The following fictional case is intended as a learning tool within the Pathology Competencies forMedical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see https://www.journals.elsevier.com/academic-pathology/news/pathology-competencies-for-medical-education-pcme.1
Primary objective
Objective SP2.1: Examples of inflammatory conditions. Give examples of specific sites and diseases in which specific pathologic diagnoses of inflammatory and/or infectious conditions are critical to treatment and prognosis.
Competency 3: Diagnostic medicine and therapeutic pathology; Topic SP: Surgical Pathology; Learning Goal 2: Immune and infectious disease.
Secondary objectives
Objective SP1.2: Differential diagnosis. List the major differential diagnoses for each type of cytology or surgical pathology specimen derived from a lesion or mass and describe appropriate further studies, both special stains and immunohistochemistry.
Competency 3: Diagnostic medicine and therapeutic pathology; Topic SP: Surgical pathology; Learning Goal 1: Role in diagnosis.
Objective IM1.3: Cytokines: Discuss with examples, the production of different cytokines by different immune cells, the roles that cytokines play in effecting the immune response, and how knowledge of cytokine action can be exploited in the treatment of disease.
Competency 1: Disease mechanisms and processes; Topic IM: Immunological mechanisms; Learning Goal 1: Immune dysfunction.
Patient presentation
A 37-year-old woman presents to her primary care physician with chief complaints of progressive shortness of breath, dry cough, and fatigue for the past 2–3 months. She also recalls having painful red bumps over both lower legs 5 months ago. There is no history of fever or weight loss. She has a 6-year history of light smoking, less than a half pack a day on average. She currently works in an office-based job in a construction company and reports no direct occupational exposures. She lives with two pet cats, has no history of incarceration, and has never traveled outside the Northeast United States. She has no relevant past medical history and is not taking any medications.
Diagnostic findings, Part 1
General physical examination reveals a well-developed and well-nourished female appearing stated age. Her vitals are within normal limits. There is no cyanosis or digital clubbing. There is no evidence of Jugular venous distention. There are currently no lesions on the lower extremity. Lungs are clear on auscultation. Cardiac auscultation reveals normal heart sounds with regular rate and rhythm. The remainder of the physical exam is normal.
Questions/discussion points, Part 1
What is the differential diagnosis based on clinical findings?
Cardiac and pulmonary organ systems are most frequently involved in the etiology of dyspnea. The more common causes of chronic dyspnea include bronchial asthma, chronic obstructive pulmonary disease (COPD), congestive heart failure, interstitial lung disease, and pneumonia. An accompanying dry cough in this patient suggests diseases affecting the airways and lung parenchyma.2
The tender, erythematous, subcutaneous nodules described by the patient bilaterally over lower extremities most likely represent an episode of erythema nodosum (EN). EN is a type of panniculitis, inflammation affecting subcutaneous fat, and the causative agents include drugs (antibiotics such as sulfonamides) and hormonal reactions, inflammatory bowel disease, and sarcoidosis in adults.3
The presence of EN in conjunction with dyspnea and dry cough in a young adult woman raises the possibility of sarcoidosis.
What is the most appropriate next step in the evaluation of this patient?
An imaging study, either a chest X-Ray (CXR), or a high-resolution computed tomography (HRCT) is the most appropriate next step in evaluation of patient with dyspnea. Currently, computed tomography (CT) represents the reference standard for the assessment of both mediastinal lymph nodes and pulmonary findings in sarcoidosis.4 In addition, pulmonary function tests (PFT's) are helpful in providing insight into underlying pathophysiology.
Diagnostic findings, Part 2
A HRCT shows bilateral perihilar opacities and pulmonary micronodules in a peri-lymphatic distribution. Pulmonary function tests reveals a restrictive pattern. A tissue biopsy to confirm the clinical suspicion of sarcoidosis was considered.
Questions/discussion points, Part 2
Describe the typical radiologic findings associated with sarcoidosis
The typical radiologic findings on CXR associated with sarcoidosis include symmetric, bilateral hilar and paratracheal lymphadenopathy, with or without concomitant parenchymal abnormalities.5 Apart from hilar and mediastinal adenopathy, the characteristic findings in HRCT are nodular infiltrates within a bronchovascular and subpleural distribution, with or without fibrotic changes.
What diagnostic tools can be used to obtain tissue sample in this scenario?
Tissue sampling is often helpful in the diagnostic evaluation of suspected sarcoidosis. Transbronchial bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has an excellent diagnostic yield in the presence of mediastinal/hilar lymphadenopathy and has replaced the traditional approach of obtaining biopsy samples via transbronchial and endobronchial routes.6 The use of a surgical lung biopsy may be warranted when the results with another procedure are not definitive and a biopsy of the mediastinal lymph nodes, the lung, or both is required.6 This can generally be done with minimally invasive procedures such as video assisted thoracoscopic surgery (VATS).
Diagnostic findings, Part 3
An EBUS-TBNA yields only inflammatory cells and is followed by a VATS lung biopsy.
Questions/discussion points, Part 3
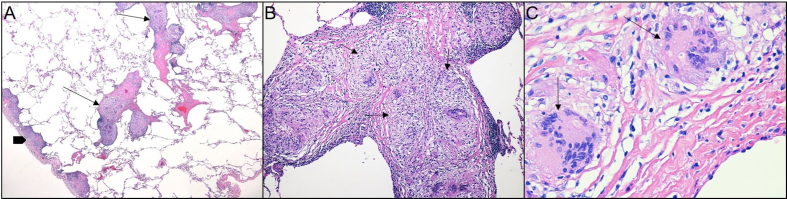
Describe the histologic findings in (Fig. 1A–C), hematoxylin and eosin (H&E) stained sections from the lung biopsy
Fig. 1.
A. A photomicrograph of the lung exhibits subpleural (block arrow) and bronchovascular nodules (line arrows) (H&E, 100x). B. The nodules (arrows) are composed of compact, well-formed, non-necrotizing granulomas with multinucleated giant cells (H&E, 200x). C. Coalesced histiocytes form multinucleated giant cells (arrows), exhibiting multiple nuclei within abundant cytoplasm (H&E, 400x).
On low power magnification (Fig. 1A) the pulmonary parenchyma with adjacent pleura is appreciated on the left side (block arrow) and multiple nodules are identified in the subpleural location, as well as around bronchovascular bundles (line arrows). These nodules represent granulomas. The term granuloma comes from the Latin word “granulum” which means “grain,” and the Greek suffix "-oma” used to refer to its nodular formation.7
Granulomatous inflammation is a form of chronic inflammation characterized by collections of activated macrophages, often with T-lymphocytes, and sometimes associated with central necrosis. The activated macrophages contain abundant cytoplasm and resemble epithelial cells for which reason they are called “epithelioid histiocytes”.8 The granulomas seen in this case are compact and well-formed and lack any central necrosis, and thus are non-necrotizing (Fig. 1B). They are also exhibiting eosinophilic, cracked hyaline-like surrounding collagen and lack a collar of lymphocytes, hence also referred to as “naked” granulomas. Some activated macrophages may coalesce to form multinucleated giant cells9 (Fig. 1C).
What is the differential diagnosis of granulomatous inflammation in lung?
The differential diagnosis of granulomas in lung can be due to infectious and non-infectious causes (see Table 1).
Table 1.
Differential diagnosis of granulomatous lung disease.
| Infectious lung diseases | |
|---|---|
| Mycobacteria | Tuberculosis and non-tuberculous mycobacteria |
| Fungal | Cryptococcus neoformans, Coccidioides immitis, Histoplasma capsulatum, Blastomyces dermatitidis |
| Non-infectious lung diseases | |
| Inflammatory | Sarcoidosis |
| Bronchocentric granulomatosis | |
| Inflammatory bowel disease | |
| Exposure/Toxins | Hypersensitivity pneumonitis |
| Hot tub lung | |
| Berylliosis | |
| Talc | |
| Drug reactions | |
| Aspiration pneumonia | |
| Vasculitis | Granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) |
| Autoimmune diseases | Rheumatoid nodule |
Infection is the most common cause of pulmonary granulomatous disease with mycobacteria and fungal organisms (Histoplasma, Cryptococcus, Coccidioides, and Blastomyces) being the common etiologic agents.8 The usual finding in Mycobacterium tuberculosis is necrotizing granulomas (Fig. 2A) and the bacilli are commonly detected in the areas of necrosis. Fungal granulomas are often suppurative with areas of necrosis and purulent exudate. Once routine hematoxylin and eosin (H&E) sections have been carefully evaluated, special stains are performed to improve diagnostic yield, Grocott methenamine silver (GMS) stain and Ziehl–Neelsen (acid fast stain) for the identification of fungi and acid-fast bacilli (AFB), respectively.8
Fig. 2.
A. A photomicrograph of lung shows granulomatous inflammation with central necrosis (asterisk) (H&E, 400x). B. A poorly formed granuloma in the interstitium (arrow) in a background of prominent chronic interstitial inflammation is suggestive of hypersensitivity pneumonitis (H&E, 200x). C. A necrotizing granuloma with central basophilic “dirty” necrosis (asterisk) is seen in this case of granulomatosis with polyangiitis (H&E, 400x). D. Pale vegetable material (arrow) is surrounded by foreign body giant cells in aspiration pneumonia (H&E, 400x).
Non-infectious pulmonary granulomas can be seen in a wide variety of disease processes and can be exposure related, as a manifestation of vasculitic granulomatous diseases and inflammatory/immune mediated. The key features of major non-infectious causes of granulomatous lung disease are summarized in Table 2.
Table 2.
Characteristic features of major non-infectious granulomatous disease.
| Diagnosis | Key features |
|---|---|
| Sarcoidosis | Prominent well formed, discrete, non-necrotizing granulomas in lymphangitic pattern |
| Berylliosis | Well-formed, compact, non-necrotizing “sarcoid-like” granulomas within the interstitium correlation with the exposure history and demonstrating a beryllium-specific immune response with beryllium lymphocyte proliferation testing, or tissue analysis may be helpful in establishing the diagnosis |
| Hypersensitivity pneumonitis | Scattered small, poorly formed granulomas or multinucleated giant cells in interstitium in a background of prominent chronic interstitial inflammation |
| Aspiration pneumonia | Aspirated material surrounded by foreign-body type granulomas or multinucleated giant cells |
| Granulomatosis with polyangiitis (GPA) | Suppurative granulomas with dirty necrosis and necrotizing vasculitis |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | Necrotizing granulomas, necrotizing vasculitis, and prominent eosinophils |
| Rheumatoid nodule | Multiple subpleural necrobiotic nodules identical to subcutaneous nodules; most patients also have subcutaneous nodules and high rheumatoid titers. |
Exposure-related granulomas
These can be seen in hypersensitivity pneumonitis (HP), which represents an immune-mediated reaction to a variety of inhaled antigens and chemical agents, and exhibit small poorly formed, non-necrotizing granulomas (Fig. 2B) in a background of chronic interstitial inflammation.10 The most common causes of HP are microbes (farmer's lung) and avian antigens (pigeon breeder's lung). Hot tub lung is associated with exposure to Mycobacterium avium complex (MAC) arising in aerosols from indoor hot tubs/spas and show non-necrotizing granulomas present in lumen of small bronchioles, interstitium and airspaces. Microorganisms must be looked for in both necrotizing and non-necrotizing granulomas, since they may be found in either.
Vasculitic granulomatous diseases
These are characterized by granulomatous inflammation, necrosis and vasculitis. Granulomatosis with polyangiitis (GPA) is a rare, systemic, immune-mediated inflammatory process characterized by suppurative granulomas with “dirty” necrosis (Fig. 2C), an appearance due to the presence of neutrophils and necrotic debris. The necrosis of GPA is often described as “geographic,” a term that refers to the map-like irregular contours.11 Eosinophilic granulomatosis with polyangiitis (EGPA), is also a systemic disorder characterized by eosinophil rich granulomatous inflammation, often involving respiratory tract with necrotizing vasculitis. This is associated with asthma in approximately 95% of the patients and peripheral eosinophilia.12 Diagnosis of these entities is significantly aided by the serum antineutrophilic cytoplasmic antibody (ANCA) test. Bronchocentric granulomatosis is characterized by necrotizing granulomas centered almost exclusively on bronchioles resulting in the partial or complete destruction of the affected airway. Approximately half of the cases occur in asthmatics presenting with allergic bronchopulmonary fungal disease, due to Aspergillus and associated with eosinophilia.
Inflammatory/immune mediated
Rheumatoid nodules in the lung are rare and are characterized by a central area of eosinophilic necrosis surrounded by palisaded histiocytes (necrobiotic granulomas).13 The diagnosis of pulmonary rheumatoid nodule can be made if an infectious etiology can be excluded and the patient is known to have rheumatoid disease.
Sarcoidosis is a common disease of unknown etiology and involves lungs and intrathoracic lymph nodes. The granulomas are usually non-necrotizing and have a tendency to localize around bronchovascular bundles and fibrous septa. In older cases, the granulomas may exhibit a peripheral rim of hyalinized fibrosis. Two other non-specific microscopic features are sometimes seen in the granulomas: (1) Schaumann bodies, laminated concretions composed of calcium and proteins; and (2) asteroid bodies (stellate inclusions enclosed within giant cells). The diagnosis of sarcoidosis requires all other causes of granulomas to be reasonably excluded.
The diagnosis of berylliosis, a rare occupational lung disease whose microscopic appearance may closely resemble that of sarcoidosis is highly dependent on an occupational history of exposure and presence of beryllium in tissues.
Granulomas can also be seen in aspiration pneumonia, which is characterized by acute and organizing inflammation and a foreign body-type granuloma with multinucleated giant cells surrounding the aspirated vegetable material (Fig. 2D). It occurs in patients with conditions that impair gag, swallowing and cough reflexes.
How do immune granulomas form? What cytokines are involved?
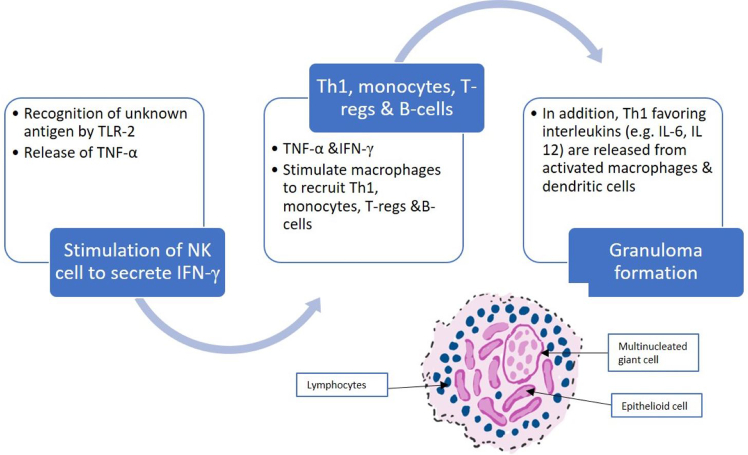
Immune granulomas are caused by a variety of agents that are capable of inducing a persistent T-cell mediated immune response (Fig. 3). The formation begins with the recognition of an airborne antigen by toll-like receptor 2-ligands (TLR-2) on alveolar macrophages and type II alveolar epithelial cells.9 Macrophage activation of TLR-2 results in the release of tumor necrosis factor-α (TNF-α), which in turn stimulates natural killer cells to release interferon-γ (INF-γ). Ultimately, the presence of TNF-α and INF-γ result in macrophage recruitment of Th1/17 cells, monocytes, regulatory T cells (Tregs), and B cells. Simultaneously TLR-2 activated type II alveolar epithelial cells, dendritic cells, and macrophages release Th1 and Th17 favoring cytokines, such as IL-6, IL-12, IL-18, IL-23, and TGF-β. The release of TNF-α, INF-γ, Th1 and Th17 favoring cytokines in the presence of decreased Treg inhibition leads to granuloma formation.9
Fig. 3.
Multiple steps occur in the process of immune granuloma formation.
The histochemical stains and cultures performed in this case were negative for microorganisms. Given the clinical, imaging and histologic findings, what is the most likely diagnosis?
The presence of discrete, well-formed, non-necrotizing granulomas within a bronchovascular distribution, negative histochemical stains for organisms, negative microbiologic cultures, and the absence of histopathologic features suggestive of other granulomatous disorders when taken in the context of patient's clinical and radiologic findings make sarcoidosis the most likely diagnosis.
Sarcoidosis is a diagnosis of exclusion and can be rendered once other infectious and non-infectious causes of granulomas are excluded.10 Second, an attempt should be made to identify features to suggest an alternate diagnosis where granulomas can be formed (See Table 1).
What is the epidemiology and etiology of sarcoidosis?
The highest prevalence of sarcoidosis is seen in populations of Nordic and African American origin. It most often occurs in young, otherwise healthy adults with an average age of onset of 47–51 years old and a peak at 30–55 years of age.14,15 While the exact etiology of sarcoidosis remains unknown, it has been proposed that the disease is driven by exposure to an unknown antigen or antigens causing a deviant immune response in genetically susceptible populations.16
What are the common presenting clinical signs and symptoms of sarcoidosis?
There are three different modes of clinical presentation: asymptomatic sarcoidosis, nonspecific constitutional symptoms, and symptoms related to specific organ involvement. Many patients are asymptomatic at presentation, and come to clinical attention because of the incidental finding of hilar lymphadenopathy in chest radiographs performed for an unrelated cause. In about two-thirds of symptomatic patients, there is gradual appearance of respiratory symptoms (shortness of breath, dry cough) or constitutional signs and symptoms (fever, fatigue, weight loss, night sweats). Eye and skin involvement occur in 25% of cases, and either may occasionally be the presenting symptom of the disease.17
Describe the extrapulmonary manifestations of sarcoidosis
Although nearly half of all patients present with the disease limited to the thorax, many extrapulmonary tissues may be involved.18 Common extrapulmonary sites include skin (erythema nodosum, painless subcutaneous nodules), and eyes (dry eyes, iritis or iridocyclitis). Erythema nodosum, as seen in this patient, is a manifestation of acute sarcoidosis that is not associated with the presence of granulomas and usually subsides within 6–8 weeks. Ocular lesions occur in 20%–30% of patients and are most serious because of the threat of blindness. Since eye involvement may be asymptomatic, every patient with sarcoidosis should undergo regular ophthalmological investigation. Sarcoidosis can also involve the heart, liver, brain, meninges, and bone marrow. All patients with sarcoidosis should have an electrocardiogram (ECG) as a part of initial work up due to risk of cardiac disease. Patients may have vitamin D deficiency or hypercalcemia and should be assessed for those as well.
What is the prognosis of sarcoidosis? What is the treatment?
Nearly two-thirds of patients with sarcoidosis have spontaneous regression of their disease. Conversely a chronic or progressive course can be seen in 10–30% of patients.19 Treatment of sarcoidosis is primarily with corticosteroids, though other immunomodulators have been trialed with limited data, and in extreme cases of pulmonary sarcoidosis, a lung transplant may be pursued.20
Teaching points
-
•
The differential diagnosis of granulomatous inflammation in lung is broad and includes both infectious and non-infectious etiologies.
-
•
Infection is the most common cause of pulmonary granulomatous disease with mycobacteria and fungal organisms being the common etiologic agents.
-
•
Microorganisms must be sought in both necrotizing and non-necrotizing granulomas and special stains should be employed to improve diagnostic yield.
-
•
Non-infectious pulmonary granulomas can be exposure related, a manifestation of vasculitic granulomatous disease and inflammatory/immune mediated.
-
•
Immune granuloma formation is cytokine driven, including macrophage release of TNF-α and INF-γ, which leads to a Th1/Th17 favored inflammatory response.
-
•
Sarcoidosis is most prevalent in populations of Nordic and African American origin, with a peak onset at 30–35 years old.
-
•
Sarcoidosis is a diagnosis of exclusion and is made in a patient with appropriate clinical and radiologic findings in which other diagnoses have been excluded, and many have a biopsy with identification of non-necrotizing granulomas.
-
•
While the true etiology of sarcoidosis is unclear, it is thought to be due to exposure to unknown antigens in a genetically susceptible person.
-
•
The majority of cases of sarcoidosis affect the lungs, but involvement may be found in almost any organ including the skin, eyes, extra-thoracic lymph nodes, liver, bone marrow, spleen, and heart.
-
•
Pulmonary sarcoidosis is characterized histologically by well formed, non-necrotizing, naked granulomas with a lymphangitic (sub pleura, pleural-septal, and bronchovascular) distribution.
-
•
Treatment of sarcoidosis is primarily with corticosteroids.
-
•
Most patients with sarcoidosis have spontaneous regression of their disease while 10–30% of patients experience chronic or progressive symptoms.
References
- 1.Knollmann-Ritschel B.E.C., Regula D.P., Borowitz M.J., Conran R., Prystowsky M.B. Pathology competencies for medical education and educational cases. Acad Pathol. 2017;4 doi: 10.1177/2374289517715040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarcoidosis CostabelU. Clinical update. Eur Respir J Suppl. 2001;32:56s–68s. [PubMed] [Google Scholar]
- 3.Schwartz A.R., Nervi J.N. Erythema nodosum: a sign of systemic disease. Am Fam Physician. 2007;75(5):695–700. [PubMed] [Google Scholar]
- 4.Silva M., Nunes H., Valeyre D., Sverzellati N. Imaging of sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):45–53. doi: 10.1007/s12016-015-8478-7. [DOI] [PubMed] [Google Scholar]
- 5.Criado E., Sanchez M., Ramirez J., et al. Pulmonary sarcoidosis: typical and atypical manifestations at high resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567–1586. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 6.Anandavelu R., Fahim A. Current diagnostic techniques in sarcoidosis. Sarcoidosis and Granulomatosis-Diagnosis and Management. 2020 doi: 10.5772/intechopen.90692. Published online July 29. [DOI] [Google Scholar]
- 7.Samsonova M., Chernyaev A. Pathology of sarcoidosis and differential diagnosis of other granulomatous diseases. Sarcoidosis and Granulomatosis-Diagnosis and Management. 2020 doi: 10.5772/intechopen.90693. Published online July 29. [DOI] [Google Scholar]
- 8.Shah K.K., Pritt B.S., Alexander M.P. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1–12. doi: 10.1016/j.jctube.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broos C.E., van Nimwegen M., Hoogsteden H.C., Hendriks R.W., Kool M., van den Blink B. granuloma formation in pulmonary sarcoidosis. Front Immunol. 2013;4:437. doi: 10.3389/fimmu.2013.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller R., Allen C.T., Barrios J.R., et al. C: a perspective from members of the pulmonary pathology society. Arch Pathol Lab Med. 2018;142(1):120–126. doi: 10.5858/arpa.2017-0138-SA. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay, S. Granulomatous lung disease. In Non-Neoplastic Pulmonary Pathology: An Algorithmic Approach to Histologic Findings in the Lung (pp. 35-121). Cambridge: Cambridge University Press. doi:10.1017/CBO9781107445079
- 12.Mitchell R. tenth ed. Elsevier/Saunders; 2017. Blood vessels. Robbins and Cotran Pathologic Basis of Disease; pp. 361–397. [Google Scholar]
- 13.Bones HorvaiA. tenth ed. Elsevier/Saunders; 2017. Joints and Soft Tissue Tumors. Robbins and Cotran Pathologic Basis of Disease; pp. 797–833. [Google Scholar]
- 14.Arkema E.V., Cozier Y.C. Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018;9(11):227–240. doi: 10.1177/2040622318790197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo D.L., Fauci A.S., Kasper D.L., Hauser S.L., Jameson J., Loscalzo J., editors. Sarcoidosis. Harrison's Principles of Internal Medicine, 20e. McGraw-Hill; New York, NY: 2012. pp. 360–365. [Google Scholar]
- 16.Saidha S., Sotirchos E.S., Eckstein C. Etiology of sarcoidosis: does infection play a role? J Biol Med. 2012;85(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- 17.Husain A. tenth ed. Elsevier/Saunders; 2017. Lung. Robbins and Cotran Pathologic Basis of Disease; pp. 495–548. [Google Scholar]
- 18.Rao D.A., Dellaripa P.F. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin N Am. 2013;39(2):277–297. doi: 10.1016/j.rdc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statement on sarcoidosis Joint statement of the American thoracic society (ATS), the European respiratory society (ERS) and the world association of sarcoidosis and other granulomatous disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, february 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 20.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]