ABSTRACT
Introduction
Drug repurposing can be a successful approach to deal with the scarcity of cost-effective therapies in situations such as the COVID-19 pandemic. Tetracyclines have previously shown efficacy in preclinical acute respiratory distress syndrome (ARDS) models and initial predictions and experimental reports suggest a direct antiviral activity against SARS-CoV2. Furthermore, a few clinical reports indicate their potential in COVID-19 patients. In addition to the scarcity and limitations of the scientific evidence, the effectiveness of tetracyclines in experimental ARDS has been proven extensively, counteracting the overt inflammatory reaction and fibrosis sequelae due to a synergic combination of pharmacological activities.
Areas covered
This paper discusses the scientific evidence behind the application of tetracyclines for ARDS/COVID-19.
Expert Opinion
The benefits of their multi-target pharmacology and their safety profile overcome the limitations, such as antibiotic activity and low commercial interest. Immunomodulatory tetracyclines and novel chemically modified non-antibiotic tetracyclines have therapeutic potential. Further drug repurposing studies in ARDS and severe COVID-19 are necessary.
KEYWORDS: Tetracyclines, COVID-19, ARDS, acute respiratory distress syndrome, immunomodulatory, repurposing, incyclinide, minocycline, doxycycline
1. Introduction
The COronaVirus Infectious Disease 2019 (COVID-19) pandemic has positioned vaccines at the top of the therapeutic arsenal. ‘Born’ to help us fight infections, their effectiveness has been demonstrated in the context of a multitude of infectious diseases, and there is no doubt of their key role in our current fight against COVID-19. However, even more remarkable is their adaptation and development to face new therapeutic challenges such as fighting cancer by targeting neoantigens, an innovative field in constant progression. This is a strikingly similar story to that of another versatile therapeutic family, the tetracycline antibiotics. With the development of second-generation tetracyclines (i.e. doxycycline and minocycline) followed the discovery of non-antibiotic properties, such as inhibition of matrix metalloproteinases (MMPs), antioxidant, immunomodulatory, and antiproliferative and antiapoptotic activities [1,2] (Figure 1). This motivated their evaluation in many noninfectious conditions with promising results, such as cancer, neurological disorders, and complex inflammatory conditions, including ARDS and tissue injury [3]. Unfortunately, clinical translation of immunomodulatory benefits has only been achieved in conditions with confirmed or suspected infectious components, such as periodontitis and rosacea. Later, non-antibiotic tetracycline analogs (Chemically Modified Tetracyclines or CMTs) were developed to explore new therapeutic indications. Based on this pharmacological potential, numerous reports have explored the possibility of repurposing tetracyclines for COVID-19 treatment.
Figure 1.
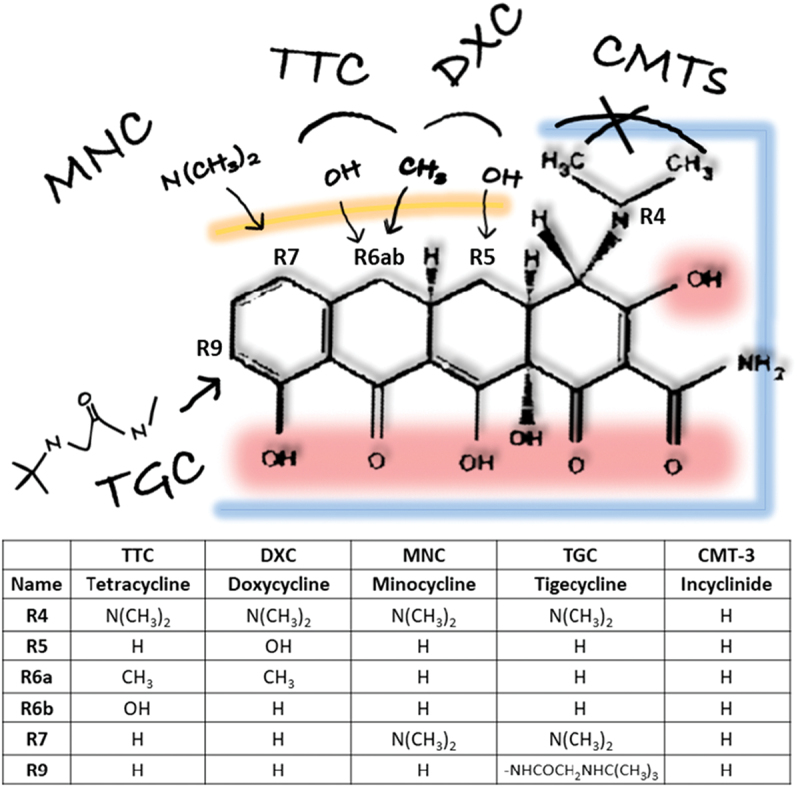
Structure–Activity Relationships of tetracycline analogs. Variations of the minimum pharmacophore with antibiotic activity (6-deoxy-6-demethyltetracycline) lead to different tetracyclines, such as 1st generation Tetracycline, 2nd (Minocycline and Doxycycline) and 3rd (Tigecycline) generations, or chemically modified tetracyclines without antibiotic activity (such as Incyclinide or CMT-3). Highlighted the areas that contribute to different activities: blue (antibiotic), red (O groups involved in antioxidant and metal chelation properties, important for MMP inhibition), and yellow (upper ring substitutions for improved pharmacokinetic profile). Detailed Structure-Activity description is reviewed elsewhere [2].
As of January 2022, COVID-19 incidence worldwide has reached the highest peak since the beginning of the pandemic, with 21 million new cases. According to WHO epidemiological update (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports), the cumulative number of cases is about to reach 350 million and the number of global reported deaths exceeds 5.5 million. Despite the success of vaccination programs in developed countries, infections are rising again in northern countries with the emergence of novel variants and the convergence with seasonal respiratory infections. In developing countries, the impact has been devastated. Severe COVID-19 cases require hospital care due to viral pneumonia progressing into ARDS [4], causing difficulty in breathing and low blood oxygen levels. In addition to direct respiratory failure (accounting for 70% of fatal COVID-19 cases), some may succumb to secondary bacterial and fungal infections. Furthermore, an aggressive inflammatory response (the ‘cytokine storm’) is strongly implicated in airway and multi-organ damage and permanent sequelae.
Histologically, lung pathological features develop from an initial acute exudative phase with proteinaceous edema, hyaline membranes, and mononuclear inflammation. From this stage, microvascular thrombosis and distinctive syncytia cells have been observed [5]. The later are large multinucleated cells originated from epithelial cells due to the fusogenic activity of the SARS-CoV2 protein. Next, a proliferative phase is characterized by type II pneumocyte hyperplasia, tissue remodeling, and septal fibrosis (organizing pneumonia). These histopathological findings, defined as Diffuse Alveolar Damage (DAD) (clock-wise depiction in Figure 2), are common to various viral infections and some non-viral pneumonia. Progression of DAD histological changes results in reduced tissue elasticity and alveolar space available for oxygenation (reduced lung compliance and hypoxemia, the defining clinical features of ARDS). Progression of DAD/ARDS leads to permanent fibrotic sequelae, secondary bacterial infection, septic shock, and respiratory and circulatory failures [6]
Figure 2.
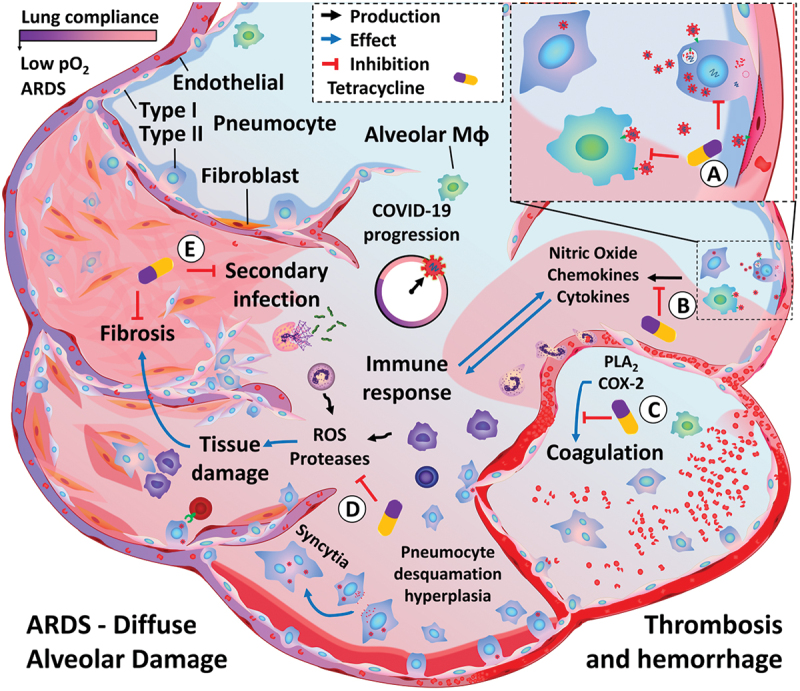
ARDS/COVID-19 progression and proposed pharmacological actions of immunomodulatory tetracyclines. Upon initial infection, SARS-CoV2 spreads to the lower respiratory tract, infecting type II pneumocytes as well as other cell populations, such as alveolar macrophages and the endothelium. Viral infection and its cytopathic effects lead to an exacerbated immune activation, which may result in increased tissue damage and fibrosis. Alterations in lung tissue architecture (defined as Diffuse Alveolar Damage) progress into ARDS, characterized by the reduction in lung compliance and impaired blood oxygenation. Additionally, complications such as thrombosis and secondary infections are frequently observed. Tetracyclines can target this pathological process at multiple levels: A) Reduce viral entry and replication. B) Reduce immune recruitment and activation. C) Inhibition of exacerbated inflammatory reaction via immunomodulatory effects and inhibition of oxidative stress and MMP, thus reducing tissue damage and fibrosis. D) Inhibition of enzymes involved in platelet function and coagulation. E) Inhibition of altered epithelial response. F) Prevention of secondary bacterial infection.
2. Current evidence
2.1. Anti-SARS-CoV2 activity
Drug repurposing can be a successful approach to accelerate the development of cost-effective therapies. This strategy is generally initiated by bioinformatic analysis to prioritize drug repurposing candidates. Based on reported activities and molecular docking with SARS-CoV2 proteins, potential antiviral activity has been predicted for several tetracycline analogs [7]. Docking studies propose binding of doxycycline to the human Adaptor-Associated Kinase 1 (AAK1) and the viral ADP-ribose phosphatase (ADPRP) [8], involved in viral endocytosis and replication, respectively [9]. It has also been proposed that tetracycline and doxycycline could act as inhibitors of ACE2-spike binding [10,11], and doxycycline and minocycline as inhibitors of the SARS-CoV-2 main protease (Mpro) [12].
In silico analysis should always be considered with caution. In fact, ambiguous experimental evidence of tetracycline’s direct antiviral effect against SARS-CoV2 has also been reported (summarized in Table 1). Doxycycline has been shown to reduce viral entry and replication in Vero E6 cells infected with SARS-CoV-2 with an IC50 of 4.5 ± 2.9 µM [13], which is compatible with the drug bioavailability profile. Whilst this value is one log10 magnitude higher than ACE2-RBD/S1 binding (offering a weak direct competition), it is in the same IC50 range (1–10 µM) calculated for inhibitor peptides derived from the ACE2 binding sequence [14]. On the contrary, another study reported an inhibitory effect for a pseudotyped virus, but not SARS-CoV2 [15]. These studies would need to be replicated in parallel using the same experimental systems or, even more relevant, on in vivo models. One of the interesting properties of tetracyclines is their amphiphilic nature, which enables them to cross biological barriers and achieve good tissue distribution. However, other amphiphilic drugs have also shown similar inhibition of SARS-CoV2 replication in vitro, which was found to be mediated by the induction of phospholipidosis, an effect that would not be sustained in vivo [16].
Table 1.
Summary of preclinical research findings
| STUDY | CONDITION | TREATMENT | REGIME/DOSE | OUTCOME | REF |
|---|---|---|---|---|---|
| In vitro (Human) | SARS-CoV-2 (IHUMI-3) infection (Vero E6) | Doxycycline | EC50 = 4.5 ± 2.9 µM | Inhibit entry and replication | [12] |
| In vitro (Human) | SARS-CoV-2 or pseudotyped virus (Vero E6 & HEK-293 T) | Doxycycline | Up to 100 µM | Inhibit pseudotyped virus, not SARS-CoV2 | [14] |
| In vitro (Human) | Epithelial (A549) reactivity | Doxycycline | Up to 30 µg/mL | Inhibit MCP-1 production (95%) and monocyte chemotaxis (55%) | [17] |
| In Vitro (Mouse) | Epithelial (LA4) reactivity | Doxycycline | Up to 30 µg/mL | Inhibit NO production (90%) | [26] |
| In Vivo (Rat) | Lung Inflammation (LPS) | CMT-3 | Preventive and therapeutic (20 mg/kg) | Inhibit inflammation, Goblet cell metaplasia and EGFR and MMP-9 expression | [42] |
| In Vivo (Mouse) | Lung Inflammation (LPS) | Doxycycline | Preventive (20 mg/kg) | Inhibit PMN inflammation | [27] |
| Ex Vivo (Horse & Human) | COPD Epithelial Lining Fluid | CMT-3 | IC50 = 20–90 μM | Inhibition of gelatinolytic activity | [29] |
| In Vivo (Mouse) | Bone Marrow Derived Macrophages | Tetracycline | Up to 30 µg/mL | Anti-inflammatory (via Casp-1-dependent IL-1β and IL-18 production) | [30] |
| In Vivo (Mouse) | ARDS (LPS/influenza A virus) | 75 mg/kg | |||
| Ex Vivo (Human) | ARDS-BALF leukocytes | Up to 30 µg/mL | |||
| In Vivo (Rat) | Lung Injury & Sepsis (Cecal Ligation and Puncture) | CMT-3 | Therapeutic (30 mg/kg) | Reduced mortality (54–33%) and pathology | [32] |
| In Vivo (Pig) | ARDS and Sepsis | CMT-3 | Preventive (200 mg/kg) | Complete prevention of septic shock and ARDS | [33] |
| In Vivo (Pig) | Lung Injury (Cardiopulmonary Bypass) | CMT-3 | Therapeutic (25 µmol/L in blood) | Prevention of Acute Lung Injury | [34] |
| In Vivo (Pig) | Lung Injury (Cardiopulmonary Bypass) | CMT-3 | Therapeutic | Inhibition of PMN recruitment, but not mononuclear infiltration | [35] |
| In Vivo (Rat) | Lung Injury & Sepsis (Cecal Ligation and Puncture) | CMT-3 | Therapeutic (30 mg/kg) | Reduced mortality and lung pathology | [36] |
| In Vivo (Porcine) | ARDS (Sepsis + Ischemia/Reperfusion) | CMT-3 | Preventive (200 mg/kg) | Prevented ARDS, coagulopathy & bowel injury | [37] |
| In Vivo (Porcine) | ARDS & Sepsis (Ischemia/Reperfusion) | CMT-3 | Therapeutic (200 mg/kg) | Pleiotropic interruption of inflammation | [38] |
| In Vivo (Rat) | Lung Injury (Mechanical Ventilation) | CMT-3 | Preventive (20 mg/kg) | Reduced of neutrophil-mediated inflammation | [39] |
| In Vivo (Sheep) | ARDS (burn + smoke inh. + barotrauma injury) | CMT-3 | Preventive (200 mg/m2) | Delayed ARDS development and prolonged survival | [40] |
| In Vivo (Rat) | Lung Injury (Transplantation) | CMT-3 | Therapeutic (30 mg/kg) | Anti-inflammatory and anti-fibrotic | [41] |
CMT-3 = Chemically Modified Tetracycline 3 (Incyclinide)
2.2. Anti-viral activity
It may not be enough time since the emergence of SARS-CoV2 to gather strong preclinical support for anti-SARS-CoV2 specific activity (Figure 2(a)), particularly for old drugs with low market interest. However, tetracyclines’ antiviral effects have been previously described for RNA viruses (Figure 2(b)), such as HIV [18], Dengue virus [19], Japanese encephalitis virus [20,21], and others. It has been suggested that tetracyclines could interact and stabilize dsRNA [22], which are involved in viral replication and activate host defense mechanisms [23], as observed with minocycline in HIV infection [24]. Tetracyclines could also attain antiviral activity indirectly. Several viral functions are associated with the host MMPs and may be susceptible to tetracyclines’ MMP inhibitory activity. Similarly, viruses exploit the mitochondrial machinery and aerobic glycolysis of infected cells. Therefore, the impact of tetracyclines on mitochondrial dynamics, mainly due to calcium buffering, could interfere with this process and contribute to their therapeutic benefit, as seen in other pathological contexts [25].
2.3. Antiproteolytic, antioxidant, and immunomodulatory activities
Tetracycline’s immunomodulatory and anti-inflammatory properties may be of greater relevance for protection against COVID-19 severe pathology than their potential antiviral activity (Figure 2(b-d)). The lung is particularly susceptible to the outcome of widespread inflammation, sequestering activated neutrophils and monocytes into the lung parenchyma. In this regard, doxycycline has been shown to reduce nitric oxide and chemokine production by lung epithelial cells [26,27], and to reduce neutrophil chemotaxis in vivo, into the alveolar lung space [28] (Figure 2(b)). Doxycycline and CMT-3 are effective in reducing the proteolytic activity derived from neutrophilic inflammation in Chronic Obstructive Pulmonary Disease (COPD) [29,30], which can prevent fibrosis sequalae in ARDS survivors (Figure 2(c)). This anti-inflammatory activity has been recently manifested for Tetracycline as well, counteracting inflammasome signaling via inhibition of caspase-1-induced IL-1β and IL-18 release, in both mouse and human leukocytes from bronchoalveolar fluid from ARDS patients [31].
Given the interest of tetracycline’s activity for lung protection (Table 1), the application of CMTs to ARDS is not completely novel [32]. Prophylactic CMT-3 has been shown to prevent the development of ARDS in models induced by sepsis [33,34] and cardiopulmonary bypass [35,36]. Therapeutic benefit has also been achieved with CMT-3 in lung injury upon established inflammation/septic events [37–39] as well as in models of ventilator-induced lung injury [40], smoke and burn injury [41] and transplantation [42]. In these contexts, CMT-3 treatment was associated with a reduction in inflammation, collagen deposition, and the histological lesions of ARDS [38]. Mechanistically, their effects could derive from the reduction in neutrophil transmigration and neutrophil-mediated inflammation, including direct inhibition of elastases, MMPs, and radical oxygen species. These radicals and enzymes, produced by the immune system during the inflammatory reaction, damage the alveolar-capillary basement membranes and the extracellular matrix and can exacerbate the preexisting pathological condition (Figure 2(c)). CMT-3 has also been shown to prevent coagulopathy associated with ARDS, an important pathological feature of COVID-19 [6]. This effect could derive from its inhibitory effects in PLA2 and COX-2, essential for platelet and endothelial functions [38] (Figure 2(d)).
2.4. Antibacterial activity and other considerations
In addition to direct immunomodulatory effects, tetracyclines can also impact altered responses of the stromal compartment (Figure 2(e)). It has been described that lung Goblet cell metaplasia and mucus hypersecretion triggered by epidermal growth factor receptor can be prevented with CMTs [43]. More specifically, a recent study has shown that CMT-3 is a great candidate to reverse the altered gene expression pattern of lung cells caused by ACE2 inhibition [44]. The authors proposed that this alteration, derived from ACE2-mediated viral entry, could contribute to lung pathology in COVID-19 and be susceptible to CMT-3 treatment. The synergic combination of the activities described above together with the lack of antibacterial activity for the novel CMTs could certainly facilitate their clinical application. On the contrary, secondary bacterial pneumonia is a frequent complication of COVID-19, involved in approximately 30% deaths. Tetracyclines have been shown to be effective and particularly useful in pneumonia and infections caused by hospital-acquired multi-resistant bacteria. Thus, rather than a limitation, their antibiotic activity could play an important role in this setting (Figure 2(f)).
Finally, for a complete understanding of the mechanisms behind the effects observed for tetracyclines in ARDS, it is also worth mentioning their ability to concentrate at sites of inflammation and tissue injury [45]. This is explained by the increased tetracycline uptake observed with increasing temperature, as well as in specific cell types, such as neutrophils and alveolar macrophages. Whilst neutrophils may play a secondary role in SARS-CoV2 pathology in the absence of bacterial co-infection, alveolar macrophages play a central role in the overt immune response. Tigecycline has been found up to 78-times more concentrated in alveolar macrophages than in blood [46]. This contributes to potentiate tetracycline’s pharmacological effects at the site of inflammation while reducing off-site effects.
3. Clinical evidence
Till date, a few studies have been reported positive results (summarized in Table 2). These have been carried out mostly in mild disease by following high-risk patients. Rapid clinical improvement was reported in four high-risk COVID-19 patients after doxycycline treatment [47]. A multicenter prospective observational study including 38 COVID-19 patients treated with tetracyclines reported a remarkable resolution of mild symptoms within the first week of treatment [48]. In a larger study, early treatment with doxycycline in 89 high-risk COVID-19 patients was associated with early clinical recovery, decreased hospitalization, and decreased mortality [49]. Doxycycline has been used for COVID-19 treatment in combination with hydroxychloroquine or lopinavir, reporting an overall 4.2% fatality rate vs 27% and 23% for monotherapy, respectively [50]. In another two studies, in combination with Ivermectin, authors reported that doxycycline helped to reduce disease progression, the time to recovery and mortality in patients with COVID-19 [51,52].
Table 2.
Summary of human studies
| STUDY | CONDITION | TREATMENT | REGIME/DOSE | OUTCOME | REF |
|---|---|---|---|---|---|
| Case-report | 4 high-risk COVID-19+ symptomatic patients with comorbid pulmonary disease | Doxycycline | Therapeutic | Rapid improvement upon treatment | [46] |
| Observational | Dermatology patients (COVID+, symptomatic) (n = 38) *No control group | Doxycycline or Minocycline | On treatment (5–200 mg/day) | Rapid symptomatic resolution, dose response | [47] |
| Retrospective | High-Risk COVID-19+ Patients (modere-severe symptoms) in Long-Term Care Facilities (n = 89). *No control group | Doxycycline | Therapeutic (100 mg/day) | 85% clinical recovery (vs 43% in another study) | [48] |
| Retrospective | 475 COVID-19 + Patients at Emergency Hospital Admission *No control group | Doxycycline + Lopinavir 400 mg or HCQ 200 mg | Therapeutic (100 mg/day) | overall case fatality rate was 4.2% | [49] |
| Randomized controlled | 140 COVID-19+ Patients (moderate-severe symptoms). Treatment+SC vs SC alone. | Doxycycline + Ivermectin 200 µg/kg | Therapeutic (100 mg/day) | Reduced time to recovery and progression to more severe disease | [50] |
| Randomized controlled trial | 400 COVID-19 symptomatic patients (mild-to-moderate). Treatment+SC vs SC alone. | Doxycycline + Ivermectin 24 mg | Therapeutic (200 mg/day) | Reduced time to recovery and progression to more severe disease | [51] |
| Randomized controlled trial | 1792 Suspected/PCR+ COVID-19. Treatment+SC vs SC alone. | Doxycycline | Therapeutic (100 mg/day) | Little benefit in self-reported recovery | [52] |
SC = Standard Care; HCQ = hydroxychloroquine
These studies have several limitations that should be considered, such as the lack of double-blinded controls, presence of comorbidities and other treatments and patients exhibiting mild symptoms instead of severe COVID-19. Thus, without proper controls, it is unclear whether their improvement is due to a beneficial pharmacological effect or the natural course of the disease. However, these limitations in their design and power to draw conclusions only stress the need for better-controlled studies. Such is the case of PRINCIPLE, a randomized, controlled, open-label trial evaluating the effects of doxycycline treatment, among others, in high-risk patients with suspected COVID-19 in the community in the UK [53]. Recently published, the study concludes that there is little evidence to support the use of doxycycline as a routine treatment for COVID-19.
4. Conclusion
In conclusion, we believe that tetracyclines offer an effective and safe repurposing strategy for severe COVID-19/ARDS. Whilst routine treatment for mild symptoms has no advantage and scientific support, their unique combination of pharmacological activities is of great interest for preventing ARDS-fibrotic sequelae. In addition, tetracyclines are safe, well known, and economically accessible, with doxycycline among the WHO essential medicines. This positions them as excellent repurposing candidates, particularly for cases arising in developing countries where successful vaccination programs have yet to be established and access to other therapeutic resources is scarce.
5. Expert opinion
The findings reported do not provide clear scientific evidence of a therapeutic effect for tetracyclines against mild SARS-CoV2 infection. However, this conclusion is only fair when we analyze the details. In particular, generating more accurate data for an old family of antibiotic drugs is quite challenging in such a short time and adverse conditions, making it impossible to overcome the methodological and regulatory problems with such a low economic interest and support. One of the biggest limitations for therapeutic developments with tetracyclines is the presence of antibiotic activity and the risk of bacterial resistance. Access to the protected non-antibiotic CMTs would help to overcome these issues and pave the way for novel indications. Unfortunately, our and other colleagues’ experience trying to access protected CMT compounds has proved quite challenging over the past years, setting a hard limitation in that direction. Additionally, we believe that clinical research is still particularly reluctant to the multi-target pharmacology exhibited by tetracyclines. These are common limitations for all novel indications, but we hope that, over time, more conclusive studies will be performed and encourage a change of this paradigm.
A key question regarding a direct anti-SARS-CoV2 activity of tetracyclines is how relevant would be that activity for their application to COVID-19. Mild disease treatments offer little benefit compared to vaccination programs. Tetracyclines may well be of no use for mild symptoms, but we believe that their evaluation in hospital settings to treat established ARDS warrants further investigation. The key developments and mechanisms described above in different preclinical models can be directly translated to COVID-19-induced ARDS but, so far, clinical studies have evaluated the very scenario facing mild and moderate symptoms. At the time of hospital intervention, the ARDS condition is over-exposed to viral infection, and many patients present low viral load. Therefore, the relevance of anti-SARS-CoV2 activity for the treatment of severe COVID-19 (ARDS) may not be essential in comparison to the many other pharmacological activities targeting ARDS consequences.
In our experience, the optimal pharmacological effect of tetracyclines is achieved in severe conditions, with an early impact on the pathology rather than long-term benefit. In ARDS, the synergic combination of anti-proteolytic, antioxidant, and immunomodulatory activities would add to the well-known antibiotic protection from secondary bacterial pneumonia. As mentioned above, ‘multi-target’ and ‘antibiotic’ are qualities that seem to be holding back the expansion of their clinical application. For too long, pharmacological strategies have aimed for ‘golden bullets’ to treat complex conditions, an attempt to avoid off-site effects that say very little about understanding how biological systems work and regulate. In this scenario, safe and well-known tetracyclines, such as minocycline and doxycycline, offer limited economic interest. The pharmaceutical industry is not likely to get on board with drug repurposing strategies involving old tetracycline antibiotics, nor had the governments the ability to support and explore new therapies, stretching all available resources in other directions.
Therefore, how is the future for pharmacological research with tetracyclines? The novel patented CMTs are attracting the interest of the pharmaceutical industry again. CMTxBiotech has licensed Incyclinide (CMT-3, a minocycline derivate) and proposed its evaluation in severe COVID-19, ARDS and sepsis (https://cmtxbiotech.com/newsroom). May this approach prove effective, it will pave the way for other indications [3] and reactivate preclinical research in the coming years, opening new avenues and answering many of the old questions.
In our opinion, characterizing their peculiar immunomodulatory actions remains as one of the most important questions and where exciting novel research is headed. Their effect in ARDS focusses on their inhibition of neutrophilic inflammation, but COVID-19 is characterized by mononuclear lung inflammation. Tetracyclines also display immunomodulatory actions in T cells and monocytes/macrophages [1]. However, in contrast to their immunosuppressive effect observed in peritoneal macrophages, it has been described that tetracyclines could potentiate the response of alveolar macrophages [54]. A certain degree of macrophage activation has been previously appreciated in vitro [55], but detailed analysis taking into account the tissue environment is required in order to explain their protective effect. We have recently observed a similar modulation in intestinal inflammation: minocycline enhances macrophage recruitment and response at the same time that accelerates their differentiation into the homeostatic macrophage phenotype and improves mucosal healing [56]. This effect is shared by doxycycline and tetracycline and is independent of the features of the disease model [57], which makes us think it represents a primary mechanism rather than a secondary effect. Since both intestinal and alveolar macrophages reside at mucosal sites and share this particular response to immunomodulatory tetracyclines, we believe that a similar protective outcome could contribute to the benefit reported in preclinical ARDS models. Considering their central role in homeostasis and disease by reprogramming tissue environment, this controversial and location-dependent effect of tetracyclines on macrophages is an exciting area of research and potential application. Progress in this direction will not only improve our understanding of tetracycline’s mechanism of action but also our biased view of the required immunomodulatory actions in order to achieve therapeutic benefit, which not always requires immunosuppression.
Article highlights
COVID-19 has a devastating impact on developing countries due to the scarcity of resources. Drug repurposing strategies are key to meeting the demand for safe and cost-effective therapies.
Tetracyclines are effectively used in bacterial pneumonia, a frequent secondary complication of COVID-19, and display activity against RNA viruses.
Specific SARS-CoV2 anti-viral activity has been proposed for tetracyclines by bioinformatic analysis and in vitro studies. Accelerated recovery has been reported in COVID-19 patients receiving tetracycline, alone or in combination therapy.
Immunomodulatory tetracyclines and non-antibiotic tetracyclines display a wide range of pharmacological activities of interest for the treatment of inflammatory conditions, such as acute respiratory distress syndrome (ARDS).
These agents protect against inflammatory associated tissue damage by ameliorating the inflammatory reaction as well as direct inhibition of matrix metalloproteinases and oxidative stress.
Repurposing tetracyclines for ARDS treatment is well supported by preclinical data and could be very beneficial for the management of severe COVID-19 and ARDS of different etiologies.
This box summarizes key points contained in the article.
Contributions
GMJ – Conceptualization, Literature Search, Visualization, Writing – Original Draft Preparation
AKE – Literature Search, Writing – Original Draft Preparation
GJ – Project Administration, Writing – Review & Editing.
GMN – Project Administration, Supervision, Writing – Review & Editing
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Garrido-Mesa N, Zarzuelo A, Gálvez J.. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2013;67(1):18–30. [DOI] [PubMed] [Google Scholar]
- 2.Fuoco D. Classification framework and chemical biology of tetracycline-structure-based drugs. Antibiot Basel Switz. 2012;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. Internet]. 2020. cited 2021 Mar 25;395:507–513. Available from;10223: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30211-7/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussani R, Schneider E, Zentilin L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. Internet]. 2020. cited 2021 Feb 22;10:766–788. Available from;5: https://www.sciencedirect.com/science/article/pii/S2211383520302999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed AM, Khalaf AM, Abdelrahim MEA, et al. Repurposing of some anti-infective drugs for COVID-19 treatment: a surveillance study supported by an in silico investigation. Int J Clin Pract. 2020;75(4):e13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putics Á, Filipowicz W, Hall J, et al. ADP-Ribose-1”-monophosphatase: a conserved coronavirus enzyme that is dispensable for viral replication in tissue culture. J Virol. Internet]. 2005. cited 2021 Jul 4;79:12721–12731. Available from;20: https://journals.asm.org/doi/full/10.1128/JVI.79.20.12721-12731.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdeva C, Wadhwa A, Kumari A, et al. In silicoPotential of approved antimalarial drugs for repurposing against COVID-19. Omics J Integr Biol. 2020;24(10):568–580. [DOI] [PubMed] [Google Scholar]
- 11.Zhao TY, Patankar NA. Tetracycline as an inhibitor to the coronavirus SARS-CoV-2. J Cell Biochem [Internet]. 2021. [cited 2021 Mar 15];jcb.29909. Available from: http://arxiv.org/abs/2008.06034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharadwaj S, Lee KE, Dwivedi VD, et al. Computational insights into tetracyclines as inhibitors against SARS-CoV-2 Mpro via combinatorial molecular simulation calculations. Life Sci. 2020;257:118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gendrot M, Andreani J, Jardot P, et al. In vitro antiviral activity of doxycycline against SARS-CoV-2. 2020;25(21):5064. 10.3390/molecules25215064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Petitjean SJL, Koehler M, et al. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. Internet]. 2020. cited 2021 Oct 22;11:4541. Available from;1: https://www.nature.com/articles/s41467-020-18319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diomede L, Baroni S, De Luigi A, et al. Doxycycline inhibition of a pseudotyped virus transduction does not translate to inhibition of SARS-CoV-2 infectivity. Viruses. 2021;13(9):1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tummino TA, Rezelj VV, Fischer B, et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science. Internet]. 2021. cited 2021 Oct 20;373:541–547. Available from;6554: https://www.science.org/doi/full/10.1126/science.abi4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szolnoky G. Further aspects of doxycycline therapy in COVID-19. Dermatol Ther [Internet]. 2020. cited 2021 Feb 22;33:e13810. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dth.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293(16):2003–2011. [DOI] [PubMed] [Google Scholar]
- 19.Rothan HA, Mohamed Z, Paydar M, et al. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014;159(4):711–718. [DOI] [PubMed] [Google Scholar]
- 20.Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. Internet]. 2008. cited 2021 Mar 15;105:1582–1595. Available from.;(5):. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1471-4159.2008.05238.x [DOI] [PubMed] [Google Scholar]
- 21.Topno R, Khan SA, Chowdhury P, et al. Pharmacodynamics of aminoglycosides and tetracycline derivatives against Japanese encephalitis virus. Asian Pac J Trop Med. 2016;9(3):241–246. [DOI] [PubMed] [Google Scholar]
- 22.Dutta K, Basu A. Use of minocycline in viral infections. Indian J Med Res. 2011;133:467–470. [PMC free article] [PubMed] [Google Scholar]
- 23.Ding S-W, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130(3):413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szeto GL, Brice AK, Yang H-C, et al. Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4 + T cells. J Infect Dis. 2010;201(8):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Martinez EM, Sanz-Blasco S, Karachitos A, et al. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem Pharmacol. 2010;79(2):239–250. [DOI] [PubMed] [Google Scholar]
- 26.Raza M, Ballering JG, Hayden JM, et al. Doxycycline decreases monocyte chemoattractant protein-1 in human lung epithelial cells. Exp Lung Res. 2006;32(1–2):15–26. [DOI] [PubMed] [Google Scholar]
- 27.Hoyt JC, Ballering J, Numanami H, et al. Doxycycline modulates nitric oxide production in murine lung epithelial cells. J Immunol Baltim Md. 2006;176:567–572. [DOI] [PubMed] [Google Scholar]
- 28.Moon A, Gil S, Gill SE, et al. Doxycycline impairs neutrophil migration to the airspaces of the lung in mice exposed to intratracheal lipopolysaccharide. J Inflamm Lond Engl. 2012;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.. Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018; 10.CD009764 10.1002/14651858.CD009764.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maisi P, Kiili M, Raulo SM, et al. MMP inhibition by chemically modified tetracycline-3 (CMT-3) in equine pulmonary epithelial lining fluid. Ann N Y Acad Sci. Internet]. 1999. cited 2021 Mar 15;878:675–677. Available from;1 INHIBITION OF: https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1999.tb07759.x [DOI] [PubMed] [Google Scholar]
- 31.Peukert K, Fox M, Schulz S, et al. Inhibition of caspase-1 with tetracycline ameliorates acute lung injury. Am J Respir Crit Care Med. 2021;204(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy SK, Kendrick D, Sadowitz BD, et al. Jack of all trades: pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS). Pharmacol Res. Internet]. 2011. cited 2021 Mar 15;64:580–589. Available from;6: https://www.sciencedirect.com/science/article/pii/S1043661811001897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg J, Halter J, Schiller HJ, et al. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. Internet]. 2003. cited 2021 Mar 15;111:185–195. Available from;2: https://www.sciencedirect.com/science/article/pii/S0022480403000891 [DOI] [PubMed] [Google Scholar]
- 34.Steinberg J, Halter J, Schiller H, et al. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock Augusta Ga. 2005;24(4):348–356. [DOI] [PubMed] [Google Scholar]
- 35.Carney David E, Lutz Charles J, Picone Anthony L, et al. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation. Internet]. 1999. cited 2021 Mar 15;100:400–406. Available from;4: https://www.ahajournals.org/doi/10.1161/01.CIR.100.4.400 [DOI] [PubMed] [Google Scholar]
- 36.McCann UG, Gatto LA, Searles B, et al. Matrix metalloproteinase inhibitor: differential effects on pulmonary neutrophil and monocyte sequestration following cardiopulmonary bypass. J Extra Corpor Technol. 1999;31(2):67–75. [PubMed] [Google Scholar]
- 37.Halter JM, Pavone LA, Steinberg JM, et al. Chemically modified tetracycline (COL-3) improves survival if given 12 but not 24 hours after cecal ligation and puncture. Shock Augusta Ga. 2006;26(6):587–591. [DOI] [PubMed] [Google Scholar]
- 38.Roy SK, Kubiak BD, Albert SP, et al. Chemically modified tetracycline 3 prevents acute respiratory distress syndrome in a porcine model of sepsis + ischemia/reperfusion–induced lung injury. Shock. Internet]. 2012. cited 2021 Mar 15;37:424–432. Available from;4: https://journals.lww.com/shockjournal/Fulltext/2012/04000/Chemically_Modified_Tetracycline_3_Prevents_Acute.13.aspx [DOI] [PubMed] [Google Scholar]
- 39.Sadowsky D, Nieman G, Barclay D, et al. Impact of chemically-modified tetracycline 3 on intertwined physiological, biochemical, and inflammatory networks in porcine sepsis/ARDS. Int J Burns Trauma Internet]. 2015. cited 2021 Mar 15;5:22–35. Available from.;:. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4448085/ [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Suk MH, Yoon DW, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol-Lung Cell Mol Physiol. Internet]. 2006. cited 2021 Mar 15;291:L580–L587. Available from;4: https://journals.physiology.org/doi/full/10.1152/ajplung.00270.2005 [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Wang D, Ballard-Croft CK, et al. A tetracycline analog improves acute respiratory distress syndrome survival in an ovine model. Ann Thorac Surg. Internet]. 2010. cited 2021 Mar 15;90:419–426. Available from;2: https://www.sciencedirect.com/science/article/pii/S0003497510009185 [DOI] [PubMed] [Google Scholar]
- 42.Yoshida S, Iwata T, Chiyo M, et al. Metalloproteinase inhibition has differential effects on alloimmunity, autoimmunity, and histopathology in the transplanted lung. Transplantation Internet]. 2007. cited 2021 Mar 15;83:799–808. Available from.;:. https://journals.lww.com/transplantjournal/Fulltext/2007/03270/Metalloproteinase_Inhibition_Has_Differential.23.aspx [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Lee SY, Bak SM, et al. Effects of matrix metalloproteinase inhibitor on LPS-induced goblet cell metaplasia. Am J Physiol - Lung Cell Mol Physiol. Internet]. 2004. cited 2021 Mar 15;287:L127–L133. Available from;1: https://koreauniv.pure.elsevier.com/en/publications/effects-of-matrix-metalloproteinase-inhibitor-on-lps-induced-gobl [DOI] [PubMed] [Google Scholar]
- 44.He B, Garmire L. Prediction of repurposed drugs for treating lung injury in COVID-19. F1000Research [Internet]. 2020. cited 2021 Jul 4;9:609. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7468567/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ong CT, Babalola CP, Nightingale CH, et al. Penetration, efflux and intracellular activity of tigecycline in human polymorphonuclear neutrophils (PMNs). J Antimicrob Chemother. 2005;56(3):498–501. [DOI] [PubMed] [Google Scholar]
- 46.Rodvold KA, Gotfried MH, Cwik M, et al. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58(6):1221–1229. [DOI] [PubMed] [Google Scholar]
- 47.Yates PA, Newman SA, Oshry LJ, et al. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis. 2020;14:1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gironi LC, Damiani G, Zavattaro E, et al. Tetracyclines in COVID-19 patients quarantined at home: literature evidence supporting real-world data from a multicenter observational study targeting inflammatory and infectious dermatoses. Dermatol Ther. 2021 Jan;34(1):e14694. 10.1111/dth.14694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam MM, Mahmud S, Rahman MM, et al. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12(8):e9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cag Y, Icten S, Isik-Goren B, et al. A novel approach to managing COVID-19 patients; results of lopinavir plus doxycycline cohort. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2021;40(2):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Hashim HA, Maulood MF, Rasheed AM, et al. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq [Internet]. Infec Dis (except HIV/AIDS); 2020. cited 2021 Mar 15]. Available from 2021 Mar 15: http://medrxiv.org/lookup/doi/10.1101/2020.10.26.20219345. [Google Scholar]
- 52.Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):3000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler CC, L-M Y, Dorward J, et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med. 2021;9(9):1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonjoch L, Gea-Sorlí S, Jordan J, et al. Minocycline inhibits peritoneal macrophages but activates alveolar macrophages in acute pancreatitis. J Physiol Biochem. 2015;71(4):839–846. [DOI] [PubMed] [Google Scholar]
- 55.Dunston CR, Griffiths HR, Lambert PA, et al. Proteomic analysis of the anti-inflammatory action of minocycline. Proteomics. 2011;11(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrido-Mesa J, Rodríguez-Nogales A, Algieri F, et al. Immunomodulatory tetracyclines shape the intestinal inflammatory response inducing mucosal healing and resolution. Br J Pharmacol. 2018;175(23):4353–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrido-Mesa J, Algieri F, Rodríguez-Nogales A, et al. Immunomodulatory tetracyclines ameliorate DNBS-colitis: impact on microRNA expression and microbiota composition. Biochem Pharmacol. 2018;155:524–536. [DOI] [PubMed] [Google Scholar]


