Abstract
COVID-19 has affected the lives of billions of people and is a causative agent for millions of deaths. After 23 months of the first reported case of COVID-19, on 25th November 2020, a new SARS-COVID-19 variant, i.e. Omicron was reported with a WHO tagline of VoC that trembled the world with its infectivity rate. This fifth VoC raised the concern about neutralising ability and adequate control of SARS-COVID-19 infection due to mass vaccination drive (nearly more than 4.7 billion individuals got vaccinated globally till December 2021). However, the present scenario of VoCs highlights the importance of vaccination and public health measures that need to be followed strictly to prevent the fatality from Omicron. The world still needs to overcome the hesitancy that poses a major barrier to the implementation of vaccination. This review highlights the SARS-COVID-19 situation and discusses in detail the mutational events that occurred at a cellular level in different variants over time. This article is dedicated to the scientific findings reported during the recent outbreak of 2019–2022 and describes their symptoms, disease, spread, treatment, and preventive action advised. The article also focuses on the treatment options available for Covid-19 and the update of Omicron by expert agencies.
Keywords: Omicron, WHO, SARS-COVID 19, mutation, vaccination, infection, virus
Introduction
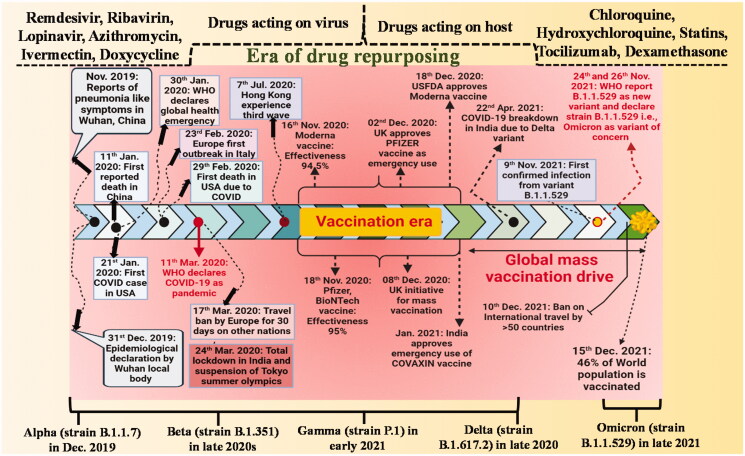
The 7th human coronavirus, SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona Virus 2), was for the first time reported in Wuhan, Hubei Province, China, after a recent pneumonia outbreak in January 2020. By the end of January, China had reported 12,167 suspected and 7736 confirmed cases, with 82 reported cases identified in 18 different countries. The number of incidences kept increasing creating havoc worldwide that forced the World Health Organisation (WHO) to proclaim it a pandemic in March 2020. SARS-CoV-2, the causative agent of COVID-19, was responsible for more than 143 million infections and the loss of 3 million and more lives globally since and around April 2021 (Figure 1).
Figure 1.
Timeline on series of events take place from origin to till date due to SARS-COVID-19.
The effort towards the design, development, and administration of new vaccines commenced to effectively protect the population against the variant of concern. SARS-CoV-2 proved to be devasting for populations across the globe, particularly in densely populated regions. Presently several novel variants of SARS-CoV-2 have been reported worldwide. WHO introduced a simplified and easy-to-say nomenclature for SARS-CoV-2 variants of concern on 31 May 2021, utilising Greek alphabets like alpha (α), beta (β), gamma (γ), and delta (δ) based on a specific mutation in their amino acid chains [1–6]. These variants had a range of mutations and most of them are linked to the S protein. The mutation leads to modification in viral activity and pathogenicities like the receptor-binding domain (RBD) modifications and resistance to natural immunity [7]. Each variant of concern is associated with a new wave of infection devastating the health of people globally. For example, the delta variant due to its capability to escape natural immunity demonstrates a higher rate of transmission as compared to the other variant of concern. Due to the greater risk of reinfection, longer duration of virulence, and more viral load, delta variant presented a heavy burden on health. At the time of the fourth wave, the infection of delta variant dominated the world that frantically generated a demand for a vaccination with different public protection measures [3–6,8].
The worrying fatigue was not even over, another novel SARS-CoV-2 variant of concern (VoC), Omicron, was reported at the earliest on 25 November 2021, which was nearly 23 months after the 1st reported case of COVID-19. Till that date, there was a global estimate of 300 million cases and 5 million deaths. The emergence of Omicron exhibited concrete difficulties on the population as the COVID-19 already filled everyone with frustration, anger, and unfavourable outcomes associated with emotional, economic, and social well-being. Unlike earlier variants of concern, which rose where natural immunity was a matter of concern, this 5th variant on the other hand originated at a stage where scientists have already developed vaccines as countermeasures. On 11 November 2021, the first case of Omicron VoC was reported in Botswana followed by the second case from Hong Kong from the person having travel history from South Africa. Following the earliest finding it was stated that the novel variant was related with the S-gene target failure on a particular PCR test, owing to certain deletion comprising of almost 69–70 amino acid sequences, which was quite analogous to the alpha variant. On 9 November 2021, a confirmed case of Omicron variant in a patient from South Africa was diagnosed at the earliest with COVID-19. Following it, the average number of cases in South Africa rose from 280 COVID-19 cases per day to 800 cases per day. The primary concerns regarding Omicron are whether it will be more contagious or serious than other variants of concern, and would it succeed in evading vaccine protection [9]? This review highlights in brief about SARS-COVID-19 situation and discusses in detail, the mutational events that occur in various variants at cellular levels. Furthermore, these mutational changes were compared with the rate of infectivity, severity, and mortality. Additionally, further discussion was done in detail about the vaccination program to counteract these events. This article is respectively dedicated to the scientific findings reported during the recent outbreak of 2019–2022 and describes the symptoms, disease, spread, treatment, and prevention. This article might be useful to attenuate the prevalence of outbreaks and provide knowledge to future generations regarding precautions needed to be taken.
History and its evolution
In December 2019, many adults in Wuhan city, Hubei’s capital city visited the local hospitals as they were suffering from severe pneumonia with unidentified cause [10]. Most of the reported cases had similar to that of patients exposed to the Huanan wholesale seafood market that trades live animals [11]. The respiratory samples of all infected individuals were sent for aetiological investigations to the reference labs by the local body organisation. The Huanan Seafood market was closed on 1st January and China immediately reported this outbreak to WHO. The virus had more than 95% similarity to bat coronavirus and 70% homology with SARS-CoV. The Seafood environmental samples collected were tested positive evidencing the origin of the virus. Mass gathering during a period of Chinese Lunar New Year might be one of the reasons for the spread of this infection. Later, on the 13th January 2020 first exported case was observed in Thailand [12]. The number of cases across the world started to rise rapidly. On 30th January 2020, the PHEIC (Public Health Emergencies of International Concern) alarm was issued by WHO. As dated 6th February 2020, WHO reported a global burden of >28 K cases along with 565 deaths in at least 25 countries. On 11 February 2020, WHO changed the name of the 2019 novel coronavirus (2019-nCoV) to SARS-CoV-2 due to its high similarity with SARS-CoV. Furthermore, on 11 March 2020, Covid-19 was declared a pandemic globally. At the beginning of June 2020, confirmed cases of COVID-19 were more than 7 million along with 400 K deaths worldwide. Globally, 200 countries and more got affected by the Covid-19 pandemic [11].
Concerning strains of COVID-19
The race to formulate effective vaccines for the protection of people started from the very first known strain of SARS-Cov-2. The vaccine development program was paced considering the devastating, harmful nature of strain for public health, particularly in the densely populated areas. Various crucial strains of SARS-Cov-2 have arrived due to mutational events even after vaccination. Moreover, FDA had also authorised an antiviral drug known as remdesivir for the management of COVID-19 in adults and children above 12 years. These new strains of SARS-Cov-2 were named and labelled using Greek alphabets, such as alpha (α), beta (β), gamma (γ), delta (δ), lambda (λ), and mu (μ), amongst which some of them were categorised in concerning the window and some under a little lesser concerned as listed in Table 1.
Table 1.
Various reported strains of concern of SARS-Cov-2.
| WHO tag | Strain tag by Pango lineage | Other name(s) | Specific mutation spot | Date of designation |
|---|---|---|---|---|
| Alpha (α) | B.1.1.7 | GRY, 20I (V1) | D614G, N501Y, P681H + S:452R, +S:484K | 18 December 2020 |
| Beta (β) | B.1.351 | GR/501Y.V2, 20H (V2) | E484K, K417N, N501Y, +S:L18F | 18 December 2020 |
| Gamma (γ) | P.1 | GR/501Y.V3, 20 J (V3) | N501Y, E484K, K417T + S:681H | 11 January 2021 |
| Delta (δ) | B.1.617.2 | G/478K.V1, 21 A, 21I, 21 J | P681R, D614G, E484Q, E484R, L452R, G142D, D111D + S:484K, +S:417N | 11 May 2021 |
| Omicron | B.1.1.529 | 21K, 21 L | 69-70del, K417N, N501Y, N679K, P681H, T478K, G142D/143-145del, T95I, +R346K | 26 November 2021 |
In the early pandemic stage, the very first mutation was identified in the SARS-CoV-2 genomic sequence was the S protein amino acid D614G whose alteration produced G614 that became the dominant form of the pandemic. This was reported in China and Germany during the starting month of 2020 and later spread worldwide. The alteration in the genomic sequence was seen in three kinds of mutations, (1) at site 241, C to T mutation in 5′ UTR, (2) at site 3037, equivalent mutation of C to T mutation, (3) at site 14,408 in RNA-dependent polymerase gene, C to T mutation. Overall, the mutation resulted in severe infectivity and faster replication in human tissue as compared to the previous strain [13,14]. Higher infection factor was directly linked with the enhanced receptor-binding domain from the attained transmutation where glycine (G) replaced aspartate (D) residue that permits the increment in the flexibility of trimeric S protein structure and also offer improved empathy [15]. Reports based on the efficacy of the Pfizer-BioNTech BNT162b2 vaccine on the new mutant variants showed a 1.7–2.0 times reduction in neutralisation potency, however, the effectiveness against the virus was much diminished [13,16]. Moreover, the Moderna mRNA-1273 vaccine also produced similar results in terms of the failure of the previously developed vaccine [17].
The alpha (α) pathological variant also known as B.1.1.7 was first reported in the United Kingdom and spread to over more than 40 countries. This pathological strain showed 8 S-protein mutations, 17 non-synonymous mutations, and D614G mutation. As per reports of WHO, out of eight spike protein mutant strains, three were notable (the deletion of two amino acids at positions 69–70, i.e. P681H and N501Y) [18]. Mutant variants showed enhanced RBD efficiency to angiotensin-converting enzyme-II (ACE2) which was like D614G mutation. In vitro studies of alpha (α) strain displayed an intermediate decrease in the neutralisation ability against Novavax and Moderna vaccines when compared to the first strain [18,19].
Similarly, another strain coded as B.1.526 was initially identified in New York, the U.S. in November 2020. This variant of SARS-CoV-2 spread at a very fast rate in New York City and its relative areas. The most notable mutation in B.1.526 was E484K and S447N observed in spike protein along with five others including T95I, D614G, A701V, D253G, L5F [20]. A study was conducted by Annavajhala et al. to check the efficiency of the monoclonal antibodies (MABs) against a pseudo-coronavirus model, including E484K and S477N. The outcome of the study showed that the S477N mutant strain of B.1.526 had nominal to negligible antigenic effect and was magnificently neutralised. On the other hand, many antibodies got unfavourable results in the neutralisation of targeting E484K mutation [21]. Such loss of neutralisation was also perceived with convalescent serum aka survivor plasma inclusive of antibodies and distinct proteins developed by the host’s defense system to the viral contagion [22]. Additionally, it was identified that mutation on E484 could be rehabilitated by the change in P, Q, or K among which almost all tend to show a reduction in the neutralisation in serum by reducing the antibody binding to the receptor-binding domain (RBD) [23]. The efficacy of vaccinated sera developed by Moderna and Pfizer-BioNTech were not affected by S477N mutation but instead resulted in a reduced neutralisation on the E484K mutation [20].
In late 2020, for the very first time Beta (β) strain also called B.1.351 was reported in South Africa and become the dominant variant in the region. As per WHO reports, specific mutations were spotted in spike protein including E484K, K417N, and N501Y. As seen previously, the B.1.526 strain showed an E484K mutation that hampers neutralisation ability. A similar observation was reported with N501Y mutation conversed in Britain strain. As studied in the pseudovirus model, all three mutations caused a rapid rate of virulence. Furthermore, B.1.351 variant evaluated a decline in neutralisation factor for both Pfizer vaccinated sera and serum convalescent sera, but the Moderna vaccine resulted in a decrease in neutralisation that still possesses the capability to sustain immunity against beta (β) strain of SARS-CoV-2 [24,25]. Recently, a couple of clinical studies were done to evaluate the efficacy of 2 more vaccines which are ChAdOx1 nCoV-19; chimpanzee adenovirus vector vaccine (replication-deficient), and NVS-CoV2373; recombinant nanoparticle vaccine against beta (β) variant. Adjuvant induced recombinant nanoparticle vaccine demonstrated 49% more efficacy towards symptomatic SARS-CoV-2 whereas the replication-deficient chimpanzee adenovirus vector vaccine showed no efficiency towards moderate SARS-CoV-2 [26,27].
In February 2020, a new strain coded as B.1.1.28 was found in Rio de Janeiro [28]. This strain also demonstrated a similar mutation on E484K as identified in B.1.526 strain. Covaxin vaccine which was developed in India had a great neutralisation ability against this variant as reported by Sapkal et al. On the other hand, Moderna and Pfizer-BioNTech vaccinated sera did not show any significant neutralisation ability to this variant [29]. The gamma (γ) strain also known as P.1 falls in the class of B.1.1.28 strain lineage and was identified in the travellers from Brazil that came to Japan. Gamma (γ) strain’s multiple mutations were observed with high infectivity factor and less neutralisation sensitivity making it the more severe and drastic form of mutation. This strain indicated three major changes within the receptor-binding domain on N501Y, K417T, and E484K that mimic the beta (β) strain found in South Africa. An experiment was performed with different monoclonal antibodies (MAB) to check the responses of gamma variant and results obtained were noted as: (1) most MABs were not able to neutralise the P.1 variant, (2) Adagio antibodies were found to be superior among other in terms of neutralisation of gamma variant, (3) zero neutralisation was found with bamlanivimab [18,30]. Moderna and Pfizer-BioNTech vaccinated sera were not able to neutralise gamma (γ) strain as like beta (β) strain [29].
B.1.617.2 variant aka delta strain was found in India during late 2020. The common mutation was identified on D111D, E484Q, D614G, P681R, and G142D in the spike protein. Inside the receptor-binding domain, three major mutations occurred at the furin cleavage site including P681R, E484Q, and L452R [31]. These transformations as a result enhanced binding to ACE2 as well as great cleavage rate of S1-S2, establishing fine transmissibility. A fresh experiment revealed that variants are inclusive of L452R mutation and escape the body’s defense system by fleeing humoral immune response and HLA-restricted responses [32]. More studies illustrated that L452R transformation had reduced affinity towards vaccine in comparison to the original variant [33]. Recently, FDA had approved an antiviral drug, i.e. remdesivir for the management of the COVID-19 as discussed earlier. A recent finding clarified that remdesivir causes the termination of RNA fabrication at three sites after incorporation. Hence the premature blockage of RNA fabrication simply abrogates further transcriptional and translational bioprocesses which are required for the development of new virions.
Recently, after 23 months from the first reported case of COVID-19, a new variant of SARS-CoV-2 had been detected in South Africa on 26 November 2021 and spread over more than 10 countries. This new variant was named Omicron and coded as B.1.1.529. It was the fifth variant of concern (VoC) as listed by WHO. Omicron had some deletion in a couple of amino acids like the alpha (α) variant and had more than 30 mutations reported. The first death from this variant was reported in Britain. Majorly it was found that the mutations were on 69-70del, K417N, N501Y, N679K, P681H, T478K, G142D/143-145del, T95I, and R346K within RBD in S protein. It was suggested that Omicron mutations might lead to enhanced transmission rate, better antibody escape, and viral infectivity. Furthermore, studies are still being conducted on this strain by various scientists across the globe [9].
Omicron mutational levels
Genetic mutation
Clinical and immunological information about the Omicron was not available to date to provide strong confirmation about various mutations in the new variant of concern. So, it was not evitable to postulate primary manifestations regarding severity, rate of transmission, and ability to escape from vaccine protection. More than 30 mutations and some deletions were seen in Omicron in which few mutations are like that of the alpha, beta, gamma, and delta variants of concern. Greater antibody escape, increase viral binding affinity and enhanced transmissibility are also reported due to these mutations and deletions. The mutagenic influence of Omicron influenced binding affinity and enhanced transmissibility. Notably, the consequences for most of the other mutations in Omicron were unknown, leaving a great deal of ambiguity regarding how the entire combination of mutations and deletions might significantly influence the sensitivity towards natural and vaccine-mediated immunity and viral activity.
Mutations in omicron spike protein
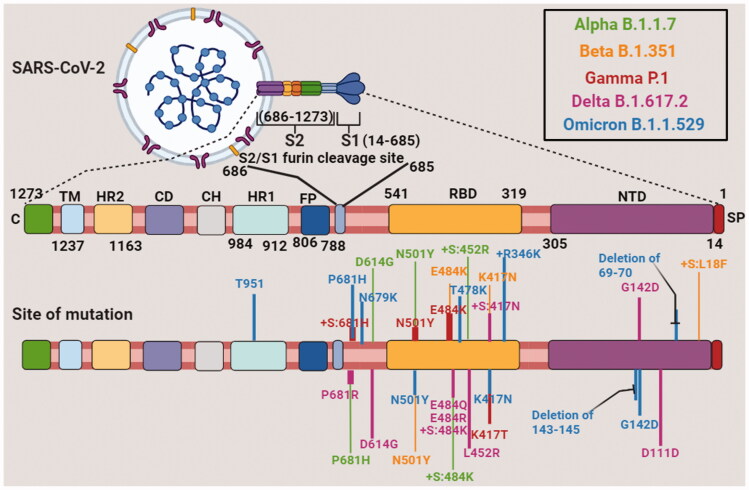
The S protein of SARS-CoV-2 binds to the ACE receptor and induces infection. In comparison with other significant variants, the S protein of Omicron was considerably mutated. 36 amino acid-altering mutations were observed in the Omicron variant. Among these mutations, 23 mutations were previously reported in the variants. Omicron possessed a new S-protein mutation in association with the other S-protein mutations already reported in earlier VoCs.
The N-terminal domain (NBD) was the first significant domain of S protein. In convalescent sera and monoclonal treatments, this N terminal domain was a target for binding and neutralising antibodies [34]. In the N-terminal domain, some deletions were found which significantly showed binding site destruction and indicated that the variant became less susceptible to antibody binding. 69, 70, 143, 144, 154, and 211 are the 6 deletions found in the N-terminal of Omicron variant (Figure 2 and Table 2). Immune evasion was the most rational explanation for these deletions. Only deletion 211 in the N-terminal domain was unique to Omicron; all others were observed in Alpha, Beta, or Delta variants. There were two possible causes for similar mutations that were observed in different strains. First was convergent evolution, which occurred when strains evolved the same mutation independent of one another, and the second was recombination, in which the exchange of genetic material takes place between two different strains. It indicated that during the evolution of Omicron, it picked up deletions from another variant [35].
Figure 2.
Sequential amino acid mutation in SARS-COVID-19 variants, i.e. Alpha, Beta, Gamma, Delta and Omicron strains. NTD: N terminal domain; RBD: receptor binding domain; FP: fusion protein; HR1: heptad repeat 1; CH: central helix; CD: connector domain; HR2: heptad repeat 2; TM: transmembrane; SP: spike protein; C or CT: cytoplasmic tail.
Table 2.
Compilation of spike protein mutations and concerning parameters in Omicron SARS-COVID 19.
| Site of S-protein mutation | Reason for concern |
|---|---|
| Asp796Tyr | Spike's sensitivity to convalescent plasma is reduced somewhat when His is substituted at this location [46]. |
| Gln498Arg | The Q498R mutation allows the virus to evade the COV2-2499 antibodies [47]. |
| Gln493Lys | The monoclonal antibody combination LY-CoV555 + LY-CoV016 fails to neutralise Q493R/K mutations [48]. In terms of binding with ACE2 and expression of the receptor binding domain (RBD) of spike, mutations at Q493 are often well-accepted. When particularly in comparison with the unmutated spike protein, Q493K imparts a higher than two log decrease in IC50 for the REGN10989/10934 set of monoclonal antibodies [49]. |
| Glu484Ala | Resistance to monoclonal antibodies 1B07 and 2B04 is mediated by the E484A mutation [50]. |
| Thr478Lys | Delta also has the T478K mutation. Resistance to neutralising antibodies has been achieved by other mutations at this region [50]. |
| Ser477Asn | Numerous monoclonal antibodies were unable to neutralise the S477N mutation in the spike protein, resulting in a level of resistance throughout the complete antibodies panel [50]. |
| Gly446Ser | In different variants other mutations at this location resulted in resistance from number of antibodies [47,50]. |
| Asn440Lys | The N440K mutation has been identified as an ‘immune escape variant’ in a virus obtained in India and is related to patient re-infection, against the human monoclonal antibody C135, in response to the selection pressureN440K was developed [51,52]. |
| Deletion at Val143-Tyr144-Tyr145 | In spike alpha variant Y144-Y145 deletion is observed. By this deletion alpha variant provides resistance to number of antibodies in NTD. Mutations in this location prevent monoclonal antibody 4A8 from binding [53,54]. |
Asp: aspartate; Tyr: tyrosine; GLN: glutamine; Arg: arginine; Lys: lysine; Glu: glutamic acid; Ala: alanine; Thr: threonine; Ser: serine; Asn: asparagine; Gly: glycine; Val: valine; NTD: N terminal domain; RBD: receptor binding domain; ACE2: angiotensin converting enzyme receptor 2.
Some mutations in the N-terminal domain of the Omicron variant consisted of G142D observed in pervasive Delta variant, T95I earlier reported in Iota variant that originated from New York. This was also previously seen in the Eta variant that originated from Nigeria. Omicron could develop resistance against antibody therapy as these mutations were observed in regional variants globally independent of each other. Thus, we could consider that Omicron had a potential impact on antibody binding ability. A genome addition of three amino acids at 214-position was also reported. Some hypothesised that the above insertion was associated with HCoV-229E, which was human cold-causing coronavirus. Dr. Roberto Patarca suggested that internal recombination and translocation of a segment in (−) strand of the UTR (5′-untranslated region) of SARS-CoV-2 were responsible for these changes and some other insertions at the 214-position. In other viruses, this site was most frequent for insertions.
Another main domain of importance in the S protein was the receptor-binding domain (RBD). This region played a key role in transmissibility and is essential for making direct contact with the ACE-2 receptor of the host. 15 amino acid substitutions were found in Omicron RBD and among these 11 amino acids were already reported in other variants. K417N, S477N, N501Y, and E484A were some important receptor binding domain mutations. Such mutations had been formerly reported in major VoC, such as Alpha and Delta. In New York City, there was an overlay between a few mutations in Omicron and mutations found in variants from wastewater. Salt bridges were formed due to the collective mutations which enhanced positive charge on a receptor-binding domain that increased binding affinity with ACE-2 receptor. The new aggressive mutations in Omicron were somewhat due to the ability of cross-species infection of SARS-CoV-2. Samples collected from wastewater showed several mutations that might be originated from dogs or rats according to published reports by Johnson et al. N501Y, Q498R, G496S, E484A, S477N, N440K, K417N, and some other mutations were also found in Omicron. This huge correlation suggested that Omicron acquired all or most of its mutations in a non-human animal before affecting African human groups. Some mutations raised around the furin cleavage site took place outside the receptor-binding and N-terminal domains. Omicron had several mutations in the furin cleavage region as observed with other variants of concern and interest. P681H, N679K, H655Y, and T547K had a role in increasing the effectiveness of furin cleavage, which was a feature of certain viruses that allowed them to split the S-protein into two subunits, hence increasing the risk of transmission. Such mutations had previously been seen in the Alpha, Gamma, Delta, and wastewater variants [36].
Other genomic mutations
The Triad
Excluding a few East African strains (A.23.1 and A.30), Omicron was derived out of the very first predominant SARS-CoV-2 variant, referred to as The Triad or even others refer as D614G. The C241U nucleotide mutation in the 5′ untranslated region, the P323L NSP12 polymerase mutation, and the most common D614G S protein mutation were all part of this variant. Enhancing the affinity among the S1 and S2 subunits post-cleavage improved the efficiency of ‘up’ formation of the receptor-binding domain and the D614G mutation enhanced the virus's infectivity. The C241U mutation within 5′ UTR had a negligible effect on sequences of protein, but for a cellular TAR binding protein linked to RNA metabolism, particularly transcription and translation efficiency established a cellular RNA binding site. The P323L mutation's function was presently unidentified, however, its presence implied replication of the virus.
There were ten homologous mutations in Omicron that did not lead to an amino acid alteration. The possibility of alterations in non-protein-coding areas, such as the C241U mutation found in the Triad, was considered during the SARS-CoV-2 investigation. Except for C241U, all 10 synonymous alterations in Omicron were distinctive. Furthermore, by modifying the structures and major recognition sequences in viral RNA essential for transcription, translation, and replication, such modifications might disrupt cis-acting regulatory sequences. As per reports of Thorne et al., similar non-coding, mutations were a result of overexpression of the N and ORF9bmRNA and proteins in the alpha variant. Such changes might alter gene expression and the virus's general biological features by changing consensus ribosome binding regions, transcription regulatory sequences, as well as other structures. The above alterations might even tend to result in the emergence of new sets of functional peptides, proteins, and RNAs, in some situations [37].
Non-structural proteins (NSPs)
The ORF1ab replication complex constitutes many non-Spike proteins. There were 16 non-structural proteins in this complex. These proteins had 11 mutations, seven of which were specific to Omicron only. Except for the Triad mutation in NSP12, all ORF1ab mutations were in ORF1a. The ORF1a proteins were essential for viral replication and the development of the double-membrane vesicle. The above finding showed that Omicron promoted the formation of double-membrane vesicles, which might lead to greater replicative ability. NSP3 was a unique gene with a wide range of functions and showed four distinct mutations. A distinct subdomain governed every function. Acidic C-terminal domain contained K38R. A deletion at position L1266I and 1265 in the nucleic acid-binding domain, and A1892T was present outside of subdomains. Many wastewater mutations had been found in NSP3, including S1087F, Kg77Q, P822G, D821A, T820N, K487Q, A465V, and 83771. It was suggested that the Omicron mutations might also influence nucleic-acid binding efficiency as well as proteolytic processing, depending on the role of these subdomains.
T492I, a unique mutation in NSP4, was thought to be crucial in the development of the double-membrane vesicle. The SARS-CoV-2 major protease breaks down the polyprotein during transcription and replication and affected another specific mutation in P132H and NSP5. In NSP4 and NSP5, notably D217G, G239S, and K35R in NSP4 along with S123C in NSP5, exhibited mutations in the wastewater variant. There were a group of deletions in NSP6 among both sites 106 and 108 that are commonly seen in variants of interest and concern. They were found in Alpha, Beta, and Gamma variants specifically in addition to several least prevalent regional variants. Additionally, the l189V mutation was also discovered that had a unique mutation. NSP6 mutations potentially performed an analogous function towards the NSP4 mutation, as it involved the development of the double-membrane replication/transcription vesicle. NSP6, on the other hand, was an immunological regulator that inhibited IRF3 phosphorylation, bound TANK binding kinase 1, and inhibited STAT1 and STAT2 activity.
The single most important significant mutation was P323L in the NSP12 RNA-dependent RNA polymerase which was mediated by circulating strains and associated with the Triad variant of mutations. ORF1b was the second component of the ORF1ab replication transcription complex and remarkably P323L was a single mutation found in it. The fact that Omicron replicates very efficiently without having significant mutations in this domain relates to the inherent replicative efficiency brought by several other mutations in the genome. All these mutations demanded functional and structural analysis. As we frequently observe, S-protein mutations were studied extensively, whereas non-S mutations were understudied. These were expected to have an analogous effect on viral functioning and need to be addressed [38].
Structural proteins
The SARS-CoV-2 genome contained accessory (regulatory) proteins as well as structural proteins in addition to NSPs. Accessory proteins were mostly immunological regulators where the Omicron variant failed to mutate. The proteins like ORF6 and ORF8 are recorded with a high mutation rate indicating Omicron's selective pressures. The Nucleocapsid (N) protein, Membrane (M) protein, Envelope (E) protein, and S protein were structural proteins. In the Omicron variant, no amino acid mutations were observed in the E protein. The M protein was a significant structural protein in the viral genome that under interferon antagonism inhibited the innate immune system and performed a function in RNA packaging. The Omicron M protein had two mutations, i.e. Q19E and D3 which are only found in the Omicron variant and influence the packaging of RNA. The multifunctional N protein, performed functions, such as immune regulation and RNA packaging. The Omicron N protein had been considerably altered, with several unique deletions along with other mutations similar as observed in other variants as well. Locations 31–33 had a distinct set of deletions. Omicron was the only significant variant with deletions in the N protein, indicating that the mutations had a significant influence on immunological escape or replication. The variation at P13L mutation was also present in Omicron, which was earlier identified in the South African C.1.2 and Peruvian C.27. The above mutations were in the N-terminal domain of the N protein. The R203K and G204R pairs in the N protein were the final set of mutations. The above group of mutations was probably the most prevalent between naturally existing variants. The RNA binding domain of N connected with its dimerisation domain initiated two mutations which were located around the core of the linker domain. The introduction of positive charge to the region was predominantly neutral because of glycine shifting to arginine at position 204. R203K and G204 mutations in Alpha and Gamma variants of concern were already reported, along with additional geographical variants, such as R.1 in Japan and C.27 in Peru. Findings revealed a significance of mutations in 199–205 regions of N protein. Single point mutations in this domain significantly improved the virus's infectivity up to 150-folds in certain instances, according to a recent study. A synergistic connection among the mutations in the N and S proteins was the more rational justification for the Omicron's enhanced replication capabilities and improved immune evasion [39].
Treatment options available for COVID-19
At the beginning of the pandemic knowledge and therapeutic management of the COVID-19 was limited and the urge to tackle this menace with experimental remedies and drug repurposing was an immediate requirement. Presently, a wide range of treatment options is available under FDA, such as antiviral drugs, anti-inflammatory agents, anti-SARS-CoV-2 monoclonal antibodies, and immunomodulators. Hospital administration uses all available prophylactic methods depending on the severity of the disease and associated risk parameters. Even antiviral medications are found to be useful and effective at different stages of viral replication. Major FDA-approved antiviral drugs that are being used are molnupiravir, paxlovid, remdesivir, hydroxychloroquine, lopinavir, and ivermectin. Additionally, these anti-viral drugs had the potential in reducing the risk of hospitalisation or death in unvaccinated adults associated with mild-to-moderate COVID-19 infection. In the later phases of the disease, the activity is carried out by pre and proinflammatory mediators due to the stimulation of the cytokine storm. The hyperinflammatory state can also be managed by the administration of anti-inflammatory agents like corticosteroids, immunomodulating remedies, or a combination of both. Moreover, anti-SARS-CoV-2 neutralising antibody products are useful in combating the illness. Convalescent plasma treatment was also tested during and against the SARS pandemic, but the lack of randomised control trials only facilitated superficial knowledge with vague information. FDA has approved this therapy under emergency use authorisation (EUA) for subjects severely affected. REGN-COV2 is a cocktail of two non-competing IgG1 antibodies, i.e. imdevimab and casirivimab that target the receptor-binding domain on S-protein and resulted in diminished viral load. Additionally, bamlanivimab and etesevimab are potential anti-spike neutralising MABs derived from convalescent plasma obtained from the COVID-19 subject. Anakinra, an interleukin-1 receptor antagonist was approved by FDA for use at the severe stage of the COVID-19. Anakinra reduced the demand for invasive mechanical ventilation and hence reduced the death rate. Baricitinib, a Janus kinase (JAK) inhibitor was considered an effective medication for COVID-19 due to its inhibitory effect on SARS-CoV-2 endocytosis in vitro and on the intracellular signalling pathways of cytokines. Nevertheless, COVID-19 patients having comorbid respiratory issues are prescribed with conventional oxygen therapy and critical monitoring with pulse oximetry. Acute hypoxemic respiratory failure is the commonest issue seen in adult subjects of COVID-19. Such subjects should be managed with enhanced respiratory therapy like non-invasive positive pressure ventilation (NIPPV), extracorporeal membrane oxygenation (ECMO), and high-flow nasal cannula (HFNC). Despite major infection prevention and public health measures to avoid the transmission of the COVID-19, vaccination in communities across the globe is extremely important. Outstanding efforts by scientists have resulted in the fabrication of novel vaccines at a very phenomenal pace to neutralise the illness globally. Vaccination stimulates the immune system to produce the natural neutralising antibodies that combat COVID-19. A thought on vaccines has been discussed further in this review. According to the WHO data, more than 4.7 billion doses of the vaccine have been administered globally with ∼58% of the world’s population [40].
Epidemiological haunt of Omicron
In the coming weeks, the Omicron variant will be dominant because of its increased rate of transmission all over the world. On 16 December 2021, confirmed cases of Omicron variant have been reported in almost 89 countries. From the second week of November, the number of COVID-19 cases drastically increased in South Africa, the birthplace of Omicron. In South Africa, the Omicron variant was dominating the overall variant reported earlier.
The incidence of COVID-19 cases brutally increased from December 6 to 12 as compared to the earlier week in the neighbouring countries of South Africa. Countries like Mozambique, Namibia, Eswatini, Zimbabwe, and Lesotho showed an increase in the number of cases by 4.4, 4.6, 5.7, 5.8, and 15.2-folds, respectively. The major reason for the sudden rise in the number of cases is still unclear but it might be due to the declaration of the variant of concern and spread of the Omicron variant resulting in an increased testing frequency [41].
The Delta variant still is responsible for more than 99.9% of COVID-19 cases globally as stated by Centres for Disease Control and Prevention (CDC) in the United States. It was also seen in almost 36 US states that the Omicron variant was detected but no death as such was reported. In most of the European countries, Delta is the dominant variant to date and Omicron is predicted to dominate within the first 2 weeks of 2022 as per the modelling predictions. There was a total of 27 confirmed cases of Omicron reported in EU/EEA on 16 December 2021 [42].
In England 41% of cases reported between 13 and 14 December are with S-gene target failure among that greater proportion is observed in London. On December 16, there were 11,708 confirmed cases of Omicron by genotyping or sequencing and 37,430 cases of S-gene target failure in the United Kingdom. Recently in mid-December 2021, a huge rise in the COVID-19 cases was reported globally. On 16 December 2021, the world has reported around 700 thousand cases per day whereas, on 16 January 2022, the cases count was found to be 2.6 million per day. The highest number of cases confirmed in a day (3.7 million) were found on 21 January 2022, denoted as the peak on the infectious wave. However, proceeding further, cases counts declined successfully which was a sigh of relief considering the situation.
Current status of vaccine in combating Omicron
Based on the clinical outcomes in England and South Africa, primary findings indicated that the efficacy of the vaccine against the variant of concern is reduced. As compared to previous VoC, the effectiveness of the vaccine against Omicron infection, hospitalisation, and symptomatic disease was comparatively much lower.
Researchers at Imperial College, London worked on comparing the risk associated with symptomatic infection of Omicron and Delta as detected in PCR. Different vaccines were scheduled, such as two doses of AstraZeneca-Vaxzervria or Pfizer BioNTech-Comirnaty with/without mRNA vaccine booster for international travellers, as well as patients with similar age, sex, locality, ethnicity, and day of infection, were studied. The above research analysis indicated vaccine efficacy between 0 and 20% after two doses and 55–80% after a booster dose. The analysis showed that the risk of infection is greater in the case of Omicron as compared to the Delta variant of concern [43].
The study included 130,867 test-negative control, 56,439 cases of Delta variant, and 581 symptomatic Omicron cases were assessed for understanding the efficacy of the vaccine. The estimated efficacy of the vaccine for Omicron was found to be 88% after 2–9 weeks and 63.5% after 25 weeks in subjects who received two doses of vaccine from Pfizer BioNTech-Comirnaty, and against Delta variant for a similar period. 15 weeks after the second dose of vaccine, the protective effect was negligible in those subjects who had received 2 doses of AstraZeneca-Vaxzevria against symptomatic infection with Omicron. Subjects who received AstraZeneca-Vaxzevria vaccine and Pfizer BioNTech-Comirnaty vaccine as a primary dose followed by Pfizer BioNTech-Comirnaty booster dose, reported the efficacy of the vaccine to be 71.4 and 75.55%, respectively [44].
A press release was posted by an insurance company named Discovery Health on primary research based on the efficacy of the vaccine against hospitalisation and infection. The efficacy of the vaccine by Pfizer BioNTech-Comirnaty against infection and hospitalisation was estimated as 33 and 70%, respectively. Details of methods and uncertainty about these data were not included [45].
Through the research and development network, WHO estimated the effect of vaccines with the support of data from cellular protection, antibody neutralisation activity, and animal model studies by management and coordination of live repository reagents that enhanced research analysis. For understanding the clinical efficacy of vaccines, regular and ideally enhanced surveillance, as well as epidemiological considerations, are required. Thus, to enhance research focussed on vaccine efficacy, the countries with reported and confirmed Omicron cases are encouraged to carry out studies regarding the effectiveness of the vaccine against severe infection and death.
Global health agencies update about omicron
On 26 November 2020, a variant of concern B.1.1.529 was brought into the picture by WHO based on guidance from the Technical Advisory Group on the Virus Evolution of WHO. This new strain was considered to be largely different from other strains and was associated with a large number of mutations, inclusive of 26–32 in S protein. Some of the mutations were concerned and linked with the escape from the defense system having a potential transmission rate. From the recent update of 16 December 2021, B.1.1.529 strain has been detected in more than 85 countries across all WHO regions.
Four major factors are directly associated with Omicron threat which are (1) transmission ability of strain, (2) effect of vaccines in the neutralisation of the strain, (3) infectivity range of strain, and, (4) public health and safety measures towards viral strain. Besides these, consistent and strong proof about Omicron having an extensive growth rate over delta strain is also seen. Omicron’s transmission rate is faster than delta strain with a doubling period of 36–72 h, posing high chances of Omicron overtaking delta strain in community transmission.
Presently, limited information is available on the clinical severity of Omicron and a broad spectrum is generally required to understand the severity profile and effect of host immunity and vaccination on severity. Hospital admission in South Africa and the United Kingdom tends to rise day by day due to a rapid increase in cases. There were possibilities for most healthcare systems to get overwhelmed quickly. In comparison to initial data, it showed that there was a decrease in neutralisation ability towards Omicron in the previously vaccinated population or patients with COVID-19 infection which showed humoral defense system evasion.
There was no peer-reviewed proof on vaccine efficiency against Omicron. Old data of vaccine effectiveness was extracted from South Africa and UK. This information required thorough evaluation with caution, as the blueprint and subject selection bias and the outcomes were collectively based on small numbers. Vaccination response from England showed a visible decrease in vaccine effectiveness towards Omicron as compared with fourth VoC after two doses of either AstraZeneca-Vaxzevria or Pfizer BioNTech-Comirnaty vaccines. Better effectiveness was observed after two weeks with a booster vaccine dose of Pfizer BioNTech-Comirnaty.
Risk assessment for Omicron must highlight related concerns with good indicative reasons. The first reason is the overall high risk of SARS-CoV-2 worldwide, second is the available data which is indicative of the Omicron’s growth rate that leads to its quick spread in population making more patients vulnerable to hospital admission. WHO’s understanding of risk assessment is still developing and will be updated as more data becomes available.
In a recent update, WHO had asked all the associated countries to make a daily check and revision of the national plans for Omicron management to minimise its spread. World Health Organisation had issued preventive guidelines worldwide and made it compulsory to wear an appropriate mask, maintain social distancing, avoid crowd attendance, and hand hygiene to minimise the COVID-19 spread among people. Public protection measures will be the master key to combat transmission along with a rapid diagnosis. Mass vaccination among the population should be the top-most priority to reduce the fatality rate and hospitalisation. Moreover, in a few nations, a booster dose of respective vaccine had also been started that has a magnificent impact on the safety of patients, a particular segment at high risk of death, crucial infection, and reinfection. All the nations were advised to report the all-initial cases linked with the fifth VoC to WHO by International Health Regulation (IHR) system. All official authorities should connect information with proof on Omicron and other mild strains along with appropriate guidance for the public in a periodically and cleanest manner by including all the aspects of known and unknown factors and the tasks being performed by dedicated authorities [45].
Future perspective
The very first case of Omicron strain was diagnosed in South Africa on 9 November 2021. The average number of Omicron cases per day rose from 280 to over 800 average cases per day. The major issue related to Omicron that still stays a matter of concern is to determine its infectivity in comparison with previous VoCs and the efficacy of previously formulated vaccines which to some extent might serve as the prime answer for its neutralisation. Since the clinical and immunological data are not adequate, it is therefore difficult to define its efficacy with evidence. The latest VoC had some deletion, e.g. 69-70del and more than 35 mutations at various positions like K417N, N501Y, P681H, T95I, etc., which are like alpha (α), beta (β), gamma (γ), and delta (δ) variants. Previous mutations of SARS-CoV-2 were known for higher binding affinity, enhanced transmissibility, and great antibody escape but effects of Omicron and differences are not yet known to a complete extent. On a thoughtful note, if the overlapping mutations of Omicron show similar effects, then it could be expected that high transmission could be encountered for mutation occurring near furin cleavage position. Increment in the COVID-19 cases in South Africa could be the best example. Omicron could become the dominant VoC if it shows a greater transmission rate. Front line clinicians in South Africa reported that the young population is trapped by Omicron, which is similar to delta strain profile, but there is no panicking concern raised due to mild symptoms of the disease.
Another issue is the immune escape behaviour of the virus in presence of vaccines and antibody neutralisation on vaccinated sera. Here, PCR testing might provide some effective clues. However, increment in the cases of reinfection can be related to mutation of Omicron in terms of immune escape. Moreover, reports on vaccines having great neutralisation power against the previous four VoCs must also be checked for Omicron through clinical trials. For example, ChAdOx1 vaccine predicted 70% neutralisation against D614G strain in the UK but the same was only 10% effective against beta (β) strain. Keeping this in mind that Omicron has several mutations and vaccines with broad-spectrum potential effects must be formulated since the effect of one might differ from the effect of the other based upon the intensity of infection. Some developed COVID-19 vaccines were effective in avoiding severe COVID-19 infection, hospital administration, and mortality for all previous VoCs and hence thought to follow a similar pattern for Omicron. Observation studies in Qatar and Kaiser were done to study the effects of vaccination against delta strain, and it was observed that the extent of immunity to avoid hospital admission was more than 90% even after 6 months of vaccine administration. Similarly, data obtained from the USA showed vaccine effect in the prevention of the infection severity in population above 65 years with good protection ability even after 6 months of vaccination.
Diagnosis of Omicron is widely done through PCR technique in South Africa. But there is no specific reason to accept as truth that present SARS-CoV-2 treatment and prevention protocol might also be similar in terms of activity against Omicron infection, except MABs.
Moreover, BA2 is a new sub-variant of Omicron (BA1) that accounts for the increase in the number of cases worldwide. According to WHO, this new subvariant has been found in around 57 countries and is responsible for about half of the Omicron cases globally. There is a sharp rise in the number of cases of new sub-variant. BA2 variant in Denmark, followed by India that replaced both Omicron and delta variant. As per the UK Health Security Agency (UKHSA) more than 1000 cases of BA2 variant have been confirmed. By British health authorities, the BA2 variant is designated as the ‘variant under investigation’. In Germany, infection with the BA2 variant is rapidly increasing as compared to the other COVID-19 variants. Denmark’s SSI conducted a study on 18,000 individuals amongst which 8000 of them showed BA2 variant to be highly transmissible as compared to the BA1 variant which is successful in evading vaccines. Unlike reports from the UK revealed that BA2 has higher transmissibility than BA1 but the primary findings suggest no evidence about the decreased effectiveness of vaccination against any of the variants. To date, no data shows that BA2 will cause more severe disease as compared to the older variants.
Most significantly, current public health measures like physical distancing, hand hygiene, mask-wearing, and avoidance of unnecessary travelling are thought to be effective against Omicron as these measures were effective in previous VoCs as well till we get a preparation with 100% efficacy.
Conclusion
Starting from Wuhan and reaching every corner of the world, SARS-CoV-2 has been demonstrated as the most devastating virus in the history of the pandemic. Despite extensive vaccination drive, social distancing, and enormous efforts to combat the pandemic, the healthcare system trembled extensively. The virus kept changing its dimension from being alpha, beta, gamma, and delta variants to the novel variant of concern (VoC) called Omicron. Natural immunity is the security system for the previous four VoC which in combination with current vaccine immunity posed a prime defense barrier. Delta variant, due to high infectivity, longer duration, high degree of reinfection along the ability to escape from the natural defense system made it the world’s most dominant COVID-19 strain. The major reason for the advent of Omicron could be the accretion of mutants in a closed circle of individuals followed by its proliferation to the larger section of the population that made it variant with a higher rate of mutation. If Omicron would have the same capacity of affecting health as the delta variant, then the new covid wave would have a more devastating impact that could drastically cost the life of humans. Previous VoCs had given the idea and importance of vaccination with social prevention measures that make a safe pathway to living a healthy life. Most significantly, current public health measures like physical distancing, hand hygiene, mask-wearing, avoiding unusual travelling, healthy diet will be effective against Omicron as these measures are effective in previous VoCs until the development of strong medications. Statements interpreted with caution can be made based on identified mutations that Omicron might spread swiftly and might escape the immune system better than previous VoCs aggravating the chance of reinfection. Moreover, cases of mild infection in the vaccinated population might still be present due to the presence of B.1.1.529. However, the vaccinated population is at lower risk of this deadly infection. These combinational safety approaches comprising of public health measures and vaccination will prove to be an effective strategy for healthy well-being.
Funding Statement
The authors acknowledge the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Govt. of India for supporting financially. The NIPER-R communication number for the review article is NIPER-R/Communication/278.
Disclosure statement
The authors declare no conflict of interest among themselves.
References
- 1.Forchette L, Sebastian W, Liu T.. A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr Med Sci. 2021;41(6):1037–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali I, Alharbi OML.. COVID-19: Disease, management, treatment, and social impact. Sci Total Environ. 2020;728:138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal M, Saraf S, Saraf S, et al. . In-line treatments and clinical initiatives to fight against COVID-19 outbreak. Respir Med. 2022;191:106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirbhate E, Pandey J, Patel VK, et al. . Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacol Rep. 2021;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mule S, Singh A, Greish K, et al. . Drug repurposing strategies and key challenges for COVID-19 management. J Drug Target. 2021;30(4):1–17. [DOI] [PubMed] [Google Scholar]
- 6.Gorain B, Choudhury H, Molugulu N, et al. . Fighting strategies against the novel coronavirus pandemic: impact on global economy. Front Public Heal. 2020;8:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevik M, Bamford CGG, Ho A.. COVID-19 pandemic–a focused review for clinicians. Clin Microbiol Infect. 2020;26(7):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lone SA, Ahmad A.. COVID-19 pandemic – an African perspective. Emerg Microbes Infect. 2020;9(1):1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim SS, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398(10317):2126–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YC, Chen CS, Chan YJ.. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83(3):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keni R, Alexander A, Nayak PG, et al. . COVID-19: emergence, spread, possible treatments, and global burden. Front Public Heal. 2020;8:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alanagreh L, Alzoughool F, Atoum M.. The human coronavirus disease covid-19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens. 2020;9(5):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou J, Xie X, Fontes-Garfias CR, et al. . The effect of SARS-CoV-2 D614G mutation on BNT162b2 vaccine-elicited neutralization. NPJ Vaccines. 2021;6(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korber B, Fischer WM, Gnanakaran S, et al. . Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 Virus. Cell. 2020;182(4):812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozono S, Zhang Y, Ode H, et al. . SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12(1):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plante JA, Liu Y, Liu J, et al. . Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Planas D, Bruel T, Grzelak L, et al. . Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27(5):917–924. [DOI] [PubMed] [Google Scholar]
- 18.Shen X, Tang H, McDanal C, et al. . SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–539.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supasa P, Zhou D, Dejnirattisai W, et al. . Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184(8):2201–2211.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, Dcosta BM, Samanovic MI, et al. B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv: the preprint server for biology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annavajhala MK, Mohri H, Wang P, et al. A Novel and Expanding SARS-CoV-2 Variant, B.1.526, Identified in New York. medRxiv: the preprint server for health sciences. 2021. [Google Scholar]
- 22.Rahul S, Aparnasai RG, Mayank H.. Convalescent plasma for treatment of COVID-19 infection. In: Kumar Anoop, editor. COVID-19 current challenges and future perspectives. Singapore: Bentham Science Publishers; 2021. [Google Scholar]
- 23.Greaney AJ, Loes AN, Crawford KHD, et al. . Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29(3):463–476.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzmina A, Khalaila Y, Voloshin O, et al. . SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29(4):522–528.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edara VV, Norwood C, Floyd K, et al. . Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe. 2021;29(4):516–521.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinde V, Bhikha S, Hoosain Z, et al. . Efficacy of NVX-CoV2373 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhi SA, Baillie V, Cutland CL, et al. . Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toovey OTR, Harvey KN, Bird PW, et al. . Introduction of Brazilian SARS-CoV-2 484K.V2 related variants into the UK. J Infect. 2021;82(5):e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Beltran WF, Lam EC, Denis K, et al. Circulating SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirotsu Y, Omata M. Discovery of SARS-CoV-2 strain of P.1 lineage harboring K417T/E484K/N501Y by whole genome sequencing in the city, Japan. medRxiv. 2021. [Google Scholar]
- 31.Cherian S, Potdar V, Jadhav S, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv. 2021:2021.04.22.440932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motozono C, Toyoda M, Zahradnik J, et al. An emerging SARS-CoV-2 mutant evading cellular immunity and increasing viral infectivity. bioRxiv. 2021:2021.04.02.438288. [Google Scholar]
- 33.Deng X, Garcia-Knight MA, Khalid MM, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv: the preprint server for health sciences. 2021. [Google Scholar]
- 34.Portelli S, Olshansky M, Rodrigues CHM, et al. . Exploring the structural distribution of genetic variation in SARS-CoV-2 with the COVID-3D online resource. Nat Genet. 2020;52(10):999–1001. [DOI] [PubMed] [Google Scholar]
- 35.Harvey WT, Carabelli AM, Jackson B, et al. . SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee AK, Ray U.. Mutation hot spots in spike protein of COVID-19 virus: Mutation in spike protein. Proc Indian Natl Sci Acad. 2020;86:1–10. [Google Scholar]
- 37.Dawood AA. Mutated COVID-19 may foretell a great risk for mankind in the future. New Microbes New Infect. 2020;35:100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benvenuto D, Angeletti S, Giovanetti M, et al. . Evolutionary analysis of SARS-CoV-2: how mutation of non-structural protein 6 (NSP6) could affect viral autophagy. J Infect. 2020;81(1):e24–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppée F, Lechien JR, Declèves A-E, et al. . Severe acute respiratory syndrome coronavirus 2: virus mutations in specific European populations. New Microbes New Infect. 2020;36:100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoury DS, Steain M, Triccas JA, et al. Analysis: A meta-analysis of early results to predict vaccine efficacy against Omicron. medRxiv. 2021. [Google Scholar]
- 41.National Institute for Communicable Diseases . South African COVID-19 weekly epidemiology brief. South Africa: National Institute for Communicable Diseases (NICD); 2020. [Google Scholar]
- 42.Assessment RR . Assessment of the further emergence and potential impact of the SARS-CoV-2 Omicron variant of concern in the context of ongoing transmission of the Delta variant of concern in the EU/EEA, 18th update Risk assessed; 2021. [Google Scholar]
- 43.England O, Ferguson N, Ghani A, et al. Report 49: growth, population distribution and immune escape of Omicron in England; 2021. p. 1–10. [Google Scholar]
- 44.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern; 2021. p. 1–16. [Google Scholar]
- 45.WHO . Brief and priority actions for member states. WHO Interim Guidelines. 2021;2021:1–8. [Google Scholar]
- 46.Kemp SA, Collier DA, Datir RP, et al. . SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starr TN, Greaney AJ, Addetia A, et al. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. bioRxiv Prepr Serv Biol. 2020:2020.11.30.405472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starr TN, Greaney Aj, Dingens AS, Bloom JD. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. bioRxiv: the preprint server for biology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen J, Baum A, Pascal KE, et al. . Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, VanBlargan LA, Bloyet L-M, et al. . Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29(3):477–488.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rani PR, Imran M, Lakshmi JV, et al. . Symptomatic reinfection of SARS-CoV-2 with spike protein variant N440K associated with immune escape. J Med Virol. 2021;93(7):4163–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisblum Y, Schmidt F, Zhang F, et al. . Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. Oct [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Zhou T, Zhang Y, et al. Antibodies with potent and broad neutralizing activity against antigenically diverse and highly transmissible SARS-CoV-2 variants. bioRxiv Prepr Serv Biol. 2021:2021.02.25.432969. [Google Scholar]
- 54.McCarthy KR, Rennick LJ, Nambulli S, et al. . Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science. 2021;371(6534):1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]