Abstract
Large vessel vasculitis (LVV) manifests as inflammation of the aorta and its major branches, and is the most common primary vasculitis in adults. LVV comprises two distinct conditions, giant cell arteritis (GCA) and Takayasu arteritis (TAK), though the phenotypic spectrum of primary LVV is complex. Non-specific symptoms often predominate and so patients with LVV present to a range of healthcare providers and settings. Rapid diagnosis, specialist referral and early treatment are key to good patient outcomes. Unfortunately, disease relapse remains common and chronic vascular complications are a source of considerable morbidity. Although accurate monitoring of disease activity is challenging, progress in vascular imaging techniques and the measurement of laboratory biomarkers may facilitate better matching of treatment intensity with disease activity. Further, advances in our understanding of disease pathophysiology have paved the way for novel biologic treatments that target important mediators of disease in both GCA and TAK. This work has highlighted the substantial heterogeneity present within LVV and the importance of an individualised therapeutic approach. Future work will focus on understanding the mechanisms of persisting vascular inflammation, which will inform the development of increasingly sophisticated imaging technologies. Together, these will enable better disease prognostication, limit treatment-associated adverse effects, and facilitate targeted development and use of novel therapies.
INTRODUCTION
Inflammation of large blood vessels, such as the aorta and its main branches, most commonly presents as one of the two primary large vessel vasculitides – giant cell arteritis (GCA) or Takayasu arteritis (TAK).1 Together, these two conditions are defined as large vessel vasculitis (LVV).
GCA is an idiopathic inflammatory condition characterised by granulomatous arteritis in temporal artery biopsy (TAB) specimens that was commonly referred to as temporal arteritis when first described almost 100 years ago.2 Later, it was observed that patients with this condition often develop constitutional symptoms and features of extravascular inflammation which was attributed to an overlap with the more common inflammatory disorder polymyalgia rheumatica (PMR).3 Several subsequent autopsy studies showed arteritis within the aorta and other great vessels4, 5 and rapid improvements in vascular imaging starting in the beginning of the 21st century have enabled an even better understanding of the extent of large vessel involvement.6, 7 It is now recognised that GCA encompasses a broad phenotypic spectrum of medium and large artery inflammation. Nomenclature has evolved to reflect this, with the terms large vessel-GCA (LV-GCA), cranial-GCA (C-GCA), and LV-GCA with cranial involvement now suggested depending on the site of inflammation (Figure 1).8
Figure 1. Disease classification and arterial involvement in large vessel vasculitis.
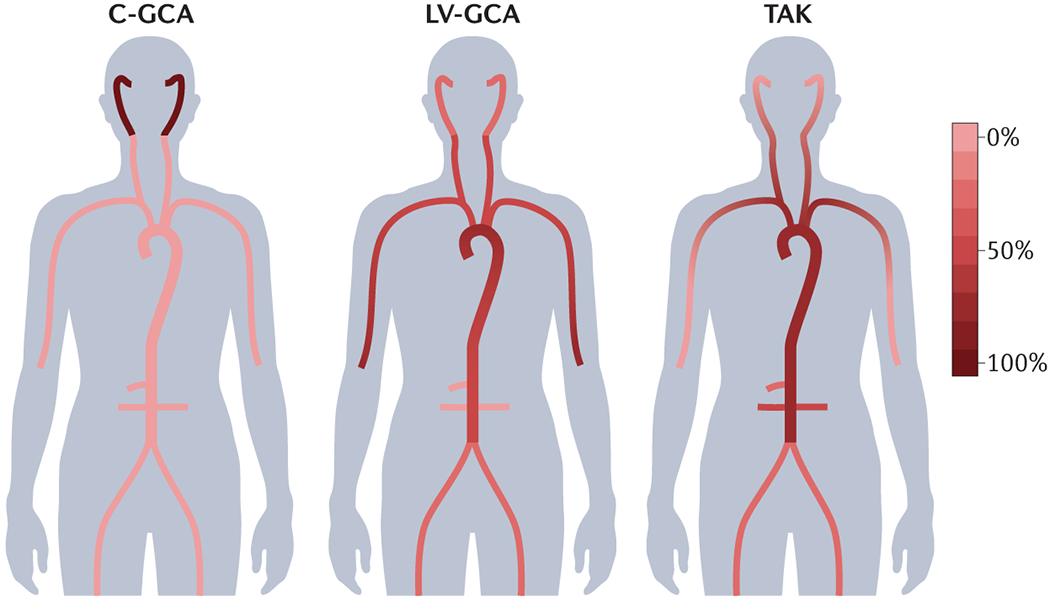
Although variation exists across the phenotypic spectrum of LVV, patterns of arterial involvement may help to distinguish GCA and TAK. Here, the scale represents typical frequency of arterial segment involvement across the LVV spectrum. LV-GCA more commonly affects the axillary arteries, whereas TAK is more likely to affect the renal and mesenteric vessels.267 Symmetrical involvement of arterial territories is typical, with the possible exception of subclavian involvement in TAK in which the left subclavian is more commonly implicated than the right.268 In addition to the vessels depicted, the vertebral arteries may be affected in both GCA and TAK. TAK may also involve the pulmonary arteries. Evidence from imaging studies and autopsy series suggests substantial overlap between cranial giant cell arteritis (C-GCA) and large-vessel giant cell arteritis (LV-GCA) such that many patients presenting with typical temporal symptoms will have evidence of large vessel involvement if this is investigated.267
TAK was first described in 1908 as a series of retinal vascular abnormalities by Japanese ophthalmologist Mikito Takayasu and colleagues.9 Its association with absent or diminished peripheral pulses led to the term ‘pulseless disease’, and autopsy studies demonstrated a pan-arteritis involving the aorta and major branches.10 Although early descriptions of the disease involved individuals of Japanese origin, TAK is now recognised to occur worldwide.
In general, both GCA and TAK are defined by granulomatous inflammation of the blood vessel wall and a maladaptive immune response to injury that promotes intimal hyperplasia, adventitial thickening and intramural vascularisation, which ultimately threaten vessel integrity and tissue perfusion. Advances in the cellular and molecular analysis of inflammatory lesions in LVV have translated into improved understanding of its pathogenesis and mechanism-based diagnostic and therapeutic approaches that can be tailored to the needs of the individual patient. These treatments are being evaluated in increasingly complex and sophisticated clinical trials, and the need for guidance on their use has driven exciting advances in vascular imaging.
Disease outcomes in LVV are generally better than in most systemic inflammatory conditions, including in small vessel vasculitis. However, LVV is not benign and constitutional symptoms such as fatigue, fever and weight loss are common and disabling. Clinical manifestations of arterial narrowing include vision loss and stroke in the short term, and limb ischaemia and heart failure in the long term. Patients with LVV also carry an increased risk of aortic aneurysm formation and rupture. Additionally, current therapeutic strategies involving prolonged immunosuppression are associated with consequences including increased risk of cardiovascular disease and infection. Although treatment strategies now enable many patients to achieve disease remission, relapse is common. Further, individuals with LVV present to a range of medical or surgical specialties and require inter-disciplinary management. As such, a working knowledge of current nomenclature, diagnostic approaches and therapeutic options is essential to providing good care to LVV patients.
This Primer provides an in-depth, global review of the epidemiology, pathophysiology, diagnosis and management of LVV, and highlights areas where ongoing and future research may be most impactful. Of note, inflammation of large vessels can also occur in a range of infectious, inflammatory, and immune diseases; these conditions are detailed in Box 1 and are outside the scope of this Primer.
Box 1. Mimics of large vessel vasculitis.
Infectious disease
Bacterial infection
Fungal infection
HIV infection
Q fever
Syphilis
Tuberculosis
Inflammatory disease
Ankylosing spondylitis
Atherosclerosis
Behçet syndrome
Clinically isolated aortitis
Cogan syndrome
Cryoglobulinaemic vasculitis
Granulomatosis with polyangiitis
IgG4-related disease
Polyarteritis nodosa
Relapsing polychondritis
Rheumatoid arthritis
Sarcoidosis
Systemic lupus erythematosus
Connective tissue disease
Ehlers–Danlos syndromes
Fibromuscular dysplasia
Loeys–Dietz syndrome
Marfan syndrome
Neurofibromatosis
Pseudoxanthoma elasticum
Congenital vascular disease
Aortic coarctation
Mid-aortic syndrome
Neoplastic disease
Erdheim–Chester disease
Adverse effects from radiotherapy
Adverse effects from immune checkpoint inhibitor therapy
EPIDEMIOLOGY
Incidence
GCA is the most common primary vasculitis worldwide, although precise estimates of incidence vary with the criteria used for case definition, which are based on either histological definition by TAB, diagnostic coding or classification criteria. GCA occurs almost exclusively in those aged >50 years and the incidence increases with age to peak in the eighth decade of life, where there is a 40-fold increase in disease risk over those aged 50–59.11–13 Women are more commonly affected than men, at a ratio of around 3:1.12–15 LV-GCA patients are younger at presentation, are more commonly female, and more often present with bilateral arterial involvement than those with C-GCA.16, 17
There is considerable global variation in GCA incidence, with estimates as high as 44 cases per 100,000 persons over the age of 50 in Northern Europe, and as low as ~0.3 per 100,000 persons over the age of 50 in Southern Asia (Figure 2).14, 18–21 Similarly, the incidence within Europe shows a marked north-south gradient and is reported to be <10 cases per 100,000 persons over the age of 50 in Mediterranean populations.11, 19 There is a particularly high prevalence amongst those of Scandinavian ancestry, both within Northern Europe and in Americans of Scandinavian descent, suggesting a shared genetic risk across these populations. Conversely, a lower reported incidence in Finland may reflect the distinct genetic ancestry in this population.22 GCA is thought to be even less common in African, Asian and Arab countries; however, formal epidemiological data in these populations are limited, potentially owing to a combination of lower disease burden, differences in access to healthcare (and thus diagnosis), or lack of study in low-income regions.
Figure 2. Global incidence of large vessel vasculitis.
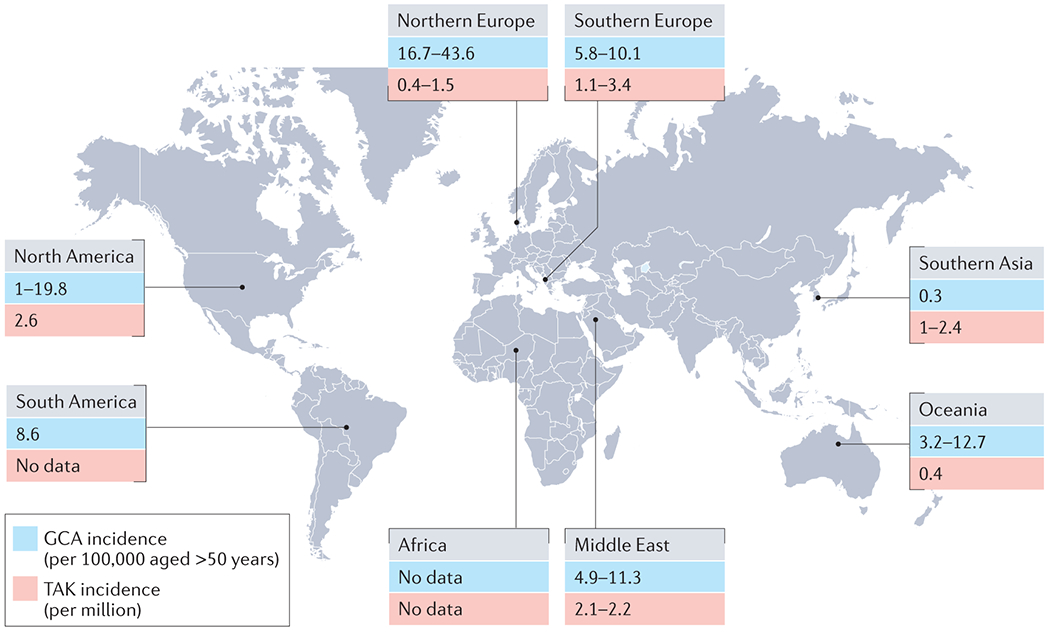
North America includes data from Alaska, USA269, Tennessee, USA270, Minnesota, USA12, 271 and Ontario, Canada272. South America includes data from Argentina273. Northern Europe includes data from Norway14, 27, 274 , the UK24, 53, Iceland275, Denmark18, 276 and Sweden26. Southern Europe includes data from Italy277, Slovenia278 and Spain11, 279. Middle East includes data from Turkey280, Israel281–283 and Kuwait284. Oceania includes data from Australia285, 286 and New Zealand287. Southern Asia includes data from Hong Kong21, Japan20, 23 and South Korea65. GCA, giant cell arteritis; TAK, Takayasu arteritis.
In Japan, where it was first described, TAK has an estimated annual incidence of 1–2 cases per million people.23 In Europe, the annual incidence ranges from 0.4 to 3.4 per million.24–27 Age of onset is usually between 10 and 40 years, and is the major epidemiological feature that distinguishes TAK from GCA, although late-onset TAK is increasingly recognised.28 TAK is also more common in women, who account for 80–90% of cases in Europeans.29 The sex ratio, however, is less skewed towards women in China, India and Thailand — where it ranges between 3:1 and 4:1 — implicating a potential role for regional environmental and genetic factors in pathogenesis.30–32 A study in Japanese patients also suggests a recent shift in sex ratio towards men.33 Notably, the pattern of disease may differ between young-onset and late-onset disease, and between men and women. Renal artery involvement, active disease with constitutional symptoms and major ischemic events such as myocardial infarction, renovascular hypertension and stroke are more common in younger patients.31, 34, 35 Involvement of the thoracic aorta and its branch vessels leading to upper limb claudication and pulse loss seems to be more common in women, whereas the renal and iliac arteries are more commonly affected in men.32, 36
Disease determinants and risk factors
The geographical and ethnic variations in GCA incidence suggest a considerable genetic contribution to disease aetiology. An association between the HLA class II region ― in particular with HLA-DRB1*04 alleles ― and GCA has been recognised for some time.37 Other studies have described links between GCA and genes encoding cytokines such as tumour necrosis factor (TNF)38 and their receptors; molecules associated with endothelial function such as intercellular adhesion molecule 1 (ICAM-1)39 and vascular endothelial growth factor (VEGF)40; regulators of innate immunity such as Toll like receptor 4 (TLR-4)41; and regulators of adaptive immunity such as the protein tyrosine phosphatase non-receptor type 22, PTPN2242. However, it was only in 2017 that the first large genome-wide association study (GWAS) in GCA ― which included >2,000 subjects of European ancestry ― confirmed a strong HLA class II association.43 This association is in keeping an underlying antigen-driven immune response in disease pathogenesis, and the predominance of CD4+ T cells within inflammatory lesions.44 The GWAS also identified risk polymorphisms in genes encoding plasminogen (PLG) and an isoform of the alpha subunit of collagen prolyl 4-hydoxylase essential for collagen biosynthesis (P4HA2), which is consistent with alterations in vascular remodelling in disease susceptibility.
In contrast to the HLA class II association observed in GCA, disease susceptibility and severity in TAK is consistently associated with inheritance of the HLA-B allele HLA-B*52:01 in populations of multiple ethnicities.45 Of note, the inflammatory lesions in TAK include a large number of CD8+ T cells, which are restricted by HLA class I polymorphisms.46, 47 Several large-scale genetic studies in the past decade have identified additional HLA and non-HLA susceptibility loci in ancestrally diverse populations,46, 48–51 which implicate a variety of pro-inflammatory, regulatory immune response and humoral pathways in disease pathogenesis. Susceptibility factors common to both GCA and TAK have also been suggested, primarily within the IL12B locus. IL12B encodes the IL-12 subunit p40, which is shared between IL-12 and IL-23 ― both of which are known to function as lineage-inducing cytokines for Th1 and Th17 cells.52
Reports of seasonal variation in GCA onset suggest that environmental factors may trigger disease in genetically susceptible individuals.53 Efforts so far have focused on identifying possible infectious triggers. Small epidemiological, clinical, and molecular studies have described potential links between GCA incidence and various organisms, including varicella-zoster virus, Chlamydia pneumoniae, Mycoplasma spp. and parvovirus B19. 54 However, as it is common for an elderly host to have encountered several infections and for there to be deposition of microbial products in tissue, these findings do not prove causality for large vessel inflammation and there is no consistent evidence of any particular micro-organism acting as a trigger for GCA.55
A higher incidence of M. tuberculosis infection has been reported in patients with TAK than in unaffected individuals, with molecular mimicry between the microbial and human 65 kDa heat shock proteins proposed as a triggering immunological event.56 However, these data suffer from epidemiological confounding and further studies are needed to support this hypothesis.57 Of note, a study from India found the frequency of tuberculosis to be 5.6% in patients with TAK, similar to the general population.30
Mortality
Data on mortality in GCA are conflicting (Box 2). In general, death in GCA is more likely due to accelerated atherosclerosis than from direct complications of the disease. Indeed, a 2017 meta-analysis demonstrated that the leading causes of death in patients with GCA were cardiovascular disease (39%, excluding deaths related to aortic aneurysm rupture), cerebrovascular disease (14%), infection (13%), and malignancy (12%), with the remaining 22% accounted for by gastrointestinal, pulmonary and renal deaths, aortic aneurysm-related deaths and deaths not specified.58 These figures are less likely to hold true in those with large-vessel complications. Indeed, the mortality in patients with ruptured aortic aneurysms as a consequence of GCA (80%) is higher than in patients without GCA (65-75%).59 A 2021 meta-analysis observed decreasing mortality rates in patients with GCA over the 50-year study period at a rate of 0.14 per 1,000 people per year.60 It may be that regular monitoring and screening for co-morbidities in patients with GCA has led to comparable mortality rates with that of the general population.61
Box 2. Mortality in giant cell arteritis (GCA).
A review of 17 studies that together included 4,733 patients with a matched, general population control group found an overall increase in mortality in GCA of ~20%.58 Importantly, subgroup analysis demonstrated that this increase was confined to hospitalised patients and no increase was observed in the community setting. In line with these results, a UK-based community study of nearly 10,000 patients with GCA demonstrated an increased mortality in the first year following diagnosis, which was not sustained at five years.288 A population-based study of >7,000 patients in Israel similarly observed increased rates of mortality within the first two years of diagnosis that was not maintained at ten years follow up and was more pronounced in those presenting at <70 years of age.289 An Italian population-based study involving 281 patients with biopsy-proven GCA found reduced survival in those with large vessel involvement at diagnosis.150 Similar results were observed in a US study of 204 patients with GCA, although in this study survival was only reduced in those with aortic manifestations, as opposed to involvement of other large vessels only.290
Mortality data for TAK are even less well-defined than those of GCA, owing to its low incidence. Overall, 10-year survival is reported to be ~90%,62–66 although this may not be that favourable given the young age at which patients are diagnosed. Several studies suggest the standardised mortality of patients with TAK is 2-fold to 3-fold higher than age-matched healthy controls.64, 66, 67 Systemic hypertension, major vascular complications, and progressive disease course were associated with increased mortality risk in these studies.
MECHANISMS/PATHOPHYSIOLOGY
Loss of arterial wall immune tolerance precedes a broad range of interlinked aberrant immunological responses involving both the innate and adaptive immune systems, which contribute to the development and progression of disease in LVV. Much of our understanding in this area comes from tissue from individuals with GCA; therefore, although mechanistic differences exist between GCA and TAK, the two conditions will be largely considered together here.
Loss of tolerance
Under physiological conditions, the walls of medium and large arteries are shielded from inflammation and autoimmunity by immune tolerance. Over the last two decades, studies in GCA have implicated several mechanisms that contribute to a loss of immune tolerance and subsequent disease induction and progression (Figure 3). Firstly, loss of anti-inflammatory T regulatory (Treg) cells leads to failed suppression of pro-inflammatory T cells in lymph nodes.68 The age-associated decline of a specialised CD8+ Treg population is mechanistically linked to mis-trafficking of intracellular vesicles. Additionally, studies have demonstrated that CD4+ Treg number and function in peripheral blood are reduced in active GCA and can be improved with IL-6 blockade.69, 70 Deficiencies in the programmed cell death 1/programmed cell death 1 ligand 1 (PD-1/PD-L1) inhibitory pathway also contribute. These deficiencies remove a natural brake of the adaptive immune system and render the artery vulnerable to autoimmune-driven inflammation. Both endothelial cells and vascular dendritic cells (DC) are naturally rich in PD-L1 and function as protective shields against activated, injurious PD-1-expressing T cells by binding PD-1 and downregulating T cell activity. In GCA, circulating and vascular DCs lack PD-L1 expression and so activated pro-inflammatory T cells are left unopposed.71, 72 Blocking the PD-1/PD-L1 pathway results in enhanced vascular inflammation, increased production of the T cell cytokines IFN-γ, IL-17 and IL-21, excessive macrophage activation and accelerated intimal hyperplasia.71, 72 Reports of large vessel inflammation developing in patients with cancer following treatment with immune checkpoint inhibitors further support the immunoinhibitory PD-1/PD-L1 pathway as a critical element of the artery’s immune tolerance.73 The third mechanism is leakiness of the endothelial barrier, which normally prevents migration of circulating cells into the vessel wall. In LVV, inflammatory cells gain access to the tunica adventitia through the adventitial vasa vasorum. In GCA, circulating monocytes produce excess matrix metalloproteases (MMPs), digest the subendothelial basal lamina layer, and enable T cells ― which are also independently capable of MMP-2 and MMP-9 production ― to infiltrate.74–77 Adventitial endothelial cells aberrantly express Jagged1, a ligand for the receptor NOTCH1, and interact with circulating CD4+ NOTCH1+ T cells,74, 78 promoting their differentiation into tissue-invasive effector cells that produce IL-17 and IFN-γ. Finally, immature neutrophils enriched in the blood of patients with GCA are potent producers of reactive oxygen species, enabling them to breach the endothelial barrier.79 Inflammation-dependent neovascularisation permits further leukocyte-endothelial cell interaction and inflammation propagation.80
Figure 3. Proposed factors contributing to a loss of immune tolerance of large arteries and initiation of inflammation in large vessel vasculitis.
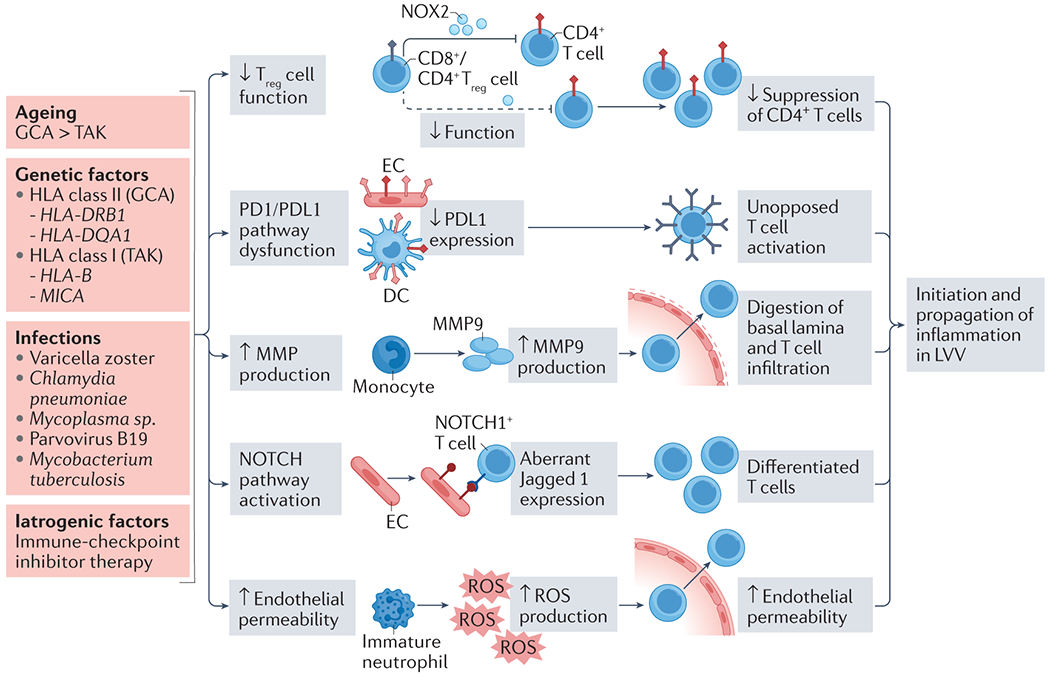
Several mechanisms contribute to loss of immune tolerance in the arterial wall, ultimately leading to the initiation of inflammation in LVV. The age-associated decline in number and function of both CD4+ and CD8+ Treg cells attenuates suppression of pro-inflammatory T cell populations. Decreased expression of PD-L1 by both dendritic cells and endothelial cells, as has been documented in GCA, removes a further check on T cell activation and pro-inflammatory cytokine release. Aberrant NOTCH pathway signalling leads to pro-inflammatory T cell differentiation. Production of several matrix metalloproteases (MMPs) is upregulated in GCA, allowing enhanced entry of inflammatory cells into the vessel wall. Reactive oxygen species produced by immature neutrophils in GCA also contribute to increased vessel wall permeability. Multiple genetic and environmental factors have been proposed which might trigger these mechanisms. In GCA, ageing is likely to play a role. Age-related reconfiguration of both the innate and adaptive immune systems — immunosenescence — and vessel wall remodelling create an environment which is susceptible to inflammation. Collaborative GWAS studies have identified both HLA and non-HLA genetic risk factors in both GCA and TAK. Links between infectious agents and LVV have been described, though no single micro-organism has been consistently implicated.
DC, dendritic cell; EC, endothelial cell; GCA, giant cell arteritis; LVV, large vessel vasculitis; MMP, matrix metalloproteinase; NOX2, NADPH oxidase 2; PD-1, programmed death-1; PD-L1, programmed death ligand-1; ROS, reactive oxygen species; TAK, Takayasu arteritis; Treg, T regulatory cell.
Loss of large vessel immune tolerance is also likely to be important in TAK, but the precise mechanisms remain elusive.81
The ageing immune system
In contrast to TAK, the incidence of GCA increases with age, suggesting that the ageing process may influence disease development. Indeed, the accrual of environmental insults over time results in epigenetic changes, with a bias towards inflammation and autoimmunity.82 Two likely synergistic mechanisms may have a role in increasing GCA risk with age. The first is immunosenescence, which is characterised by a reduction in naïve T cell and Treg cell numbers, production of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and reduced cellular responsiveness to inflammatory signals.83 The second is age-related vascular wall remodelling, defined by a reduction in number and function of vascular smooth muscle cells (VSMCs), degeneration of the media, calcium deposition, thickening of the intima and biochemical modification of matrix proteins, collectively leading to loss of elasticity and pliability.83 Unopposed, these processes create the ideal environment for chronic inflammation to dominate. Although no single infective trigger has been demonstrated in GCA, persistent or cumulative infection with pathogens and chronic antigenic stimulation could lead to loss of antigen-independent control by T cells and activation of vascular DCs.83, 84
Vascular inflammation
Once immune tolerance is lost in LVV, a cascade of pro-inflammatory mediators leads to progressive tissue damage. Vascular DCs are recognised as instigators of pathogenesis given their position at the adventitia-media interface and their sensitivity to TLR activation and reduced PD-L1 expression in GCA patients.85 Once vascular DCs are stimulated, they migrate and occupy the vessel wall,86 recruiting and retaining further innate and adaptive immune cells; in parallel, infiltrating monocytes differentiate into macrophages and multinucleate giant cells. This inflammatory process can persist for years even when disease is perceived as quiescent clinically.87 This concept of persistent vasculitis that is difficult to detect and quantify is supported by the clinical evolution of disease, with aneurysm formation and progressive arterial occlusion occurring decades after the initial diagnosis of GCA or TAK.
T cells recruited to and settling in the vessel wall produce a broad spectrum of effector cytokines, which coordinate immune and vascular cells in tissue destruction and wall remodelling (Figure 4). In granulomatous TAK and GCA lesions, T cells have a functional bias towards T helper 1 (Th1) and T helper 17 (Th17) cells.88, 89 Th1 cells are important sites of IFN-γ production which drives a low-grade inflammatory process involving macrophage activation and recruitment. Stimulated macrophages amplify inflammation and injury through releasing an array of effector molecules including cytokines such as IL-6, IL-12, IL-23, IL-1; growth factors such as VEGF and platelet derived growth factor (PDGF); and MMPs such as MMP-9, MMP-7 and MMP-2. Notably, VEGF plays a role in priming endothelial cells, which promotes further T cell influx and drives vascular remodelling, intimal thickening and neovascularisation.74, 90
Figure 4. Mediators of inflammation in large vessel vasculitis.
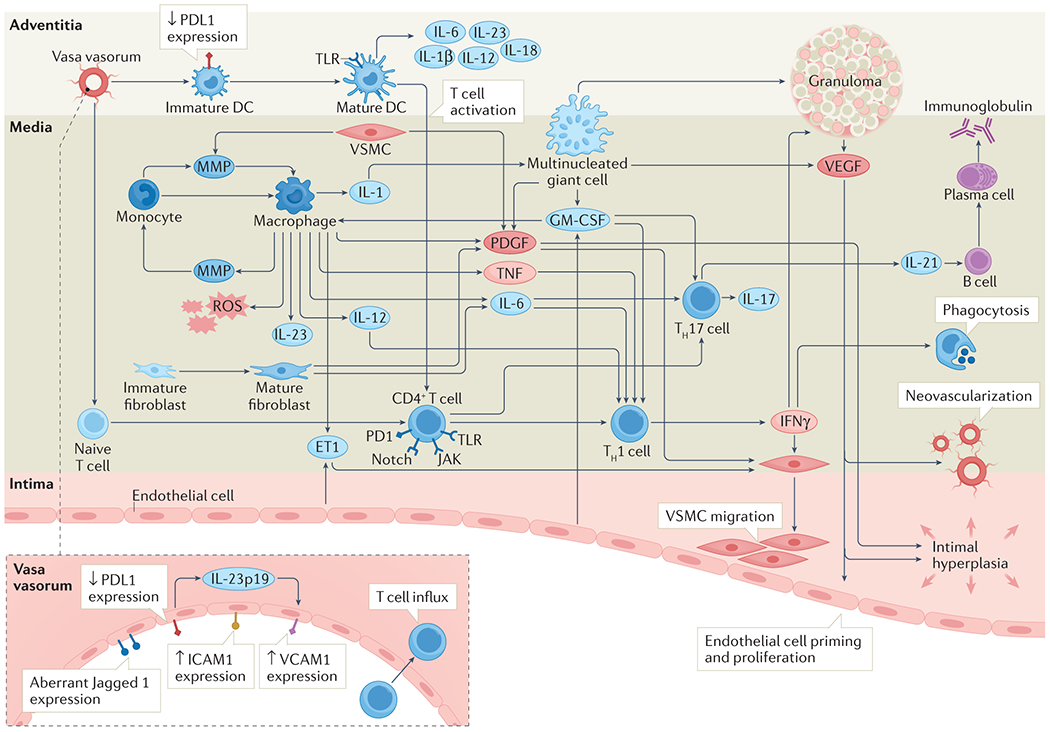
Once immune tolerance has been overcome, a cascade of pro-inflammatory mediators leads to progressive tissue damage. Stimulated dendritic cells act as instigators by recruiting and retaining pro-inflammatory cells including monocytes and T cells. Monocytes differentiate into macrophages which amplify inflammation through release of an assortment of effector molecules. Recruited T cells differentiate into Th1 cells and Th17 cells, further driving the inflammatory cascade through release of cytokines including IFN-γ (Th1) and IL-17/IL-21 (Th17). Vascular inflammation is propagated by neo-vascularisation within the vessel wall which sustains the inflammatory milieu and allows further influx of inflammatory cells. Ultimately, persistent inflammation and attempted remodelling lead to vessel wall damage including intimal hyperplasia and fibrosis, with clinical manifestations including arterial stenosis, occlusion and aneurysm formation.
DC, dendritic cell; EC, endothelial cell; ET-1, endothelin-1; GM-CSF, granulocyte macrophage colony stimulating factor; ICAM-1, intercellular adhesion molecule 1; 1IFN-γ, interferon-gamma; IL-, interleukin; JAK, Janus kinase; MMP, matrix metalloproteinase; PDGF, platelet derived growth factor; PD-1, programmed death-1; PD-L1, programmed death ligand-1; ROS, reactive oxygen species; TLR, toll-like receptor; TNF-α, tumour necrosis factor alpha; VCAM-1, vascular cell adhesion molecule 1; VSMC, vascular smooth muscle cell.
Granulocyte macrophage colony stimulating factor (GM-CSF) is an upstream mediator of Th1 and Th17 cells and is largely expressed by macrophages and endothelial cells. Inhibition of the GM-CSF receptor pathway in mouse models and explanted human tissue results in suppression of vessel wall T cell infiltration and reductions in both intimal thickness and neovascularisation, suggesting a potent interplay between GM-CSF and the Th1 axis.91–93 One of the important differences between GCA and TAK is the glucocorticoid responsiveness of T cell-mediated inflammation; in GCA, Th17 cells are more sensitive to glucocorticoid treatment than Th1-dependent responses;94, 95 conversely, in TAK, Th1 cells seem more glucocorticoid-responsive than Th17 cells.94, 96
A consistent finding in LVV vasculitic infiltrates is a broad spectrum of T cell effector cytokines beyond IFN-γ and IL-17, including IL-9, IL-21, and IL-22.91, 97, 98 It remains unclear whether a cytokine hierarchy exists, what the mechanisms for the induction of these cytokines are, whether they derive from a common cellular source or from functionally distinct T cell subsets, and whether they have distinguishing pathological roles.
Mechanistic studies have implicated the NOTCH, Janus kinase-signal transducer and activator of transcription (JAK-STAT) and mammalian target of rapamycin (mTOR) signalling pathways as being important in both GCA and TAK pathogenesis.99, 100 In humanised mouse models of LVV, blocking NOTCH signalling reduced T cell activity, downregulating both Th1 and Th17 pathways.78 Transcriptomic analysis of arterial tissue has indicated a critical, pro-inflammatory role for JAK-STAT signalling in GCA, and treatment of immunodeficient mice carrying engrafted, inflamed human arteries with small molecule JAK-STAT inhibitors is highly effective in suppressing vasculitis and the production of associated cytokines.101 mTOR complex 1 (mTORC1) activation plays a crucial role in polarising T cells towards a pro-inflammatory, effector cell status and has been demonstrated within the endothelium of the aortic wall and in Th1 and Th17 cells derived from inflammatory lesions in both GCA and TAK,102, 103 implicating mTOR signalling as a universal pathogenic pathway in LVV. Further, immunophenotyping using DNA methylation profiling has identified a pathogenic role for the calcineurin/nuclear factor of activated T cells (NFAT) pathway — another potential target for future therapeutics.82
In addition to differences in glucocorticoid-responsiveness within the Th17 axis, another distinguishing pathological feature between GCA and TAK is the composition of vessel wall infiltrates. Both share an abundance of highly activated T cells and macrophages organised into granulomata;86, 90 however, in TAK, aortic wall infiltrates contain a relatively large population of cytotoxic CD8+ T cells — reflecting the association of TAK with HLA I class polymorphisms — and natural killer (NK) cells. CD8+ T cells account for ~15% of infiltrating cells in aortic TAK lesions and are also seen in greater numbers in the circulation of patients with TAK compared with healthy controls.47, 104 It should be noted, however, that studies have also demonstrated elevated circulating CD8+ T cells in patients with GCA and CD8+ T cells have also been noted within diseased temporal artery tissue — a finding that is associated with a more aggressive disease phenotype.105 In contrast to GCA, CD16+ NK cells may represent up to 20% of all immune cells in TAK lesions,47 suggesting a pathogenic role for cytotoxicity in mediating vessel wall injury; however, histological examination in TAK most often occurs years after disease onset as opposed to early examination of TAB in GCA, which may account for some of the above differences.
Vascular injury and remodelling
Persistent intramural inflammation leads to structural changes within the diseased vessel wall including neovascularisation, which sustains resident vascular inflammation and enables further recruitment of pro-inflammatory leucocytes.80 Ultimately, a maladaptive vascular repair process is initiated whereby stromal cell populations ―primarily endothelial cells, VSMCs and fibroblasts ― expand and differentiate to drive laminar necrosis, intimal hyperplasia and fibrosis.106 VSMCs are thought to be key players in this process, undergoing phenotypic modulation by resident macrophages and Th1 cells through PDGF and endothelin-1 signalling.107, 108 Activated VSMCs proliferate and invade the intima where they deposit extracellular matrix proteins. The resultant intimal expansion leads to eventual luminal stenosis and ischemic complications.
Within the last few years studies have highlighted the role of mast cells in the pathogenesis of TAK lesions. In a series of in vitro and in vivo experiments using serum and aortic tissue from both healthy controls and patients with TAK, mast cells were responsible for increased vessel wall permeability, neovascularisation, and fibrosis; these cells represent a potential therapeutic target.109
Extravascular systemic inflammation
Emerging data suggest that vascular inflammation in LVV is often combined with an extravascular systemic inflammatory component and that these may operate independently with regards to disease mechanisms, clinical phenotypes, and therapeutic responses. This systemic inflammatory response in LVV is characterised by a florid acute phase reaction manifesting as anaemia and thrombocytosis, liver function abnormalities, and marked elevations in the erythrocyte sedimentation rate (ESR) and levels of C-reactive protein (CRP) in the blood, with a clinical phenotype of fever, malaise and myalgia. Acute phase proteins are produced by hepatocytes in response to stimulation by cytokines including IL-6 and others, though the triggers for unleashing this cytokine cluster remain unknown. The ease of measuring ESR and CRP enables swift assessment of this extravascular component; however, these metrics cannot measure the burden of inflammation within the vessel wall.
B cells in large vessel vasculitis
Chronic tissue inflammation is associated with the formation of tertiary lymphoid organs and, in LVV, these are exemplified by the accumulation of lymphoid aggregates in the perivascular tissue of atherosclerotic arteries and the aneurysmal aortic wall.110, 111 B-cell clusters have been reported in the adventitial layer of TAK-affected aorta, whereas organised B-cell infiltrates have also been confirmed within the aneurysmal aortic wall of patients with LV-GCA.111, 112 Varying in complexity, these structures are rich in both T cells and B cells and may have both pro-inflammatory and anti-inflammatory functions. Systemic inflammation in GCA is associated with changes in circulating B cell numbers and their ability to produce IL-6.113 A potential pathogenic role of autoantibodies has been suggested in TAK following the identification of endothelial cell autoantigens in these patients.114 A role for B cells in TAK pathogenesis is also supported by the findings of a large GWAS study published in 2021.51 Additionally, work in TAK has highlighted a novel follicular helper T cell signature which may promote B cell activation and function.115
DIAGNOSIS, SCREENING AND PREVENTION
No validated diagnostic criteria exist for GCA or TAK. Historically, diagnosis of GCA was based on a constellation of symptoms, ideally with histologic confirmation of vasculitis. Incorporation of vascular imaging into diagnostic assessment may complement or even replace tissue diagnosis in C-GCA and is generally considered mandatory to diagnose LV-GCA and TAK. In 2018, the European Alliance of Associations for Rheumatology (EULAR, previously the European League Against Rheumatism) proposed management recommendations for LVV that advocated for multidisciplinary diagnostic evaluation by specialists.8 Given the potential for irreversible vision loss associated with diagnostic delay, fast-track referral pathways have been developed for patients with GCA and demonstrate improved clinical outcomes and reduced healthcare costs.116
Presentation and initial investigation
Clinical features of LVV can occur owing to vascular inflammation, ischaemia, or both (Box 3). In some cases, a diagnosis of LVV is suspected in an asymptomatic patient based on findings from vascular examination or imaging studies.35 Vision disturbance requires urgent ophthalmological assessment to reduce rates of permanent vision loss,117 and treatment initiation at time of referral is recommended if the diagnosis of LVV is strongly suspected and always when sight is threatened.116
Box 3. Clinical features of giant cell arteritis & Takayasu arteritis.
Systemic symptoms
Anorexia
Arthralgia
Fatigue
Lethargy
Low-grade fever
Myalgia
Sweats
Weight loss
Symptoms of tissue/organ ischaemia
Abdominal paina
Chest paina
Coughb
Dyspneaa
Headacheb
Jaw claudicationb
Lightheadednessa
Limb claudicationa
Neck painb
Neurological deficit
Scalp tendernessb
Tongue claudicationb
Vision disturbanceb
Examination findings
Aortic regurgitationa
Carotidyniaa
Discrepancy between right and left arm BP
Hypertensiona
Ophthalmic abnormalitiesb
Reduced or absent pulsesa
Scalp tendernessb
Tender and/or thickened temporal arteriesb
Vascular bruits
amore prevalent in TAK; bmore prevalent in GCA291;BP, blood pressure
Initial investigations (Table 1) are influenced by presenting features, physician preference and availability of imaging modalities (Figure 5). As the presenting features of LVV may be non-specific, these should aim to exclude mimics such as infection or malignancy (Box 1). Elevated levels of inflammatory markers such as ESR or CRP can be observed in most patients with active disease, although may be more modestly elevated in TAK compared with GCA. 35, 118, 119
Table 1.
Laboratory investigations for large vessel vasculitis
| Investigation | Rationale |
|---|---|
| Recommended for all | |
| Full blood count (FBC) | A ‘reactive’ FBC (for example, thrombocytosis, normochromic normocytic anaemia or leukocytosis) may reflect systemic inflammatory processes |
| Urea & electrolytes test (U&E) | Although LVV rarely affects kidney function directly, baseline results may help inform treatment |
| Liver function test (LFT) | Non-specific abnormalities such as transaminitis or isolated raised alkaline phosphatase may be observed |
| Serum albumin test | May be reduced owing to systemic inflammatory process and can track recovery |
| C-reactive protein (CRP) test | Non-specific marker of inflammation |
| Erythrocyte sedimentation rate (ESR) | Non-specific marker of inflammation |
| Additional tests not recommended for all | |
| Anti-neutrophil cytoplasm antibodies (ANCA) test | Useful to exclude small vessel vasculitis if part of differential diagnosis |
| Anti-nuclear antibodies (ANA) test | Non-specific, but useful to exclude alternate systemic inflammatory conditions if part of differential diagnosis |
| Rheumatoid factor (RF)/anti-cyclic citrullinated peptides (anti-CCP) test | Useful to exclude rheumatoid arthritis if part of differential diagnosis; may detect cryoglobulinaemia |
| Complement test | May be elevated as part of the inflammatory response; low complement C3 and/or C4 suggest alternative diagnoses such as systemic lupus erythematosus, cryoglobulinaemia and bacterial endocarditis |
| Cryoglobulin test | Useful to exclude cryoglobulinaemia, which may present with systemic features and mimic large vessel inflammation |
| Quantitative serum immunoglobulin tests | Useful to exclude monoclonal gammopathy and IgG4-related disease, which may present with systemic symptoms and large vessel inflammation |
| Protein electrophoresis | Useful to exclude monoclonal gammopathy |
| Microbial investigations | Used if infection is suspected clinically; hepatitis serology is useful if polyarteritis nodosa in differential diagnosis |
Figure 5. Investigation and diagnosis of large vessel vasculitis.
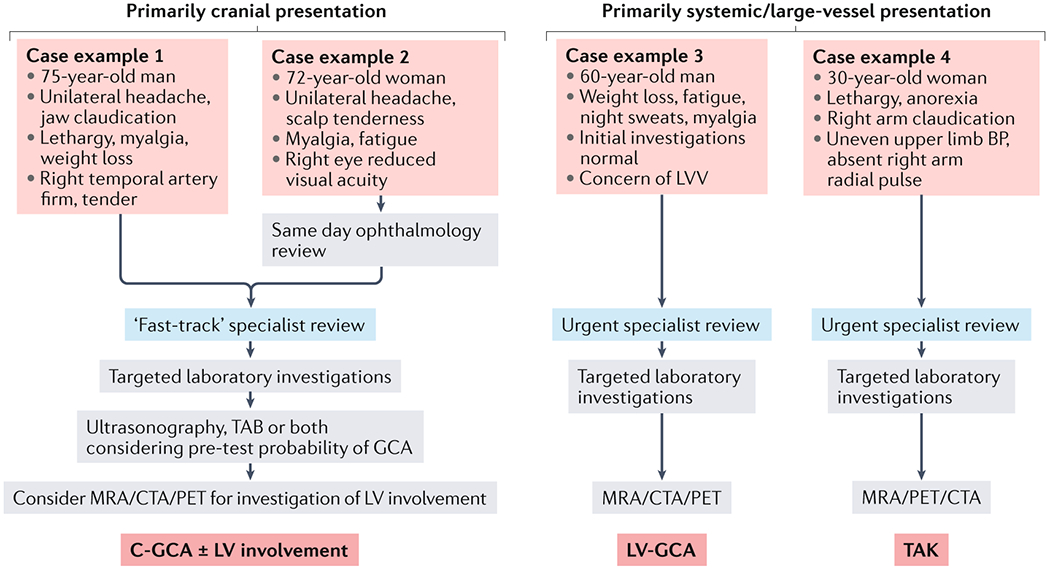
Schematic outlining a simplified approach to the investigation and diagnosis of different large vessel vasculitis (LVV) clinical syndromes. Typical features of cranial giant cell arteritis (C-GCA) (case examples 1 and 2) include headache and jaw and scalp pain, together with constitutional symptoms. Visual disturbance (case example 2) should prompt rapid ophthalmological review. The diagnostic approach to a patient with a primarily cranial presentation of LVV should consider the pre-test probability of C-GCA, which will inform whether ultrasonography or temporal arterial biopsy (TAB) is the most appropriate initial investigation116. Co-existing involvement of the aorta and associated great vessels should be considered in all patients with C-GCA. Case examples 3 and 4 depict more non-specific disease presentations typical of large vessel giant cell arteritis (LV-GCA) (case examples 3) and TAK (case examples 4). In these cases, imaging with either MRA, CTA and/or PET is required. BP, blood pressure; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; PET, positron emission tomography; TAK, Takayasu arteritis.
Imaging versus histological diagnosis
TAB is a useful investigation for suspected C-GCA or LV-GCA with cranial involvement. Previously considered the gold-standard for diagnosis, advances in the reliability of vascular imaging techniques have meant that reliance on TAB in some centres has declined.116 Indeed, several high-quality studies have demonstrated equivalent diagnostic accuracy between imaging and TAB.116 Additionally, at least in the case of ultrasonography, imaging is more cost-effective and less invasive.120 The clinical pre-test probability of GCA should be taken into account when considering which investigation might best suit the individual.116, 121 Ultrasonography alone may be sufficient to both exclude GCA in cases of low pre-test probability and confirm GCA in cases of high pre-test probability. TAB is recommended in those cases with an uncertain pre-test probability or in which ultrasonography has failed to confirm the diagnosis. The slight shift in focus towards imaging for diagnosis has been accelerated by the increased recognition of large vessel involvement in GCA — something that TAB fails to identify.6 Despite this, TAB is still an important consideration in the diagnostic pathway of C-GCA and in many parts of the world, particularly North America, remains the recommended first line investigation in suspected C-GCA.122, 123 TAB has no role in the diagnosis of TAK, in which temporal artery involvement is unusual. Histological diagnosis of TAK is only possible in exceptional circumstances or in the post-operative setting, such as following aortic valve replacement.
Choice of initial imaging modality
Multiple imaging modalities are available to assess extent and severity of LVV, including ultrasonography, MRI, CT and 18F-fluorodeoxyglucose (FDG) PET. Each modality has advantages and disadvantages and use is typically guided by the clinical scenario and local expertise. It is recommended that imaging of the aorta and major branches is considered in all patients, even in those with a primarily cranial presentation, as the presence of great vessel involvement may influence treatment strategy and prognosis (Figure 5). Of note, the diagnostic accuracy of the imaging modalities described declines quickly following treatment with glucocorticoids and imaging is best performed within one week of starting therapy.120, 121, 123 Accordingly, the use of imaging for disease monitoring presents many challenges and is considered separately (Box 4).
Box 4. Advantages and disadvantages of different imaging modalities for large vessel vasculitis (LVV) monitoring.
Assessing the response to treatment and monitoring vascular complications are important aspects of long-term disease management in LVV and can be achieved with various non-invasive imaging techniques.292 Interval ultrasonography is rarely used for disease monitoring owing to operator dependence and reliance on the involvement of accessible vessels. MRI has the potential to be a useful tool, particularly as lack of radiation exposure enables interval scanning. Vessel wall-based metrics including mural thickness, increased mural signal and mural enhancement following administration of contrast agents may inform ongoing disease activity, though further study is required.293 In a prospective study in 84 patients, correlation with clinical assessment of disease activity was less reliable with MR angiography than with PET; however, these modalities offered complementary information.294 Vascular damage, including areas of previously identified stenosis or dilation, may be best monitored with MR angiography, with scoring systems now capable of quantifying vascular damage longitudinally.257, 258 CT angiography (CTA) may also be used for monitoring vascular damage, although it is less able to detect active disease once treatment has started.295 CTA may be more useful when combined with PET, and although hybrid PET–CT is associated with more radiation exposure than CTA alone, its use may be justified by the additional functional information gained. One study investigated PET–CT as a disease monitoring tool in 56 patients with LVV and a control group consisting of 59 individuals including healthy volunteers, disease mimics and patients with hyperlipidaemia. They found a sensitivity of 85% and specificity of 83% for distinguishing active vasculitis from comparators.129 However, PET–CT did detect active inflammation in 58% of patients who were in clinically-determined remission, suggesting either an inability to distinguish active disease from vascular remodeling and atherosclerosis, or the presence of low-grade disease. This phenomenon has also been noted with other imaging modalities and remains a source of intense investigation. Such drawbacks mean that the role of PET–CT in disease monitoring remains far less established than its role in diagnosis. Hybrid PET–MR overcomes many of the problems associated with PET–CT and may provide a more detailed assessment of disease activity with reduced radiation exposure (~20% of PET–CT).135, 136 PET–MR use is increasing in other cardiovascular disorders including coronary artery disease, cardiac sarcoidosis and cardiomyopathy.296, 297 Data to support longitudinal PET–MR scanning over other imaging modalities are limited, but early results suggest feasibility and further research is ongoing.135, 298
Ultrasonography
In suspected C-GCA, ultrasonography is considered by many to be the initial investigation of choice.121 Demonstration of features including a thickened temporal artery wall (halo sign) and a vessel which remains visible following compression of the lumen (compression sign) provides a diagnostic sensitivity of 77% and specificity of 96%.124 Although ultrasonography is useful for assessing the temporal and axillary arteries ― two common sites of inflammation in GCA ― its use to detect pathology in the aorta is limited. However, assessment of the carotid and subclavian vessels by ultrasonography may have utility in TAK.125
Ultrasonography is safe, inexpensive, and widely available, although differences in performance and data interpretation can lead to reduced inter-reporter reliability. The Role of Ultrasound Compared with Biopsy of Temporal Arteries in the Diagnosis and Treatment of GCA (TABUL) study, the largest study of its kind, recruited 381 patients with a suspected new diagnosis of GCA to undergo both ultrasonography (axillary and temporal) and TAB within 10 days of starting treatment. Ultrasonography had superior sensitivity over TAB (54% vs. 39%) but inferior specificity compared with clinical diagnosis of GCA as the reference standard (81% vs. 100%).120 The lower-than-expected diagnostic accuracy of ultrasonography in this study may relate to the inexperience of some operators. Indeed, sensitivity improved by 17% once operators had completed at least 10 scans. However, the diagnostic sensitivity of TAB is also operator dependent, influenced both by specimen adequacy and the expertise of the reporting pathologist.120 Where diagnostic uncertainty exists, there may be a role for both ultrasonography and TAB.116, 120
MRI
Although prone to less inter-operator variability than ultrasonography, MRI is more expensive and less widely available. MRI provides a thorough assessment of the vessel wall and can accurately identify luminal abnormalities when combined with magnetic resonance angiography (MRA). MRI–MRA is generally considered first-line imaging for suspected TAK as it requires no radiation exposure and these patients are generally younger and may require interval scans, although few data support its accuracy.121, 126 MRI–MRA may be appropriate first-line imaging for suspected LV-GCA; however, there is little to support its superiority over CT or PET. When ultrasonography is unavailable in suspected C-GCA, high-resolution MRI of the cranial arteries provides comparable diagnostic accuracy.124
CT angiography
CT angiography (CTA) is quicker and more widely available than MRI, with a sensitivity of 73% and specificity of 78% for diagnosing LV-GCA.127 EULAR do not recommend its use for cranial disease and although it is an option for suspected large vessel disease, the ability of CTA to identify vessel wall oedema and inflammation is probably inferior to MRI.121 Further, obligatory radiation exposure makes CTA less favorable for younger patients with TAK. CT may be a useful initial investigation in situations where LVV is one of several possible diagnoses. In such cases ― for example, pyrexia of unknown origin ― CT may be the preferred imaging modality either alone or in combination with PET.
PET
FDG-PET imaging provides a functional map of large vessel inflammation. Contiguous, high-grade vascular FDG uptake affecting multiple arterial territories is typical of active LVV (Figure 7).128 Alternative causes of vascular FDG uptake, primarily atherosclerosis, can introduce diagnostic uncertainty, and several quantification methods have been proposed to distinguish LVV from atheroma and other mimics.128 Areas of maximal FDG uptake are typically referenced against background uptake values such as those from the liver or venous bloodpool, with cumulative arterial territory scores such as the PET Vasculitis Activity Score (PETVAS) used to reflect disease burden.129 A 2015 meta-analysis of the diagnostic efficacy of PET across 11 studies ― 4 in GCA (57 patients) and 7 in TAK (191 patients) ― demonstrated pooled sensitivities and specificities of 90% and 98% for GCA, respectively, and 87% and 73% for TAK.130 Evidence suggests that PET may also be useful to detect vascular pathology in the cranial arteries131, 132 and that baseline PET metrics may have a role in predicting disease course.133
Figure 7. Longitudinal follow-up imaging using 18F-fluorodeoxyglucose (FDG) PET.
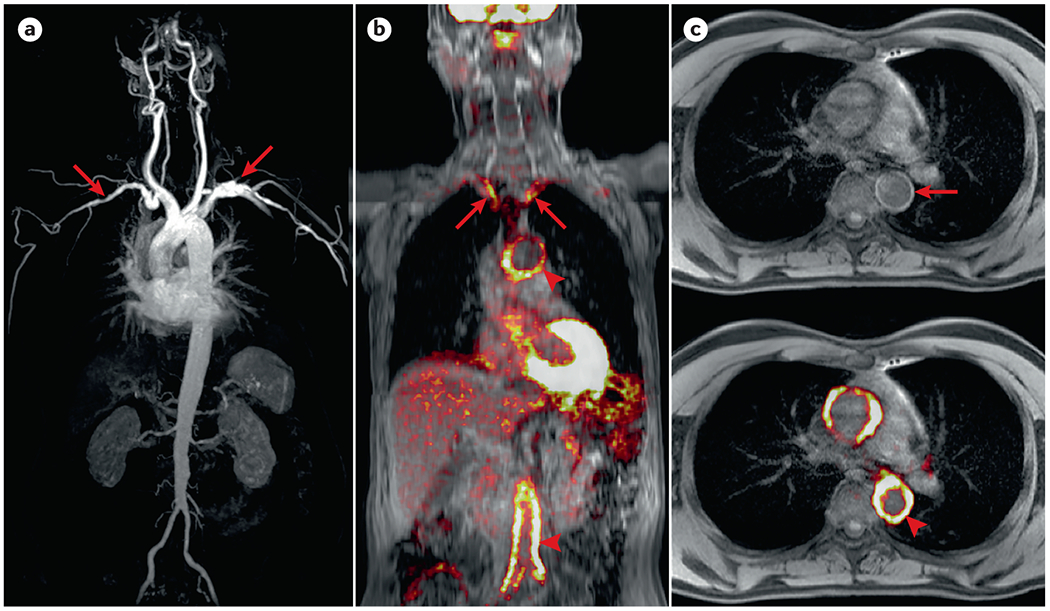
Images show a 68-year-old female patient with large vessel giant cell arteritis (LV-GCA) at time of diagnosis (A) and at 6 (B) and 12 months (C) follow-up points during treatment with tapered glucocorticoids and tocilizumab. FDG uptake is seen throughout the aorta and subclavian arteries bilaterally at diagnosis and is attenuated at each time-point thereafter.
The drawbacks of PET include limited access, high cost, and long procedure times. Further, vascular FDG uptake is attenuated rapidly following treatment initiation. A study examining the diagnostic accuracy of PET following the introduction of high-dose prednisolone in 24 patients with active LVV showed FDG signal was reduced after 3 days of treatment; although the signal at this time-point was still diagnostic in 100% of patients, by 10 days this figure had fallen to 36%.134
PET also requires a second imaging modality to map the low-definition functional image. Traditionally this has been CT, which enables impressive structural imaging data to be collected simultaneously with the functional data, albeit with considerable radiation exposure. More recently, hybrid scanners combining PET with MRI (PET–MR) have demonstrated promising results produced with a fraction of the radiation exposure of CT (Figure 6).135, 136 Further studies will determine if hybrid PET–MR is a useful diagnostic tool in LVV. Additionally, advances in PET radiotracers may enable active vascular inflammation to be distinguished from other pathologies such as atherosclerosis.137 Radioligands with specific affinity for activated macrophages such as 11C-(R)-PK11195 have shown promise in small studies demonstrating the ability to track inflammation and differentiate active LVV from inactive disease.138 PET may be of particular value in cases of diagnostic uncertainty ― for example, to exclude occult malignancy ― whether combined with CT or MRI.
Figure 6. Positron emission tomography/magnetic resonance (PET/MR) imaging in large vessel vasculitis.
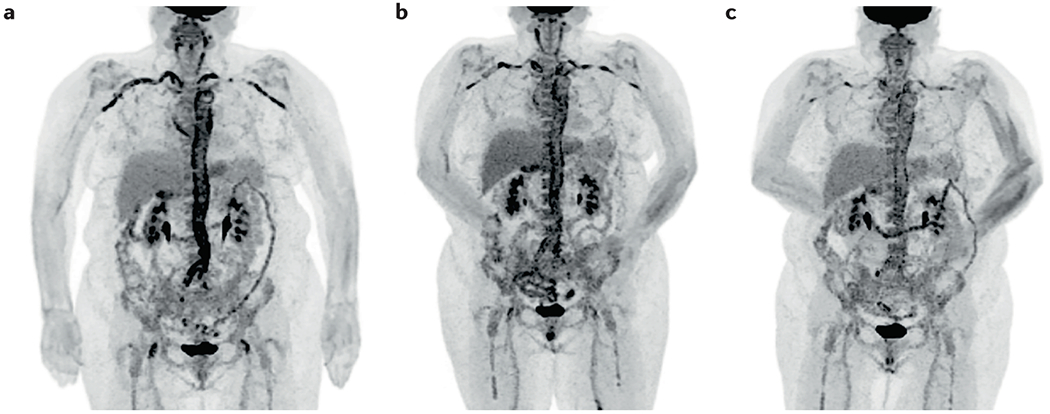
(A) Whole body magnetic resonance angiography (MRA) showing luminal subclavian abnormalities (arrows) in a patient with Takayasu arteritis (TAK). (B) Fused coronal PET–MR showing 18F-fluorodeoxyglucose (FDG) uptake involving subclavian arteries (arrows), aortic arch, and distal aorta (arrowheads) in a patient with large vessel giant cell arteritis (LV-GCA). (C) Axial T1-VIBE MRI, which provides rapid, high-definition imaging, with and without fused PET, showing mural thickening (arrow) and FDG uptake (arrowhead) within the thoracic aorta of a patient with LV-GCA.
Disease relapse
Risk of relapse is high and remains elevated for years after diagnosis of LVV. EULAR guidelines define major relapse as the recurrence of clinically active disease alongside features of ischaemia or radiologically confirmed aortic inflammation, and minor relapse as the recurrence of disease not fulfilling these criteria.8
Relapse risk in GCA has been reported as ~30–75% over the disease course and is particularly high within the first two years following diagnosis.139, 140 A retrospective US cohort study of 286 patients with biopsy-proven GCA reported a relapse rate of 74% over a median period of 5.1 years, with female patients and those with pre-existing hypertension and diabetes at greatest risk.141 Involvement of the aorta and major branches seems to confer an increased relapse risk in GCA.16 For patients with TAK, disease relapse rates are ~20% at 1 year and ~50% at 10 years142 and male sex, elevated CRP and carotidynia at presentation are associated with higher relapse risk.142 Accurate disease monitoring is key to the early recognition and treatment of relapse; such tools are important for tracking persisting, low-grade inflammation that has been demonstrated in pre-clinical and clinical studies, but does not meet the criteria for relapse and may be clinically silent.86, 87
Disease monitoring
Disease monitoring is crucial to accurately match treatment intensity with disease activity. Although several disease activity assessment tools have been proposed, none have been widely accepted for use either clinically or for research purposes. Consequently, escalation and de-escalation of treatment is based on a combination of clinical assessment, laboratory investigations and imaging.
Clinical assessment
Accurate monitoring of disease activity by clinical assessment alone can be challenging in the later phases of LVV. Symptoms such as fatigue and pain may reflect active inflammation or be consequences of established vascular disease, treatment, anxiety, or a separate disease process entirely. Similarly, arm claudication ― a symptom of LVV — may be modifiable with treatment if owing to active vessel inflammation or may be chronic and treatment-refractory if related to vascular damage. Rigorous assessment at presentation and care continuity within the same clinical team are important to recognise subsequent disease progression expeditiously.
Laboratory markers
CRP and ESR are often used for disease monitoring but may not correlate with clinical or vascular disease activity once treatment has started. In a study of biopsy-proven GCA, 24 of 25 patients had a normal ESR by day 28 of glucocorticoid treatment.143 15 patients relapsed with a total of 31 relapses; of these patients, 42% had a normal ESR at time of relapse. In this study, IL-6 was a more sensitive marker of active disease than ESR and IL-6 remained high in 67% of patients achieving complete clinical remission, whereas ESR was high in only 12.5%, supporting low-grade inflammation. In a study of 112 patients with LVV — 56 with GCA, 56 with TAK — the researchers found no correlation with ESR and only a modest correlation between CRP and outcome measures, including physician and patient reported outcomes and PET imaging.144
New biomarkers of LVV disease activity with better performance characteristics compared with clinical and imaging-based based reference standards are urgently needed. Advances in our understanding of disease pathogenesis have identified potential candidates; for example, in 2003, one study demonstrated a correlation between TAK disease activity and MMP-3 and MMP-9.145 Another study found that circulating PTX3 was higher in patients with clinically active TAK than in inactive disease, healthy controls and acute infection, and elevated levels of circulating PTX3 distinguished active disease from inactive disease better than CRP or ESR.146 Elevated PTX3 levels also correlated with active GCA, particularly in those with recent optic nerve ischaemia.147 Several other candidate biomarkers remain under investigation, including serum amyloid A, osteopontin, aminoterminal pro-B-type natriuretic peptide (NT-pro-BNP) and calprotectin; however, none have been incorporated into widespread clinical use. Potential novel biomarkers may have a role beyond diagnosis and disease monitoring, including prognostication and assessment of vascular and end-organ damage, although further work is required.148
Imaging
The ideal imaging modality for disease monitoring in LVV should be safe, widely available, cost-effective and able to distinguish persisting vascular inflammation from vascular remodeling and alternative conditions — most notably atherosclerosis. There is no current consensus on how frequently imaging should be performed in this setting and decisions should be made on an individual basis. The advantages and disadvantages of different imaging modalities for LVV disease monitoring are highlighted in Box 4. This is an area of unmet need as highlighted by the 2018 EULAR LVV research agenda.121
Disease activity assessment tools
A robust disease severity scoring system for LVV is needed. Although several assessment tools exist, these are mostly used as endpoints in clinical trials rather than for clinical purposes (Table 2). Unfortunately, there is no well-defined reference standard of disease activity against which new tools may be compared, presenting a major challenge for clinical trials.
Table 2.
Disease activity assessment tools
| Tool | GCA or TAK | Description | Validated in LVV |
|---|---|---|---|
| Birmingham Vasculitis Activity Score (BVAS)263 | Both | Designed to quantify disease activity for any vasculitis syndrome but only successfully validated in small vessel vasculitis and remains less applicable to LVV. | No |
| National Institutes of Health (NIH) criteria264 | TAK | Combines clinical assessment, laboratory investigations and imaging; 74% correlation with physician global assessment (PGA).265 | No |
| Disease extent index-Takayasu (DEI.Tak) 265 | TAK | Detailed in certain aspects such as cardiovascular examination findings. Does not consider imaging or laboratory investigations and cannot easily distinguish active disease from established vascular complications. | Yes |
| Indian Takayasu Clinical Activity Score (ITAS) and ITAS-activity (ITAS-A)266 | TAK | Similar to DEI.Tak but with even greater weighting applied to cardiovascular involvement. ITAS-A also considers C-reactive protein and erythrocyte sedimentation rate. Validation in 177 patients showed good inter-rater reliability but correlation with PGA was limited. | Yes |
GCA, giant cell arteritis; TAK, Takayasu arteritis; LVV, large vessel vasculitis.
Disease complications
Unchecked vascular inflammation may lead to a range of disease complications in LVV. Vision loss is the most feared complication of GCA in the short-term and occurs in ~15–20% of patients.149 Anterior ischemic optic neuropathy (AION) is the most common pathology contributing to vision loss and may be halted by prompt initiation of glucocorticoids. Symptoms such as diplopia and blurred vision may improve with treatment; however, complete monocular vision loss is unlikely to recover and the goal of therapy in this case is to prevent bilateral vision loss. Encouragingly, vision loss is far less common during disease relapse compared with initial presentation — an important consideration during treatment reduction or withdrawal.139
Large vessel involvement in GCA is associated with higher mortality, a potentially greater risk of relapse and higher cumulative glucocorticoid exposure.16, 150 A 2019 retrospective analysis comparing 183 patients with LVV aged 50–60 years with 183 patients aged >60 years found patients in the younger group had a higher incidence of aortic and peripheral vascular involvement and required more treatment than older patients.151 Similarly, in a cohort of 332 GCA patients, 14% of those with large vessel involvement at diagnosis had developed aortic aneurysms within ~4 years, compared with 5% of those with cranial GCA at outset.16 In a large UK study, the risk of aortic aneurysm formation in GCA was 2–fold higher than in matched controls.152 Further, owing in large part to a continued reliance on glucocorticoids, complications of treatment remain a considerable cause of morbidity in GCA with adverse effects occurring in >80% of treated patients.153
TAK is more commonly associated with large vessel complications than GCA.154 Complications, in order of frequency, include new arterial occlusion (42%), stroke or transient ischaemic attack (20%), new or worsening aneurysm (11%), end-stage kidney disease (10%), myocardial infarction (6%), heart failure (6%) and aortic regurgitation (5%).142 These are more likely in those with progressive disease, thoracic aorta involvement and in those with retinopathy.142
MANAGEMENT
There are two stages in the pharmacological treatment of LVV. Induction of disease remission, which aims to suppress initial vascular inflammation and typically requires high doses of glucocorticoids, and remission maintenance (Figure 8). The evidence base for treatment is more robust for GCA, whereas the treatment of TAK is largely based on expert opinion.
Figure 8. Management of large vessel vasculitis.
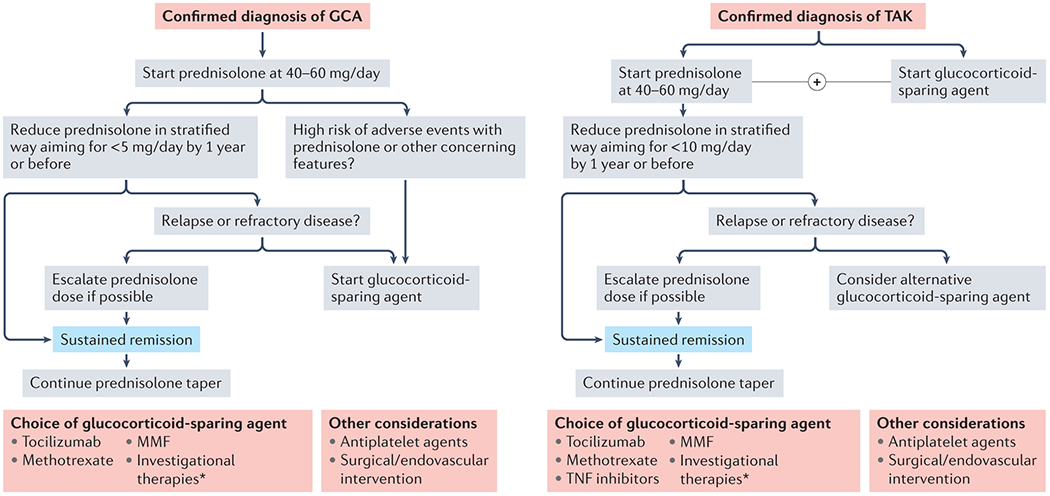
Schematic outlining a simplified approach to the management of large vessel vasculitis (LVV). Despite the associated adverse effects, glucocorticoids remain the mainstay of treatment for both giant cell arteritis (GCA) and Takayasu arteritis (TAK). Addition of a glucocorticoid-sparing agent is recommended from the outset in TAK, and may be considered in some with GCA based on clinical features. Choice of glucocorticoid-sparing agent is largely dictated by physician preference. *Several novel therapeutic agents are currently under investigation in GCA and TAK and are outlined in box 5.
Remission induction
Although never subjected to evaluation in randomised controlled trials, glucocorticoids are the mainstay of treatment for remission induction in LVV. Glucocorticoids induce rapid symptom relief and reduce the risk of vision loss in GCA. The optimal initial dose of glucocorticoids and their route of administration has not been thoroughly investigated, but is usually 40–60 mg of oral prednisolone or equivalent per day, as recommended by EULAR and ACR guidelines for both GCA and TAK.8, 123 For a more rapid and intensive, patients with GCA-related sight-threatening symptoms may be given pulsed intravenous methylprednisolone; however, there is little evidence to support this approach and it may increase the risk of glucocorticoid-related complications, as seen with its use in other vasculitides.155 Low initial oral prednisolone doses may be considered (25–30 mg/day) in select patients with TAK with a lower risk of complications; for example, those without lesions that threaten arterial flow. TAK may present without clinical, serological or imaging-based evidence of disease activity; in such patients, the benefit of treatment with glucocorticoids or other disease-modifying therapies is unknown.
An open-label study of 18 patients with GCA tested the ability of the IL-6 receptor antagonist tocilizumab to induce disease remission following three intravenous pulses of methylprednisolone.156 78% of patients achieved remission within 24 weeks and 72% were relapse-free at week 52. Five out of 18 patients (28%) stopped treatment owing to non-response or tocilizumab-related adverse events. Although tocilizumab monotherapy may induce disease-remission following brief glucocorticoid exposure, remission induction is slow and persisting disease activity may lead to ongoing symptoms or irreversible complications such as AION, as developed by one patient during the study. Thus, tocilizumab monotherapy cannot currently be recommended for remission induction.
Remission maintenance
Disease remission in LVV is defined as the absence of any clinical features attributable to active disease, normalisation of laboratory parameters and a halt in the progression of vascular imaging abnormalities.8
Glucocorticoids
Once initial disease control is achieved, glucocorticoids are tapered to reduce adverse effects, usually after 2–4 weeks. The optimal pace of tapering has not been established and probably varies between patients. In general, to achieve a compromise between relapse risk and glucocorticoid-related adverse effects — which are common157 and particularly common in elderly patients153 — it is recommended that tapering should aim to achieve 15–20 mg of prednisolone (or equivalent) per day after 2–3 months and ≤5 mg/day after 1 year. Glucocorticoid tapering is usually slower for TAK due to the greater propensity for relapse, and a target dose of ≤10 mg/day should be achieved at 1 year.158
Although tapering is needed to reduce glucocorticoid-related adverse effects, LVV relapses in 34–75% of patients when glucocorticoids are reduced,159 most commonly at doses of prednisolone below 20 mg/day.140 In general, glucocorticoid minimisation results in higher relapse rates160 and clinical trials have shown that only ~20% of patients with GCA in placebo arms maintain sustained remission at 1 year after an aggressive glucocorticoid taper and early discontinuation at 22–26 weeks.161, 162 Most patients require longer treatment periods. In a RCT published in 2017, two different tapering regimens were compared, with discontinuation of glucocorticoids at 26 or 52 weeks. At one year, relapses had occurred in 68% and 49% of patients, respectively.162 With respect to TAK, a rapid glucocorticoid taper resulted in relapses in ~60–80% of patients at the end of follow-up.163, 164
Glucocorticoid monotherapy may be considered as an option for maintaining disease-remission in GCA as ~40% of patients can reach the target of ≤5 mg/day at one year, a dose considered safe.165 When used in this way, glucocorticoid treatment should be continued for a minimum of 2 years.8 Conversely, glucocorticoid monotherapy is less effective in TAK.142, 163, 164 As TAK evolves as a more chronic and relapsing disease than GCA, the addition of disease-modifying therapy early is recommended.8
Disease-modifying or glucocorticoid-sparing treatments
Most published guidelines recommend the use of a disease-modifying agent in patients with GCA who have relapsing or refractory disease, or in those with an increased risk of glucocorticoid-related adverse effects.8 Increasingly, physicians are opting for these treatments earlier in the GCA treatment pathway, with some adopting initial combination therapy as standard practice in patients with large vessel involvement.123 Indeed, this approach has been incorporated within the latest ACR guidelines.123 In TAK, the combination of glucocorticoids and a glucocorticoid-sparing agent is considered first-line owing to the potential for higher relapse rates and disease progression in those treated with glucocorticoids alone.8, 30, 166 Novel biologic agents are now available for use in LVV in addition to traditional broad-spectrum glucocorticoid-sparing agents.
Methotrexate (MTX) has been tested in three randomised, double-blind, placebo-controlled trials in patients with newly diagnosed GCA.167–169 An individual patient-level meta-analysis of all three studies demonstrated a reduced risk of disease relapse and reduced cumulative glucocorticoid exposure in those treated with MTX compared with glucocorticoids alone;170 however, a second meta-analysis did not replicate this finding.171 MTX doses used in these trials were generally low (7.5–15 mg/week) and higher doses, although not formally tested, have been used in clinical practice. Observational, real-life data also support an effect of MTX on reducing GCA disease relapses and glucocorticoid dose.172
The glucocorticoid-sparing activity of several other immunosuppressive agents has been reported in low-quality studies (mostly retrospective or case series) including leflunomide,173 mycophenolate,174 dapsone175 and cyclophosphamide.176, 177 In a small, randomised trial, cyclosporin did not show significant glucocorticoid-sparing activity, and azathioprine showed a glucocorticoid-sparing effect in a mixed population of patients with GCA and PMR.176
TAK is often more difficult to control than GCA due to more frequent relapses. No RCT of broad-spectrum immunosuppressive agents has been performed in TAK. MTX, azathioprine, mycophenolate and leflunomide have all been reported as potentially useful.158, 178 Unless other therapies fail, cyclophosphamide is not generally recommended in TAK because of its adverse effects on fertility. Physician expertise, patient preferences, comorbidity and adverse effects usually dictate choice of treatment.
Targeted biologics therapies for GCA
Improved understanding of specific disease pathways involved in the pathogenesis of LVV has paved the way for targeted biologics therapies (Figure 4), some of which have demonstrated efficacy in phase II and phase III clinical trials and others that are currently under investigation.
After a promising phase II trial,179 the efficacy of the IL-6 receptor-blocking humanised monoclonal antibody tocilizumab has been demonstrated in the phase III Giant-Cell Arteritis Actemra (GiACTA) trial, which included both newly diagnosed and relapsing patients with GCA.162 Treatment with tocilizumab resulted in a significantly increased proportion of patients in sustained remission at week 52, a longer time to disease flare, decreased cumulative glucocorticoid doses, and improvements in quality of life over placebo.162, 180 Weekly dosing achieved better disease control than dosing every other week, particularly in relapsing and refractory cases.162
A number of observational clinical studies, including a higher proportion of relapsing patients with GCA than previous clinical trials, have used tocilizumab as an add-on therapy.181 These patients had fewer disease flares than those in the GiACTA trial, possibly because low-dose glucocorticoid or concomitant immunosuppressive treatments were not discontinued in a substantial proportion of patients.181, 182 However, one study also showed more infections in tocilizumab-treated patients.183
Tocilizumab has been a major therapeutic advance and is now licensed for the treatment of GCA in both the US and Europe. However, >40% of patients are unable to maintain disease-remission despite adherence to recommended glucocorticoid tapering, and extended follow-up data show that only 40% of initial responders maintain treatment-free disease remission after 3 years. This is supported by observational data.182, 184 Thus, tocilizumab may need to be continued for longer than the 52 weeks initially assessed in the GiACTA trial and other options are needed.185
Routinely measured acute phase reactants are abrogated by tocilizumab.186 This could be disadvantageous as there is the potential for undetected, low-grade large vessel inflammation with tocilizumab use and glucocorticoid minimisation. Indeed, case reports have demonstrated histologically active vasculitis despite clinically quiescent disease and suppressed acute phase reactants in those receiving tocilizumab.187, 188 In these cases, imaging biomarkers may be useful.189–191 Until more long-term follow-up data are available, many health care providers reserve tocilizumab for patients with, or at risk of, glucocorticoid-related adverse effects or patients with relapsing disease.
Mavrilimumab is a fully humanised monoclonal antibody targeting the GM-CSF receptor α subunit. Expression of GM-CSF and its receptor are increased in GCA tissue and preliminary results in functional models suggest a role for GM-CSF in key pathogenic aspects of GCA including dendritic cell activation, T cell differentiation, pro-inflammatory macrophage activation and angiogenesis.93 A 2021 phase II study demonstrated that mavrilimumab alongside a 26-week prednisolone taper was superior to placebo plus a 26-week prednisolone taper for increasing the time to disease flare. Sustained disease remission at week 26 was achieved in 83% of mavrilimumab recipients and in 50% of those receiving placebo.192 It is noteworthy that acute phase reactants retain their clinical value under mavrilimumab treatment and therefore mavrilimumab has promise as a novel therapeutic option for patients with GCA, although efficacy and safety need to be confirmed in larger trials.
Abatacept is a fusion protein comprised of cytotoxic T-lymphocyte protein 4 (CTLA-4) and the Fc region of IgG1 that inhibits CD28-mediated T-cell activation. In a phase II RCT recruiting patients with active disease, after an initial 3-month combination treatment with glucocorticoids and abatacept, patients in remission were randomised to continue abatacept or receive placebo in addition to standardised glucocorticoid taper with discontinuation at 28 weeks. Relapse-free survival at 12 months was slightly higher in the abatacept arm than placebo (48% versus 31%).193 The efficacy of abatacept is currently being explored in an investigator-sponsored phase III RCT.194
TNF-α is strongly expressed in GCA lesions, is elevated in serum from patients with a strong acute phase response and remains elevated in relapsing patients.195, 196 TNF inhibitors including infliximab, etanercept and adalimumab have been subjected to RCT evaluation in newly diagnosed patients with GCA and have failed to demonstrate significant benefits.161, 197, 198 These data underline that a biomarker of disease activity may not necessarily be a viable therapeutic target and TNF inhibitors are not recommended for patients with GCA.8
Ongoing phase II/III trials
Novel models using murine engraftment of human arterial tissue followed by induction of LVV-like inflammation now enable assessment of therapeutic strategies specific to large vessels.199 Work using such models has suggested a potential role for JAK inhibitors in GCA.101 The JAK1 inhibitor upadacitinib is now being evaluated for the treatment of GCA in a randomised multi-centre, double-blinded, placebo-controlled trial.200 Several other phase II and phase III trials in patients with GCA are ongoing and results are eagerly awaited (Box 5).
Box 5. Ongoing studies in giant cell arteritis (GCA) and Takayasu arteritis (TAK) GCA.
An investigator-sponsored phase III trial testing the efficacy of the CD28-mediated T cell activation inhibitor abatacept in GCA following demonstration of improved relapse-free survival in a phase II trial.194
An investigator-sponsored phase III trial with the recombinant IL-1 receptor antagonist anakinra.299 IL-1 is strongly expressed in GCA195, 300 and may have an important role at multiple steps in the pathogenesis cascade.
A phase III randomised controlled trial (RCT) blocking IL-17 with secukinumab.301 IL-17 expression is increased in GCA and rapidly decreases with glucocorticoid treatment, indicating that IL-17 suppression by high dose glucocorticoids may underline their beneficial effects.95
A phase II RCT evaluating guselkumab, a monoclonal antibody which neutralises the IL-23p19 subunit.302 IL-23, a heterodimer composed of p40 and p19 subunits, is a relevant cytokine in maintaining the Th17 differentiation pathway in GCA. The IL-23p19 subunit is expressed in excess over its partner IL12/23p40303 and may have independent pro-inflammatory activities.304
A small, open label, investigator-sponsored, phase II RCT of ustekinumab, a monoclonal antibody against p40.305 IL-12/23p40 is expressed at low levels in GCA lesions303 and blocking IL-12p40 may reduce the activity of molecules related to Th1 and Th17 differentiation in GCA lesions.303 Non-controlled studies regarding the effect have been inconclusive.306, 307
A phase III, multi-centre trial of the efficacy of the JAK1 inhibitor upadacitinib.200
An open-label trial of the endothelin receptor antagonist bosentan in GCA has been proposed but is not yet recruiting.308 In vitro data suggest a potential role for endothelin receptor antagonism as a means of inhibiting vascular smooth muscle cell proliferation in LVV.108
An investigator-sponsored phase III trial comparing tocilizumab, a monoclonal antibody against the IL-6 receptor, and methotrexate (MTX).309
Phase III clinical trials of sirukumab and sarilumab (both of which target IL-6 activity) in patients with GCA were initiated but terminated early by the sponsor. Preliminary data with sirukumab showed positive trends.310
TAK
An open-label, randomised study comparing MTX with the JAK1/3 inhibitor, tofacitinib, in patients with mild to moderate TAK.311
A phase III, multicentre RCT of the efficacy of the JAK1 inhibitor upadacitinib.312
A phase III RCT targeting the IL12/23p40 subunit with ustekinumab; proposed following promising case series results.313
A multicentre phase II RCT comparing tocilizumab with infliximab in patients with refractory or relapsing TAK (not yet recruiting).314 This study will hopefully provide clarification regarding the efficacy of different biologic therapies in this patient group.
Targeted biologic therapies for TAK
As TAK is less common than GCA and assessment of disease activity may be more difficult, there are fewer clinical trials in these patients. The efficacy of tocilizumab was tested in a RCT including 36 patients with relapsing TAK.163 Although the primary endpoint (time to relapse) did not reach statistical significance between treatment arms, there were favourable trends and no safety concerns were raised. Extended follow-up of this trial,201 observational studies and case series support a sustained benefit of tocilizumab in TAK.202–204 TNF inhibitors are used in clinical practice for those with refractory disease, and increasingly in some centres as first-line glucocorticoid-sparing therapy, although no RCT data support their efficacy.158, 205 Retrospective analyses suggest better outcomes in patients with TAK receiving biologic therapies than broad-spectrum immunosuppressive agents.202–205 A multi-centre study led by the French Takayasu Network examining outcomes in 209 patients with TAK treated with either tocilizumab or TNF inhibitors found no difference in rates of complete remission at 6 months (~70%) and prevention of relapse.206 Abatacept was tested in a phase II RCT and, in contrast to GCA, failed to demonstrate any benefit over placebo in patients with TAK.164 Case series and non-controlled small studies have reported satisfactory responses to different agents including ustekinumab,207, 208 rituximab,209 and JAK inhibitors.210 Several other agents remain under investigation (Box 5).
Revascularisation and aneurysm repair
Revascularisation procedures have an important role in the management of patients with TAK. They may be necessary when vascular lesions cause complications such as uncontrolled renovascular hypertension; are organ-threatening, for example, in the case of critical carotid or vertebral stenosis; or if they persist despite optimal pharmacological treatment.8, 158 Percutaneous angioplasty and open surgical approaches are both possible and outcomes are broadly similar.211 Simple balloon angioplasty may be preferable to stenting as in-stent stenosis seems to be more frequent than in atherosclerotic lesions in observational studies.212, 213 The use of paclitaxel-coated balloon renal artery angioplasty is being evaluated in a RCT.214 Immunomodulatory therapy should be optimised before any attempted revascularization and procedures should ideally be performed in patients in established disease remission.215 Reduced patency, restenosis and complications are more frequent when manipulating arteries with active disease.215
Revascularisation is infrequently needed in GCA, a disease with a lower incidence of stenosis than TAK. Its use for limb-artery stenoses has been reported.216, 217 Percutaneous angioplasty should be considered in patients with stroke or transient ischaemic attacks owing to proximal carotid or vertebral stenoses.218, 219 Aortic aneurysm repair may be needed in both TAK and GCA and requires joint long-term management with cardiothoracic surgeons.8, 158
Cardiovascular disease risk
Chronic low-grade inflammation and prolonged glucocorticoid exposure contribute to an increased risk of cardiovascular disease in LVV. This is due in part to the development of risk factors such as hypertension, diabetes and hypercholesterolaemia, which should be managed according to standard guidelines. Population studies have demonstrated an increased risk of myocardial infarction, stroke and atherosclerotic peripheral vascular disease in GCA versus healthy controls.220 A Canadian retrospective cohort study comparing 1,141 patients with GCA and 200,000 healthy controls aged >65 years without pre-existing cardiovascular disease showed the adjusted hazard ratio (HR) for the composite endpoint of coronary artery disease, stroke, peripheral vascular disease, aortic aneurysm or aortic dissection was 2.1 in GCA.221 These findings were replicated in a smaller and more carefully matched study, which suggested a HR of 1.8 for myocardial infarction and 2.0 for stroke in GCA.222 In contrast, a UK data linkage study examined cardiovascular outcomes in >10,000 patients with either PMR, GCA or both, and >100,000 matched controls and found no difference in cardiovascular disease incidence, although follow-up was limited to ~3 years.223
Cardiovascular disease may be more readily observed in younger patients with TAK owing to their longer life expectancy,224, 225 although supporting data are more limited than in GCA. Arterial stiffness, an independent predictor of all-cause and cardiovascular mortality, is increased in patients with TAK compared with healthy controls.226, 227 Another study found an increased burden of carotid atherosclerotic plaque in 30 patients with TAK compared with 50 matched healthy controls.228 Plaque burden was similar to a third group of patients with systemic lupus erythematosus.
Although antiplatelet agents have been used in some centres, current evidence does not support their routine use in GCA.229 Prophylactic aspirin prescription is more common in TAK and is supported by a small, retrospective study that reported a reduction in ischemic events.230 However, >90% of patients included had existing cardiovascular disease. Accordingly, anti-platelet agents should be considered on an individualised basis in both GCA and TAK, for example in patients with coronary arteritis, a history of amaurosis or symptomatic supra-aortic disease. As novel treatments continue to improve outcomes, cardiovascular risk reduction will become increasingly important, particularly in young patients.
QUALITY OF LIFE
Several studies have demonstrated impaired quality of life as a consequence of LVV231–233 comparable to that in rheumatoid arthritis.232 This may be due to the effects of active disease, disease complications or the adverse effects of immunosuppressive therapies. Quality of life effects are unique to each affected patient (Box 6 and Box 7). For example, in many with C-GCA concerns about vision loss dominate.234 In patients with large vessel involvement, the adverse effects on quality of life seem consistent between GCA and TAK despite the age difference between cohorts.233
Box 6. Patient experience – before diagnosis.
On the evening of Christmas Eve 2018 I was very tired and felt as if I had a virus. The next day, during a walk on the beach promenade, I had to rest at several benches. In retrospect, I had pain in exactly the place where everyone who knows about it would say: ‘that person has temporal arteritis’.
I was unwell at home for a long time, eventually seeing my family doctor in February when the coughing showed no signs of abating and I still felt very unwell. The symptoms lessened with a course of antibiotics but a week later, they were worse. I had no energy and no appetite. At night I woke up coughing and had nightly sweats. My head was constantly ‘bunged up’. A nose spray with steroids made no difference. My hand was often in the temporal arteritis position and I developed a rash on my back and chest.
I was referred to a bowel clinic on suspicion of cancer, and from there to a general medicine clinic. By this time, my family thought I was dying, and my family doctor was also very concerned. In March, I was diagnosed with anaemia and type 2 diabetes. I also had vision disturbances referred to as visual migraines. By the time of my appointment at the general medicine clinic, I was aching all over with what the consultant said was polymyalgia rheumatica. The consultant had to help me onto the couch and said that my spine revealed how much weight I had lost.
Box 7. Patient experience – following diagnosis.
The specialist at the general medicine clinic referred me for a PET scan and I was diagnosed with large vessel vasculitis with thickening in the aorta, which I had never heard of before. Soon after my diagnosis, a colleague said to me: ‘My step-mother had that and almost lost her kidney and my friend was on chemo for another type of vasculitis.’ Suddenly, I had a life-challenging disease. I read leaflets about vasculitis but there were none for large vessel vasculitis because it is so rare. I tried hard to get well, to be physically active and to eat well, but was exhausted most of the time and often fell asleep. I had trouble walking up stairs and showering and dressing were sometimes so tiring that I went back to bed.
For the polymyalgia rheumatica symptoms, the steroids worked wonders. I was euphoric — out of pain within two hours after the first dose — but soon after this, my lips and fingers tingled, my body was restless and my mind was racing and out of focus. I could not concentrate, could not organise myself, and spoke rapidly. I was hungry, then very tired. Then, the dip: 4 hours where I just did not care about anything. This happened every single day after taking the steroid medication. I also had the round face, the round stomach, the fatty lump on my arm and pouches of fat in odd places.
Throughout the period of this illness, I saw a counsellor. I do not know how I would have managed without her. She helped me face how hard it was to be ill, how much it changed me, how I struggled to work and how to keep going. I was able to talk about how out of control I felt, as if I was going mad. My grown-up daughter was also amazing in her support. She helped me to let go and accept that all I had to do was to focus on getting well, instead of thinking I was useless, incompetent and without a role in life.
I now feel strongly reassured by my rheumatologist, who supports me to remain calm about the continuing effects of taking steroids, which at this moment I feel I might probably be taking for ever. Without this support I would be very lost, as I still struggle to associate my symptoms with my disease.
Qualitative studies have attempted to determine the specific patient-reported outcomes that are most influenced by LVV.234, 235 A study in patients with TAK in both the USA and Turkey suggested that almost all areas of day-to-day life were affected including employment, family life, finance and self-care.235 During periods of active disease, fatigue and pain were the dominant factors reducing quality of life, whereas during remission, the emotional burden of disease was more substantial. Functional impairment in this young patient group should not be underestimated; in a US cohort of 30 patients with TAK with a median age of 27 years at diagnosis, >60% had difficulty with routine activities of daily living, and 23% were unable to work due to disability.154
Recognising that standardised health questionnaires may not accurately capture the complexities of LVV disease effects, efforts are ongoing to construct disease-specific, patient-reported outcome measures in both GCA and TAK. A Delphi exercise conducted by the Outcome Measures in Rheumatology (OMERACT) group evaluated which disease-related items were of most value to both clinicians and patients when determining disease activity in LVV with the aim of creating a multi-dimensional tool for use in future studies.236 The outcomes identified as most important to patients were fatigue, pain and the emotional impact of disease.
Owing to the absence of any one single reliable measure of disease activity in LVV, assessments of quality of life and other patient-reported outcomes have been evaluated as potential disease biomarkers for use both clinically and in trials. In a US-based prospective cohort study of 112 participants — 56 with GCA, 56 with TAK — patient global assessment of disease activity scores independently associated with clinically active disease.233 This study demonstrated a complex relationship between other patient-reported outcomes and clinical outcomes based on laboratory analysis, imaging, and physician’s assessment. Accordingly, composite measures of disease activity, combining clinical and patient-reported outcomes including quality of life assessment may provide a more accurate reflection of disease activity.
Understanding that attainment of disease remission is only one aspect of a patient’s disease burden may be an important step towards improving the patient journey in the longer term. Interestingly, the GiACTA trial reported that attainment of remission by pharmacological therapy only modestly affected quality of life indices.162 Non-pharmacological interventions including exercise and psychological therapy may improve quality of life — demonstrated in other rheumatological conditions237 — as could supporting access to employment where possible.238 Future work should continue to focus on what matters most to patients in order to provide sustained improvements in quality of life.
OUTLOOK
The past decade has seen substantial advances in our understanding and ability to manage LVV. However, morbidity remains high; in GCA, vision loss is frequent and in TAK, premature mortality is a continued concern.239 Further, adverse effects from immunosuppressive therapy, particularly glucocorticoids, are an unresolved dilemma. Key aspirations for the next decade will include improved understanding of pathogenesis, earlier diagnosis and more targeted therapeutic approaches underpinned by clinical trial data.
Molecular and cellular studies of the arterial wall in LVV are improving understanding of disease pathogenesis. Recognition of the differences between GCA and TAK, with clear definition of shared and disease-specific pathogenic mechanisms will be critical for targeted treatment strategies.84 Access to tissue is a considerable challenge for these studies, although the use of TAB has accelerated progress in GCA, including identification of the importance of NOTCH ligand Jagged1.240 The ageing immune system is pertinent to GCA, with defects in both the PD-1/PD-L1 immunoinhibitory checkpoint72 and immunosuppressive function of CD8+ Treg cells reported.241 Further definition of the relative importance of lesion CD4+ and CD8+ T cells in LVV104 and investigation of persistent tissue-resident T cells will help direct novel treatment approaches.100, 101
The role of additional cell types in the various stages of LVV — for example, NK cells46 and suppressor neutrophils242 — and their potential as therapeutic targets merits further study. Indeed, the role of both B cells and of the vascular endothelium in the pathogenesis of GCA and TAK has received renewed attention. Antibodies directed against endothelial protein C receptor and scavenger receptor class B member 1 may induce endothelial cell activation.114 However, the importance of the endothelium in facilitating leukocyte trafficking into the arterial wall, and how this might be targeted therapeutically, remains to be determined.
Collaborative GWAS studies have yielded pathogenic insights and revealed potential therapeutic targets. Alongside identification of novel disease susceptibility loci, prominent roles for NK cells, monocytes, macrophages, T cells and potentially B cells have been reported.46, 51, 52, 243 A large multi-ancestral GWAS for TAK identified candidate disease susceptibility loci and devised a new genetic risk score in addition to reinforcing and extending the identification of HLA risk factors and non-HLA susceptibility loci.51 A TAK risk locus identified in IL6 was shown to influence the monocyte anti-inflammatory gene GPNMB through chromatin looping and recruitment of an epigenetic repressive complex244 and further functional analyses of genetic variants identified are now required.
Detection of low-grade arterial wall inflammation in LVV remains sub-optimal, especially in the presence of normalised acute phase proteins. There is an urgent need for novel plasma and imaging biomarkers capable of sensitively and specifically identifying active disease, monitoring treatment response, and distinguishing vascular and extravascular components of disease.148, 245 Collaborative effort will facilitate collection of samples in sufficient numbers and diversity for application in novel technologies able to identify biomarkers and pathogenic pathways in complex autoimmune diseases. These include proteomic and metabolomic platforms, alongside genomic approaches such as single-cell and single-nucleus RNA-sequencing. Although individual novel biomarkers may be useful, interest is focused on the use of clusters as biomarkers, for example groups of metabolites.246 Indeed, a logistic regression model based on a group of eight cytokines has been reported to accurately distinguish active and inactive TAK247. Further, microRNA (miRNA) screening has revealed overexpression of pro-synthetic miRNAs and underexpression of contractile miRNAs in TAB samples from patients with GCA.248
Advances in imaging technology, including the advent of total body PET, novel PET tracers, hybrid PET–MR scanners and high-resolution MRI, offer important opportunities for cardiovascular imaging. PET–CT has proved a sensitive and specific method for LVV diagnosis, although its role in patient follow-up is not well defined and recent studies have identified important caveats that suggest additional PET tracers are required for this purpose.8, 249–251 Issues also surround the interpretation of persistent, MRI-detected arterial wall enhancement in LVV patients with apparent treatment-induced clinical remission.189 Various PET tracers are under investigation for their potential use in vascular inflammation imaging.252 Although much of this work is centred around atherosclerosis, it may ultimately translate to vasculitis. PET tracers explored for use in LVV include ligands for translocator protein (TSPO)253, 254 and, more recently, the somatostatin receptor type 2 ligands 68Ga-Dotatate and 18F-FET-βAG-TOCA as part of an on-going PET–MR clinical study.255, 256 The need to minimise radiation exposure, particularly in young patients, remains paramount. New PET scanners limiting exposure times and increasing use of MRI are important steps in this direction.
One outstanding imaging challenge is the need to develop standardised and validated quantification techniques for non-invasive imaging,249 such as those reported for MRI257, 258 and PET imaging.250 Composite imaging scores suitable for use in patient monitoring and as defined end points in clinical trials are urgently needed.
Multi-national studies will accelerate progress and indeed pooling of multi-centre imaging data has led to improved understanding of LVV phenotypic clusters259, 260 that will likely lead to the recognition of additional LVV subgroups.151 Stratification followed by prospective monitoring to investigate distinct patterns of risk and complications will ultimately enable personalised treatment approaches. Determining homogeneous disease subgroups is essential for future clinical trials.
The paucity of randomised, placebo-controlled clinical trials in LVV is well-recognised,261 but the landscape is changing (Box 5). Novel trial designs, such as that proposed for BIOVAS (https://fundingawards.nihr.ac.uk/award/17/83/01), may prove valuable. An important hurdle in LVV clinical trials is the lack of widely accepted methods for grading disease activity, remission, and damage. The OMERACT group are currently developing a core set of domains and outcome measures to address this issue.262
Although considerable challenges remain, progress is good and prospects have never been better. Advances will soon facilitate earlier diagnosis, better define disease remission, reduce morbidity, and may enable development of glucocorticoid-free therapeutic protocols with the aim of achieving reliable, relapse-free treatment withdrawal for patients with LVV.
Acknowledgements
D.P. and M.K. are funded by Clinical Academic Fellowships from the Chief Scientist Office, Scotland (CAF/19/01 and CAF/21/05, respectively). M.C.C. is funded by the Ministerio de Ciencia e Innovación/AEI//10.13039/501100011033 (PID2020-114909RB-I00) and the International Vasculitis Foundation. N.D. is supported by a Senior Clinical Research Fellowship from the Chief Scientist Office (SCAF/19/02). J.C.M., S.P.M. and T.Y. acknowledge infrastructure support from the Imperial National Institute for Health Research (NIHR) Biomedical Research Centre. P.C.G. is supported by the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K and Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Horton BT, Magath TB and Brown GE. Arteritis of the temporal vessels: a previously undescribed form. Archives of internal medicine. 1934;53:400–409. [Google Scholar]
- 3.Kogstad OA. Polymyalgia rheumatica and its relation to arteritis temporalis. Acta Med Scand. 1965;178:591–8. [PubMed] [Google Scholar]
- 4.Gilmour JR. Giant cell chronic arteritis. J Pathol Bacteriol. 1941;53:263–277. [Google Scholar]
- 5.Hamrin B, Jonsson N and Hellsten S. Polymyalgia Arteritica. Annals of the rheumatic diseases. 1968;27:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L and Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131–7. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt WA, Natusch A, Moller DE, Vorpahl K and Gromnica-Ihle E. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol. 2002;20:309–18. [PubMed] [Google Scholar]
- 8.Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, Cassie R, Cid MC, Dasgupta B, Dejaco C, Hatemi G, Hollinger N, Mahr A, Mollan SP, Mukhtyar C, Ponte C, Salvarani C, Sivakumar R, Tian X, Tomasson G, Turesson C, Schmidt W, Villiger PM, Watts R, Young C and Luqmani RA. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Annals of the rheumatic diseases. 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 9.Takayasu M A case with peculiar changes of the retinal central vessels (in Japanese). Acta Soc Ophthal Jpn. 1908;12:554–555. [Google Scholar]
- 10.Nasu T Pathology of pulseless disease. A systematic study and critical review of twenty-one autopsy cases reported in Japan. Angiology. 1963;14:225–42. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Gay MA, Miranda-Filloy JA, Lopez-Diaz MJ, Perez-Alvarez R, Gonzalez-Juanatey C, Sanchez-Andrade A, Martin J and Llorca J. Giant cell arteritis in northwestern Spain: a 25-year epidemiologic study. Medicine (Baltimore). 2007;86:61–8. [DOI] [PubMed] [Google Scholar]
- 12.Salvarani C, Crowson CS, O’Fallon WM, Hunder GG and Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis and rheumatism. 2004;51:264–8. [DOI] [PubMed] [Google Scholar]
- 13.Kermani TA, Schafer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL and Warrington KJ. Increase in age at onset of giant cell arteritis: a population-based study. Annals of the rheumatic diseases. 2010;69:780–1. [DOI] [PubMed] [Google Scholar]
- 14.Gran JT and Myklebust G. The incidence of polymyalgia rheumatica and temporal arteritis in the county of Aust Agder, south Norway: a prospective study 1987–94. The Journal of rheumatology. 1997;24:1739–43. [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J and Llorca J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis and rheumatism. 2009;61:1454–61. [DOI] [PubMed] [Google Scholar]
- 16.Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL and Warrington KJ. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford). 2015;54:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A and Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford). 2008;47:96–101. [DOI] [PubMed] [Google Scholar]
- 18.Boesen P and Sorensen SF. Giant cell arteritis, temporal arteritis, and polymyalgia rheumatica in a Danish county. A prospective investigation, 1982-1985. Arthritis and rheumatism. 1987;30:294–9. [DOI] [PubMed] [Google Scholar]
- 19.Salvarani C, Macchioni P, Zizzi F, Mantovani W, Rossi F, Castri C, Capozzoli N, Baricchi R, Boiardi L, Chiaravalloti F and et al. Epidemiologic and immunogenetic aspects of polymyalgia rheumatica and giant cell arteritis in northern Italy. Arthritis and rheumatism. 1991;34:351–6. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Yano T, Matsumoto Y, Numano F, Nakajima N, Yasuda K, Yutani C, Nakayama T, Tamakoshi A, Kawamura T, Ohno Y, Inaba Y and Hashimoto H. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis and rheumatism. 2003;49:594–8. [DOI] [PubMed] [Google Scholar]
- 21.Tam S and Wong TC. Temporal arteritis in Hong Kong. Int J Rheum Dis. 2008;11:163–169. [Google Scholar]
- 22.Sharma A, Mohammad AJ and Turesson C. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: A systematic literature review. Semin Arthritis Rheum. 2020;50:1040–1048. [DOI] [PubMed] [Google Scholar]
- 23.Koide K Takayasu arteritis in Japan. Heart Vessels Suppl. 1992;7:48–54. [DOI] [PubMed] [Google Scholar]
- 24.Watts R, Al-Taiar A, Mooney J, Scott D and Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology (Oxford). 2009;48:1008–11. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer L, Faurschou M and Baslund B. A population-based study of Takayasu s arteritis in eastern Denmark. Clinical and experimental rheumatology. 2011;29:S40–2. [PubMed] [Google Scholar]
- 26.Mohammad AJ and Mandl T. Takayasu arteritis in southern Sweden. The Journal of rheumatology. 2015;42:853–8. [DOI] [PubMed] [Google Scholar]
- 27.Gudbrandsson B, Molberg O, Garen T and Palm O. Prevalence, Incidence, and Disease Characteristics of Takayasu Arteritis by Ethnic Background: Data From a Large, Population-Based Cohort Resident in Southern Norway. Arthritis Care Res (Hoboken). 2017;69:278–285. [DOI] [PubMed] [Google Scholar]
- 28.Arnaud L, Haroche J, Limal N, Toledano D, Gambotti L, Chalumeau NC, Boutin D, Cacoub P, Cluzel P, Koskas F, Kieffer E, Piette JC and Amoura Z. Takayasu arteritis in France: a single-center retrospective study of 82 cases comparing white, North African, and black patients. Medicine (Baltimore). 2010;89:1–17. [DOI] [PubMed] [Google Scholar]
- 29.Rutter M, Bowley J, Lanyon PC, Grainge MJ and Pearce FA. A Systematic Review and Meta-Analysis of the Incidence Rate of Takayasu Arteritis. Rheumatology (Oxford). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel R, Danda D, Joseph G, Ravindran R, Kumar S, Jayaseelan V, Jayaseelan L and Bacon P. Long-term outcome of 251 patients with Takayasu arteritis on combination immunosuppressant therapy: Single centre experience from a large tertiary care teaching hospital in Southern India. Semin Arthritis Rheum. 2018;47:718–726. [DOI] [PubMed] [Google Scholar]
- 31.Danda D, Goel R, Joseph G, Kumar ST, Nair A, Ravindran R, Jeyaseelan L, Merkel PA and Grayson PC. Clinical course of 602 patients with Takayasu’s arteritis: comparison between Childhood-onset versus adult onset disease. Rheumatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang W, Zhou M, Lu PYJ, Li Y and Chen Y. An Observational Study of Sex Differences in Takayasu Arteritis in China: Implications for Worldwide Regional Differences. Ann Vasc Surg. 2020;66:309–317. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe Y, Miyata T and Tanemoto K. Current Clinical Features of New Patients With Takayasu Arteritis Observed From Cross-Country Research in Japan: Age and Sex Specificity. Circulation. 2015;132:1701–9. [DOI] [PubMed] [Google Scholar]
- 34.Aeschlimann FA, Barra L, Alsolaimani R, Benseler SM, Hebert D, Khalidi N, Laxer RM, Noone D, Pagnoux C, Twilt M and Yeung RSM. Presentation and Disease Course of Childhood-Onset Versus Adult-Onset Takayasu Arteritis. Arthritis & rheumatology (Hoboken, NJ). 2019;71:315–323. [DOI] [PubMed] [Google Scholar]
- 35.Quinn KA, Gribbons KB, Carette S, Cuthbertson D, Khalidi NA, Koening CL, Langford CA, McAlear CA, Monach PA, Moreland LW, Pagnoux C, Seo P, Sreih AG, Warrington KJ, Ytterberg SR, Novakovich E, Merkel PA and Grayson PC. Patterns of clinical presentation in Takayasu’s arteritis. Semin Arthritis Rheum. 2020;50:576–581. [DOI] [PubMed] [Google Scholar]
- 36.Tomelleri A, Campochiaro C, Sartorelli S, Cavalli G, De Luca G, Baldissera E and Dagna L. Gender differences in clinical presentation and vascular pattern in patients with Takayasu arteritis. Scand J Rheumatol. 2019;48:482–490. [DOI] [PubMed] [Google Scholar]
- 37.Carmona FD, Gonzalez-Gay MA and Martin J. Genetic component of giant cell arteritis. Rheumatology. 2014;53:6–18. [DOI] [PubMed] [Google Scholar]
- 38.Mattey DL, Hajeer AH, Dababneh A, Thomson W, González-Gay MA, García-Porrúa C and Ollier WE. Association of giant cell arteritis and polymyalgia rheumatica with different tumor necrosis factor microsatellite polymorphisms. Arthritis and rheumatism. 2000;43:1749–55. [DOI] [PubMed] [Google Scholar]
- 39.Salvarani C, Casali B, Boiardi L, Ranzi A, Macchioni P, Nicoli D, Farnetti E, Brini M and Portioli I. Intercellular adhesion molecule 1 gene polymorphisms in polymyalgia rheumatica/giant cell arteritis: association with disease risk and severity. The Journal of rheumatology. 2000;27:1215–21. [PubMed] [Google Scholar]
- 40.Rueda B, Lopez-Nevot MA, Lopez-Diaz MJ, Garcia-Porrua C, Martín J and Gonzalez-Gay MA. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteritis. The Journal of rheumatology. 2005;32:1737–41. [PubMed] [Google Scholar]
- 41.Palomino-Morales R, Torres O, Vazquez-Rodriguez TR, Morado IC, Castañeda S, Callejas-Rubio JL, Miranda-Filloy JA, Fernandez-Gutierrez B, Martin J and Gonzalez-Gay MA. Association between toll-like receptor 4 gene polymorphism and biopsy-proven giant cell arteritis. The Journal of rheumatology. 2009;36:1501–6. [DOI] [PubMed] [Google Scholar]
- 42.Serrano A, Márquez A, Mackie SL, Carmona FD, Solans R, Miranda-Filloy JA, Hernández-Rodríguez J, Cid MC, Castañeda S, Morado IC, Narváez J, Blanco R, Sopeña B, García-Villanueva MJ, Monfort J, Ortego-Centeno N, Unzurrunzaga A, Marí-Alfonso B, Sánchez Martín J, de Miguel E, Magro C, Raya E, Braun N, Latus J, Molberg O, Lie BA, Moosig F, Witte T, Morgan AW, González-Gay MA and Martín J. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Annals of the rheumatic diseases. 2013;72:1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmona FD, Vaglio A, Mackie SL, Hernandez-Rodriguez J, Monach PA, Castaneda S, Solans R, Morado IC, Narvaez J, Ramentol-Sintas M, Pease CT, Dasgupta B, Watts R, Khalidi N, Langford CA, Ytterberg S, Boiardi L, Beretta L, Govoni M, Emmi G, Bonatti F, Cimmino MA, Witte T, Neumann T, Holle J, Schonau V, Sailler L, Papo T, Haroche J, Mahr A, Mouthon L, Molberg O, Diamantopoulos AP, Voskuyl A, Brouwer E, Daikeler T, Berger CT, Molloy ES, O’Neill L, Blockmans D, Lie BA, McLaren P, Vyse TJ, Wijmenga C, Allanore Y, Koeleman BPC, Spanish CGAG, Consortium U, Vasculitis Clinical Research C, Barrett JH, Cid MC, Salvarani C, Merkel PA, Morgan AW, Gonzalez-Gay MA and Martin J A Genome-wide Association Study Identifies Risk Alleles in Plasminogen and P4HA2 Associated with Giant Cell Arteritis. Am J Hum Genet. 2017;100:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weyand C and Goronzy J. Immune mechanisms in medium and large-vessel vasculitis. Nature reviews Rheumatology. 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renauer P and Sawalha AH. The genetics of Takayasu arteritis. Presse medicale. 2017;46:e179–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terao C, Yoshifuji H, Matsumura T, Naruse TK, Ishii T, Nakaoka Y, Kirino Y, Matsuo K, Origuchi T, Shimizu M, Maejima Y, Amiya E, Tamura N, Kawaguchi T, Takahashi M, Setoh K, Ohmura K, Watanabe R, Horita T, Atsumi T, Matsukura M, Miyata T, Kochi Y, Suda T, Tanemoto K, Meguro A, Okada Y, Ogimoto A, Yamamoto M, Takahashi H, Nakayamada S, Saito K, Kuwana M, Mizuki N, Tabara Y, Ueda A, Komuro I, Kimura A, Isobe M, Mimori T and Matsuda F. Genetic determinants and an epistasis of LILRA3 and HLA-B*52 in Takayasu arteritis. Proc Natl Acad Sci U S A. 2018;115:13045–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seko Y, Minota S, Kawasaki A, Shinkai Y, Maeda K, Yagita H, Okumura K, Sato O, Takagi A, Tada Y and et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest. 1994;93:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renauer PA, Saruhan-Direskeneli G, Coit P, Adler A, Aksu K, Keser G, Alibaz-Oner F, Aydin SZ, Kamali S, Inanc M, Carette S, Cuthbertson D, Hoffman GS, Akar S, Onen F, Akkoc N, Khalidi NA, Koening C, Karadag O, Kiraz S, Langford CA, Maksimowicz-McKinnon K, McAlear CA, Ozbalkan Z, Ates A, Karaaslan Y, Duzgun N, Monach PA, Ozer HT, Erken E, Ozturk MA, Yazici A, Cefle A, Onat AM, Kisacik B, Pagnoux C, Kasifoglu T, Seyahi E, Fresko I, Seo P, Sreih AG, Warrington KJ, Ytterberg SR, Cobankara V, Cunninghame-Graham DS, Vyse TJ, Pamuk ON, Tunc SE, Dalkilic E, Bicakcigil M, Yentur SP, Wren JD, Merkel PA, Direskeneli H and Sawalha AH. Identification of Susceptibility Loci in IL6, RPS9/LILRB3, and an Intergenic Locus on Chromosome 21q22 in Takayasu Arteritis in a Genome-Wide Association Study. Arthritis & rheumatology (Hoboken, NJ). 2015;67:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saruhan-Direskeneli G, Hughes T, Aksu K, Keser G, Coit P, Aydin SZ, Alibaz-Oner F, Kamali S, Inanc M, Carette S, Hoffman GS, Akar S, Onen F, Akkoc N, Khalidi NA, Koening C, Karadag O, Kiraz S, Langford CA, McAlear CA, Ozbalkan Z, Ates A, Karaaslan Y, Maksimowicz-McKinnon K, Monach PA, Ozer HT, Seyahi E, Fresko I, Cefle A, Seo P, Warrington KJ, Ozturk MA, Ytterberg SR, Cobankara V, Onat AM, Guthridge JM, James JA, Tunc E, Duzgun N, Bicakcigil M, Yentur SP, Merkel PA, Direskeneli H and Sawalha AH. Identification of multiple genetic susceptibility loci in Takayasu arteritis. American journal of human genetics. 2013;93:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terao C, Yoshifuji H, Kimura A, Matsumura T, Ohmura K, Takahashi M, Shimizu M, Kawaguchi T, Chen Z, Naruse TK, Sato-Otsubo A, Ebana Y, Maejima Y, Kinoshita H, Murakami K, Kawabata D, Wada Y, Narita I, Tazaki J, Kawaguchi Y, Yamanaka H, Yurugi K, Miura Y, Maekawa T, Ogawa S, Komuro I, Nagai R, Yamada R, Tabara Y, Isobe M, Mimori T and Matsuda F. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. American journal of human genetics. 2013;93:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz-Fernandez L, Saruhan-Direskeneli G, Alibaz-Oner F, Kaymaz-Tahra S, Coit P, Kong X, Kiprianos AP, Maughan RT, Aydin SZ, Aksu K, Keser G, Kamali S, Inanc M, Springer J, Akar S, Onen F, Akkoc N, Khalidi NA, Koening C, Karadag O, Kiraz S, Forbess L, Langford CA, McAlear CA, Ozbalkan Z, Yavuz S, Cetin GY, Alpay-Kanitez N, Chung S, Ates A, Karaaslan Y, McKinnon-Maksimowicz K, Monach PA, Ozer HTE, Seyahi E, Fresko I, Cefle A, Seo P, Warrington KJ, Ozturk MA, Ytterberg SR, Cobankara V, Onat AM, Duzgun N, Bicakcigil M, Yentur SP, Lally L, Manfredi AA, Baldissera E, Erken E, Yazici A, Kisacik B, Kasifoglu T, Dalkilic E, Cuthbertson D, Pagnoux C, Sreih A, Reales G, Wallace C, Wren JD, Cunninghame-Graham DS, Vyse TJ, Sun Y, Chen H, Grayson PC, Tombetti E, Jiang L, Mason JC, Merkel PA, Direskeneli H and Sawalha AH. Identification of susceptibility loci for Takayasu arteritis through a large multi-ancestral genome-wide association study. American journal of human genetics. 2021;108:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carmona FD, Coit P, Saruhan-Direskeneli G, Hernandez-Rodriguez J, Cid MC, Solans R, Castaneda S, Vaglio A, Direskeneli H, Merkel PA, Boiardi L, Salvarani C, Gonzalez-Gay MA, Martin J, Sawalha AH, Spanish GCASG, Italian GCASG, Turkish Takayasu Study G and Vasculitis Clinical Research C. Analysis of the common genetic component of large-vessel vasculitides through a meta-Immunochip strategy. Sci Rep. 2017;7:43953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smeeth L, Cook C and Hall AJ. Incidence of diagnosed polymyalgia rheumatica and temporal arteritis in the United Kingdom, 1990-2001. Annals of the rheumatic diseases. 2006;65:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordborg E and Nordborg C. Giant cell arteritis: epidemiological clues to its pathogenesis and an update on its treatment. Rheumatology (Oxford). 2003;42:413–21. [DOI] [PubMed] [Google Scholar]
- 55.Ostrowski RA, Metgud S, Tehrani R and Jay WM. Varicella Zoster Virus in Giant Cell Arteritis: A Review of Current Medical Literature. Neuroophthalmology. 2019;43:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar Chauhan S, Kumar Tripathy N, Sinha N, Singh M and Nityanand S. Cellular and humoral immune responses to mycobacterial heat shock protein-65 and its human homologue in Takayasu’s arteritis. Clin Exp Immunol. 2004;138:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedreira ALS and Santiago MB. Association between Takayasu arteritis and latent or active Mycobacterium tuberculosis infection: a systematic review. Clinical rheumatology. 2020;39:1019–1026. [DOI] [PubMed] [Google Scholar]
- 58.Hill CL, Black RJ, Nossent JC, Ruediger C, Nguyen L, Ninan JV and Lester S. Risk of mortality in patients with giant cell arteritis: A systematic review and meta-analysis. Semin Arthritis Rheum. 2017;46:513–519. [DOI] [PubMed] [Google Scholar]
- 59.Richards BL, March L and Gabriel SE. Epidemiology of large-vessel vasculidities. Best Pract Res Clin Rheumatol. 2010;24:871–83. [DOI] [PubMed] [Google Scholar]
- 60.Li KJ, Semenov D, Turk M and Pope J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis research & therapy. 2021;23:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gran JT, Myklebust G, Wilsgaard T and Jacobsen BK. Survival in polymyalgia rheumatica and temporal arteritis: a study of 398 cases and matched population controls. Rheumatology (Oxford). 2001;40:1238–42. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Zhang H, Jiang X, Zou Y, Qin F, Song L, Guan T, Wu H, Xu L, Liu Y, Zhou X, Bian J, Hui R and Zheng D. Clinical manifestations and longterm outcome for patients with Takayasu arteritis in China. J Rheumatol. 2014;41:2439–46. [DOI] [PubMed] [Google Scholar]
- 63.Soto ME, Espinola N, Flores-Suarez LF and Reyes PA. Takayasu arteritis: clinical features in 110 Mexican Mestizo patients and cardiovascular impact on survival and prognosis. Clinical and experimental rheumatology. 2008;26:S9–15. [PubMed] [Google Scholar]
- 64.Schmidt J, Kermani TA, Bacani AK, Crowson CS, Cooper LT, Matteson EL and Warrington KJ. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc. 2013;88:822–30. [DOI] [PubMed] [Google Scholar]
- 65.Park SJ, Kim HJ, Park H, Hann HJ, Kim KH, Han S, Kim Y and Ahn HS. Incidence, prevalence, mortality and causes of death in Takayasu Arteritis in Korea - A nationwide, population-based study. International journal of cardiology. 2017;235:100–104. [DOI] [PubMed] [Google Scholar]
- 66.Mirouse A, Biard L, Comarmond C, Lambert M, Mekinian A, Ferfar Y, Kahn JE, Benhamou Y, Chiche L, Koskas F, Cluzel P, Hachulla E, Messas E, Cacoub P, Mirault T, Resche-Rigon M, Saadoun D and French Takayasu n. Overall survival and mortality risk factors in Takayasu’s arteritis: A multicenter study of 318 patients. J Autoimmun. 2019;96:35–39. [DOI] [PubMed] [Google Scholar]
- 67.Goel R, Chandan JS, Thayakaran R, Adderley NJ, Nirantharakumar K and Harper L. Cardiovascular and Renal Morbidity in Takayasu Arteritis: A Population-Based Retrospective Cohort Study From the United Kingdom. Arthritis & rheumatology. 2021;73:504–511. [DOI] [PubMed] [Google Scholar]
- 68.Jin K, Wen Z, Wu B, Zhang H, Qiu J, Wang Y, Warrington KJ, Berry GJ, Goronzy JJ and Weyand CM. NOTCH-induced rerouting of endosomal trafficking disables regulatory T cells in vasculitis. The Journal of clinical investigation. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyabe C, Miyabe Y, Strle K, Kim ND, Stone JH, Luster AD and Unizony S. An expanded population of pathogenic regulatory T cells in giant cell arteritis is abrogated by IL-6 blockade therapy. Annals of the rheumatic diseases. 2017;76:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samson M, Greigert H, Ciudad M, Gerard C, Ghesquière T, Trad M, Corbera-Bellalta M, Genet C, Ouandji S, Cladière C, Thebault M, Ly KH, Liozon E, Maurier F, Bienvenu B, Terrier B, Guillevin L, Charles P, Quipourt V, Devilliers H, Gabrielle PH, Creuzot-Garcher C, Tarris G, Martin L, Saas P, Audia S, Cid MC and Bonnotte B. Improvement of Treg immune response after treatment with tocilizumab in giant cell arteritis. Clin Transl Immunology. 2021;10:e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weyand CM, Berry GJ and Goronzy JJ. The immunoinhibitory PD-1/PD-L1 pathway in inflammatory blood vessel disease. J Leukoc Biol. 2018;103:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Watanabe R, Berry GJ, Vaglio A, Liao YJ, Warrington KJ, Goronzy JJ and Weyand CM. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci U S A. 2017;114:E970–e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daxini A, Cronin K and Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37:2579–2584. [DOI] [PubMed] [Google Scholar]
- 74.Wen Z, Shen Y, Berry G, Shahram F, Li Y, Watanabe R, Liao YJ, Goronzy JJ and Weyand CM. The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe R, Maeda T, Zhang H, Berry GJ, Zeisbrich M, Brockett R, Greenstein AE, Tian L, Goronzy JJ and Weyand CM. MMP (Matrix Metalloprotease)-9-Producing Monocytes Enable T Cells to Invade the Vessel Wall and Cause Vasculitis. Circulation research. 2018;123:700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segarra M, García-Martínez A, Sánchez M, Hernández-Rodríguez J, Lozano E, Grau JM and Cid MC. Gelatinase expression and proteolytic activity in giant-cell arteritis. Annals of the rheumatic diseases. 2007;66:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, Urbano-Márquez A, Yamada KM and Cid MC. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. Faseb j. 2005;19:1875–7. [DOI] [PubMed] [Google Scholar]
- 78.Piggott K, Deng J, Warrington K, Younge B, Kubo JT, Desai M, Goronzy JJ and Weyand CM. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Ai Z, Khoyratty T, Zec K, Eames HL, van Grinsven E, Hudak A, Morris S, Ahern D, Monaco C, Eruslanov EB, Luqmani R and Udalova IA. ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cid MC, Cebrián M, Font C, Coll-Vinent B, Hernández-Rodríguez J, Esparza J, Urbano-Márquez A and Grau JM. Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte-endothelial cell interactions. Arthritis and rheumatism. 2000;43:184–94. [DOI] [PubMed] [Google Scholar]
- 81.Tombetti E and Mason JC. Takayasu arteritis: advanced understanding is leading to new horizons. Rheumatology (Oxford). 2019;58:206–219. [DOI] [PubMed] [Google Scholar]
- 82.Coit P, De Lott LB, Nan B, Elner VM and Sawalha AH. DNA methylation analysis of the temporal artery microenvironment in giant cell arteritis. Annals of the rheumatic diseases. 2016;75:1196–202. [DOI] [PubMed] [Google Scholar]
- 83.Mohan SV, Liao YJ, Kim JW, Goronzy JJ and Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe R, Berry GJ, Liang DH, Goronzy JJ and Weyand CM. Pathogenesis of Giant Cell Arteritis and Takayasu Arteritis-Similarities and Differences. Curr Rheumatol Rep. 2020;22:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ and Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weyand CM and Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nature reviews Rheumatology. 2013;9:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maleszewski JJ, Younge BR, Fritzlen JT, Hunder GG, Goronzy JJ, Warrington KJ and Weyand CM. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. 2017;30:788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weyand CM and Goronzy JJ. Medium- and large-vessel vasculitis. The New England journal of medicine. 2003;349:160–9. [DOI] [PubMed] [Google Scholar]
- 89.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ and Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaiser M, Younge B, Björnsson J, Goronzy JJ and Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terrier B, Geri G, Chaara W, Allenbach Y, Rosenzwajg M, Costedoat-Chalumeau N, Fouret P, Musset L, Benveniste O, Six A, Klatzmann D, Saadoun D and Cacoub P. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis and rheumatism. 2012;64:2001–11. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe R, Zhang H, Maeda T, Akiyama M, Gandhi R, Paolini J, Berry G and Weyand C. GM-CSF Is a Pro-Inflammatory Cytokine in Experimental Vasculitis of Medium and Large Arteries. Arthritis & rheumatology (Hoboken, NJ). 2019;71. [Google Scholar]
- 93.Corbera-Bellalta M, Alba-Rovira R, Muralidharan S, Espigol-Frigole G, Rios-Garces R, Marco-Hernandez J, Denuc A, Kamberovic F, Pérez-Galán P, Joesph A, D’Andrea A, Bondensgaard K, Cid MC and Paolini JF. Blocking GM-CSF receptor α with mavrilimumab reduces infiltrating cells, pro-inflammatory markers, and neoangiogenesis in ex-vivo cultured arteries from patients with giant cell arteritis. Annals of the rheumatic diseases. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng J, Younge BR, Olshen RA, Goronzy JJ and Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Espígol-Frigolé G, Corbera-Bellalta M, Planas-Rigol E, Lozano E, Segarra M, García-Martínez A, Prieto-González S, Hernández-Rodríguez J, Grau JM, Rahman MU and Cid MC. Increased IL-17A expression in temporal artery lesions is a predictor of sustained response to glucocorticoid treatment in patients with giant-cell arteritis. Annals of the rheumatic diseases. 2013;72:1481–7. [DOI] [PubMed] [Google Scholar]
- 96.Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, Terrier B, Geri G, Rosenzwajg M, Klatzmann D, Fourret P, Cluzel P, Chiche L, Gaudric J, Koskas F and Cacoub P. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis & rheumatology (Hoboken, NJ). 2015;67:1353–60. [DOI] [PubMed] [Google Scholar]
- 97.Ciccia F, Rizzo A, Guggino G, Cavazza A, Alessandro R, Maugeri R, Cannizzaro A, Boiardi L, Iacopino DG, Salvarani C and Triolo G. Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford). 2015;54:1596–604. [DOI] [PubMed] [Google Scholar]
- 98.Zerbini A, Muratore F, Boiardi L, Ciccia F, Bonacini M, Belloni L, Cavazza A, Cimino L, Moramarco A, Alessandro R, Rizzo A, Parmeggiani M, Salvarani C and Croci S. Increased expression of interleukin-22 in patients with giant cell arteritis. Rheumatology (Oxford). 2018;57:64–72. [DOI] [PubMed] [Google Scholar]
- 99.Corbera-Bellalta M, Planas-Rigol E, Lozano E, Terrades-García N, Alba MA, Prieto-González S, García-Martínez A, Albero R, Enjuanes A, Espígol-Frigolé G, Hernández-Rodríguez J, Roux-Lombard P, Ferlin WG, Dayer JM, Kosco-Vilbois MH and Cid MC. Blocking interferon γ reduces expression of chemokines CXCL9, CXCL10 and CXCL11 and decreases macrophage infiltration in ex vivo cultured arteries from patients with giant cell arteritis. Annals of the rheumatic diseases. 2016;75:1177–86. [DOI] [PubMed] [Google Scholar]
- 100.Régnier P, Le Joncour A, Maciejewski-Duval A, Desbois AC, Comarmond C, Rosenzwajg M, Klatzmann D, Cacoub P and Saadoun D. Targeting JAK/STAT pathway in Takayasu’s arteritis. Annals of the rheumatic diseases. 2020;79:951–959. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ and Weyand CM. Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis. Circulation. 2018;137:1934–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maciejewski-Duval A, Comarmond C, Leroyer A, Zaidan M, Le Joncour A, Desbois AC, Fouret JP, Koskas F, Cluzel P, Garrido M, Cacoub P and Saadoun D. mTOR pathway activation in large vessel vasculitis. J Autoimmun. 2018;94:99–109. [DOI] [PubMed] [Google Scholar]
- 103.Hadjadj J, Canaud G, Mirault T, Samson M, Bruneval P, Régent A, Goulvestre C, Witko-Sarsat V, Costedoat-Chalumeau N, Guillevin L, Mouthon L and Terrier B. mTOR pathway is activated in endothelial cells from patients with Takayasu arteritis and is modulated by serum immunoglobulin G. Rheumatology (Oxford). 2018;57:1011–1020. [DOI] [PubMed] [Google Scholar]
- 104.Kurata A, Saito A, Hashimoto H, Fujita K, Ohno SI, Kamma H, Nagao T, Kobayashi S, Yamashina A and Kuroda M. Difference in immunohistochemical characteristics between Takayasu arteritis and giant cell arteritis: It may be better to distinguish them in the same age. Mod Rheumatol. 2019;29:992–1001. [DOI] [PubMed] [Google Scholar]
- 105.Samson M, Ly KH, Tournier B, Janikashvili N, Trad M, Ciudad M, Gautheron A, Devilliers H, Quipourt V, Maurier F, Meaux-Ruault N, Magy-Bertrand N, Manckoundia P, Ornetti P, Maillefert JF, Besancenot JF, Ferrand C, Mesturoux L, Labrousse F, Fauchais AL, Saas P, Martin L, Audia S and Bonnotte B. Involvement and prognosis value of CD8(+) T cells in giant cell arteritis. J Autoimmun. 2016;72:73–83. [DOI] [PubMed] [Google Scholar]
- 106.Kaiser M, Weyand CM, Björnsson J and Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis and rheumatism. 1998;41:623–33. [DOI] [PubMed] [Google Scholar]
- 107.Lozano E, Segarra M, García-Martínez A, Hernández-Rodríguez J and Cid MC. Imatinib mesylate inhibits in vitro and ex vivo biological responses related to vascular occlusion in giant cell arteritis. Annals of the rheumatic diseases. 2008;67:1581–8. [DOI] [PubMed] [Google Scholar]
- 108.Planas-Rigol E, Terrades-Garcia N, Corbera-Bellalta M, Lozano E, Alba MA, Segarra M, Espígol-Frigolé G, Prieto-González S, Hernández-Rodríguez J, Preciado S, Lavilla R and Cid MC. Endothelin-1 promotes vascular smooth muscle cell migration across the artery wall: a mechanism contributing to vascular remodelling and intimal hyperplasia in giant-cell arteritis. Annals of the rheumatic diseases. 2017;76:1624–1634. [DOI] [PubMed] [Google Scholar]
- 109.Le Joncour A, Desbois AC, Leroyer AS, Tellier E, Régnier P, Maciejewski-Duval A, Comarmond C, Barete S, Arock M, Bruneval P, Launay JM, Fouret P, Blank U, Rosenzwajg M, Klatzmann D, Jarraya M, Chiche L, Koskas F, Cacoub P, Kaplanski G and Saadoun D. Mast cells drive pathologic vascular lesions in Takayasu arteritis. J Allergy Clin Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 110.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, Ialenti A, Sabir SR, McInnes IB, Brewer JM, Garside P, Weber C, Lehmann T, Teupser D, Habenicht L, Beer M, Grabner R, Maffia P, Weih F and Habenicht AJ. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin β Receptors. Immunity. 2015;42:1100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graver JC, Boots AMH, Haacke EA, Diepstra A, Brouwer E and Sandovici M. Massive B-Cell Infiltration and Organization Into Artery Tertiary Lymphoid Organs in the Aorta of Large Vessel Giant Cell Arteritis. Front Immunol. 2019;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inder SJ, Bobryshev YV, Cherian SM, Wang AY, Lord RS, Masuda K and Yutani C. Immunophenotypic analysis of the aortic wall in Takayasu’s arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno-inflammatory reactions. Cardiovasc Surg. 2000;8:141–8. [DOI] [PubMed] [Google Scholar]
- 113.van der Geest KS, Abdulahad WH, Chalan P, Rutgers A, Horst G, Huitema MG, Roffel MP, Roozendaal C, Kluin PM, Bos NA, Boots AM and Brouwer E. Disturbed B cell homeostasis in newly diagnosed giant cell arteritis and polymyalgia rheumatica. Arthritis & rheumatology (Hoboken, NJ). 2014;66:1927–38. [DOI] [PubMed] [Google Scholar]
- 114.Mutoh T, Shirai T, Ishii T, Shirota Y, Fujishima F, Takahashi F, Kakuta Y, Kanazawa Y, Masamune A, Saiki Y, Harigae H and Fujii H. Identification of two major autoantigens negatively regulating endothelial activation in Takayasu arteritis. Nat Commun. 2020;11:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Desbois AC, Régnier P, Quiniou V, Lejoncour A, Maciejewski-Duval A, Comarmond C, Vallet H, Rosenzwag M, Darrasse-Jèze G, Derian N, Pouchot J, Samson M, Bienvenu B, Fouret P, Koskas F, Garrido M, Sène D, Bruneval P, Cacoub P, Klatzmann D and Saadoun D. Specific Follicular Helper T Cell Signature in Takayasu Arteritis. Arthritis & rheumatology (Hoboken, NJ). 2021;73:1233–1243. [DOI] [PubMed] [Google Scholar]
- 116.Mackie SL, Dejaco C, Appenzeller S, Camellino D, Duftner C, Gonzalez-Chiappe S, Mahr A, Mukhtyar C, Reynolds G, de Souza AWS, Brouwer E, Bukhari M, Buttgereit F, Byrne D, Cid MC, Cimmino M, Direskeneli H, Gilbert K, Kermani TA, Khan A, Lanyon P, Luqmani R, Mallen C, Mason JC, Matteson EL, Merkel PA, Mollan S, Neill L, Sullivan EO, Sandovici M, Schmidt WA, Watts R, Whitlock M, Yacyshyn E, Ytterberg S and Dasgupta B. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology (Oxford). 2020;59:e1–e23. [DOI] [PubMed] [Google Scholar]
- 117.Diamantopoulos AP, Haugeberg G, Lindland A and Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford). 2016;55:66–70. [DOI] [PubMed] [Google Scholar]
- 118.Parikh M, Miller NR, Lee AG, Savino PJ, Vacarezza MN, Cornblath W, Eggenberger E, Antonio-Santos A, Golnik K, Kardon R and Wall M. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology. 2006;113:1842–5. [DOI] [PubMed] [Google Scholar]
- 119.Furuta S, Cousins C, Chaudhry A and Jayne D. Clinical features and radiological findings in large vessel vasculitis: are Takayasu arteritis and giant cell arteritis 2 different diseases or a single entity? J Rheumatol. 2015;42:300–8. [DOI] [PubMed] [Google Scholar]
- 120.Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, Dasgupta B, Diamantopoulos AP, Forrester-Barker W, Hamilton W, Masters S, McDonald B, McNally E, Pease C, Piper J, Salmon J, Wailoo A, Wolfe K and Hutchings A. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20:1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, Brouwer E, Cimmino MA, Clark E, Dasgupta B, Diamantopoulos AP, Direskeneli H, Iagnocco A, Klink T, Neill L, Ponte C, Salvarani C, Slart R, Whitlock M and Schmidt WA. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Annals of the rheumatic diseases. 2018;77:636–643. [DOI] [PubMed] [Google Scholar]
- 122.Turesson C, Borjesson O, Larsson K, Mohammad AJ and Knight A. Swedish Society of Rheumatology 2018 guidelines for investigation, treatment, and follow-up of giant cell arteritis. Scand J Rheumatol. 2019;48:259–265. [DOI] [PubMed] [Google Scholar]
- 123.Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, Archer AM, Conn DL, Full KA, Grayson PC, Ibarra MF, Imundo LF, Kim S, Merkel PA, Rhee RL, Seo P, Stone JH, Sule S, Sundel RP, Vitobaldi OI, Warner A, Byram K, Dua AB, Husainat N, James KE, Kalot MA, Lin YC, Springer JM, Turgunbaev M, Villa-Forte A, Turner AS and Mustafa RA. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis & rheumatology (Hoboken, NJ). 2021;73:1349–1365. [DOI] [PubMed] [Google Scholar]
- 124.Duftner C, Dejaco C, Sepriano A, Falzon L, Schmidt WA and Ramiro S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barra L, Kanji T, Malette J and Pagnoux C. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmun Rev. 2018;17:175–187. [DOI] [PubMed] [Google Scholar]
- 126.Yamada I, Nakagawa T, Himeno Y, Kobayashi Y, Numano F and Shibuya H. Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three-dimensional MR angiography. J Magn Reson Imaging. 2000;11:481–7. [DOI] [PubMed] [Google Scholar]
- 127.Lariviere D, Benali K, Coustet B, Pasi N, Hyafil F, Klein I, Chauchard M, Alexandra JF, Goulenok T, Dossier A, Dieude P, Papo T and Sacre K. Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: A real-life prospective study. Medicine (Baltimore). 2016;95:e4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stellingwerff MD, Brouwer E, Lensen KDF, Rutgers A, Arends S, van der Geest KSM, Glaudemans A and Slart R. Different Scoring Methods of FDG PET/CT in Giant Cell Arteritis: Need for Standardization. Medicine (Baltimore). 2015;94:e1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, Malayeri AA, Merkel PA, Novakovich E, Bluemke DA and Ahlman MA. (18) F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis & rheumatology (Hoboken, NJ). 2018;70:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O and Mekinian A. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore). 2015;94:e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sammel AM, Hsiao E, Schembri G, Nguyen K, Brewer J, Schrieber L, Janssen B, Youssef P, Fraser CL, Bailey E, Bailey DL, Roach P and Laurent R. Diagnostic Accuracy of Positron Emission Tomography/Computed Tomography of the Head, Neck, and Chest for Giant Cell Arteritis: A Prospective, Double-Blind, Cross-Sectional Study. Arthritis & rheumatology (Hoboken, NJ). 2019;71:1319–1328. [DOI] [PubMed] [Google Scholar]
- 132.Nielsen BD, Hansen IT, Kramer S, Haraldsen A, Hjorthaug K, Bogsrud TV, Ejlersen JA, Stolle LB, Keller KK, Therkildsen P, Hauge EM and Gormsen LC. Simple dichotomous assessment of cranial artery inflammation by conventional 18F-FDG PET/CT shows high accuracy for the diagnosis of giant cell arteritis: a case-control study. European journal of nuclear medicine and molecular imaging. 2019;46:184–193. [DOI] [PubMed] [Google Scholar]
- 133.Dellavedova L, Carletto M, Faggioli P, Sciascera A, Del Sole A, Mazzone A and Maffioli LS. The prognostic value of baseline (18)F-FDG PET/CT in steroid-naïve large-vessel vasculitis: introduction of volume-based parameters. European journal of nuclear medicine and molecular imaging. 2016;43:340–348. [DOI] [PubMed] [Google Scholar]
- 134.Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P and Hauge EM. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. European journal of nuclear medicine and molecular imaging. 2018;45:1119–1128. [DOI] [PubMed] [Google Scholar]
- 135.Einspieler I, Thurmel K, Pyka T, Eiber M, Wolfram S, Moog P, Reeps C and Essler M. Imaging large vessel vasculitis with fully integrated PET/MRI: a pilot study. European journal of nuclear medicine and molecular imaging. 2015;42:1012–24. [DOI] [PubMed] [Google Scholar]
- 136.Martin O, Schaarschmidt BM, Kirchner J, Suntharalingam S, Grueneisen J, Demircioglu A, Heusch P, Quick HH, Forsting M, Antoch G, Herrmann K and Umutlu L. PET/MRI Versus PET/CT for Whole-Body Staging: Results from a Single-Center Observational Study on 1,003 Sequential Examinations. J Nucl Med. 2020;61:1131–1136. [DOI] [PubMed] [Google Scholar]
- 137.Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY and Cai W. ImmunoPET: Concept, Design, and Applications. Chem Rev. 2020;120:3787–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, Rimoldi OE, Mason JC and Camici PG. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. Journal of the American College of Cardiology. 2010;56:653–61. [DOI] [PubMed] [Google Scholar]
- 139.Kermani TA, Warrington KJ, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Maksimowicz-McKinnon K, McAlear CA, Monach PA, Seo P, Merkel PA and Ytterberg SR. Disease Relapses among Patients with Giant Cell Arteritis: A Prospective, Longitudinal Cohort Study. The Journal of rheumatology. 2015;42:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, Espigol-Frigole G, Butjosa M, Hernandez-Rodriguez J and Cid MC. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore). 2014;93:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Labarca C, Koster MJ, Crowson CS, Makol A, Ytterberg SR, Matteson EL and Warrington KJ. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford). 2016;55:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn JE, Benhamou Y, Chiche L, Koskas F, Cluzel P, Hachulla E, Messas E, Resche-Rigon M, Cacoub P, Mirault T and Saadoun D. Long-Term Outcomes and Prognostic Factors of Complications in Takayasu Arteritis: A Multicenter Study of 318 Patients. Circulation. 2017;136:1114–1122. [DOI] [PubMed] [Google Scholar]
- 143.Weyand CM, Fulbright JW, Hunder GG, Evans JM and Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis and rheumatism. 2000;43:1041–8. [DOI] [PubMed] [Google Scholar]
- 144.Rimland CA, Quinn KA, Rosenblum JS, Schwartz MN, Gribbons KB, Novakovich E, Sreih AG, Merkel PA, Ahlman MA and Grayson PC. Outcome Measures in Large-Vessel Vasculitis: Relationship Between Patient, Physician, Imaging, and Laboratory-Based Assessments. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsuyama A, Sakai N, Ishigami M, Hiraoka H, Kashine S, Hirata A, Nakamura T, Yamashita S and Matsuzawa Y. Matrix metalloproteinases as novel disease markers in Takayasu arteritis. Circulation. 2003;108:1469–73. [DOI] [PubMed] [Google Scholar]
- 146.Dagna L, Salvo F, Tiraboschi M, Bozzolo EP, Franchini S, Doglioni C, Manfredi AA, Baldissera E and Sabbadini MG. Pentraxin-3 as a marker of disease activity in Takayasu arteritis. Annals of internal medicine. 2011;155:425–33. [DOI] [PubMed] [Google Scholar]
- 147.Baldini M, Maugeri N, Ramirez GA, Giacomassi C, Castiglioni A, Prieto-González S, Corbera-Bellalta M, Di Comite G, Papa I, Dell’antonio G, Ammirati E, Cuccovillo I, Vecchio V, Mantovani A, Rovere-Querini P, Sabbadini MG, Cid MC and Manfredi AA. Selective up-regulation of the soluble pattern-recognition receptor pentraxin 3 and of vascular endothelial growth factor in giant cell arteritis: relevance for recent optic nerve ischemia. Arthritis and rheumatism. 2012;64:854–65. [DOI] [PubMed] [Google Scholar]
- 148.Tombetti E, Hysa E, Mason JC, Cimmino MA and Camellino D. Blood Biomarkers for Monitoring and Prognosis of Large Vessel Vasculitides. Curr Rheumatol Rep. 2021;23:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soriano A, Muratore F, Pipitone N, Boiardi L, Cimino L and Salvarani C. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nature reviews Rheumatology. 2017;13:476–484. [DOI] [PubMed] [Google Scholar]
- 150.Macchioni P, Boiardi L, Muratore F, Restuccia G, Cavazza A, Pipitone N, Catanoso M, Mancuso P, Luberto F, Giorgi Rossi P and Salvarani C. Survival predictors in biopsy-proven giant cell arteritis: a northern Italian population-based study. Rheumatology (Oxford). 2019;58:609–616. [DOI] [PubMed] [Google Scholar]
- 151.Delaval L, Daumas A, Samson M, Ebbo M, De Boysson H, Liozon E, Dupuy H, Puyade M, Blockmans D, Benhamou Y, Sacre K, Berezne A, Devilliers H, Pugnet G, Maurier F, Zenone T, de Moreuil C, Lifermann F, Arnaud L, Espitia O, Deroux A, Grobost V, Lazaro E, Agard C, Balageas A, Bouiller K, Durel CA, Humbert S, Rieu V, Roriz M, Souchaud-Debouverie O, Vinzio S, Nguyen Y, Regent A, Guillevin L and Terrier B. Large-vessel vasculitis diagnosed between 50 and 60years: Case-control study based on 183 cases and 183 controls aged over 60years. Autoimmun Rev. 2019;18:714–720. [DOI] [PubMed] [Google Scholar]
- 152.Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, Hamilton W, Emin A, Culliford D and Luqmani RA. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Annals of the rheumatic diseases. 2015;74:129–35. [DOI] [PubMed] [Google Scholar]
- 153.Proven A, Gabriel SE, Orces C, O’Fallon WM and Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. [DOI] [PubMed] [Google Scholar]
- 154.Maksimowicz-McKinnon K, Clark TM and Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000–9. [DOI] [PubMed] [Google Scholar]
- 155.Chanouzas D, McGregor JAG, Nightingale P, Salama AD, Szpirt WM, Basu N, Morgan MD, Poulton CJ, Draibe JB, Krarup E, Dospinescu P, Dale JA, Pendergraft WF, Lee K, Egfjord M, Hogan SL and Harper L. Intravenous pulse methylprednisolone for induction of remission in severe ANCA associated Vasculitis: a multi-center retrospective cohort study. BMC Nephrol. 2019;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Christ L, Scholtz G, Seitz L, Sarbu AC, Amsler J, Bütikofer L, Tappeiner C, Kollert F, Reichenbach S and Villiger PM. Tocilizumab Monotherapy after Ultra-Short Glucocorticoid Administration in Giant Cell Arteritis: a proof-of-concept trial. Lancet Rheumatology. 2021;In press. [DOI] [PubMed] [Google Scholar]
- 157.Wilson JC, Sarsour K, Collinson N, Tuckwell K, Musselman D, Klearman M, Napalkov P, Jick SS, Stone JH and Meier CR. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): A nested case-control analysis. Semin Arthritis Rheum. 2017;46:819–827. [DOI] [PubMed] [Google Scholar]
- 158.Águeda AF, Monti S, Luqmani RA, Buttgereit F, Cid M, Dasgupta B, Dejaco C, Mahr A, Ponte C, Salvarani C, Schmidt W and Hellmich B. Management of Takayasu arteritis: a systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis. RMD Open. 2019;5:e001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mainbourg S, Addario A, Samson M, Puéchal X, François M, Durupt S, Gueyffier F, Cucherat M, Durieu I, Reynaud Q and Lega JC. Prevalence of Giant Cell Arteritis Relapse in Patients Treated With Glucocorticoids: A Meta-Analysis. Arthritis Care Res (Hoboken). 2020;72:838–849. [DOI] [PubMed] [Google Scholar]
- 160.Mukhtyar C, Cate H, Graham C, Merry P, Mills K, Misra A and Jones C. Development of an evidence-based regimen of prednisolone to treat giant cell arteritis - the Norwich regimen. Rheumatol Adv Pract. 2019;3:rkz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, Salvarani C, Xu W, Visvanathan S and Rahman MU. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Annals of internal medicine. 2007;146:621–30. [DOI] [PubMed] [Google Scholar]
- 162.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, Salvarani C, Schett G, Schulze-Koops H, Spiera R, Unizony SH and Collinson N. Trial of Tocilizumab in Giant-Cell Arteritis. The New England journal of medicine. 2017;377:317–328. [DOI] [PubMed] [Google Scholar]
- 163.Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, Nomura A, Yoshida S and Nishimoto N. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Annals of the rheumatic diseases. 2018;77:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, Seo P, Moreland LW, Weisman M, Koening CL, Sreih AG, Spiera R, McAlear CA, Warrington KJ, Pagnoux C, McKinnon K, Forbess LJ, Hoffman GS, Borchin R, Krischer JP and Merkel PA. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Takayasu Arteritis. Arthritis & rheumatology (Hoboken, NJ). 2017;69:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Strehl C, Bijlsma JW, de Wit M, Boers M, Caeyers N, Cutolo M, Dasgupta B, Dixon WG, Geenen R, Huizinga TW, Kent A, de Thurah AL, Listing J, Mariette X, Ray DW, Scherer HU, Seror R, Spies CM, Tarp S, Wiek D, Winthrop KL and Buttgereit F. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Annals of the rheumatic diseases. 2016;75:952–7. [DOI] [PubMed] [Google Scholar]
- 166.Barra L, Liang P, Benseler SM, Cabral DA, Fifi-Mah A, Li Y, Milman N, Twilt M, Yacyshyn E and Pagnoux C. Variations in the clinical practice of physicians managing Takayasu arteritis: a nationwide survey. Open Access Rheumatol. 2017;9:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A and Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 2001;134:106–14. [DOI] [PubMed] [Google Scholar]
- 168.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, Cohen P, Calabrese LH, Dickler H, Merkel PA, Fortin P, Flynn JA, Locker GA, Easley KA, Schned E, Hunder GG, Sneller MC, Tuggle C, Swanson H, Hernandez-Rodriguez J, Lopez-Soto A, Bork D, Hoffman DB, Kalunian K, Klashman D, Wilke WS, Scheetz RJ, Mandell BF, Fessler BJ, Kosmorsky G, Prayson R, Luqmani RA, Nuki G, McRorie E, Sherrer Y, Baca S, Walsh B, Ferland D, Soubrier M, Choi HK, Gross W, Segal AM, Ludivico C and Puechal X. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis and rheumatism. 2002;46:1309–18. [DOI] [PubMed] [Google Scholar]
- 169.Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG and Paget SA. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol. 2001;19:495–501. [PubMed] [Google Scholar]
- 170.Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP and Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis and rheumatism. 2007;56:2789–97. [DOI] [PubMed] [Google Scholar]
- 171.Gérard AL, Simon-Tillaux N, Yordanov Y, Cacoub P, Tubach F, Saadoun D and Dechartres A. Efficacy and safety of steroid-sparing treatments in giant cell arteritis according to the glucocorticoids tapering regimen: A systematic review and meta-analysis. Eur J Intern Med. 2021;88:96–103. [DOI] [PubMed] [Google Scholar]
- 172.Koster MJ, Yeruva K, Crowson CS, Muratore F, Labarca C and Warrington KJ. Efficacy of Methotrexate in Real-world Management of Giant Cell Arteritis: A Case-control Study. The Journal of rheumatology. 2019;46:501–508. [DOI] [PubMed] [Google Scholar]
- 173.Diamantopoulos AP, Hetland H and Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int. 2013;2013:120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karabayas M, Dospinescu P, Fluck N, Kidder D, Fordyce G, Hollick RJ, De Bari C and Basu N. Evaluation of adjunctive mycophenolate for large vessel giant cell arteritis. Rheumatol Adv Pract. 2020;4:rkaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ly KH, Dalmay F, Gondran G, Palat S, Bezanahary H, Cypierre A, Fauchais AL and Liozon E. Steroid-sparing effect and toxicity of dapsone treatment in giant cell arteritis: A single-center, retrospective study of 70 patients. Medicine (Baltimore). 2016;95:e4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Monti S, Águeda AF, Luqmani RA, Buttgereit F, Cid M, Dejaco C, Mahr A, Ponte C, Salvarani C, Schmidt W and Hellmich B. Systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis: focus on giant cell arteritis. RMD Open. 2019;5:e001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Boysson H, Boutemy J, Creveuil C, Ollivier Y, Letellier P, Pagnoux C and Bienvenu B. Is there a place for cyclophosphamide in the treatment of giant-cell arteritis? A case series and systematic review. Semin Arthritis Rheum. 2013;43:105–12. [DOI] [PubMed] [Google Scholar]
- 178.Misra DP, Wakhlu A, Agarwal V and Danda D. Recent advances in the management of Takayasu arteritis. Int J Rheum Dis. 2019;22 Suppl 1:60–68. [DOI] [PubMed] [Google Scholar]
- 179.Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, Bütikofer L, Seitz M and Reichenbach S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2016;387:1921–7. [DOI] [PubMed] [Google Scholar]
- 180.Strand V, Dimonaco S, Tuckwell K, Klearman M, Collinson N and Stone JH. Health-related quality of life in patients with giant cell arteritis treated with tocilizumab in a phase 3 randomised controlled trial. Arthritis Res Ther. 2019;21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Calderón-Goercke M, Loricera J, Aldasoro V, Castañeda S, Villa I, Humbría A, Moriano C, Romero-Yuste S, Narváez J, Gómez-Arango C, Pérez-Pampín E, Melero R, Becerra-Fernández E, Revenga M, Álvarez-Rivas N, Galisteo C, Sivera F, Olivé-Marqués A, Álvarez Del Buergo M, Marena-Rojas L, Fernández-López C, Navarro F, Raya E, Galindez-Agirregoikoa E, Arca B, Solans-Laqué R, Conesa A, Hidalgo C, Vázquez C, Román-Ivorra JA, Lluch P, Manrique-Arija S, Vela P, De Miguel E, Torres-Martín C, Nieto JC, Ordas-Calvo C, Salgado-Pérez E, Luna-Gomez C, Toyos-Sáenz de Miera FJ, Fernández-Llanio N, García A, Larena C, Palmou-Fontana N, Calvo-Río V, Prieto-Peña D, González-Vela C, Corrales A, Varela-García M, Aurrecoechea E, Dos Santos R, García-Manzanares Á, Ortego N, Fernández S, Ortiz-Sanjuán F, Corteguera M, Hernández JL, González-Gay M and Blanco R. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin Arthritis Rheum. 2019;49:126–135. [DOI] [PubMed] [Google Scholar]
- 182.Unizony S, McCulley TJ, Spiera R, Pei J, Sidiropoulos PN, Best JH, Birchwood C, Pavlov A and Stone JH. Clinical outcomes of patients with giant cell arteritis treated with tocilizumab in real-world clinical practice: decreased incidence of new visual manifestations. Arthritis Res Ther. 2021;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Calderón-Goercke M, Castañeda S, Aldasoro V, Villa I, Prieto-Peña D, Atienza-Mateo B, Patiño E, Moriano C, Romero-Yuste S, Narváez J, Gómez-Arango C, Pérez-Pampín E, Melero R, Becerra-Fernández E, Revenga M, Álvarez-Rivas N, Galisteo C, Sivera F, Olivé-Marqués A, Álvarez Del Buergo M, Marena-Rojas L, Fernández-López C, Navarro F, Raya E, Galindez-Agirregoikoa E, Arca B, Solans-Laqué R, Conesa A, Hidalgo C, Vázquez C, Román-Ivorra JA, Loricera J, Lluch P, Manrique-Arija S, Vela P, De Miguel E, Torres-Martín C, Nieto JC, Ordas-Calvo C, Salgado-Pérez E, Luna-Gomez C, Toyos-Sáenz de Miera FJ, Fernández-Llanio N, García A, Larena C, González-Vela C, Corrales A, Varela-García M, Aurrecoechea E, Dos Santos R, García-Manzanares Á, Ortego N, Fernández S, Ortiz-Sanjuán F, Corteguera M, Hernández JL, González-Gay M and Blanco R. Tocilizumab in giant cell arteritis: differences between the GiACTA trial and a multicentre series of patients from the clinical practice. Clin Exp Rheumatol. 2020;38 Suppl 124:112–119. [PubMed] [Google Scholar]
- 184.Clément J, Duffau P, Constans J, Schaeverbeke T, Viallard JF, Barcat D, Vernhes JP, Sailler L and Bonnet F. Real-world Risk of Relapse of Giant Cell Arteritis Treated With Tocilizumab: A Retrospective Analysis of 43 Patients. The Journal of rheumatology. 2021. [DOI] [PubMed] [Google Scholar]
- 185.Stone JH, Han J, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, Salvarani C, Spiera R, Unizony SH and Bao M. Long-term effect of tocilizumab in patients with giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatology. 2021;In press. [DOI] [PubMed] [Google Scholar]
- 186.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, Salvarani C, Schulze-Koops H, Schett G, Spiera R, Unizony SH and Collinson N. Glucocorticoid Dosages and Acute-Phase Reactant Levels at Giant Cell Arteritis Flare in a Randomized Trial of Tocilizumab. Arthritis & rheumatology (Hoboken, NJ). 2019;71:1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, Stone JR and Stone JH. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken). 2012;64:1720–9. [DOI] [PubMed] [Google Scholar]
- 188.Xenitidis T, Horger M, Zeh G, Kanz L and Henes JC. Sustained inflammation of the aortic wall despite tocilizumab treatment in two cases of Takayasu arteritis Rheumatology (Oxford) England; 2013(52): 1729–31. [DOI] [PubMed] [Google Scholar]
- 189.Reichenbach S, Adler S, Bonel H, Cullmann JL, Kuchen S, Bütikofer L, Seitz M and Villiger PM. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford). 2018;57:982–986. [DOI] [PubMed] [Google Scholar]
- 190.Schönau V, Roth J, Tascilar K, Corte G, Manger B, Rech J, Schmidt D, Cavallaro A, Uder M, Crescentini F, Boiardi L, Casali M, Spaggiari L, Galli E, Kuwert T, Versari A, Salvarani C, Schett G and Muratore F. Resolution of vascular inflammation in patients with new-onset giant cell arteritis: data from the RIGA study. Rheumatology (Oxford). 2021. [DOI] [PubMed] [Google Scholar]
- 191.Sebastian A, Kayani A, Prieto-Pena D, Tomelleri A, Whitlock M, Mo J, van der Geest N and Dasgupta B. Efficacy and safety of tocilizumab in giant cell arteritis: a single centre NHS experience using imaging (ultrasound and PET-CT) as a diagnostic and monitoring tool. RMD Open. 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cid MC, Unizony S, Pupim L, Fang F, Pirello J, Ren A, Samant M, Zhou T and Paolini JP. Mavrilimumab (anti GM-CSF receptor α monoclonal antibody) reduces risk of flare and increases sustained remission in a phase 2 trial of patients with giant cell arteritis. Annals of the rheumatic diseases. 2021;80:31.33004335 [Google Scholar]
- 193.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, Seo P, Moreland LW, Weisman M, Koening CL, Sreih AG, Spiera R, McAlear CA, Warrington KJ, Pagnoux C, McKinnon K, Forbess LJ, Hoffman GS, Borchin R, Krischer JP and Merkel PA. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Giant Cell Arteritis. Arthritis & rheumatology (Hoboken, NJ). 2017;69:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.NCT04474847. ClinicalTrials.gov. 2021.
- 195.Hernández-Rodríguez J, Segarra M, Vilardell C, Sánchez M, García-Martínez A, Esteban MJ, Queralt C, Grau JM, Urbano-Márquez A, Palacín A, Colomer D and Cid MC. Tissue production of pro-inflammatory cytokines (IL-1beta, TNFalpha and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology (Oxford). 2004;43:294–301. [DOI] [PubMed] [Google Scholar]
- 196.García-Martínez A, Hernández-Rodríguez J, Espígol-Frigolé G, Prieto-González S, Butjosa M, Segarra M, Lozano E and Cid MC. Clinical relevance of persistently elevated circulating cytokines (tumor necrosis factor alpha and interleukin-6) in the long-term followup of patients with giant cell arteritis. Arthritis Care Res (Hoboken). 2010;62:835–41. [DOI] [PubMed] [Google Scholar]
- 197.Martínez-Taboada VM, Rodríguez-Valverde V, Carreño L, López-Longo J, Figueroa M, Belzunegui J, Mola EM and Bonilla G. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Annals of the rheumatic diseases. 2008;67:625–30. [DOI] [PubMed] [Google Scholar]
- 198.Seror R, Baron G, Hachulla E, Debandt M, Larroche C, Puéchal X, Maurier F, de Wazieres B, Quéméneur T, Ravaud P and Mariette X. Adalimumab for steroid sparing in patients with giant-cell arteritis: results of a multicentre randomised controlled trial. Annals of the rheumatic diseases. 2014;73:2074–81. [DOI] [PubMed] [Google Scholar]
- 199.Brack A, Geisler A, Martinez-Taboada VM, Younge BR, Goronzy JJ and Weyand CM. Giant cell vasculitis is a T cell-dependent disease. Mol Med. 1997;3:530–43. [PMC free article] [PubMed] [Google Scholar]
- 200.NCT03725202. 2021.
- 201.Nakaoka Y, Isobe M, Tanaka Y, Ishii T, Ooka S, Niiro H, Tamura N, Banno S, Yoshifuji H, Sakata Y, Kawakami A, Atsumi T, Furuta S, Kohsaka H, Suzuki K, Hara R, Maejima Y, Tsukamoto H, Takasaki Y, Yamashita K, Okada N, Yamakido S, Takei S, Yokota S and Nishimoto N. Long-term efficacy and safety of tocilizumab in refractory Takayasu arteritis: final results of the randomized controlled phase 3 TAKT study. Rheumatology (Oxford). 2020;59:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mekinian A, Comarmond C, Resche-Rigon M, Mirault T, Kahn JE, Lambert M, Sibilia J, Néel A, Cohen P, Hie M, Berthier S, Marie I, Lavigne C, Anne Vandenhende M, Muller G, Amoura Z, Devilliers H, Abad S, Hamidou M, Guillevin L, Dhote R, Godeau B, Messas E, Cacoub P, Fain O and Saadoun D. Efficacy of Biological-Targeted Treatments in Takayasu Arteritis: Multicenter, Retrospective Study of 49 Patients. Circulation. 2015;132:1693–700. [DOI] [PubMed] [Google Scholar]
- 203.Misra DP, Rathore U, Patro P, Agarwal V and Sharma A. Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis-a systematic review and meta-analysis. Clin Rheumatol. 2021:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Youngstein T, Peters JE, Hamdulay SS, Mewar D, Price-Forbes A, Lloyd M, Jeffery R, Kinderlerer AR and Mason JC. Serial analysis of clinical and imaging indices reveals prolonged efficacy of TNF-α and IL-6 receptor targeted therapies in refractory Takayasu arteritis. Clin Exp Rheumatol. 2014;32:S11–8. [PubMed] [Google Scholar]
- 205.Gudbrandsson B, Molberg Ø and Palm Ø. TNF inhibitors appear to inhibit disease progression and improve outcome in Takayasu arteritis; an observational, population-based time trend study. Arthritis Res Ther. 2017;19:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mekinian A, Biard L, Dagna L, Novikov P, Salvarani C, Espita O, Sciscia S, Michaud M, Lambert M, Hernández-Rodríguez J, Schleinitz N, Awisat A, Puéchal X, Aouba A, Pons HM, Smitienko I, Gaultier JB, Edwige LM, Benhamou Y, Perlat A, Jego P, Goulenok T, Sacre K, Lioger B, Nolan H, Broner J, Dufrost V, Sene T, Seguier J, Maurier F, Berthier S, Belot A, Frikha F, Denis G, Audemard-Verger A, Pault IK, Humbert S, Woaye-Hune P, Tomelleri A, Baldissera E, Kuwana M, Logullo A, Gaches F, Zeminsky P, Galli E, Alvarado M, Luigi PB, Francesco M, Vautier M, Campochiaro C, Moiseev S, Cacoub P, Fain O and Saadoun D. Efficacy and safety of TNF-α antagonists and tocilizumab in Takayasu arteritis: Multicenter retrospective study of 209 patients. Rheumatology (Oxford). 2021. [DOI] [PubMed] [Google Scholar]
- 207.Terao C, Yoshifuji H, Nakajima T, Yukawa N, Matsuda F and Mimori T. Ustekinumab as a therapeutic option for Takayasu arteritis: from genetic findings to clinical application. Scand J Rheumatol. 2016;45:80–82. [DOI] [PubMed] [Google Scholar]
- 208.Yachoui R, Kreidy M, Siorek M and Sehgal R. Successful treatment with ustekinumab for corticosteroid- and immunosuppressant-resistant Takayasu’s arteritis. Scand J Rheumatol. 2018;47:246–247. [DOI] [PubMed] [Google Scholar]
- 209.Pazzola G, Muratore F, Pipitone N, Crescentini F, Cacoub P, Boiardi L, Spaggiari L, Comarmond C, Croci S, Saadoun D and Salvarani C. Rituximab therapy for Takayasu arteritis: a seven patients experience and a review of the literature. Rheumatology (Oxford). 2018;57:1151–1155. [DOI] [PubMed] [Google Scholar]
- 210.Kuwabara S, Tanimura S, Matsumoto S, Nakamura H and Horita T. Successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis Annals of the rheumatic diseases England; 2020(79): 1125–1126. [DOI] [PubMed] [Google Scholar]
- 211.Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, Mignot C, Schoindre Y, Chiche L, Hatron PY, Emmerich J and Cacoub P. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. 2012;125:813–9. [DOI] [PubMed] [Google Scholar]
- 212.Park HS, Do YS, Park KB, Kim DK, Choo SW, Shin SW, Cho SK, Hyun D and Choo IW. Long term results of endovascular treatment in renal arterial stenosis from Takayasu arteritis: angioplasty versus stent placement. Eur J Radiol. 2013;82:1913–8. [DOI] [PubMed] [Google Scholar]
- 213.Jeong HS, Jung JH, Song GG, Choi SJ and Hong SJ. Endovascular balloon angioplasty versus stenting in patients with Takayasu arteritis: A meta-analysis. Medicine (Baltimore). 2017;96:e7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.NCT04366596. 2021.
- 215.Perera AH, Youngstein T, Gibbs RG, Jackson JE, Wolfe JH and Mason JC. Optimizing the outcome of vascular intervention for Takayasu arteritis. Br J Surg. 2014;101:43–50. [DOI] [PubMed] [Google Scholar]
- 216.Assie C, Janvresse A, Plissonnier D, Levesque H and Marie I. Long-term follow-up of upper and lower extremity vasculitis related to giant cell arteritis: a series of 36 patients. Medicine (Baltimore). 2011;90:40–51. [DOI] [PubMed] [Google Scholar]
- 217.Le Hello C, Auboire L, Berger L, Gouicem D, Barrellier MT and Duthois S. Symptomatic lower-limb giant-cell arteritis: Characteristics, management and long-term outcome. J Med Vasc. 2017;42:148–156. [DOI] [PubMed] [Google Scholar]
- 218.Alba MA, Espígol-Frigolé G, Prieto-González S, Tavera-Bahillo I, García-Martínez A, Butjosa M, Hernández-Rodríguez J and Cid MC. Central nervous system vasculitis: still more questions than answers. Curr Neuropharmacol. 2011;9:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guerrero AM, Sierra-Hidalgo F, Calleja P, Navia P, Campollo J and Díaz-Guzmán J. Intracranial internal carotid artery angioplasthy and stenting in giant cell arteritis. J Neuroimaging. 2015;25:307–309. [DOI] [PubMed] [Google Scholar]
- 220.Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK and Merkel PA. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Annals of internal medicine. 2014;160:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ray JG, Mamdani MM and Geerts WH. Giant cell arteritis and cardiovascular disease in older adults. Heart (British Cardiac Society). 2005;91:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Amiri N, De Vera M, Choi HK, Sayre EC and Avina-Zubieta JA. Increased risk of cardiovascular disease in giant cell arteritis: a general population-based study. Rheumatology (Oxford). 2016;55:33–40. [DOI] [PubMed] [Google Scholar]
- 223.Pujades-Rodriguez M, Duyx B, Thomas SL, Stogiannis D, Smeeth L and Hemingway H. Associations between polymyalgia rheumatica and giant cell arteritis and 12 cardiovascular diseases. Heart (British Cardiac Society). 2016;102:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pujades-Rodriguez M, Morgan AW, Cubbon RM and Wu J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: A population-based cohort study. PLoS Med. 2020;17:e1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mirouse A, Biard L, Comarmond C, Lambert M, Mekinian A, Ferfar Y, Kahn JE, Benhamou Y, Chiche L, Koskas F, Cluzel P, Hachulla E, Messas E, Cacoub P, Mirault T, Resche-Rigon M and Saadoun D. Overall survival and mortality risk factors in Takayasu’s arteritis: A multicenter study of 318 patients. J Autoimmun. 2019;96:35–39. [DOI] [PubMed] [Google Scholar]
- 226.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR and Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ng WF, Fantin F, Ng C, Dockery F, Schiff R, Davies KA, Rajkumar C and Mason JC. Takayasu’s arteritis: a cause of prolonged arterial stiffness. Rheumatology (Oxford). 2006;45:741–5. [DOI] [PubMed] [Google Scholar]
- 228.Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S and Yazici H. Atherosclerosis in Takayasu arteritis. Annals of the rheumatic diseases. 2006;65:1202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Narváez J, Bernad B, Gómez-Vaquero C, García-Gómez C, Roig-Vilaseca D, Juanola X, Rodriguez-Moreno J, Nolla JM and Valverde J. Impact of antiplatelet therapy in the development of severe ischemic complications and in the outcome of patients with giant cell arteritis. Clin Exp Rheumatol. 2008;26:S57–62. [PubMed] [Google Scholar]
- 230.de Souza AW, Machado NP, Pereira VM, Arraes AE, Reis Neto ET, Mariz HA and Sato EI. Antiplatelet therapy for the prevention of arterial ischemic events in takayasu arteritis. Circ J. 2010;74:1236–41. [DOI] [PubMed] [Google Scholar]
- 231.Abularrage CJ, Slidell MB, Sidawy AN, Kreishman P, Amdur RL and Arora S. Quality of life of patients with Takayasu’s arteritis. J Vasc Surg. 2008;47:131–6; discussion 136-7. [DOI] [PubMed] [Google Scholar]
- 232.Akar S, Can G, Binicier O, Aksu K, Akinci B, Solmaz D, Birlik M, Keser G, Akkoc N and Onen F. Quality of life in patients with Takayasu’s arteritis is impaired and comparable with rheumatoid arthritis and ankylosing spondylitis patients. Clin Rheumatol. 2008;27:859–65. [DOI] [PubMed] [Google Scholar]
- 233.Rimland CA, Quinn KA, Rosenblum JS, Schwartz MN, Bates Gribbons K, Novakovich E, Sreih AG, Merkel PA, Ahlman MA and Grayson PC. Outcome Measures in Large Vessel Vasculitis: Relationship Between Patient-, Physician-, Imaging-, and Laboratory-Based Assessments. Arthritis Care Res (Hoboken). 2020;72:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hellmann DB, Uhlfelder ML, Stone JH, Jenckes MW, Cid MC, Guillevin L, Moreland L, Dellaripa PF, Hoffman GS, Merkel PA, Spiera R, Brown L, Hernández-Rodríguez J and Rubin HR. Domains of health-related quality of life important to patients with giant cell arteritis. Arthritis and rheumatism. 2003;49:819–25. [DOI] [PubMed] [Google Scholar]
- 235.Sreih AG, Alibaz-Oner F, Easley E, Davis T, Mumcu G, Milman N, Robson J, Direskeneli H, Merkel PA and Cronholm P. Health-related outcomes of importance to patients with Takayasu’s arteritis. Clin Exp Rheumatol. 2018;36 Suppl 111:51–57. [PubMed] [Google Scholar]
- 236.Aydin SZ, Direskeneli H and Merkel PA. Assessment of Disease Activity in Large-vessel Vasculitis: Results of an International Delphi Exercise. The Journal of rheumatology. 2017;44:1928–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aitken M and Basu N. Improving quality of life in vasculitis patients. Rheumatology (Oxford). 2020;59:iii132–iii135. [DOI] [PubMed] [Google Scholar]
- 238.Barra L, Borchin RL, Burroughs C, Casey GC, McAlear CA, Sreih AG, Young K, Merkel PA and Pagnoux C. Impact of vasculitis on employment and income. Clin Exp Rheumatol. 2018;36 Suppl 111:58–64. [PMC free article] [PubMed] [Google Scholar]
- 239.Koster MJ, Warrington KJ and Matteson EL. Morbidity and Mortality of Large-Vessel Vasculitides. Curr Rheumatol Rep. 2020;22:86. [DOI] [PubMed] [Google Scholar]
- 240.Piggott K, Deng J, Warrington K, Younge B, Kubo JT, Desai M, Goronzy JJ and Weyand CM. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ and Weyand CM. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126:1953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nadkarni S, Dalli J, Hollywood J, Mason JC, Dasgupta B and Perretti M. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circulation research. 2014;114:242–8. [DOI] [PubMed] [Google Scholar]
- 243.Carmona FD, Vaglio A, Mackie SL, Hernandez-Rodriguez J, Monach PA, Castaneda S, Solans R, Morado IC, Narvaez J, Ramentol-Sintas M, Pease CT, Dasgupta B, Watts R, Khalidi N, Langford CA, Ytterberg S, Boiardi L, Beretta L, Govoni M, Emmi G, Bonatti F, Cimmino MA, Witte T, Neumann T, Holle J, Schonau V, Sailler L, Papo T, Haroche J, Mahr A, Mouthon L, Molberg O, Diamantopoulos AP, Voskuyl A, Brouwer E, Daikeler T, Berger CT, Molloy ES, O’Neill L, Blockmans D, Lie BA, McLaren P, Vyse TJ, Wijmenga C, Allanore Y, Koeleman BP, Spanish CGAG, Consortium U, Vasculitis Clinical Research C, Barrett JH, Cid MC, Salvarani C, Merkel PA, Morgan AW, Gonzalez-Gay MAand Martin J. A Genome-wide Association Study Identifies Risk Alleles in Plasminogen and P4HA2 Associated with Giant Cell Arteritis. American journal of human genetics. 2017;100:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kong X and Sawalha AH. Takayasu arteritis risk locus in IL6 represses the anti-inflammatory gene GPNMB through chromatin looping and recruiting MEF2-HDAC complex. Ann Rheum Dis. 2019;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Keser G, Aksu K and Direskeneli H. Discrepancies between vascular and systemic inflammation in large vessel vasculitis: an important problem revisited. Rheumatology (Oxford). 2018;57:784–790. [DOI] [PubMed] [Google Scholar]
- 246.Guleria A, Misra DP, Rawat A, Dubey D, Khetrapal CL, Bacon P, Misra R and Kumar D. NMR-Based Serum Metabolomics Discriminates Takayasu Arteritis from Healthy Individuals: A Proof-of-Principle Study. J Proteome Res. 2015;14:3372–81. [DOI] [PubMed] [Google Scholar]
- 247.Cui X, Qin F, Song L, Wang T, Geng B, Zhang W, Jin L, Wang W, Li S, Tian X, Zhang H and Cai J. Novel Biomarkers for the Precisive Diagnosis and Activity Classification of Takayasu Arteritis. Circ Genom Precis Med. 2019;12:e002080. [DOI] [PubMed] [Google Scholar]
- 248.Bolha L, Pizem J, Frank-Bertoncelj M, Hocevar A, Tomsic M and Jurcic V. Identification of microRNAs and their target gene networks implicated in arterial wall remodelling in giant cell arteritis. Rheumatology (Oxford). 2020;59:3540–3552. [DOI] [PubMed] [Google Scholar]
- 249.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, Brouwer E, Cimmino MA, Clark E, Dasgupta B, Diamantopoulos AP, Direskeneli H, Iagnocco A, Klink T, Neill L, Ponte C, Salvarani C, Slart R, Whitlock M and Schmidt WA. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–643. [DOI] [PubMed] [Google Scholar]
- 250.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, Malayeri AA, Merkel PA, Novakovich E, Bluemke DA and Ahlman MA. 18F-Fluorodeoxyglucose-Positron Emission Tomography as an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients with Large Vessel Vasculitis. Arthritis Rheumatol. 2018;70:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Youngstein T, Tombetti E, Mukherjee J, Barwick TD, Al-Nahhas A, Humphreys E, Nash J, Andrews J, Incerti E, Tombolini E, Salerno A, Sartorelli S, Ramirez GA, Papa M, Sabbadini MG, Gianolli L, De Cobelli F, Fallanca F, Baldissera E, Manfredi AA, Picchio M and Mason JC. FDG Uptake by Prosthetic Arterial Grafts in Large Vessel Vasculitis Is Not Specific for Active Disease. Jacc. 2017;10:1042–1052. [DOI] [PubMed] [Google Scholar]
- 252.Ćorović A, Wall C, Mason JC, Rudd JHF and Tarkin JM. Novel Positron Emission Tomography Tracers for Imaging Vascular Inflammation. Curr Cardiol Rep. 2020;22:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lamare F, Hinz R, Gaemperli O, Pugliese F, Mason JC, Spinks T, Camici PG and Rimoldi OE. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med. 2011;52:33–9. [DOI] [PubMed] [Google Scholar]
- 254.Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Rimoldi OE, Mason JC and Camici PG. Imaging of vascular inflammation with [11C]-PK11195 and PET/CT angiography. J Am Coll Cardiol. 2010;56:33–39. [DOI] [PubMed] [Google Scholar]
- 255.Tarkin JM, Wall C, Gopalan D, Aloj L, Manavaki R, Fryer TD, Aboagye EO, Bennett MR, Peters JE, Rudd JHF and Mason JC. Novel Approach to Imaging Active Takayasu Arteritis Using Somatostatin Receptor Positron Emission Tomography/Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2020;13:e010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.NCT04071691. 2021.
- 257.Tombetti E, Godi C, Ambrosi A, Doyle F, Jacobs A, Kiprianos AP, Youngstein T, Bechman K, Manfredi AA, Ariff B and Mason JC. Novel Angiographic Scores for evaluation of Large Vessel Vasculitis. Sci Rep. 2018;8:15979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nakagomi D, Cousins C, Sznajd J, Furuta S, Mohammad AJ, Luqmani R and Jayne D. Development of a score for assessment of radiologic damage in large-vessel vasculitis (Combined Arteritis Damage Score, CARDS). Clin Exp Rheumatol. 2017;35 Suppl 103:139–145. [PubMed] [Google Scholar]
- 259.Goel R, Gribbons KB, Carette S, Cuthbertson D, Hoffman GS, Joseph G, Khalidi NA, Koening CL, Kumar S, Langford C, Maksimowicz-McKinnon K, McAlear CA, Monach PA, Moreland LW, Nair A, Pagnoux C, Quinn KA, Ravindran R, Seo P, Sreih AG, Warrington KJ, Ytterberg SR, Merkel PA, Danda D and Grayson PC. Derivation of an angiographically based classification system in Takayasu’s arteritis: an observational study from India and North America. Rheumatology (Oxford). 2020;59:1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gribbons KB, Ponte C, Carette S, Craven A, Cuthbertson D, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Maksimowicz-McKinnon K, McAlear CA, Monach PA, Moreland LW, Pagnoux C, Quinn KA, Robson JC, Seo P, Sreih AG, Suppiah R, Warrington KJ, Ytterberg SR, Luqmani R, Watts R, Merkel PA and Grayson PC. Patterns of Arterial Disease in Takayasu’s Arteritis and Giant Cell Arteritis. Arthritis care & research. 2020;72:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tarzi RM, Mason JC and Pusey CD. Issues in trial design for ANCA-associated and large-vessel vasculitis. Nature reviews. 2014;10:502–10. [DOI] [PubMed] [Google Scholar]
- 262.Sreih AG, Alibaz-Oner F, Kermani TA, Aydin SZ, Cronholm PF, Davis T, Easley E, Gul A, Mahr A, McAlear CA, Milman N, Robson JC, Tomasson G, Direskeneli H and Merkel PA. Development of a Core Set of Outcome Measures for Large-vessel Vasculitis: Report from OMERACT 2016. The Journal of rheumatology. 2017;44:1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C and Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Qjm. 1994;87:671–8. [PubMed] [Google Scholar]
- 264.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M and Hoffman GS. Takayasu arteritis. Annals of internal medicine. 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 265.Aydin SZ, Yilmaz N, Akar S, Aksu K, Kamali S, Yucel E, Karadag O, Bicakcigil M, Ozer H, Kiraz S, Onen F, Inanc M, Keser G, Akkoc N and Direskeneli H. Assessment of disease activity and progression in Takayasu’s arteritis with Disease Extent Index-Takayasu. Rheumatology (Oxford). 2010;49:1889–93. [DOI] [PubMed] [Google Scholar]
- 266.Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM, Jeyaseelan L, Lawrence A and Bacon PA. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology (Oxford). 2013;52:1795–801. [DOI] [PubMed] [Google Scholar]
- 267.Gribbons KB, Ponte C, Craven A, Robson JC, Suppiah R, Luqmani R, Watts R, Merkel PA and Grayson PC. Diagnostic Assessment Strategies and Disease Subsets in Giant Cell Arteritis: Data From an International Observational Cohort. Arthritis & rheumatology (Hoboken, NJ). 2020;72:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, Khalidi NA, Langford CA, Monach PA, Seo P, Warrington KJ, Ytterberg SR, Hoffman GS and Merkel PA. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Annals of the rheumatic diseases. 2012;71:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mader TH, Werner RP, Chamberlain DG and Doornbos D. Giant cell arteritis in Alaska Natives. Can J Ophthalmol. 2009;44:53–6. [DOI] [PubMed] [Google Scholar]
- 270.Smith CA, Fidler WJ and Pinals RS. The epidemiology of giant cell arteritis. Report of a ten-year study in Shelby County, Tennessee. Arthritis and rheumatism. 1983;26:1214–9. [DOI] [PubMed] [Google Scholar]
- 271.Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ and Hunder GG. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore). 1985;64:89–99. [PubMed] [Google Scholar]
- 272.Ing EB, Lahaie Luna G, Pagnoux C, Baer PA, Wang D, Benard-Seguin E, Godra I, Godra A, Munoz DG, McReelis K and Ten Hove M. The incidence of giant cell arteritis in Ontario, Canada. Can J Ophthalmol. 2019;54:119–124. [DOI] [PubMed] [Google Scholar]
- 273.Martinez P, Mollerach F, Vergara F, Gandino I, Scolnik M, Catoggio L, Rosa J and Soriano E. Incidence and Prevalence of Polymyalgia Rheumatic and Giant Cell Arteritis: A 15-Year Study in a Health Care Management Organization [abstract]. Arthritis & rheumatology (Hoboken, NJ). 2016;68. [Google Scholar]
- 274.Brekke LK, Diamantopoulos AP, Fevang BT, Aβmus J, Esperø E and Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972-2012: a retrospective cohort study. Arthritis Res Ther. 2017;19:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tomasson G, Bjornsson J, Zhang Y, Gudnason V and Merkel PA. Cardiovascular risk factors and incident giant cell arteritis: a population-based cohort study. Scand J Rheumatol. 2019;48:213–217. [DOI] [PubMed] [Google Scholar]
- 276.Dreyer L, Faurschou M and Baslund B. A population-based study of Takayasús arteritis in eastern Denmark. Clin Exp Rheumatol. 2011;29:S40–2. [PubMed] [Google Scholar]
- 277.Catanoso M, Macchioni P, Boiardi L, Muratore F, Restuccia G, Cavazza A, Pipitone N, Mancuso P, Luberto F and Salvarani C. Incidence, Prevalence, and Survival of Biopsy-Proven Giant Cell Arteritis in Northern Italy During a 26-Year Period. Arthritis Care Res (Hoboken). 2017;69:430–438. [DOI] [PubMed] [Google Scholar]
- 278.Pucelj NP, Hočevar A, Ješe R, Rotar Ž, Hawlina M, Fakin A, Pižem J and Tomšič M. The incidence of giant cell arteritis in Slovenia. Clin Rheumatol. 2019;38:285–290. [DOI] [PubMed] [Google Scholar]
- 279.Romero-Gómez C, Aguilar-García JA, García-de-Lucas MD, Cotos-Canca R, Olalla-Sierra J, García-Alegría JJ and Hernández-Rodríguez J. Epidemiological study of primary systemic vasculitides among adults in southern Spain and review of the main epidemiological studies. Clin Exp Rheumatol. 2015;33:S-11-8. [PubMed] [Google Scholar]
- 280.Saritas F, Donmez S, Direskeneli H and Pamuk ON. The epidemiology of Takayasu arteritis: a hospital-based study from northwestern part of Turkey. Rheumatol Int. 2016;36:911–6. [DOI] [PubMed] [Google Scholar]
- 281.Friedman G, Friedman B and Benbassat J. Epidemiology of temporal arteritis in Israel. Isr J Med Sci. 1982;18:241–4. [PubMed] [Google Scholar]
- 282.Bas-Lando M, Breuer GS, Berkun Y, Mates M, Sonnenblick M and Nesher G. The incidence of giant cell arteritis in Jerusalem over a 25-year period: annual and seasonal fluctuations. Clin Exp Rheumatol. 2007;25:S15–7. [PubMed] [Google Scholar]
- 283.Nesher G, Ben-Chetrit E, Mazal B and Breuer GS. The Incidence of Primary Systemic Vasculitis in Jerusalem: A 20-year Hospital-based Retrospective Study. The Journal of rheumatology. 2016;43:1072–7. [DOI] [PubMed] [Google Scholar]
- 284.el-Reshaid K, Varro J, al-Duwairi Q and Anim JT. Takayasu’s arteritis in Kuwait. J Trop Med Hyg. 1995;98:299–305. [PubMed] [Google Scholar]
- 285.Dunstan E, Lester SL, Rischmueller M, Dodd T, Black R, Ahern M, Cleland LG, Roberts-Thomson P and Hill CL. Epidemiology of biopsy-proven giant cell arteritis in South Australia. Intern Med J. 2014;44:32–9. [DOI] [PubMed] [Google Scholar]
- 286.Makin K, Isbel M and Nossent J. Frequency, presentation, and outcome of Takayasu arteritis in Western Australia. Mod Rheumatol. 2017;27:1019–1023. [DOI] [PubMed] [Google Scholar]
- 287.Abdul-Rahman AM, Molteno AC and Bevin TH. The epidemiology of giant cell arteritis in Otago, New Zealand: a 9-year analysis. N Z Med J. 2011;124:44–52. [PubMed] [Google Scholar]
- 288.Li L, Neogi T and Jick S. Mortality in Patients With Giant Cell Arteritis: A Cohort Study in UK Primary Care. Arthritis Care Res (Hoboken). 2018;70:1251–1256. [DOI] [PubMed] [Google Scholar]
- 289.Ben-Shabat N, Tiosano S, Shovman O, Comaneshter D, Shoenfeld Y, Cohen AD and Amital H. Mortality among Patients with Giant Cell Arteritis: A Large-scale Population-based Cohort Study. The Journal of rheumatology. 2020;47:1385–1391. [DOI] [PubMed] [Google Scholar]
- 290.Kermani TA, Warrington KJ, Crowson CS, Ytterberg SR, Hunder GG, Gabriel SE and Matteson EL. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Annals of the rheumatic diseases. 2013;72:1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Michailidou D, Rosenblum JS, Rimland CA, Marko J, Ahlman MA and Grayson PC. Clinical symptoms and associated vascular imaging findings in Takayasu’s arteritis compared to giant cell arteritis. Annals of the rheumatic diseases. 2020;79:262–267. [DOI] [PubMed] [Google Scholar]
- 292.Uy CP, Tarkin JM, Gopalan D, Barwick TD, Tombetti E, Youngstein T and Mason JC. The Impact of Integrated Noninvasive Imaging in the Management of Takayasu Arteritis. JACC Cardiovascular imaging. 2021;14:495–500. [DOI] [PubMed] [Google Scholar]
- 293.Spira D, Xenitidis T, Henes J and Horger M. MRI parametric monitoring of biological therapies in primary large vessel vasculitides: a pilot study. Br J Radiol. 2016;89:20150892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, Bagheri AA, Merkel PA, Novakovich E and Grayson PC. Comparison of magnetic resonance angiography and (18)F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Annals of the rheumatic diseases. 2018;77:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Prieto-González S, García-Martínez A, Tavera-Bahillo I, Hernández-Rodríguez J, Gutiérrez-Chacoff J, Alba MA, Murgia G, Espígol-Frigolé G, Sánchez M, Arguis P and Cid MC. Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Medicine (Baltimore). 2015;94:e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dweck MR, Abgral R, Trivieri MG, Robson PM, Karakatsanis N, Mani V, Palmisano A, Miller MA, Lala A, Chang HL, Sanz J, Contreras J, Narula J, Fuster V, Padilla M, Fayad ZA and Kovacic JC. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2018;11:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Abgral R, Dweck MR, Trivieri MG, Robson PM, Karakatsanis N, Mani V, Padilla M, Miller M, Lala A, Sanz J, Narula J, Fuster V, Contreras J, Kovacic JC and Fayad ZA. Clinical Utility of Combined FDG-PET/MR to Assess Myocardial Disease. JACC Cardiovascular imaging. 2017;10:594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Laurent C, Ricard L, Fain O, Buvat I, Adedjouma A, Soussan M and Mekinian A. PET/MRI in large-vessel vasculitis: clinical value for diagnosis and assessment of disease activity. Sci Rep. 2019;9:12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.NCT02902731. 2020.
- 300.Weyand CM, Hicok KC, Hunder GG and Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Annals of internal medicine. 1994;121:484–91. [DOI] [PubMed] [Google Scholar]
- 301.NCT04930094. 2021.
- 302.NCT04633447. 2021.
- 303.Espígol-Frigolé G, Planas-Rigol E, Lozano E, Corbera-Bellalta M, Terrades-García N, Prieto-González S, García-Martínez A, Hernández-Rodríguez J, Grau JM and Cid MC. Expression and Function of IL12/23 Related Cytokine Subunits (p35, p40, and p19) in Giant-Cell Arteritis Lesions: Contribution of p40 to Th1- and Th17-Mediated Inflammatory Pathways. Front Immunol. 2018;9:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Espígol-Frigolé G, Planas-Rigol E, Ohnuki H, Salvucci O, Kwak H, Ravichandran S, Luke B, Cid MC and Tosato G. Identification of IL-23p19 as an endothelial proinflammatory peptide that promotes gp130-STAT3 signaling. Sci Signal. 2016;9:ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.NCT03711448. 2021.
- 306.Conway R, O’Neill L, Gallagher P, McCarthy GM, Murphy CC, Veale DJ, Fearon U and Molloy ES. Ustekinumab for refractory giant cell arteritis: A prospective 52-week trial. Semin Arthritis Rheum. 2018;48:523–528. [DOI] [PubMed] [Google Scholar]
- 307.Matza MA, Fernandes AD, Stone JH and Unizony SH. Ustekinumab for the Treatment of Giant Cell Arteritis. Arthritis Care Res (Hoboken). 2020. [DOI] [PubMed] [Google Scholar]
- 308.NCT03841734. 2021.
- 309.NCT03892785. 2021.
- 310.Schmidt WA, Dasgupta B, Luqmani R, Unizony SH, Blockmans D, Lai Z, Kurrasch RH, Lazic I, Brown K and Rao R. A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Sirukumab in the Treatment of Giant Cell Arteritis. Rheumatol Ther. 2020;7:793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.NCT04299971. 2021.
- 312.NCT04161898. 2021.
- 313.NCT04882072. 2021.
- 314.NCT04564001. 2020.


