Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Italy, and co‐rapporteur Member State, France, for the pesticide active substance oxamyl and the assessment of applications for maximum residue levels (MRLs) are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative uses of oxamyl as a nematicide on potato and tobacco (field use), on tomato (permanent greenhouse), on cucurbits (edible and inedible peel), pepper, aubergine and plants nurseries of the above‐mentioned crops on soil bed preparation (permanent greenhouse). The reliable end points, appropriate for use in regulatory risk assessment and the proposed MRLs, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: oxamyl, peer review, risk assessment, pesticide, nematicide, article 12 confirmatory data
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Oxamyl is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Italy, and co‐rapporteur Member State (co‐RMS), France, received an application from DuPont Production Agriscience Deutschland GmbH (current name Corteva Agriscience) for the renewal of approval of the active substance oxamyl. In addition, DuPont Production Agriscience Deutschland GmbH (currently Corteva Agriscience) submitted for the assessment of confirmatory data following the review of maximum residue levels (MRLs), as referred to in Article 12 of Regulation (EC) No 396/2005.
An initial evaluation of the dossier on oxamyl was provided by the RMS in the renewal assessment report (RAR), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The representative uses of oxamyl as a nematicide on potato and tobacco (field use) and tomato (permanent greenhouse) as well as on solarisation (soil bed preparation in permanent greenhouse) for growing of tomato, cucurbits (edible and inedible peel), pepper, aubergine and plants nurseries of the above‐mentioned crops, as proposed at EU level results in a sufficient nematicidal efficacy against the target organisms.
In the area of identity, physical–chemical properties and analytical methods, there were not any critical areas of concern.
In the area of mammalian toxicology, the representativeness of the material tested in the toxicological studies with regard to the proposed technical specification could not be concluded in the absence of the assessment of the toxicological relevance of all impurities (issue that could not be finalised and data gap). Additionally, exceedance of the (A)AOEL for the operators’ exposure estimates for all uses was identified as a critical area of concern.
In the area of residues, several data gaps were identified and the residue definition for risk assessment proposed as oxamyl only in primary and rotational crops is provisional. The consumer dietary risk assessment for the representative uses could not therefore be concluded (issue not finalised). The preliminary chronic consumer risk assessment did not indicate exceedance of the acceptable daily intake (ADI) (90% of the ADI (GEMS/Food G06)) whilst a significant exceedance of the acute reference dose (ARfD) was noted for all the representative uses – 1,538% (potatoes; children), 1,223% (watermelons, children), 656% (cucumbers, children) and 581% (tomatoes, children). Furthermore, as IN‐D2708 and IN‐A2213 are groundwater metabolites that exceeded the concentration of 0.75 µg/L, the consumer risk assessment through drinking water has been performed regarding these compounds and the TMDI accounted for 33% and 251% of the ADI for adults, 98% and 753% of the ADI for children and 147% and 1129% of the ADI for infants, for IN‐A2213 and IN‐D2708, respectively. This is identified as a critical area of concern. Moreover, a global exposure of the consumers to IN‐A2213 and IN‐D2708 through dietary intake and drinking water in compliance with the Guidance document (European Commission, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001) could not be carried out in view of the identified data gaps.
As an outcome of the renewal review, specifically as for the lowered toxicological reference values for oxamyl, a screening assessment for all MRLs in place indicated a TMDI corresponding to 1,240% of the ADI (NL toddler) and a large exceedance of the ARfD for several commodities (top 3: 1,538% potatoes, 1,517% melons, 1,385% pears). The confirmatory data identified during the review of MRLs have been addressed or become obsolete.
The data available on environmental fate and behaviour were sufficient to carry out the required environmental exposure assessments at EU level for the representative uses, with the notable exception that information was not available regarding the effect of ozonation on the degradation of oxamyl that might be present in surface and the effect of water treatments processes on the nature of residues of oxamyl transformation products that might be present in surface and groundwater, when surface water or groundwater are abstracted for the production of drinking water. Consequently, the consumer risk assessment through drinking water could not be finalised. Furthermore, a critical area of concern was identified for the potential groundwater contamination by oxamyl and relevant metabolites.
In the area of ecotoxicology, the representativeness of the material tested in the (eco)toxicological studies with regard to the proposed technical specification could not be concluded in the absence of the assessment of the (eco)toxicological relevance of all impurities. Although no critical area of concern was concluded, a high risk for birds, mammals, aquatic organisms, bees, non‐target arthropods other than bees and soil organisms was indicated for some of the representative field uses, whereas low risk was identified for non‐target terrestrial plants and microorganism involved in biological methods for sewage treatment. Furthermore, the risk assessment for birds and mammals could not be finalised for several exposure scenarios. For the uses in permanent greenhouses, low risk was concluded for all groups of non‐target organisms.
Based on the available data and assessment, it is concluded that oxamyl does not meet the criteria for endocrine disruption for humans and non‐target organisms through oestrogen, androgen, thyroid and steroidogenic (EATS)‐modalities as set in point 3.6.5. and point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
Background
Commission Implementing Regulation (EU) No 844/2012 1 , as amended by Commission Implementing Regulation (EU) No 2018/1659 2 (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009 3 . This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS, Italy, and co‐RMS, France, received an application from DuPont Production Agriscience Deutschland GmbH (current name Corteva Agriscience) for the renewal of approval of the active substance oxamyl. In addition, DuPont Production Agriscience Deutschland GmbH (currently Corteva Agriscience) submitted application for the assessment of the confirmatory data following the review of MRLs according to Article 12 of Regulation (EC) No 396/2005 4 . Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (France), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on oxamyl in the RAR, which was received by EFSA on 15 October 2019 (Italy, 2020).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, DuPont Production Agriscience Deutschland GmbH (current name Corteva Agriscience), for consultation and comments on 8 September 2020. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 8 November 2020. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant’s response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 28 January 2021. On the basis of the comments received, the applicant’s response to the comments and the RMS’s evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA’s further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment and on the confirmatory data following the review of MRLs according to Article 12 of Regulation (EC) No 396/2005 took place with Member States via a written procedure in January–February 2022.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of oxamyl as a nematicide on potato, tobacco (field use), tomato (permanent greenhouse), cucurbits (edible and inedible peel), pepper, aubergine and plants nurseries of the above‐mentioned crops (permanent greenhouse application), as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion. MRLs were assessed in potato, tobacco (field use), tomato, cucurbits (edible and inedible peel), pepper, aubergine and plants nurseries of the above‐mentioned crops (soil bed preparation in permanent greenhouses).
A list of the relevant end points for the active substance and the formulation is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for oxamyl according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2022), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (28 January 2021);
the evaluation table (28 March 2022);
the report(s) of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Italy, 2021), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Oxamyl is the ISO common name for methyl (EZ)‐2‐(dimethylamino)‐N‐[(methylcarbamoyl)oxy]‐2‐oxothioacetimidate (IUPAC).
The representative formulated products for the evaluation were ‘Oxamyl 10GR’ a granule formulation (GR) containing 100 g/kg oxamyl, and ‘Oxamyl 10SL’ a soluble concentrate (SL) containing 100 g/L oxamyl.
The representative uses evaluated for ‘Oxamyl 10GR’ were tractor mounted broadcast and in‐furrow granule applications on soil for the control of nematicides before planting of potato crops in the central European zone and before planting of tobacco crops in southern European zone. The representative uses evaluated for ‘Oxamyl 10SL’ were drip irrigation on soil in permanently closed greenhouses, for the control of nematicides on tomato crop and for the control of nematodes in soil beds designated for tomato, cucurbits (edible and inedible peel), pepper and aubergine crops. Full details of the good agricultural practices (GAPs) can be found in the list of end points in Appendix B.
Data were submitted to conclude that the representative uses of oxamyl proposed at EU level result in sufficient nematocidal efficacy against the target organisms, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
A data gap has been identified for a transparent evaluation by the RMS of the search of the scientific peer‐reviewed open literature on the active substance and its relevant metabolites, dealing with side effects on health, the environment and non‐target species and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion (European Commission, 2000a,b, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001).
The proposed specification for oxamyl was based on batch data from industrial plant production. Oxamyl is produced as a technical concentrate (TK) containing 41.8–42.9% oxamyl (equal to 392.1–402.0 g/kg pure oxamyl). The current reference specification was based on data from a solid technical material that was artificially precipitated in the laboratory from the oxamyl technical concentrate and subsequently collected and dried. This precipitation process resulted in lower quantities of certain impurities in the precipitated technical material relative to those present in the oxamyl technical concentrate from which they were derived and relative to the impurities specified on theoretical dry weight calculations. The RMS proposed to change the current reference specification to the specification calculated on a theoretical dry weight, it should be noted that the RMS proposal was based on current and older batch data, whilst EFSA is of the opinion that only current batch data should be considered. The proposed updated minimum purity based on the theoretically calculated dry technical material is 936 g/kg, which is lower than the minimum purity in the current reference specification (970 g/kg). It should be noted that even if the proposed updated minimum purity was based on data from the laboratory precipitated oxamyl solid technical material, a lower minimum purity, compared to the current reference specification, equal to 947 g/kg would be derived. The batches used in the (eco)toxicological assessment do not support the original and updated reference specification (See Sections 2 and 5). It should be noted that evaluation of the toxicological relevance of several impurities is not finalised (See Section 2) and as a consequence, new data such as spectral data, content of the impurities before and after the storage of the formulation and methods for analysis of the relevant impurities in the formulation might be required. The manufactured technical concentrate meets the requirements of the existing FAO specification (342/TK, April 2008, belonging to the material of DuPont Crop Protection (USA)) in terms of minimum purity.
The main data regarding the identity of oxamyl and its physical and chemical properties are given in Appendix B.
Adequate methods are available for the generation of data required for the risk assessment except for analytical methods used in the toxicological studies, for which an RMS’s assessment is missing (data gap). Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulations and for the determination of the respective impurities in the technical material.
Oxamyl residues can be monitored in food and feed of plant origin by the quick, easy, cheap, effective and safe (QuEChERS) method (high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS)) with a limit of quantification (LOQ) of 0.01 mg/kg in the four major crop groups and dried tobacco leaf. However, it should be noted that the matrix effects were not studied in the original method and in the independent laboratory validation (ILV) (data gap). In addition, efficiency of the extraction procedure used was not verified (data gap). Oxamyl residues in food of animal origin can be determined by the QuEChERS method using HPLC–MS/MS with LOQ of 0.01 mg/kg in all animal matrices, it should be noted that the absence of matrix effects and the extraction efficiency was not verified for the monitoring method and its ILV study. A single oxamyl liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) method with LOQ of 0.01 mg/kg was also provided for monitoring residues in food of animal origin, yet the absence of matrix effects and the extraction efficiency was not verified for the original single oxamyl method and its ILV study. However, pending the decision on setting MRLs for animal products, no relevant data gaps were currently set for the residue monitoring method in food of animal origin.
Oxamyl residues in soil and air can be monitored by HPLC–MS/MS with LOQs of 0.001 mg/kg and 0.25 μg/m3, respectively. Oxamyl residues in surface water, ground water and drinking water can be analysed by HPLC–MS/MS with an LOQ of 0.1 μg/L. It should be noted that calibration data for its ILV study were not provided (data gap).
Oxamyl residues in body tissues can be determined by using the monitoring methods for residue in food of animal origin, note that the absence of matrix effects was not verified for all matrixes in the multiresidue monitoring method and in the single oxamyl monitoring method for residue in food of animal origin (data gap). Oxamyl residues in body fluids can be monitored by HPLC–MS/MS with LOQ of 0.05 mg/kg. It should be noted that the absence of matrix effects was not verified (data gap).
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion (European Commission, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001, 2012; EFSA PPR Panel, 2012; EFSA 2014b; ECHA, 2017). Oxamyl was discussed at the Pesticides Peer Review Experts’ Meeting PREV TC 60 in September 2021.
Based on the available information, the batches used in the critical toxicological studies cannot be considered as covering the proposed and the original technical specification (critical area of concern). According to the first peer review (EFSA, 2005), no toxicologically relevant impurities were identified in Annex 1 technical specification. Based on current available data, the toxicological relevance of several impurities in the proposed technical specification cannot be concluded (data gap).
Oral absorption of oxamyl accounts for > 90% of the given dose. Excretion mainly occurs via the urinary route, whilst faeces and expired air are minor elimination routes. In the rat, oxamyl widely distributes into organs and tissues, with the highest levels being reached in whole blood, heart, liver, kidney, lungs, spleen and gastrointestinal tract. There is no evidence of bioaccumulation.
In rats, oxamyl is rapidly and extensively metabolised by hydrolysis to IN‐A2213, an oxime. Main urinary elimination occurs in the form of IN‐A2213 glucuronide following glucurono‐ conjugation. Besides hydrolysis, the parent compound can undergo enzymatic conversion via the N,N‐dimethyl‐ carbonocyanidic amide (IN‐N0079) to N,N’‐dimethyloxamic acid (IN‐D2708) and N‐methyloxamic acid (IN‐KP532). Minor unidentified metabolites are considered to be conjugates of demethylated (e.g. IN‐L2953) or IN‐D2708. No major metabolic inter‐species differences have been observed. Also, no unique human metabolites have been identified in the in vitro comparative metabolism study using mouse, rat, rabbit, dog and human hepatocytes.
Oxamyl has high acute oral toxicity (possibly triggering the CLP criteria for classification (ECHA, 2017) as Acute Tox. 1; H300) and high acute inhalation toxicity (harmonised classification 5 : Acute Tox. 2; H330), but relatively low acute dermal toxicity (harmonised classification5: Acute Tox. 4; H312). It is not an eye or skin irritant, or a phototoxicant. The lack of a skin sensitising effect of oxamyl was agreed 6 based on the weight of the evidence.
Short‐term dietary exposure to oxamyl results in the rat: in decreased body weight gain and absolute organ weight, and blood in urine; in the dog: in clinical signs among females and decreased plasma and brain cholinesterase activity among males; in the rabbit: in decreased plasma erythrocyte and brain cholinesterase activity. The relevant overall oral subchronic NOAEL is 1 mg/kg bw per day based on the combined assessment of two 12‐month dog studies.
The weight of the in vitro and in vivo genotoxicity studies supports that oxamyl is unlikely to be genotoxic in humans.
In a long‐term dietary exposure study in rats, the critical systemic effects were ovary atrophy in females (basis for setting the relevant systemic NOAEL at 1.32 mg/kg per day), and lower body weight and body weight gain, clinical signs as well as plasma cholinesterase inhibition in males (NOAEL: 1.97 mg/kg per day). In mice, decreased body weight was the basis for setting the long‐term NOAEL at 4.2 mg/kg bw per day.
Oxamyl showed no evidence of oncogenicity at any dose in the long‐term rat and mouse studies. The overall weight of the evidence suggests that oxamyl does not raise any concern for carcinogenicity.
With respect to reproductive toxicity, the same NOAEL from a two‐generation rat study of 1.43 mg/kg bw per day applied to both parental toxicity (decreased body weight and body weight gain, reduced food consumption and efficiency during treatment in both males and females of both generations) and offspring toxicity (significant treatment‐related reduction in pup mean body weights). The relevant reproductive NOAEL was agreed at 4.22 mg/kg bw per day, based on decreased pup survival and decreased litter size in both generations.
In a rat developmental toxicity study with oxamyl, the maternal and developmental NOAELs were 0.5 mg/kg bw per day based on reduced food consumption and body weight gain and tremors in dams and decreased fetal weight in offspring. Signs of maternal (decreased body weight gain) and developmental (increased resorption) toxicity of oxamyl were also detected in rabbits at (slightly) higher doses.
Acute neurotoxicity is the most sensitive adverse effect of oxamyl. In rats, the acute oral (by gavage) neurotoxicity NOAEL was identified at 0.1 mg/kg bw for males/females based on clinical signs and significant reductions in brain, plasma and erythrocyte cholinesterase activity on day 1, decreases in body weight, perturbations in Functional Observation Battery (FOB), including exophthalmos, and in motor activity parameters at 1 mg/kg bw. Similar effects were also observed in a rat subchronic oral (via the diet) neurotoxicity study where the NOAEL was set at higher doses than the acute NOAEL (10 ppm, corresponding to 0.67 mg/kg bw per day) on the basis of dose‐related, although not statistically significant, increase in exophthalmos at 30 ppm (corresponding to 1.69 mg/kg bw per day). This finding indicates that a cumulative effect over time is unlikely as a consequence of subchronic administration of oxamyl, rather adaptation might occur. A few limited experimental evidence available indicates potential slightly higher sensitivity of pre‐weanling pups compared to adults, to oxamyl‐induced inhibition of brain cholinesterase in rats. 7 Some literature data with other carbamates are also suggestive of a higher susceptibility of pups to this adverse effect. However, in the absence of robust experimental data, it is unclear if age‐related differences in sensitivity, with higher sensitivity possibly displayed by rat pups compared to adult rats might occur. On this basis, an outstanding data gap for a DNT study8 (likely via gavage) was agreed by the experts though the RMS expressed some doubts that a DNT study would change the overall picture.
All toxicological reference values (TRVs) for oxamyl are based on the same key study, 8 i.e. the rat acute oral neurotoxicity study, with an NOAEL of 0.1 mg/kg bw based on neurotoxicity findings. Specifically, the acceptable daily intake (ADI), the acute reference dose (ARfD), the acceptable operator exposure level (AOEL) and the acute AOEL (AAOEL) have been set at 0.0001 mg/kg bw (per day). Compared with the TRVs agreed for the first approval (European Commission, 2011a, 2011b), these newly agreed TRVs have been decreased by 10‐fold. In particular, an extra‐factor of 10 has been added to the standard uncertainty factor (UF) of 100 to cover the DNT data gap, with the overall UF resulting to be 1,000 9 . Even though significant differences across species in the susceptibility to oxamyl are not expected, it was also acknowledged that a DNT effect would likely result from an acute exposure to oxamyl, and that a specific subgroup of the population may be particularly sensitive to this effect. The conservative approach proposed is also consistent with previous EFSA evaluations where the same 10‐fold factor was used to cover the DNT data gap. The RMS and another expert considered instead the 10‐fold extra factor overly conservative due to the presence of some data, though uncertain, regarding some age‐related toxicity.
Regarding dermal absorption, in the absence of an acceptable study in line with the EFSA Guidance, a default value of 10% was agreed for the concentrate of Oxamyl 10GR (the granular formulation is applied as concentrate without dilution). For Oxamyl 10SL, dermal absorption of 5.4% was agreed 10 on the basis of the in vitro study on human skin samples.
For Oxamyl 10GR (use on potato), operator exposure estimates with EFSA model (2014) exceed largely the (A)AOEL. Based on a field study, the use of the highest individual exposure values, together with additional protection factors for the use of gloves and respiratory protective equipment (RPE) during mixing/loading and application, the refined exposure estimates were still exceeding the AOEL (137%). It is noted that the RMS had a diverging view, supporting additional protection factors for the use of a closed cabin (which is only relevant for high crop application in the EFSA model) and closed transfer system. It is acknowledged that the use of a close transfer system may reduce the exposure, but the exact percent decrease cannot be defined in the absence of a validated protection factor set for this technological equipment. Workers’ exposure is not considered after in furrow application of granules. For residents and bystanders, the exposure to oxamyl vapours is estimated, according to the EFSA model, to exceed the A(AOEL) (by 230% and 1,070% in adult and child, respectively). However, the resident and bystander exposure to vapour is considered unlikely in view of the physico‐chemical properties of the active substance and the application technique (in furrow or soil incorporation) of oxamyl granules for both potato and tobacco use.
For Oxamyl 10SL, the operator exposure estimates during mixing and loading are above the AOEL (min 112%) and AAOEL (min 389%) for all uses, even with the use of personal protective equipment (PPE) including RPE. The RMS proposed to apply an additional protection factor for the use of a closed transfer system. It is noted that there is no EU‐validated protection factor in place for this technological equipment and it is not expected that acute exposure will be reduced to a value below the AAOEL. Predicted exposure levels for workers are below the A(AOEL) considering contact with soil residues during inspection activities. For bystanders and residents, it was noted that the scenario of application by drip irrigation in greenhouses is not included in the EFSA model (EFSA, 2014b) 11 . In principle, emission from the ventilation systems of permanent closed greenhouses could be a source of exposure to vapours for bystanders and residents. However, considering indoor application by drip irrigation and low volatility of the substance, significant exposure of residents and bystanders is not expected.
For the metabolites identified in residues (see Section 3) IN‐A2213, IN‐D2708, IN‐QKT34 and IN‐N0079, an in vitro acetylcholinesterase (AChE) inhibition study confirmed their lower potencies in respect to the parent. IN‐A2213 (also groundwater metabolite) was identified as a major rat metabolite, less acutely toxic than oxamyl. Regarding genotoxicity, IN‐A2213 did not show any potential for mutagenicity in bacterial cells and mammalian cells; in addition, it showed no potential to induce chromosome aberration (both structural and numerical) in the in vitro mammalian cell micronucleus test. Being a major rat metabolite, the reference values of the parent can be applied as a worst‐case scenario. 12 IN‐D2708 (also a groundwater metabolite) was also identified as a major rat metabolites and shown to be less acutely toxic than the parent. Regarding genotoxicity, IN‐D2708 showed an inconclusive results in an Ames test, whilst no potential to induce gene mutation in mammalian cells and chromosome aberration in the in vitro micronucleus test. Being a major rat metabolite, the reference values of the parent can be applied as a worst‐case scenario also to IN‐D27088. IN‐QKT34 being a glucoside conjugate of IN‐A2213, it is considered covered by the parent and same reference values can be applied.8 For IN‐N0079, the ADME study in rats with oxamyl indicated that it is an intermediate of IN‐D2708 and IN‐KP532. Regarding genotoxicity, only an Ames test was available (negative) and the quantitative structure–activity relationship (QSAR) analysis provided for gene mutation and chromosome aberration end points showed both negative and positive predictions. Regarding general toxicity, studies available indicated that IN‐N0079 is less acutely toxic than the parent and also less toxic than oxamyl from the 10‐day and 90‐day oral studies in the rat. The majority of experts agreed that the reference values of the parent can be applied also to IN‐N0079 mainly in consideration of the fact it is an intermediate of IN‐D2708 and IN‐KP532.8 Regarding the metabolite thiocyanate, the reported QSAR analysis would support the lack of genotoxic potential (see also Section 3). No data on general toxicity are available for this metabolite.
3. Residues
The assessment in the residue section is based on the following guidance documents (OECD, 2009, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001; European Commission, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001, 2011a, 2011b; JMPR, 2004, 2007).
3.1. Representative use residues
Studies investigating the metabolism of oxamyl in fruit crops (tomatoes) following foliar and soil treatment and in root crops (potatoes) following soil treatment were considered as guideline compliant to support the representative uses on these crops. In tomato fruit following foliar and soil treatments, parent oxamyl was recovered at only up to 3% total radioactive residue (TRR) (0.028 mg eq./kg) and metabolite IN‐D2708 was found to be the predominant compound of the total radioactive residues with up to 21% TRR (0.2 mg eq./kg). Besides, the total radioactive residues were constituted of several metabolites (IN‐A2213, IN‐N0079 and IN‐QKT‐34) that occurred each at a level below 10% of the TRR but at relevant absolute concentrations (> 0.05 mg eq./kg) along with polar compounds that accounted for up to 23% TRR and suggesting the incorporation of a significant fraction of the radioactive residues into natural plant constituents. In potato tubers following soil treatment, the parent oxamyl was never detected whilst IN‐D2708 was by far the predominant component of the total residues (71% TRR; 0.61 mg eq./kg). The rest of the radioactivity was characterised as polar compounds. Also, in potato foliage, the parent compound was hardly detected (1% TRR) and IN‐QKT34 was recovered at significant levels (45.7% TRR; 0.69 mg eq./kg) besides polar compounds (13.5% TRR). Additional metabolism studies on fruit crops, pulses and oilseeds crops, on potatoes and tobacco leaves were non‐GAP compliant and provided supportive information only. Based on the supportive metabolism data on tobacco leaves and the overall metabolic pattern of oxamyl depicted in the leafy parts of potatoes and tomatoes, a new guideline‐compliant metabolism study specifically on tobacco leaves is not needed and the metabolism of oxamyl can be considered as sufficiently depicted for the representative uses. However, in case additional uses on leafy crops are intended to be authorised, a fully guideline compliant metabolism study on a crop representative of the leafy crops should be provided.
Although it is acknowledged that the parent oxamyl was recovered at proportions below 10% TRR in tomato fruit and not detected at all in the potato tuber, it is proposed to set the residue definition for monitoring by default as oxamyl alone. From a risk assessment point of view, the predominant compounds of the total residues identified in tomato fruit (IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079) and in potato tuber (IN‐D2708) are major rat metabolites, and therefore, the toxicological reference values of the parent compound are applicable to these compounds (see Section 2). Since these metabolites may globally contribute significantly to the toxicological and dietary burden in view of their much higher concentration occurrence compared to the parent compound as observed from the metabolism data, their potential inclusion in the risk assessment residue definition should be envisaged. Complete residue data sets on tomatoes and potatoes compliant with the respective GAPs for the determination of the residues of the compounds (IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079) relevant for these crops should therefore be provided (data gap). Meanwhile, the residue definition for risk assessment is provisionally set as oxamyl only. The proposed residue definitions apply to the representative uses only. Finally, it is also noted that since IN‐QKT34 is the major compound of the total residues identified in potato foliage, it is recommended that for any future use on root crops with the upper leafy parts that can be fed to animals (e.g. sugar beet and turnips tops and leaves), sufficient residue trials may be needed to determine the magnitude of residues of this metabolite in the leafy parts of the root crops to conduct the livestock dietary burden calculation.
A study simulating normal processing practices by using the representative hydrolytic conditions of pasteurisation, baking/brewing/boiling and sterilisation showed that with increasing temperature oxamyl degraded significantly into IN‐A2213 at baking/boiling (41.42% of the applied radioactivity (AR)) and was completely degraded into this compound at sterilisation. Oxamyl remained stable at pasteurisation. Pending the finalisation of the plant residue definition for risk assessment, additional hydrolysis studies may be required to determine the nature of the residues of all compounds included in the residue definition. Studies investigating the magnitude of residues of oxamyl in processed commodities were provided for potatoes only.
Although not triggered (field soil DT90 for Oxamyl: 31.4 days; for IN‐A2213: 58 days and for IN‐D2708: 30.1 days), confined rotational crops’ metabolism studies were provided and showed a similar metabolic pattern as depicted in primary crops with an extensive degradation of oxamyl. From these studies, there are also indications that when rotational crops are planted at the shortest plant back interval (PBI) of 30 days, residues of oxamyl and several metabolites (IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079) are recovered at quantifiable levels in root crops, leafy crops and cereals, small grain. The available rotational crops’ field trials determined the residues of parent oxamyl at a level below the LOQ of 0.01 mg/kg at PBIs of 80 and 120 days for leafy crops, root crops, cereals and at PBIs from 30 to 120 days for leafy and root crops. Additional rotational crops’ field trials covering the NEU and SEU zones are required to analyse the residues of oxamyl and of the metabolites IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079 in the edible parts of the representative rotational crops at the shortest PBI of 30 days (data gap). Meanwhile, the same residue definitions for monitoring and risk assessment as proposed for primary crops also apply to the rotational crops.
Besides the requested complete GAP‐compliant residue data sets on potatoes and tomatoes for the determination of the relevant compounds identified in these crops (see data gap here above), sufficient GAP‐compliant residue trials on potatoes should be provided to confirm that the residues of oxamyl are below the LOQ as observed from the metabolism data (data gap). The use on solarisation was supported by two residue trials, respectively, on cherry tomatoes and courgettes. To comply with the current data requirements, at least two additional GAP compliant residue trials analysing for oxamyl residues, respectively, on tomatoes and cucumbers (with a possible extrapolation to fruiting vegetables, except sweet corn) should be provided to confirm that no quantifiable residues are expected in fruiting vegetables when oxamyl is applied in accordance with the representative use on solarisation (data gap). Sufficient acceptable residue trials analysing for oxamyl residues were available for the representative uses on tomatoes and tobacco. All the requested residue trials in primary and rotational crops should be supported by sufficiently validated analytical methods and acceptable storage stability data.
In the metabolism studies conducted in poultry and ruminants with the [1‐14C] oxamyl, the parent compound was shown to be extensively metabolised when fed to hens and goats as residues as oxamyl and its structurally related metabolites were not detected in any of the tissues, milk or eggs. The major metabolite found in both lactating goats and laying hens matrices was the non‐specific compound thiocyanate that accounted for up to 31% TRR and 48.5% TRR in ruminant tissues and milk, respectively. The genotoxicity potential of thiocyanate could be ruled out (see Section 2). Further characterisation of the residues in tissues demonstrated fragmentation of oxamyl into numerous polar components, many of these being released through proteolytic digestion suggesting incorporation of the radioactivity into natural components. The in vitro rumen fluid metabolism study also demonstrated that oxamyl was extensively metabolised prior to absorption in the goats. The livestock dietary burden will have to be recalculated based on the requested field residue trials on potatoes and rotational crops to conclude whether the residue levels of oxamyl and the relevant metabolites may lead to a significant dietary intake (> 0.004 mg/kg bw per day). Meanwhile, residue definitions are not proposed for products of animal origin.
From the metabolism data in plants, IN‐D2708 was shown to be the predominant compound of the total residues in potato tuber grown as a primary crop and in cereal grain when cereals, small grain are grown in rotation. Since the log Pow of IN‐D2708 is below 3, a fish metabolism study dosed with this compound is therefore not required. This assessment should be confirmed considering the data gaps set for residue trials analysing for all the relevant compounds in potatoes as a primary crop and in rotational crops.
The data requirement to determine the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom is not triggered for the representative uses on tomatoes, potatoes and tobacco as these crops have low or no melliferous capacity (low attractivity for pollen collection and not relevant at all for nectar collection). However, and pending upon the magnitude of residues of the relevant metabolites in rotational crops (see data gap above), the residues in pollen and bee products might need to be addressed.
In view of the identified data gaps to finalise the residue definitions for risk assessment in primary and in rotational crops, a provisional consumer dietary intake calculation has been carried out considering the risk assessment input values for oxamyl at the LOQ (0.01 mg/kg) of the method for the representative uses, respectively, on potatoes, tomatoes and fruiting vegetables following solarisation according to the EFSA PRIMo rev. 3.1 model. The calculated chronic intake accounted for 90% of the ADI (GEMS/Food G06) whilst a large exceedance of the ARfD was noted for all the representative uses – 1,538% (potatoes; children), 1,223% (watermelons, children), 656% (cucumbers, children) and 581% (tomatoes, children). According to EFSA PRIMo rev. 2A model, the calculated chronic intake accounted for 83.5% of the ADI (WHO Cluster diet B) whilst the highest acute intake was 1538% of the ARfD (potatoes; children), 1,516% (melons, children), 630% (peppers, children) and 581.5% (tomatoes, children). Furthermore, as IN‐D2708 and IN‐A2213 are also groundwater metabolites that exceeded the concentration of 0.75 µg/L, the consumer risk assessment through drinking water has been performed regarding these compounds and the TMDI accounted for 33% and 251% of the ADI for adults, 98% and 753% of the ADI for children and 147% and 1129% of the ADI for infants, for IN‐A2213 and IN‐D2708, respectively. This is considered a critical area of concern. Moreover, a global exposure of the consumers to IN‐A2213 and IN‐D2708 through dietary intake and drinking water in compliance with the Guidance document (European Commission, 2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001) could not be carried out in view of the identified data gaps. Finally, the consumer risk assessment is not finalised regarding the unknown nature of residues that might be present in drinking water, consequent to water treatment processes following abstraction of surface water or groundwater that might contain oxamyl and its metabolites (see Section 4).
3.2. Confirmatory data under Article 12 MRL review
An MRL application has been submitted to address the confirmatory data identified during the MRL review (Art.12) (EFSA, 2010). The data gaps identified for a metabolism study with radioactive marker representative for the use of oxamyl by drip irrigation in fruits and fruiting vegetables and for a study demonstrating storage stability of oxamyl residues in commodities with high acid content have been addressed in the draft renewal assessment report. The data gap for four additional residues trials on oranges and four additional residues trials on mandarins compliant with southern outdoor GAPs for these crops is considered as obsolete as the use on citrus is no longer supported.
As an outcome of the renewal review, specifically as for the lowered toxicological reference values for oxamyl, a screening assessment for all MRLs in place indicated a TMDI corresponding to 1,240% of the ADI (NL toddler) and a large exceedance of the ARfD for several commodities (top 3: 1,538% potatoes, 1,517% melons, 1,385% pears).
4. Environmental fate and behaviour
Oxamyl was discussed at the Pesticides Peer Review Experts’ Meeting TC 61 in September 2021.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, oxamyl exhibited low to medium persistence, forming the major (> 10% applied radioactivity (AR)) metabolites IN‐A2213 (max. 51% AR) which exhibited low to moderate persistence, and IN‐D2708 (max. 78% AR), which exhibited low to medium persistence (see Appendix C for the wording in relation to DT and Koc ‘classes’ exhibited by each compound assessed). For oxamyl, no clear pH dependency of the soil degradation can be established, and then, a data gap is identified for investigating the potential pH dependency of the soil degradation of oxamyl in acid soils. Mineralisation of the [1‐14C] radiolabel to carbon dioxide accounted for 17.9–35.2% AR at 90 days. The formation of unextractable residues (not extracted by acetonitrile/water or methanol/water) for this radiolabel accounted for 15.3–19.3% AR at 90 days. In an anaerobic soil incubation, oxamyl exhibited low persistence forming no novel metabolites. In a laboratory soil photolysis study, oxamyl degraded more rapidly than in the dark control forming no major metabolites.
For oxamyl, only one soil at 20°C was available for deriving adsorption end points, and then, a data gap was identified for reliable batch adsorption experiments at 20°C on at least three additional soils. Considering this data gap, it was agreed to perform two sets of calculations for the exposure assessment using for oxamyl the default worst‐case Kfoc values of 10 mL/g and 10,000 mL/g. Metabolites IN‐A2213 and IN‐D2708 exhibited very high soil mobility. It was concluded that the adsorption of all these compounds was not pH dependent.
In satisfactory field dissipation studies carried out at one site in the Netherlands and one in the United Kingdom (incorporated application in bare soil plots), oxamyl exhibited very low to low persistence. Sample analyses were carried out also for metabolites IN‐A2213 and IN‐D2708. Field study DegT50 values for oxamyl were derived following normalisation to FOCUS reference conditions (20°C and pF2 soil moisture) following the EFSA (2014a) DegT50 guidance. The field data end points were combined with laboratory values to derive modelling end points for oxamyl and its metabolites IN‐A2213 and IN‐D2708.
In laboratory incubations in dark aerobic natural sediment water systems, oxamyl exhibited very low to low persistence, partitioning to sediment and forming the major metabolites IN‐A2213 (max. 48.8% AR in water, exhibiting low to moderate persistence), IN‐D2708 (max. 66.8% AR in water and max. 12% AR in sediment exhibiting high persistence), IN‐N0079 (max. 52.9% AR in water exhibiting low persistence), IN‐T2921 (max. 11.4% AR in water exhibiting moderate persistence). The unextractable sediment fraction (not extracted by methanol/water and methanol after separation from the water phase) was a sink for the [1‐14C] radiolabel, accounting for 9–18% AR at study end (100 days). Mineralisation of this radiolabel accounted for 28–61% AR at the end of the study. The rate of decline of oxamyl in a laboratory sterile pH 5 buffered aqueous photolysis experiment was similar relative to that occurred in the dark aerobic sediment water incubations. The transformation product formed was IN‐N0079 (max. 67.6% AR).
The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for the metabolites IN‐A2213, IN‐D2708, IN‐N0079, and IN‐T2921 using the FOCUS (FOCUS, 2001) step 1 and step 2 approach (version 3.2 of the Steps 1–2 in FOCUS calculator).
For the field uses (Oxamyl 10GR) for the active substance oxamyl appropriate step 3 (FOCUS, 2001) and step 4 calculations were available. 13 The step 4 calculations appropriately followed the FOCUS (FOCUS, 2007) guidance, regarding no‐spray buffer zones of up to 25 m being implemented for the runoff scenarios (representing an 88–95% drift reduction). The SWAN tool (version 5.0.1) was appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that whilst run‐off mitigation is included in the step 4 calculations available, the FOCUS (FOCUS, 2007) report acknowledges that for substances with KFoc < 2,000 mL/g (i.e. oxamyl), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature than for more strongly adsorbed compounds. For the greenhouse uses (Oxamyl 10SL) for the active substance oxamyl appropriate step 3 (FOCUS, 2001) were available13 for the drainage scenarios.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.413 for the active substance oxamyl and its metabolites IN‐A2213 and IN‐D2708. As only one Kfoc value was available for oxamyl, two sets of calculations were performed using the default worst‐case Kfoc values of 10 mL/g and 10,000 mL/g.
For the field uses (Oxamyl 10GR):
-
–
For the representative use on potato:
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at four out of nine scenarios for oxamyl and metabolite IN‐D2708 and at one out of nine scenarios for IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be < 0.1 µg/L at all scenarios for oxamyl, IN‐D2708 and IN‐A2213.
-
–
For the representative use on tobacco (in‐furrow application):
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at one out of two scenarios for oxamyl and IN‐D2708 and < 0.1 µg/L at all scenarios for IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be < 0.1 µg/L at all scenarios for oxamyl, IN‐D2708 and IN‐A2213.
-
–
For the representative use on tobacco (broadcast application):
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at one out of two scenarios for oxamyl, IN‐D2708 and IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at one out of two scenarios for IN‐D2708 and < 0.1 µg/L at all scenarios for oxamyl and IN‐A2213.
For the greenhouse uses (Oxamyl 10SL):
-
–
For the representative use on tomato:
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at three out of five scenarios for oxamyl, IN‐D2708 and IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be < 0.1 µg/L at all scenarios for oxamyl, and > 0.1 µg/L at two out of five scenarios for IN‐D2708 and at one out of five scenarios for IN‐A2213.
-
–
For the representative use solarisation:
following July application.
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at three out of five scenarios for oxamyl, at four out of five scenarios for IN‐D2708 and at two out of five scenarios for IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at two out of five scenarios for IN‐D2708 and < 0.1 µg/L at all scenarios for oxamyl and IN‐A2213.
following August application.
using a Kfoc of 10 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at four out of five scenarios for oxamyl and IN‐D2708 and at three out of five scenarios for IN‐A2213;
using a Kfoc of 10,000 mL/g for oxamyl, concentrations were estimated to be > 0.1 µg/L at three out of five scenarios for IN‐D2708 and < 0.1 µg/L at all scenarios for oxamyl and IN‐A2213.
It should be noted that concentrations in groundwater were > 0.75 µg/L for metabolite IN‐D2708 (representative use on tobacco (broadcast application), using a Kfoc of 10 mL/g for oxamyl; representative use on tomato using both Kfoc of 10 mL/g and 10,000 mL/g for oxamyl; representative use solarisation, following July application using a Kfoc of 10 mL/g for oxamyl; representative use solarisation, following August application using both Kfoc of 10 mL/g and 10,000 mL/g for oxamyl) and for metabolite IN‐A2213 (only in one scenario for the representative use on tomato using Kfoc of 10 mL/g). None of the metabolites exceed 10 µg/L for all uses.
It should be acknowledged that an attempt was made to perform the groundwater exposure assessment for the solarisation use with the aim to mimic the peculiar conditions of this representative use (i.e. very high temperature and no irrigation), but the approach used was not considered acceptable.
The applicant provided information to address the effect of chlorination on the degradation of oxamyl that might be present in surface water, when surface water is abstracted for drinking water; however, a transparent evaluation of this information and information on the effect of ozonation on the degradation of oxamyl were not provided. Furthermore, information to address the effect of water treatment processes on the nature of oxamyl transformation products that can be present in surface water and groundwater when surface water or groundwater are abstracted for drinking water was not available. This has led to the identification of a data gap and results in the consumer risk assessment not being finalised (see Section 3). The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix B. A key to the persistence and mobility class wording used, relating these words to numerical DT and Koc end point values can be found in Appendix C.
5. Ecotoxicology
The risk assessment was based on European Commission (2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001,2011a, 2011b), SETAC (2000a, 2000b, 2002a, 2002b, 2003, 2010, 2011, 2001), EFSA (2009, 2013) and EFSA PPR Panel (2013).
Some specific aspects related to the environmental risk assessment of oxamyl were discussed at the Pesticides Peer Review Experts’ meeting PREV 63 in September 2021 (EFSA, 2022).
Based on the available information, the batches used in the critical (eco)toxicological studies cannot be considered as covering the proposed technical specification (issue not finalised). Based on current available data, the (eco)toxicological relevance of several impurities in the proposed technical specification cannot be concluded (data gap).
The applicant clarified that the greenhouse uses in tomatoes and solarisation are indicated for permanent structures. For those uses, low risk could be concluded for birds and mammals, bees, non‐target arthropod other than bees, soil organisms and non‐target terrestrial plants based on the limited exposure to oxamyl. The aquatic risk assessment for the uses in permanent greenhouses was conducted by using exposure estimates as for the field uses (see Section 4).
Suitable acute and reproductive data with oxamyl were available for birds and mammals. The acute and reproductive end points for birds and mammals, respectively, were discussed and agreed during the experts’ meeting. 14
The risk assessment for birds and wild mammals considered the exposure scenarios for granular applications that are described in EFSA (2009). 15 Considering the low nutritional value of oxamyl granules and their shape and colour, no quantitative risk assessment was deemed necessary for two of the scenarios, that is, birds and mammals ingesting granules as source of food and birds and mammals mistaking granules for small seeds. For the relevant exposure routes (i.e. birds ingesting granules as grit, birds ingesting granules when eating soil‐contaminated food, birds consuming seedling or ground‐dwelling invertebrates contaminated with oxamyl residues), several refinement options and mitigation measures to address the risks identified at tier 1 for several exposure scenarios were discussed at the experts’ meeting. 16 The use of a time‐weighted average factor of 0.53 to refine the risk to birds and mammals exposed to oxamyl when eating soil contaminated food was agreed by the experts.
Based on the available data and risk assessment, a high acute and long‐term risk was concluded for birds upon ingestion of granules as grit for all representative field uses of oxamyl. Specifically, upon consideration of two field monitoring studies, acute and long‐term risks were characterised considering the 90th percentile number of granules left of the soil surface after application. Moreover, as agreed at the experts’ meeting, separate risk assessments for the centre of the field and end of rows were conducted. Based on these calculations, a high risk was identified for the broadcast uses on tobacco (all scenarios) and for the in‐furrow applications, when considering end of rows for tobacco (i.e. cultivated and uncultivated headlands) and potato (uncultivated headlands). The applicant proposed label phrases to mitigate the risk. Although the experts did not question the validity of such mitigation measures, it was not demonstrated that they were sufficient to resolve the risk to birds consuming granules as grit.
The risk assessment for birds ingesting granules when eating soil‐contaminated food was discussed by the experts. A low acute and long‐term risk was concluded for all representative field uses at tier 1, considering 5 cm as the minimum depth of granule incorporation as indicated in the GAP and a time‐weighted factor of 0.53 for the long‐term scenario. The experts considered that the available residue studies could not be used to estimate residue values to address the risk for herbivorous birds consuming seedlings contaminated with oxamyl residues from granular applications (data gap and issue not finalised for the representative field uses). Oxamyl residues were detected in ground‐dwelling arthropods. Therefore, the experts concluded that the risk to insectivorous birds consuming ground‐dwelling arthropods should be addressed, despite this scenario not being directly addressed in EFSA (2009).2 Moreover, the experts at the meeting considered the risk to insectivorous birds consuming ground‐dwelling arthropods to be covered by the vermivore risk assessment, since the available evidence suggested that residue levels measured in earthworms were higher than in arthropods. Nonetheless, the acute risk for earthworm‐eating birds could not be addressed in the absence of reliable residue values in earthworms (data gap and issue not finalised for the representative field uses). A high chronic risk was concluded for earthworm‐eating birds for the uses in tobacco whereas a low risk was indicated for the uses in potato. A low acute and long‐term risk from exposure via contaminated water was indicated for all representative field uses at tier 1, using FOCUS Step 3 PECsw.
A low acute and long‐term risk was concluded for mammals ingesting granules when eating soil‐contaminated food at tier 1 for all field uses. For this, the same depth of incorporation and dissipation as for birds was considered. The risk for herbivorous mammals consuming seedlings contaminated with oxamyl residues from granular applications could not be addressed (data gap and issue not finalised for the representative field uses). The risk to mammals from consumption of ground‐dwelling arthropod was covered by the vermivore scenario. The acute risk for earthworm‐eating mammals could not be addressed in the absence of reliable residue values in earthworms (data gap and issue not finalised for the representative field uses). A low chronic risk for earthworm‐eating mammals was concluded for the uses in potato and tobacco at 1 × 3,000 g a.s./ha (in ‐furrow applications) whereas a high risk was identified for the uses in tobacco 1 × 5,500 g a.s./ha (broadcast applications). A low acute and long‐term risk from exposure via contaminated water was concluded for all representative field uses at tier 1.
An assessment of the dietary risk posed by plant metabolites was not required for either birds or mammals.
Several valid studies with oxamyl (either alone or as formulated representative product) were available, covering the relevant aquatic taxa. A number of reliable studies with aquatic invertebrates (identified as the most sensitive taxa) were available and a species sensitivity distribution approach could be followed based on that data. Experts at the meeting agreed to consider an assessment factor of 5 and the acute risk assessment for aquatic invertebrates was refined with the median 5% hazardous concentration. 17 The chronic end point for aquatic invertebrates was also agreed at the meeting. There were no reliable studies for sediment‐dwelling organisms (data gap and issue not finalised for the representative field uses since for the greenhouse uses, exposure of this taxa to oxamyl is expected to be limited).
For fish (acute and chronic), algae and aquatic macrophytes, a low risk from oxamyl was concluded for all representative uses, except for the uses on potato, for which a high acute and chronic risk for fish was indicated at FOCUS Step 3a PECsw values in one out of eight scenarios (R3). The risk could not be refined further. For aquatic invertebrates, a low acute and chronic risk was indicated for the uses in solarisation. For the uses on tomato in permanent greenhouses, FOCUS Step 3a PECsw values exceeded the acute and chronic regulatory acceptable concentrations, indicating a high risk for aquatic invertebrates. The risk could not be further refined since FOCUS Step 3d values were considered only supportive. However, a low risk could be concluded at the end for those uses on the basis of the limited exposure. For the broadcast applications on tobacco, a low acute risk to aquatic invertebrates was concluded whereas a high chronic risk was indicated. 18 A high acute and chronic risk to aquatic invertebrates was concluded for the in‐furrow applications of oxamyl on tobacco (R3 scenario) and potatoes (R3 scenario for the acute risk and six out of eight scenarios for the chronic risk assessment) at FOCUS Step 3a PECsw values.
Acute studies with relevant surface water metabolites (IN‐A2213, IN‐D2708, IN‐N0079 and IN‐T2921) were available for fish and aquatic invertebrates. In addition, a chronic study with IN‐D2708 was submitted for aquatic invertebrates. Based on the available information, a low risk could be concluded for all surface water metabolites for the representative uses of oxamyl. In the absence of reliable data, the risk to sediment‐dwelling organisms for the only relevant metabolite in sediment IN‐2708 could not be assessed (data gap and issue not finalised for the representative field uses of oxamyl).
Acute (contact and oral) toxicity data on honeybees were available for the active substance and one of the representative formulations, Oxamyl 10SL. Chronic larval (both single and repeated exposure designs) and adult toxicity were investigated using the active substance. Additionally, an acute contact and oral study was submitted with the bumble bee Bombus terrestris. A semi‐field (tunnel) study with B. terrestris for Oxamyl 10GR was also submitted; however, it was only considered for residue values in pollen and nectar as the biological results were deemed not reliable.
A high acute (oral and contact) and chronic risk (adults and larvae) to honeybees was concluded for all field uses of oxamyl based on EFSA (2013). 19 For all representative field uses, a high risk for bumble bees was indicated upon acute (oral and contact) exposure. 20 The refinements proposed by the applicant were not accepted. 21 A suitable assessment of accumulative and sublethal effects (e.g. hypopharyngeal glands) was not available (data gap for the field representative uses). Furthermore, no risk assessment was performed to address the oral exposure via contaminated water (data gap for the field representative uses). Acute oral studies for honey and bumble bees were available with the relevant plant metabolites (IN‐A2213, IN‐2708 and IN‐N0079). However, no quantitative risk assessment to plant metabolites was available. Since the parent assessment resulted in a high risk, a data gap was identified for further risk assessment to show a low risk from the metabolites (data gap for the field representative uses). Finally, toxicity data were not available for solitary bees.
For non‐target arthropods other than bees, tier 1 (glass plate) and extended laboratory species were available with the standard test species Aphidius rhopalosiphi and Typhlodromus pyri for Oxamyl 10SL. An extended laboratory study to assess the effects of dust of Oxamyl 10GR on A. rhopalosiphi was available. Additionally, extended laboratory tests were conducted with the soil‐dwelling species Aleochara bilineata, Pardosa sp. and Poecilus cupreus for both representative formulations and an aged residue study was performed with A. bilineata for Oxamyl 10GR. Considering, the toxicity studies and risk assessment, a low in‐field risk was concluded for the field uses of oxamyl on potato and tobacco applied in‐furrow. In contrast, a high in‐field risk was indicated for the broadcast application on tobacco since the concentration tested in the extended laboratory and aged‐residue studies on the most sensitive species, A. bilineata, did not cover the predicted environmental concentration in soil. A high off‐field risk was indicated for all field uses since the proposed refinement of dust deposition was not accepted during the peer review.
Reproduction studies with the earthworm species Eisenia fetida were available for Oxamyl 10GR, Oxamyl 10SL and all relevant soil metabolites (IN‐A2213 and IN‐D2708). The tier 1 risk assessment indicated a high risk for the field uses of oxamyl on potato and tobacco. To refine the risk assessment for such uses, three field studies conducted in Central Europe were available. Several shortcomings were identified in all three studies; however, they were considered as supportive evidence in a qualitatively weight of evidence approach. Taking into account that the value of the toxicity:exposure ratio (TER) was slightly below the trigger value for the uses on potatoes and the outcome of the supporting field studies, a low risk could be concluded for such uses. However, for the uses on tobacco (both in‐furrow and broadcast applications), the risk could not be refined given that TER values were much lower than the trigger value. For the soil metabolites, a low risk was concluded for all representative uses.
To assess the risk to soil macroorganisms other than earthworms, toxicity studies with Folsomia candida and Hypoaspis aculeifer were available with the active substance and the relevant soil metabolites. A high risk was indicated at tier 1 for F. candida for all field uses of oxamyl and for H. aculeifer for the field uses on tobacco. To refine the risk, a population modelling study on F. candida and a field study on Collembola were submitted for Oxamyl 10GR. Both studies were discussed at the Pesticides Peer Review Experts’ meeting. 22 The experts agreed that the population modelling study could not be used based on the uncertainties identified. However, the experts considered that the field study demonstrated the recovery of collembolan populations after the application of Oxamyl 10GR. The rate applied in the study only covered the intended uses on potato; therefore, a low risk to soil macroorganisms was concluded only for the field uses on potatoes whereas a high risk was indicated for the uses on tobacco (both in‐furrow and broadcast applications). Regarding the soil metabolites, a low risk was concluded for all representative uses.
The risk for soil microorganisms, non‐target terrestrial plants and organisms involved in biological methods for sewage treatment was considered low for all representative uses of oxamyl.
6. Endocrine disruption properties
The endocrine disruption potential of oxamyl was discussed at the Pesticides Peer Review Expert Meeting TC 60 (Joint Mammalian toxicology–Ecotoxicology session) in September 2021.
With regard to the assessment of the endocrine disruption potential of oxamyl for humans, the data set for the T modality was adequate and in line with the ECHA‐EFSA guidance. Two pubertal and thyroid function studies in rats further complemented the data package. Based on the evidence, no pattern of adversity indicating perturbation of the T modality was observed. Therefore, the ED criteria for the T modality are not met.
The data set for the EAS modalities was incomplete based on level 5 studies. However, in line with the ECHA‐EFSA guidance (ECHA and EFSA, 2018), the EAS‐mediated endocrine activity for the EAS modality was complete and fully investigated. Therefore, the data set for the EAS modality was overall considered as complete. There was no indication in the data set of perturbations of the EAS modality. The applicable scenario is Scenario 2a (ii): ED criteria are not met because no endocrine activity and no adversity have been observed for EAS modalities.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, for the T‐modality, a level 3 test according to OECD TG 231 (Amphibian Metamorphosis Assay) was available. No effect was observed on all the measured parameters. A slight increase in animals presenting mild follicular cell hypertrophy was reported at the highest dose. However, that change was an isolated finding in the entire data set for non‐target organisms, including mammals. Therefore, in the absence of any other effect, it was concluded that the endocrine activity through the T‐modality is considered negative, and therefore, T‐mediated adversity is unlikely for non‐target organisms other than mammals.
For the EAS‐modalities, a Fish Short‐Term Reproduction Assay (FSTRA) according to OECD TG 229 was available. There was no effect on any of the measured parameters. In addition, there were no other positive mechanistic findings in the available data set, i.e. ToxCast data. Overall, the endocrine activity through the EAS‐modality was considered negative.
Based on the available data and assessment, it is concluded that oxamyl does not meet the criteria for endocrine disruption for both humans and non‐target organisms through EATS modalities as set in point 3.6.5 and point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3–4)
Table 1.
Soil
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Oxamyl | High risk to soil organisms (a) |
| IN‐A2213 (Oxime) | Low risk to soil organisms |
| IN‐D2708 (DMOA) | Low risk to soil organisms |
A high risk was concluded for the field uses on tobacco (in‐furrow and broadcast applications). A low risk was concluded for the uses on potatoes and for the uses in permanent greenhouses (tomatoes and on solarisation).
Table 2.
Groundwater (a)
| Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses (b) Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
| Oxamyl |
Yes For all representative uses with a Kfoc of 10 mL/g: Potato: 4/9 FOCUS scenarios (< 0.1–0.359 μg/L) Tobacco (in‐furrow): 1/2 FOCUS scenarios (0.128–0.403 μg/L) Tobacco (broadcast): 1/2 FOCUS scenarios (0.128–0.403 μg/L) Tomato: 3/5 FOCUS scenarios (< 0.1–1.814 μg/L) Solarisation – July application: 3/5 FOCUS scenarios (< 0.1–1.002 μg/L) Solarisation – August application: 4/5 FOCUS scenarios (< 0.1–1.747 μg/L) No For all representative uses with a Kfoc of 10,000 mL/g |
Yes | – | – | Yes |
| IN‐A2213 (Oxime) |
Yes Potato – Kfoc of 10 mL/g: 1/9 FOCUS scenarios (< 0.1–0.191 μg/L) Tobacco (broadcast) – Kfoc of 10 mL/g: 1/2 FOCUS scenarios (< 0.1–0.171 μg/L) Tomato – Kfoc of 10 mL/g: 3/5 FOCUS scenarios (< 0.1–0.98 μg/L) Tomato – Kfoc of 10,000 mL/g: 1/5 FOCUS scenarios (< 0.1–0.156 μg/L) Solarisation – Kfoc of 10 mL/g – July application: 2/5 FOCUS scenarios (< 0.1–0.396 μg/L) Solarisation – Kfoc of 10 mL/g – August application: 3/5 FOCUS scenarios (< 0.1–0.719 μg/L) No For representative uses in potato, tobacco (in‐furrow), tobacco (broadcast), solarisation – July application, solarisation – August application with a Kfoc of 10,000 mL/g |
No |
No Acute oral LD50: 1,100 mg/kg bw per day Unlikely to be genotoxic Major rat metabolite: ADI of the parent 0.0001 mg/kg bw per day can be applied |
Yes Consumer intake is triggered: TMDI accounted for 33% of the ADI for adults, 98% of the ADI for children and 147% of the ADI for infants. |
Not triggered |
| IN‐D2708 (DMOA) |
Yes Potato – Kfoc of 10 mL/g: 4/9 FOCUS scenarios (< 0.1–0.291 μg/L) Tobacco (in‐furrow) – Kfoc of 10 mL/g: 1/2 FOCUS scenarios (0.239–0.630 μg/L) Tobacco (broadcast) – Kfoc of 10 mL/g: 1/2 FOCUS scenarios (0.722–1.686 μg/L) Use on tobacco (broadcast) – Kfoc of 10,000 mL/g: 1/2 FOCUS scenarios (< 0.1–0.377 μg/L) Tomato – Kfoc of 10 mL/g: 3/5 FOCUS scenarios (< 0.1–5.046 μg/L) Tomato – Kfoc of 10,000 mL/g: 2/5 FOCUS scenarios (< 0.1–0.789 μg/L) Solarisation – Kfoc of 10 mL/g – July application: 4/5 FOCUS scenarios (< 0.1–4.381 μg/L) Solarisation – Kfoc of 10,000 mL/g – July application: 2/5 FOCUS scenarios (< 0.1–0.702 μg/L) Solarisation – Kfoc of 10 mL/g – August application: 4/5 FOCUS scenarios (< 0.1–7.527 μg/L) Solarisation – Kfoc of 10,000 mL/g – August application: 3/5 FOCUS scenarios (< 0.1–3.785 μg/L) No For representative uses in potato and tobacco (in‐furrow) with a Kfoc of 10,000 mL/g |
No |
No Rat acute oral LD50 > 3,540 mg/kg bw Unlikely to be genotoxic Major rat metabolite, ADI of the parent 0.0001 mg/kg bw per day can be applied |
Yes Consumer intake is triggered: TMDI accounted for 251% of the ADI for adults, 753% of the ADI for children and 1,129% of the ADI for infants |
Yes for the representative use(s) assessed |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Oxamyl | High risk to aquatic organisms (a) , (b) |
| IN‐A2213 (Oxime) | Low risk to aquatic organisms |
| IN‐D2708 (DMOA) | Low risk to aquatic organisms (b) |
| IN‐N0079 | Low risk to aquatic organisms |
| IN‐T2921 | Low risk to aquatic organisms |
A high risk was concluded for all representative field uses on potatoes and tobacco (in‐furrow and broadcast applications), whereas a low risk was indicated for the uses in permanent greenhouses (tomatoes and on solarisation).
Except for the sediment‐dwelling organisms for which the risk assessment could not be finalised for the field uses of oxamyl.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Oxamyl | Harmonised classification: Acute Tox. 2; H330 |
| Rat LC50 inhalation = 0.056 mg/L (nose only) |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant’s proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remain the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
No appropriate conditions are proposed for the representative uses evaluated.
9. Concerns and related data gaps
9.1. Concerns and related data gaps for the representative uses evaluated
9.1.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 23 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
-
The material used in the critical (eco)toxicological studies could not be compared with the proposed technical material specification.
-
The consumer dietary risk assessment could not be concluded since the risk assessment residue definition for primary and rotational crops could not be finalised in view of the identified data gaps. Furthermore, the consumer risk assessment is also not finalised with regard to the unknown nature of residues that might be present in drinking water, consequent to water treatment following abstraction of surface water that might contain the active substance and its metabolites (see Sections 3 and 4).
Complete residue data sets on tomatoes and potatoes compliant with the respective GAPs for the determination of the residues of the compounds that are relevant for these crops (IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079) (relevant for the uses in tomatoes and potatoes; see Section 3).
Additional rotational crops field trials covering the NEU and SEU zones for the determination of the residues of oxamyl and of the metabolites IN‐D2708, IN‐A2213, IN‐QKT34 (IN‐A2213 glucoside conjugate) and IN‐N0079 in the edible parts of the representative rotational crops at the shortest PBI of 30 days (relevant for the uses in tomatoes, potatoes, tobacco and use on solarisation; see Section 3).
Sufficient GAP‐compliant residue trials on potatoes to confirm that the residues of oxamyl are below the LOQ as observed from the metabolism data (relevant for the use in potatoes; see Section 3).
To comply with the current data requirements, at least two additional GAP compliant residue trials analysing for oxamyl residues, respectively, on tomatoes and cucumbers to confirm that no quantifiable residues are expected in fruiting vegetables when oxamyl is applied in accordance with the representative use on solarisation (relevant for the use on solarisation; see Section 3).
Information to address the effect of water treatment processes on the nature of residues of that have the potential to be present in surface and groundwater, when surface or ground water are abstracted for drinking water was not available. Probably in the first instance, a consideration of the processes of ozonation (for parent and metabolites) and chlorination (for metabolites) would appear appropriate. Should this consideration indicate that novel compounds might be expected to be formed from water treatment, the risk to human or animal health through the consumption of drinking water containing them would need to be addressed (relevant to comply with the conditions of approval, not dependent of any specific use, see Section 4).
-
The risk for herbivorous birds and mammals consuming seedlings contaminated with oxamyl residues from granular applications could not be finalised for the representative field uses (potatoes in‐furrow and tobacco in‐furrow and broadcast applications (see Section 5)).
Suitable data on residues in weed seedlings contaminated from granular applications were not available.
-
The acute risk for earthworm‐eating birds could not be addressed for the representative field uses) (see Section 5).
Suitable data on residues in earthworms from granular applications were not available.
-
The risk to sediment‐dwelling organisms to oxamyl and the metabolite IN‐D2708 could not be addressed for the representative field uses (see Section 5).
Suitable data from granular applications were not available.
9.1.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Non‐dietary exposure estimates exceed the (A)AOEL for the operators (relevant for all representative uses, see Section 2).
Based on the representative uses, the preliminary chronic consumer dietary risk assessment did not indicate exceedance of the ADI (90% of the ADI (GEMS/Food G06)) whilst a large exceedance of the ARfD was noted for all the representative uses – 1,538% (potatoes; children), 1,223% (watermelons, children), 656% (cucumbers, children) and 581% (tomatoes, children) when oxamyl residues at the LOQ of 0.01mg/kg were considered. Furthermore, as IN‐D2708 and IN‐A2213 are also groundwater metabolites that exceeded the concentration of 0.75 µg/L, the consumer risk assessment through drinking water has been performed regarding these compounds and the TMDI accounted for 33% and 251% of the ADI for adults, 98% and 753% of the ADI for children and 147% and 1,129% of the ADI for infants, for IN‐A2213 and IN‐D2708, respectively (relevant for all representative uses; see Sections 3 and 4).
Potential groundwater contamination by oxamyl and relevant metabolites
batch adsorption/desorption experiments with oxamyl at 20°C on at least three additional soils were missing (relevant for all representative uses evaluated; see Section 4).
9.1.3. Overview of the concerns identified for each representative use considered (Table 5)
Table 5.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use |
Potato In‐furrow |
Tobacco In‐furrow |
Tobacco Broadcast |
Tomato Drip irrigation |
Solarisation Drip irrigation |
|
|---|---|---|---|---|---|---|
| Field | Field | Field | Greenhouse | Greenhouse | ||
| Operator risk | Risk identified | X2* | X2* | X2* | X2* | X2* |
| Assessment not finalised | ||||||
| Worker risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Resident/bystander risk | Risk identified | |||||
| Assessment not finalised | ||||||
| Consumer risk | Risk identified | X2 | X2 | X2 | ||
| Assessment not finalised | X2 | X2 | X2 | X2 | X2 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X | X | X | ||
| Assessment not finalised | X3,4 | X3,4 | X3,4 | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X | X | X | ||
| Assessment not finalised | ||||||
| Risk to aquatic organisms | Risk identified |
X (6/9 FOCUS scenarios) |
X (1/1 FOCUS scenarios) |
X (1/1 FOCUS scenarios) | ||
| Assessment not finalised | X5 | X5 | X5 | |||
| Groundwater exposure to active substance | Legal parametric value breached |
X4 (4/9 FOCUS scenarios) |
X4 (1/2 FOCUS scenarios) |
X4 (1/2 FOCUS scenarios) |
X4 (3/5 FOCUS scenarios) |
X4 (4/5 FOCUS scenarios) |
| Assessment not finalised | ||||||
| Groundwater exposure to metabolites | Legal parametric value breached (a) |
X4 (4/9 FOCUS scenarios) |
X4 (1/2 FOCUS scenarios) |
X4 (1/2 FOCUS scenarios) |
X4 (3/5 FOCUS scenarios) |
X4 (4/5 FOCUS scenarios) |
| Parametric value of 10 µg/L (b) breached | ||||||
| Assessment not finalised | ||||||
The superscript numbers relate to the numbered points indicated in Sections 9.1.1 and 9.1.2. Where there is no superscript number, see Sections 2–7 for further information.
The RMS proposed other mitigation measures, not validated at EU level, to reduce the estimated exposure of operators. The use of a closed transfer system is likely to reduce operator exposure, but the exact percent decrease cannot be defined in the absence of a validated protection factor set for this system. The protection factor from ‘closed cabins’ proposed to reduce exposure during application is not considered applicable since it is only relevant for applications on high crops in the EFSA calculator.
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections:
11.
A Developmental NeuroToxicity study (DNT; likely via gavage) to address potential age‐related differences in sensitivity to Oxamyl neurotoxicity is missing (relevant for all representative uses evaluated; see Section 2).
RMS to report the description, validation and assessment of all analytical methods used in support of toxicology studies (relevant for all representative uses evaluated; see Section 1).
Data assessing the matrix effects for the monitoring method for oxamyl residues in body fluids, the multiresidue and single monitoring methods in food of animal origin and their ILVs (proposed to be used for monitoring of body tissues) and the multiresidue monitoring methods in food and feed of plant origin (relevant for all representative uses evaluated; see Section 1).
Data assessing the extraction efficiency for multiresidue monitoring method in food and feed of plant origin (relevant for all representative uses evaluated; see Section 1).
Calibration data for the ILV study in water (relevant for all representative uses evaluated; see Section 1).
Data on the potential pH dependency of the soil degradation of oxamyl in acid soils were not available (relevant for all representative uses evaluated; see Section 4).
Data on the degradation of oxamyl in chlorinated water should be reported and assessed by the RMS (relevant for all representative uses evaluated; see Section 4).
Data were not available to address the risk to honeybees from accumulative and sublethal effects (e.g. effects on hypopharyngeal glands), via exposure to contaminated water and via exposure to metabolites formed in pollen and nectar (relevant for all field uses, see Section 5).
The scientific peer‐reviewed open literature conducted by the applicant on the active substance and its relevant metabolites, dealing with side effects on health, the environment and non‐target species–and published within the 10 years before the date of submission of the dossier, was not reported and assessed by the RMS in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011; relevant for all representative uses evaluated and for Sections 2, 3, 4 and 5).
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
Wavelength
- ε
decadic molar extinction coefficient
- AMA
Amphibian Metamorphosis Assay
- a.s.
active substance
- AChE
Acetylcholinesterase
- ADI
acceptable daily intake
- AAOEL
acute acceptable operator exposure level
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- AR
androgen receptor
- ARfD
acute reference dose
- ARSTTA
Stably Transfected Human Androgen Receptor Activation Assay
- AST
aspartate aminotransferase (SGOT)
- AUC
area under the blood concentration/time curve
- AV
avoidance factor
- bw
body weight
- ChE
Cholinesterase
- CI
confidence interval
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EAS
oestrogen, androgen and steroidogenesis modalities
- EC50
effective concentration
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- ErC50
effective concentration (growth rate)
- EUROPOEM
European Predictive Operator Exposure Model
- f(twa)
Time‐weighted average factor
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- FIR
food intake rate
- FOB
functional observation battery
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- FSTRA
Fish Short‐Term Reproduction Assay
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GCPF
Global Crop Protection Federation (formerly known as International Group of National Associations of Manufacturers of Agrochemical Products; GIFAP)
- GGT
gamma glutamyl transferase
- GM
geometric mean
- GS
growth stage
- GSH
Glutathione
- Hb
Haemoglobin
- Hct
Haematocrit
- HGPRT
hypoxanthine‐guanine phosphoribosyl transferase
- HPLC
high‐pressure liquid chromatography
or high‐performance liquid chromatography
- HPLC‐MS
high‐pressure liquid chromatography–mass spectrometry
- HPG
hypopharyngeal glands
- HQ
hazard quotient
- HQcontact
hazard quotient for contact exposure
- HR
hazard rate
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
Independent Laboratory Validation
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
Intravenous
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- Kdoc
organic carbon linear adsorption coefficient
- KFoc
Freundlich organic carbon adsorption coefficient
- LAGDA
Larval Amphibian Growth and Development Test
- LC
liquid chromatography
- LC50
lethal concentration, median
- LC‐MS
liquid chromatography–mass spectrometry
- LC‐MS‐MS
liquid chromatography with tandem mass spectrometry
- LD50
lethal dose, median; dosis letalis media
- LDD50
lethal dietary dose; median
- LDH
lactate dehydrogenase
- LH
luteinising hormone
- LOAEL
lowest observable adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- M/L
mixing and loading
- MAF
multiple application factor
- MCH
mean corpuscular haemoglobin
- MCHC
mean corpuscular haemoglobin concentration
- MCV
mean corpuscular volume
- MEOGRT
Medaka Extended One‐Generation Reproduction Test
- M&K
Maximisation test of Magnusson & Kligman
- mm
millimetre (also used for mean measured concentrations)
- mN
milli‐Newton
- MOA
mode of action
- MRL
maximum residue level
- MS
mass spectrometry
- MSDS
material safety data sheet
- MTD
maximum tolerated dose
- MWHC
maximum water‐holding capacity
- NESTI
national estimated short‐term intake
- NOAEC
no observed adverse effect concentration
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NOEL
no observed effect level
- NPD
nitrogen–phosphorus detector
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
Pascal
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PECsw
predicted environmental concentration in surface water
- pF2
pF value of 2 (suction pressure that defines field capacity soil moisture)
- Pow
partition coefficient between n‐octanol and water
- PPE
personal protective equipment
- Ppm
parts per million (10–6)
- QSAR
quantitative structure–activity relationship
- r2
coefficient of determination
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- RPE
respiratory protective equipment
- SC
suspension concentrate
- SD
standard deviation
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- t1/2
half‐life (define method of estimation)
- TER
toxicity exposure ratio
- TERA
toxicity exposure ratio for acute exposure
- TERLT
toxicity exposure ratio following chronic exposure
- TERST
toxicity exposure ratio following repeated exposure
- TK
technical concentrate
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- TWA
time‐weighted average
- UF
uncertainty factor
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for oxamyl according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
| Properties | Conclusion (a) | |
|---|---|---|
| CMR | Carcinogenicity (C) | Oxamyl is not considered to be carcinogenic, mutagenic or toxic for reproduction. |
| Mutagenicity (M) | ||
| Toxic for Reproduction (R) | ||
| Endocrine disrupting properties | Oxamyl is not considered to meet the criteria for endocrine disruption for human health and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II of Regulation No 1107/2009, as amended by Commission Regulation (EU) 2018/605. | |
| POP |
Persistence |
Oxamyl is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009. |
|
Bioaccumulation | ||
|
Long‐range transport | ||
| PBT |
Persistence |
Oxamyl is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. |
|
Bioaccumulation | ||
|
Toxicity | ||
| vPvB |
Persistence |
Oxamyl not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the representative formulation
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7296
Appendix C – Wording EFSA used in Section 4 of this conclusion, in relation to DT and Koc ‘classes’ exhibited by each compound assessed
| Wording | DT50 normalised to 20°C for laboratory incubations 24 or not normalised DT50 for field studies (SFO equivalent, when biphasic, the DT90 was divided by 3.32 to estimate the DT50 when deciding on the wording to use) |
|---|---|
| Very low persistence | < 1 day |
| Low persistence | 1 to < 10 days |
| Moderate persistence | 10 to < 60 days |
| Medium persistence | 60 to < 100 days |
| High persistence | 100 days to < 1 year |
| Very high persistence | A year or more |
Note these classes and descriptions are unrelated to any persistence class associated with the active substance cut‐off criteria in Annex II of Regulation (EC) No 1107/2009. For consideration made in relation to Annex II, see Appendix A.
| Wording | Koc (either KFoc or Kdoc) mL/g |
|---|---|
| Very high mobility | 0–50 |
| High mobility | 51–150 |
| Medium mobility | 151–500 |
| Low mobility | 501–2,000 |
| Slight mobility | 2,001–5,000 |
| Immobile | > 5,000 |
Based on McCall et al. (1980)
Appendix D – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula |
|---|---|---|
| IN‐A2213 |
methyl (1Z)‐2‐(dimethylamino)‐N‐hydroxy‐2‐oxoethanimidothioate ucodep>O)C(=N\O)\SC KIDWGGCIROEJJW‐XQRVVYSFSA‐N |
|
| IN‐D2708 |
(dimethylamino)(oxo)acetic acid ucodep>O)C(=O)O YKFGLGXRUVEMNF‐UHFFFAOYSA‐N |
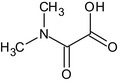
|
| IN‐N0079 |
[(cyanocarbonyl)azanediyl]dimethane CN(C)C(=O)C#N DNRRZLQWEDPRRM‐UHFFFAOYSA‐N |

|
| IN‐QKT34 (IN‐A2213 glucoside) |
1‐O‐{(Z)‐[2‐(dimethylamino)‐1‐(methylsulfanyl)‐2‐oxoethylidene]amino}hexopyranose CN(C)C(=O)C(=N\OC1OC(CO)C(O)C(O)C1O)\SC BVJZJNMSARVECQ‐XFXZXTDPSA‐N |

|
| IN‐T2921 |
N 1,N 1‐dimethylethanediamide NC(=O)C(=O)N(C)C ZWGBGUVGGJJWMF‐UHFFFAOYSA‐N |

|
| IN‐KP532 |
(methylamino)(oxo)acetic acid CNC(=O)C(=O)O FMNLVGDFBCBTJZ‐UHFFFAOYSA‐N |
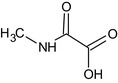
|
| IN‐L2953 |
2‐(hydroxyamino)‐N‐methyl‐2‐(methylsulfanyl)acetamide CNC(=O)C(NO)SC QFGADSMFUVRTSK‐UHFFFAOYSA‐N |
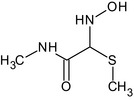
|
| Thiocyanate |
sodium thiocyanate [Na+].[S‐]C#N VGTPCRGMBIAPIM‐UHFFFAOYSA‐M |

|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Federica Castoldi A, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Herrero Nogareda L, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance oxamyl. EFSA Journal 2022;20(5):7296, 36 pp. 10.2903/j.efsa.2022.7296
Requestor: European Commission
Question numbers: EFSA‐Q‐2019‐00650; EFSA‐Q‐2020‐00545
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the rapporteur Member State, Italy, for the preparatory work on this scientific output.
Legal notice: This scientific output, approved on 30 March 2022, supersedes the previous output published on 31 March 2005 (EFSA, 2005).
Approved: 30 March 2022
Note
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine‐disrupting properties introduced by Regulation (EU) 2018/605.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Please refer to the Pesticide Peer Review Experts’ TC 60 (discussion point 2.2.) (EFSA, 2021).
For full details of the experts’ discussion, please refer to experts’ consultation point 2.8 in the Meeting Report of the Pesticides Peer Review Experts’ TC 60 in September 2021 (EFSA 2021).
The same study and NOAEL were also selected by EFSA in 2005 for setting the oxamyl TRVs at 0.001 mg/kg bw (per day) (EFSA, 2005; European Commission, 2009).
For full details of the experts’ discussion, please refer to experts’ consultation point 2.12 in the Meeting Report of the Pesticides Peer Review Experts’ TC 60 in September 2021 (EFSA 2021).
For full details of the experts’ discussion, please refer to experts’ consultation point 2.13 of the Pesticides Peer Review Experts’ TC 60 in June 2021 (EFSA 2021).
For full details of the experts’ discussion, please refer to experts’ consultation point 2.14 of the Pesticides Peer Review Experts’ TC 60 in June 2021 (EFSA, 2021).
For full details of the experts’ discussion, please refer to experts’ consultation point 2.11 of the Pesticides Peer Review Experts’ TC 60 in September 2021 (EFSA, 2021).
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
See experts’ consultation points 5.2 and 5.3 at the Pesticide Peer Review Experts’ Meeting 63 (September 2021) (EFSA, 2021).
The risk for granular formulations considers the following exposure routes: (i) birds/mammals ingesting granules as a source of food; (ii) birds/mammals may ingest granules as grit; (iii) birds/mammals mistaking granules for small seeds; (iv) birds/mammals ingesting granules when they eat food contaminated with soil; (v) birds/mammals consuming food contaminated with residues resulting from granular applications.
See experts’ consultation points 5.1 and 5.4 at the Pesticide Peer Review Experts’ Meeting 63 (September 2021) (EFSA, 2021).
See experts’ consultation point 5.5 at the Pesticide Peer Review Experts’ Meeting 63 (September 2021) (EFSA, 2021).
For the representative uses on tobacco and potatoes, the risk for fish and aquatic invertebrates could only be resolved using mitigation measures (see Table 5 for further details).
For all representative field uses, a high acute risk via contact exposure was concluded for the ‘field margin’ scenario whereas a high acute risk via oral exposure was indicated for all scenarios except for the ‘treated crop’. A high chronic risk to larvae and adults was concluded for all scenarios with the exception of the ‘treated crop’ scenario for larvae (for all representative field margins).
For all representative field uses, a high acute risk via contact exposure was concluded for the ‘field margin’ scenario whereas a high acute risk via oral exposure was indicated for all scenarios.
A dust deposition study on potatoes to refine the acute risk to honeybees and bumble bees via contact exposure was considered not reliable. The available honeybee semi‐field and bumble bee semi‐field and field studies were considered not suitable for risk assessment. Residues in Phacelia tanacetifolia were not used in the risk assessment as the crop is not representative of the GAP uses. An extended honey bee colony feeding study based on Oomen et al. (1992) and OECD (2007) was available to evaluate effects on honey bee brood; however, the study was finally deemed by itself not sufficient to refine the chronic risk to honey bee brood for the representative field uses.
See experts’ consultation point 5.6 at the Pesticide Peer Review Experts’ Meeting 63 (September 2021) (EFSA, 2021).
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
For laboratory soil incubations, normalisation was also to field capacity soil moisture (pF2/10kPa). For laboratory sediment water system incubations, the whole system DT values were used.
References
- ECHA (European Chemicals Agency) , 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5; Available online: https://echa.europa.eu/guidance‐documents/guidance‐on‐clp
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2005. Conclusion regarding the peer review of the pesticide risk assessment of the active substance. EFSA Journal 2005;3(1):26r, 78 pp. 10.2903/j.efsa.2005.26r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Review of the existing maximum residue levels (MRLs) for oxamyl according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2010;8(10):1830, 34 pp. 10.2903/j.efsa.2010.1830 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance oxamyl. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council. Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011a. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. pp. 1–46.
- European Commission , 2011b. Review report for the active substance oxamyl. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 17 June 2011 in view of the inclusion of oxamyl in Annex I of Council Directive 91/414/EEC. SANCO/10212/05‐Final. rev 1, 17 June 2011, 8 pp.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005-v. 2.0, 434 pp, as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- Italy , 2020. Renewal Assessment Report (RAR) on the active substance oxamyl prepared by the rapporteur Member State Italy, in the framework of Commission Implementing Regulation (EU) No 844/2012, June 2020. Available online: www.efsa.europa.eu
- Italy , 2021. Revised Renewal Assessment Report (RAR) on Oxamyl prepared by the rapporteur Member State Italy in the framework of Commission Implementing Regulation (EU) No 844/2012, November 2021. Available online: www.efsa.europa.eu
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp. [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues) , 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland. 18–27 September 2007, 164 pp. [Google Scholar]
- McCall PJ, Laskowski DA, Swann RL and Dishburger HJ, 1980. Measurements of sorption coefficients of organic chemicals and their use in environmental fate analysis. In: Test Protocols for Environmental Fate and Movement of Toxicants. In: Proceedings of the 94th Annual Meeting of the American Association of Official Analytical Chemists (AOAC). October 21–22, Washington, DC, pp. 89–109. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2009. Guidance document on overview of residue chemistry studies. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011, (Organisation for Economic Co‐operation and Development). In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org [Google Scholar]
- Oomen PA, De Ruliter A and van der Steen J, 1992. Method for honey bee brood feeding tests with insect growth‐regulating insecticides. Bulletin OEPP/EPPO Bulletin, 22, 613–616.
- SETAC (Society of Environmental Toxicology and Chemistry) , Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet MC, Lewis G, Oomen PA, Schmuck R and Vogt H (eds.), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2 workshop.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation



