43.1%]; HR, 0.53 [95% CI, 0.39-0.72]; P < .001; annual event rate difference, −4.8% per year [95% CI, −8.0% to −1.6%]; Table 2 and Figure 2A). Graphical examination suggested the possibility of varying effects over time, so the proportional hazards assumption was checked using the Kolmogorov-type supremum test (P = .05). A post hoc sensitivity analysis using flexible parametric survival models with either constant or time-varying HRs was therefore performed. The flexible model with constant HRs generated an estimated HR of 0.54 (95% CI, 0.39-0.74), which was very similar to that obtained using the Cox model. There was some evidence that the time-varying HR was a better fit to the study data than a constant HR (eFigure 2 in Supplement 2; P = .02 for model fit with time-varying HR vs constant HR), but both consistently estimated a significant risk reduction with methylprednisolone.
Table 2. Primary and Secondary Outcomes in a Study of the Effect of Oral Methylprednisolone on Kidney Function Decline in Patients With IgA Nephropathy.
| Outcome | Methylprednisolone (n = 257)a,b | Placebo (n = 246)a,b | Rate difference (95% CI), %b | Hazard ratio (95% CI)c | P valuec | ||
|---|---|---|---|---|---|---|---|
| No. of events | Annual event rate (95% CI), % | No. of events | Annual event rate (95% CI), % | ||||
| Primary | |||||||
| 40% eGFR reduction, kidney failure, or death due to kidney diseased,e | 74 | 7.3 (5.7 to 9.4) | 106 | 12.1 (9.7 to 15.1) | −4.8 (−8.0 to −1.6) | 0.53 (0.39 to 0.72) | <.001 |
| Secondary | |||||||
| 30% eGFR reduction, kidney failure, or all-cause death | 86 | 8.4 (6.7 to 10.6) | 113 | 12.8 (10.3 to 15.8) | −4.4 (−7.7 to −1.0) | 0.56 (0.42 to 0.75) | <.001 |
| 40% eGFR reduction, kidney failure, or all-cause death | 78 | 7.7 (6.1 to 9.8) | 106 | 12.2 (9.8 to 15.2) | −4.5 (−7.7 to −1.2) | 0.56 (0.42 to 0.76) | <.001 |
| 50% eGFR reduction, kidney failure, or all-cause death | 71 | 7.0 (5.5 to 9.1) | 94 | 10.8 (8.6 to 13.7) | −3.8 (−6.9 to −0.7) | 0.62 (0.46 to 0.85) | .003 |
| Kidney failure requiring dialysis/transplant | 50 | 4.9 (3.7 to 6.6) | 67 | 7.8 (5.9 to 10.2) | −2.9 (−5.4 to −0.3) | 0.59 (0.40 to 0.87) | .008 |
| eGFR reduction | |||||||
| 30% | 67 | 6.7 (5.2 to 8.7) | 98 | 11.4 (9.1 to 14.3) | −4.7 (−7.8 to −1.6) | 0.47 (0.34 to 0.65) | <.001 |
| 40% | 57 | 5.8 (4.4 to 7.7) | 91 | 10.9 (8.6 to 13.7) | −5.0 (−8.0 to −2.0) | 0.44 (0.31 to 0.62) | <.001 |
| 50% | 49 | 5.0 (3.7 to 6.7) | 76 | 9.1 (7.0 to 11.7) | −4.1 (−6.8 to −1.3) | 0.52 (0.36 to 0.74) | <.001 |
| Death due to kidney failuref | 1 | 0 | 1 | 0 | 0 | NA | NA |
| Death due to any cause | 6 | 0.5 (0.2 to 1.3) | 3 | 0.3 (0.1 to 1.0) | 0.2 (−0.4 to 0.8) | 2.62 (0.53 to 13.05) | .24 |
| Rate of eGFR decline, mL/min/1.73 m2/y | Mean (95% CI)g | Mean difference (95% CI)g | P valueg | ||||
| Using all visits | −2.50 (−3.56 to −1.44) | −4.97 (−6.07 to −3.87) | 2.46 (0.94 to 3.99) | .002 | |||
| Excluding values from those receiving high-exposure treatment | −2.18 (−3.16 to −1.20) | −4.94 (−6.01 to −3.87) | 2.76 (1.32 to 4.21) | <.001 | |||
| Excluding values from those receiving treatment | −2.11 (−3.03 to −1.20) | −4.76 (−5.81 to −3.72) | 2.65 (1.27 to 4.03) | <.001 | |||
| Time-averaged proteinuria, g/d | 1.70 (1.54 to 1.86) | 2.39 (2.15 to 2.63) | −0.69 (−0.98 to −0.41) | <.001 | |||
Median (IQR) follow-up was 3.5 (2.4-6.2) years.
Yearly event rates (per 100 patients) and rate differences were obtained from a Poisson model adjusted for the stratification factors as fixed effects but without site as a random effect.
Hazard ratios and corresponding P values were obtained from a Cox model adjusted for stratification factors as fixed effects and site as a random effect.
Persistent ≥40% estimated glomerular filtration rate (eGFR) reduction was confirmed by a repeated reading at least 30 days later.
Kidney failure requiring maintenance dialysis or kidney transplant.
Too few events to derive CIs, estimates of effect, and P values.
Means, mean differences, and corresponding P values were obtained from a linear model adjusted for stratification factors as fixed effects and site as a random effect. Yearly event rates (per 100 patients) and rate differences were obtained from a Poisson model adjusted for site as a random effect and proteinuria, eGFR, and kidney biopsy findings as fixed effects.
Figure 2. Time From Randomization to First Outcome in a Study of the Effect of Oral Methylprednisolone on Kidney Function Decline in Patients With IgA Nephropathy.
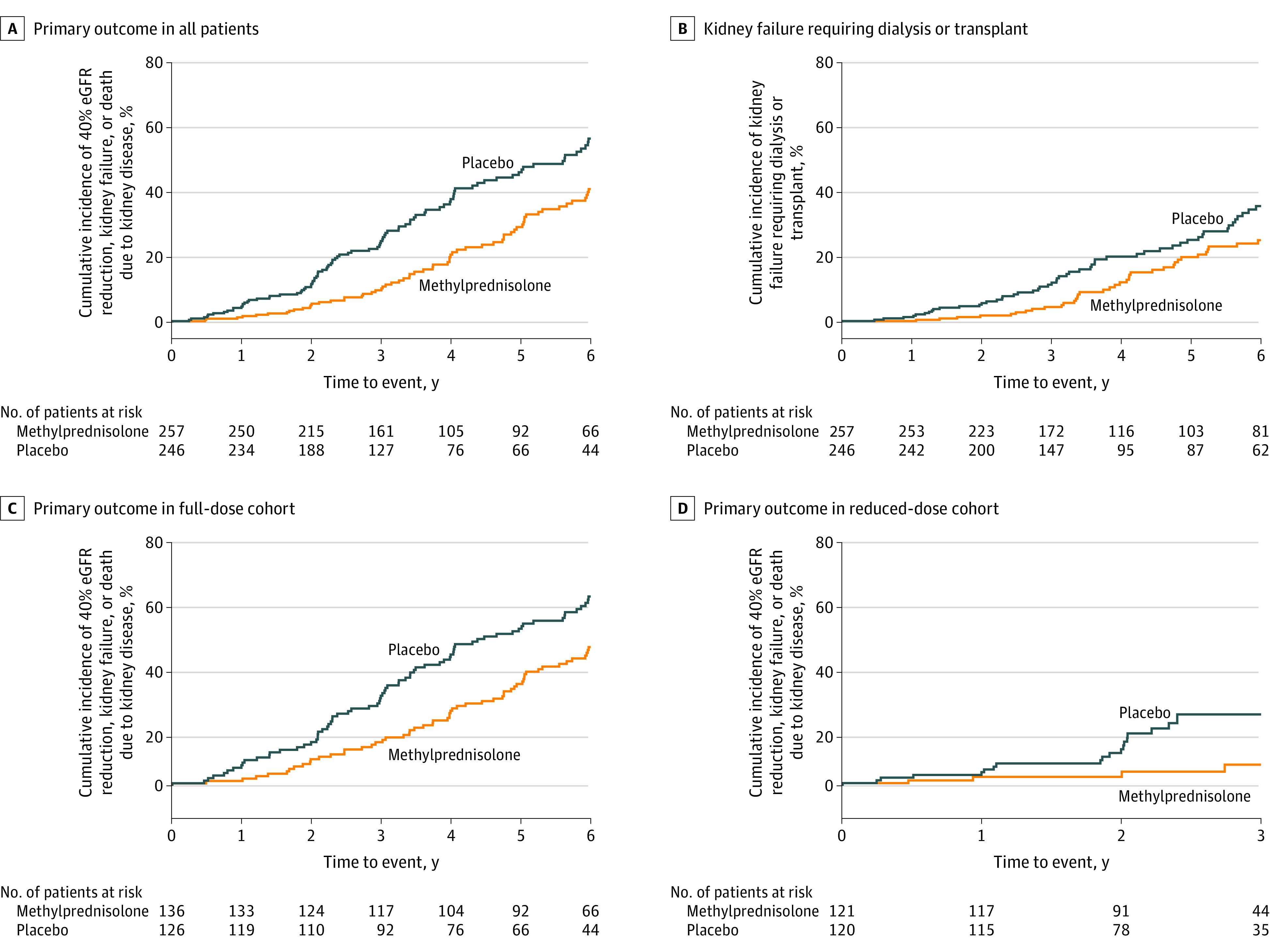
Component outcome of 40% eGFR reduction is shown in eFigure 4 in Supplement 2; the component outcome of death is not shown because there were only 2 events (after a median [IQR] follow-up of 3.5 [2.4-6.2] y). Hazard ratios, CIs, and P values were estimated using the Cox proportional hazards regression models, stratified according to randomization factors (site, ethnicity, baseline proteinuria, baseline eGFR, and kidney biopsy findings). Included in these analyses are all the participants who were randomized and received at least 1 dose of methylprednisolone or placebo. Analyses were censored at the date when patients died (for causes other than death due to kidney failure), were lost to follow-up, or withdrew from the study or at the end of study visit, whichever occurred first. The log-rank P values were <.001 for the primary outcome in all patients, .03 for kidney failure requiring dialysis or transplant, .002 for the primary outcome in the full-dose cohort, and .004 for the primary outcome in the reduced-dose cohort.
In subgroup analyses in which each methylprednisolone dose group was compared with the participants in the placebo group randomized at that time, the effect size showed no significant difference across methylprednisolone dose regimens (full dose: HR, 0.58 [95% CI, 0.41-0.81]; reduced dose: HR, 0.27 [95% CI, 0.11-0.65]; P for heterogeneity = .11; Figure 2C and D) and most other prespecified subgroups (Figure 3), except for significantly greater benefit in non-Chinese participants (HR, 0.24 [95% CI, 0.10-0.56]) compared with Chinese participants (HR, 0.61 [95% CI, 0.44-0.84]) (P for heterogeneity = .046).
Figure 3. Primary Outcome in a Study of the Effect of Oral Methylprednisolone on Kidney Function Decline in Patients With IgA Nephropathy.
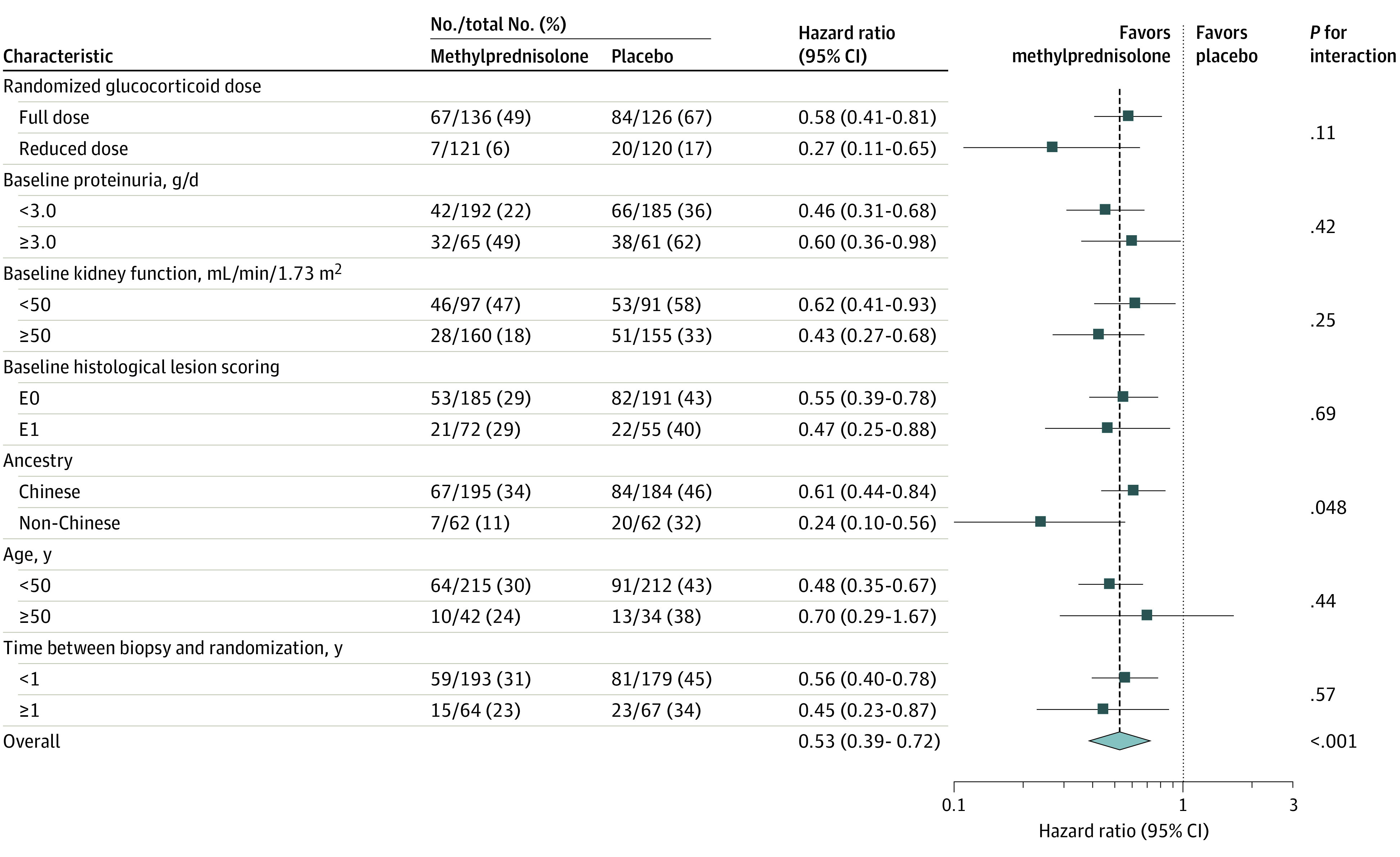
Hazard ratios and CIs were calculated with a Cox proportional hazards model with stratification according to region, baseline proteinuria, baseline estimated glomerular filtration rate, and kidney biopsy findings, with factors for trial group, subgroup, and the interaction between trial group and the subgroup variable. Ancestry was reported by the investigators. Proteinuria was 24-hour urinary protein.
Secondary Outcomes
The effect of the intervention on secondary outcomes is shown in Table 2. There was 1 death (0.4%) due to kidney failure in each group and 6 (2.3%) vs 3 (1.2%) deaths from any cause in the methylprednisolone vs placebo groups (P = .24). The risk of kidney failure requiring dialysis or transplant was significantly lower in the methylprednisolone group than the placebo group (50 [19.5%] vs 67 [27.2%]; HR, 0.59 [95% CI, 0.40-0.87]; P = .008; annual event rate difference, −2.9% per year [95% CI, −5.4% to −0.3%]; Table 2 and Figure 2B).
Time-averaged mean 24-hour urine protein excretion was significantly lower during follow-up in the methylprednisolone group vs the placebo group (1.70 g/d [95% CI, 1.54-1.86] vs 2.39 g/d [95% CI, 2.15-2.63]; between-group difference, −0.69 g/d [95% CI, −0.98 to −0.41]; P < .001) (eFigure 3A in Supplement 2). The difference was mostly seen relatively early and was no longer apparent by 3 years of follow-up. The results were similar for the full-dose protocol (1.80 g/d [95% CI, 1.57-2.03] vs 2.38 g/d [95% CI, 2.07-2.68]; between-group difference, −0.58 g/d [95% CI, −0.96 to −0.19]; P = .003) and the reduced-dose protocol (1.58 g/d [95% CI, 1.36-1.80] vs 2.41 g/d [95% CI, 2.04-2.78]; between-group difference, −0.83 g/d [95% CI, −1.25 to −0.40]; P < .001), without evidence of heterogeneity between the full-dose and reduced-dose protocols (P for heterogeneity = .39).
The annual rate of loss of kidney function was 2.50 mL/min/1.73 m2 per year in participants randomized to the methylprednisolone group compared with 4.97 mL/min/1.73 m2 per year in the placebo group (mean difference, 2.46 mL/min/1.73 m2 per year [ 95% CI, 0.94-3.99]; P = .002; eFigure 3B in Supplement 2). The effect on loss of kidney function was similar when eGFR measurements during therapy (months 1-6) were excluded (mean difference, 2.65 mL/min/1.73 m2 per year [95% CI, 1.27-4.03]; P < .001). The effects showed no significant difference (P for heterogeneity = .77) between the full-dose (2.73 mL/min/1.73 m2 per year [95% CI, 0.66-4.81]) and reduced-dose (2.29 mL/min/1.73 m2 per year [95% CI, 0.13-4.44]) regimens compared with participants randomized to receive placebo during the relevant study phase.
Adverse Events
Serious adverse events were reported in 28 participants (10.9%) randomized to the methylprednisolone group compared with 7 (2.8%) in the placebo group (eTable 4 in Supplement 2), mostly due to an excess of hospitalizations (25 vs 7) and serious infections (17 vs 3). The excess was primarily observed with the full-dose methylprednisolone regimen compared with participants randomized to receive placebo during that study period (22 vs 4), rather than the reduced-dose regimen compared with participants in the placebo group randomized at that time (6 vs 3). Four serious adverse events were fatal, all of which were in the methylprednisolone group (1.6%), and infection related, including 3 in the full-dose protocol (2.2%) and 1 in the low-dose protocol (0.8%).
Discussion
In this randomized clinical trial of patients with IgA nephropathy and persistent proteinuria of 1 g per day or greater, a 6- to 9-month course of treatment with oral methylprednisolone, compared with placebo, significantly reduced the risk of the composite outcome of decline in kidney function, kidney failure requiring dialysis or transplant, or death due to kidney disease. The observed kidney benefits showed no significant heterogeneity of treatment effect between a reduced-dose regimen and the original full-dose protocol. However, the incidence of serious adverse events was increased with oral methylprednisolone and the reduced-dose regimen had fewer adverse events compared with the full-dose regimen. Overall, these data suggest that a 6- to 9-month course of oral corticosteroids effectively protected kidney function in people with IgA nephropathy and that this benefit can be realized with a reduced-dose protocol with a lower risk of adverse events.
Results from this trial are consistent with pooled analyses of a heterogenous group of small studies of different glucocorticoid regimens in populations with IgA nephropathy, although these trials were not individually powered to detect benefits for clinically important outcomes.8,9 In contrast, the STOP-IgAN trial compared immunosuppression based on corticosteroids with standard care, but did not find kidney benefits in either the original randomized trial10 or during long-term follow-up,3 although higher rates of adverse events and infections were also seen. The reasons for these discrepant findings are not clear, but may relate to the slower rate of loss of kidney function observed among participants in that trial (including the control group), the different interventions used, or other patient characteristics. Although our study included a large percentage of participants from China, for whom different prognosis and/or therapeutic response have been proposed,16 there was no evidence of loss of efficacy in kidney benefit in non-Chinese participants.
Glucocorticoids are nonspecific immunosuppressive agents and are likely to produce benefit through their effects on the demonstrated autoimmune pathogenesis of IgA nephropathy.17 Glucocorticoids had also long been known to increase the risk of infections, as was observed with the original full-dose protocol used in this trial. The increased risk was able to be mitigated with a reduction in dosage and the addition of antibiotic prophylaxis with sulfamethoxazole-trimethoprim, such that a lower incidence of serious adverse events was observed in the reduced-dose groups after these changes were made. Various more specific strategies for immunomodulation have been proposed for people with IgA nephropathy, including systemic or intestinal B cell–focused interventions, complement inhibitors, and others.18,19,20 These therapies may have a superior risk-benefit profile, and this is being assessed in ongoing clinical trials.
This trial had a number of strengths. To our knowledge, it was the largest trial in IgA nephropathy completed to date and it provided more precise evidence than previous trials about the benefits and risks of this treatment. The double-blind nature of the intervention as well as the long and rigorous follow-up reduced the risk of bias, while the international multicenter involvement increased generalizability.
Limitations
This trial had several limitations. First, the majority of participants were from China, although the prespecified subgroup analyses found that the benefits were at least comparable in non-Chinese participants. Second, the dose of methylprednisolone used in the original protocol increased the risk of adverse events so that the study treatment was stopped and the trial was modified and transitioned to a lower-dose regimen, with participants recruited to that point unblinded, and a transitional analysis was published. The consistency in the findings among these participants, and those subsequently randomized to receive the lower-dose regimen, suggests that this process is unlikely to have meaningfully biased the results of the trial. Third, although clear reductions in the risk of major kidney outcomes were identified, the reduction in proteinuria was no longer apparent by 36 months after randomization, and post hoc analyses suggest that the benefit on other outcomes may diminish over time. Furthermore, many participants continued to lose kidney function over time, suggesting that additional longer-term therapies may also be required to optimize kidney outcomes in IgA nephropathy.
Conclusions
Among patients with IgA nephropathy at high risk of progression, treatment with oral methylprednisolone for 6 to 9 months, compared with placebo, significantly reduced the risk of the composite outcome of kidney function decline, kidney failure, or death due to kidney disease. However, the incidence of serious adverse events was increased with oral methylprednisolone, mainly with high-dose therapy.
Trial protocol and statistical analysis plan
eTable 1. Detailed Timeline of the TESTING Trial
eTable 2. Inclusion and Exclusion Criteria
eTable 3. Comparison of Baseline Characteristics Between Participants Randomized to the Full-dose and Reduced-dose Protocols
eTable 4. Serious Adverse Events by Randomized Group Overall, and for the Full and Reduced Methylprednisolone Dosage Regimens
eFigure 1. Summary of sequence of Events of the TESTING Trial
eFigure 2. Graphical Comparison Between Fixed Hazard Ratio and Time-Varying Hazard Ratio Using Kolmogorov-type Supremum Test
eFigure 3. Proteinuria and eGFR Reduction by Randomized Group Over Time
Nonauthor collaborators
Data sharing statement
References
- 1.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414-430. doi: 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 2.Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: core curriculum 2021. Am J Kidney Dis. 2021;78(3):429-441. doi: 10.1053/j.ajkd.2021.01.024 [DOI] [PubMed] [Google Scholar]
- 3.Rauen T, Wied S, Fitzner C, et al. ; STOP-IgAN Investigators . After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int. 2020;98(4):1044-1052. doi: 10.1016/j.kint.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 4.Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9(3):e91756. doi: 10.1371/journal.pone.0091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maillard N, Wyatt RJ, Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26(7):1503-1512. doi: 10.1681/ASN.2014101000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22(10):1795-1803. doi: 10.1681/ASN.2011050464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016;387(10032):2036-2048. doi: 10.1016/S0140-6736(16)00272-5 [DOI] [PubMed] [Google Scholar]
- 8.Lv J, Xu D, Perkovic V, et al. ; TESTING Study Group . Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol. 2012;23(6):1108-1116. doi: 10.1681/ASN.2011111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natale P, Palmer SC, Ruospo M, et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev. 2020;3:CD003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauen T, Eitner F, Fitzner C, et al. ; STOP-IgAN Investigators . Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373(23):2225-2236. doi: 10.1056/NEJMoa1415463 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 12.Wong MG, Lv J, Hladunewich MA, et al. ; TESTING Study Group . The therapeutic evaluation of steroids in IgA nephropathy global (TESTING) study: trial design and baseline characteristics. Am J Nephrol. 2021;52(10-11):827-836. doi: 10.1159/000519812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billot L, Monaghan H, Wong MG, Perkovic V. Statistical analysis plan for the Therapeutic Evaluation of Steroids in Iga Nephropathy Global (TESTING) study. OSFPreprints. Preprint posted August 3, 2021. doi:10.31219/osf.io/d7qrw
- 14.Lv J, Zhang H, Wong MG, et al. ; TESTING Study Group . Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318(5):432-442. doi: 10.1001/jama.2017.9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trimarchi H, Barratt J, Cattran DC, et al. ; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants . Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014-1021. doi: 10.1016/j.kint.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 16.Barbour SJ, Cattran DC, Kim SJ, et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013;84(5):1017-1024. doi: 10.1038/ki.2013.210 [DOI] [PubMed] [Google Scholar]
- 17.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402-2414. doi: 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 18.Selvaskandan H, Cheung CK, Muto M, Barratt J. New strategies and perspectives on managing IgA nephropathy. Clin Exp Nephrol. 2019;23(5):577-588. doi: 10.1007/s10157-019-01700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep. 2020;5(11):2032-2041. doi: 10.1016/j.ekir.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellström BC, Barratt J, Cook H, et al. ; NEFIGAN Trial Investigators . Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389(10084):2117-2127. doi: 10.1016/S0140-6736(17)30550-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eTable 1. Detailed Timeline of the TESTING Trial
eTable 2. Inclusion and Exclusion Criteria
eTable 3. Comparison of Baseline Characteristics Between Participants Randomized to the Full-dose and Reduced-dose Protocols
eTable 4. Serious Adverse Events by Randomized Group Overall, and for the Full and Reduced Methylprednisolone Dosage Regimens
eFigure 1. Summary of sequence of Events of the TESTING Trial
eFigure 2. Graphical Comparison Between Fixed Hazard Ratio and Time-Varying Hazard Ratio Using Kolmogorov-type Supremum Test
eFigure 3. Proteinuria and eGFR Reduction by Randomized Group Over Time
Nonauthor collaborators
Data sharing statement