1. Introduction
The coronavirus disease (COVID-19) pandemic, caused by the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), marked its debut in 2019. To date, this single-stranded ribonucleic acid (RNA) virus has caused more than 5,485,911 deaths worldwide [1,2]. Unfortunately, medications targeting RNA respiratory viruses have had limited therapeutic benefit in practice and pose a significant burden in the management of COVID-19 patients [3,4]. Since then, multiple COVID-19 vaccines have entered the market, including mRNA-based vaccines, such as the Moderna mRNA-1273 and the Pfizer-BioNTech BNT162b2 vaccine, and viral vector vaccines, such as the Oxford/AstraZeneca AZD1222 (ChAdOx1) vaccine, administered under emergency authorized use [5]. Despite the protection they provide, these vaccines only offer short-term immunization and subsequent ‘booster’ doses are vital [6]. Further limitations of vaccine use lie in their efficacy with emerging SARS-CoV-2 variants and protection of immunosuppressed patients [7]. This stresses the urgency of investigating and implementing efficient and safe antivirals that will, in conjunction with the vaccines, offer the population protection against the COVID-19 disease.
There are multiple products on the market currently for the management of COVID-19, with fusion inhibitors, protease inhibitors and transcription inhibitor antivirals showing promising results [8]. The official recommendations from the National Institutes of Health for non-hospitalized patients are Paxlovid, Remdesivir and Sotrovimab, in addition to Molnupiravir [9].
Firstly, Paxlovid (PF-07321332/Nirmatrelvir and ritonavir), was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) on 31st December 2021 and has evidenced reduced hospitalization rates in mild and moderate COVID-19 disease [10,11]. Secondly, Remdesivir (Molecular cell), an experimental used for Ebola infection in 2016, has also been approved by the MHRA in May 2020 to be used in COVID-19 pneumonia, who fit the clinical criteria, for up to 5 days, and 10 days for those severely immunocompromised [8,12,13]. Sotrovimab (Xevudy, developed by GSK), a single 500 mg IV dose of monoclonal antibody, was found to reduce hospitalization by 79% in COVID-19 disease, when administered over 30 mins and was approved for individuals aged 12 and above, weighing over 40 kg [14]. Finally, Molnupiravir, approved by the MHRA on 4th November 2021, is a broad acting oral antiviral used to treat multiple viruses including Influenza and Ebola, and more recently SARS-CoV-2 [7]. This review will highlight Molnupiravir’s impact on the treatment of COVID-19, through analyzing latest clinical trial data and possible limitations of this drug.
Molnupiravir’s discovery began in 2013 with the aim to treat the New World alphavirus, the Venezuelan equine encephalitis virus [7]. This novel drug inhibits the RNA-dependent RNA polymerase (RdRp) enzyme which subsequently results in RNA virus replication errors.
Figure 1 illustrates the different methods of action antivirals can interfere in the SARS-CoV-2 cycle; like Remdesivir, Molnupiravir acts on step 5, RNA replication [8]. Although similar in reducing reproduction and pathogenicity of the virus, Molnupiravir is thought to be effective in treating even Remdesivir-resistant patients [6]. In a silico study using computational analysis, 45 analogs of molnupiravir against SARS-CoV-2 RdRp were evaluated; certain analogs showed the strongest inhibition of up to 7.3 kcal/mol, namely compound C17 with isobutyric acid ester, and monophosphate forms. This study identified key pharmacological properties that may explain the efficacy of molnuprivair [15]. Additionally, it is important to note that although the recent B.1.1.529 (omicron) variant contains mutations in the RdRp enzyme, a 2022 in vitro study confirmed that Malnupiravir’s efficacy was still preserved [16].
Figure 1.
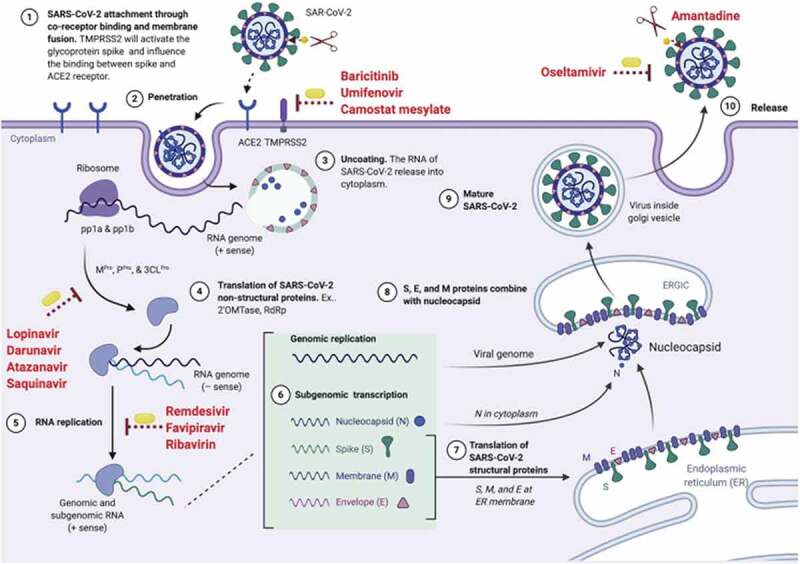
The life cycle of the SARS-CoV-2 virus and the mechanism of action of the antivirals. reproduced from [8] with permission of Elsevier.
Once absorbed, Malnupiravir is converted in the plasma to EIDD-1931, the active nucleoside analogue [6]. EIDD-1931 was found to be widely bioavailable in the lungs and successfully converted into its triphosphate form in the central nervous system in pharmacokinetic and distribution studies in mice, rats, ferrets and dogs [7]. Pre-clinical studies at Emory University showed that molnupiravir yielded no significant chromosomal damage or genotoxicity. However, the Therapeutic Goods Administration of Australia reported reversible, dose-related bone marrow toxicity in dogs and bone and cartilage toxicity in rats after 3 months of use [17].
During the phase I trial, Molnupiravir was well tolerated, with more adverse effects reported in the control group of the double-blinded study. Additionally, 93.3% of adverse events were classified as mild. No serious adverse events were reported and other investigations, such as basic observations and electrocardiography, were unremarkable [7]. Common side effects of the drug have been nausea, headaches and diarrhea. Other reported effects include hot flushes, flu-like symptoms and pain in the limbs and back [6]. It is tolerated well orally, with food affecting rate of absorption of the antiviral, however not its extent [18].
Considering the use of molnupiravir in pregnancy, there are currently no studies investigating its impact regarding human intrauterine development or neonatal complications, but reproductive and maternal toxicity were shown in animal trials. Hence the manufacturer suggests women of childbearing age should utilize effective contraception while on treatment, and for at least four days post treatment [19,20]. Similarly, because of the limited data, no drug interactions have been identified to date. The only contraindication noted was hypersensitivity to the drug’s active substance or excipients [17].
A 2021 open-label, dose escalating randomized controlled trial assessed three doses of 300 mg, 600 mg and 800 mg. The antiviral was safe and well-tolerated, with no serious adverse events reported. For phase II evaluation, a dose of 800 mg twice daily for 5 days was recommended [21]. However, when another phase II trial was carried out, there was a reduced viral RNA clearance time (RNA negativity) when this dosage and frequency was followed, in contrast to the placebo (p = 0.01) [22]. The drug is currently in phase 3 trials and further data is needed to confirm the drug’s full efficacy in treating SARS-CoV-2. However, in interim analysis of their Phase 3 MOVe-OUT trial (MK-4482-002) (NCT04575597), Merck declared that Molnupiravir reduced the risk of hospitalization or death by approximately 50%. On day 29, 7.3% (28/385) of patients were hospitalized or died, in comparison to 14.1% (53/377) in the placebo group (p = 0.0012). 8 patients had died in the placebo group, in contrast to none in the drug group. These results have led to the development and mass production of Molnupiravir, with countries including the United Kingdom have approved for use [23]. Similarly, the Food and Drug Administration in the United States issued an emergency use authorization for this drug [24]. However, despite European Medicines Agency’s approval of Molnupiravir, there are still conflicting concerns regarding its efficacy as France has canceled their order for the drug. This again, further reinforces the need for and importance of further clinical trials [20,25].
2. Conclusion
In conclusion, Molnupiravir has been shown to potentially halving all hospitalization and deaths in mild to moderate COVID-19 cases. As an oral antiviral that can be taken at home, Molnupiravir provides a promising advancement in our fight against COVID-19, alongside vaccination.
3. Expert opinion
This effective drug holds the advantage of being one of many limited SARS-CoV-2 treatments that can be taken orally. Subsequently, patients do not need to attend hospitals to have drugs administered intravenously, protecting both the patient and others, that may be immunocompromised, in the hospitals. Additionally, phase 3 interim analysis, although limited, has already shown promising results, with a significant impact on this condition’s prognosis. Despite the promising data for efficacy and reduction in hospitalization, it is important to take into consideration that most physicians are bounded by approved guidelines, and local guidelines of their constituent hospital/practice. This would be key for policymakers, and government as they endeavor to flatten to the epidemic curve to reduce the burden on hospitals against the waves of COVID-19 disease. However, these pressures do not come without their shortcomings; phase 3/4 clinical trial data, as many of these drugs are approved for emergency use over that past 6–12 months, are not enough to observe any side effects. Confounding factors may also be identified. Additionally, performing meta-analysis on various trials will ultimately provide reliable data, to guide both clinical and policy decisions. There are many factors that will dictate how Molnupiravir will be incorporated into SARS-CoV-2 treatment, and beyond scientific data, this will depend on availability, supply chain, funding and political decisions. However, in the short to medium term, the authors predict that this efficacious drug will be widely approved across the globe, given its promising results quoted above.
Funding Statement
This manuscript was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ludwig S, Zarbock A.. Coronaviruses and SARS-CoV-2: a brief overview. Anesth Analg. 2020;131(1): 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Dashboard . Johns Hopkins University & Medicine; 2022. [cited 2022 Jan 16. Available from: https://coronavirus.jhu.edu/map.html [Google Scholar]
- 3.Srinivas P, Sacha GL, Koval C. Antivirals for COVID-19. Cleve Clin J Med. 2020. Oct 7. DOI: 10.3949/ccjm.87a.ccc030. [DOI] [PubMed] [Google Scholar]
- 4.Emergency Use Authorization Declaration [Internet] . Federal Register. 2020. [cited 2022 Jan 2]. Available from: https://www.federalregister.gov/documents/2020/04/01/2020-06905/emergency-use-authorization-declaration
- 5.Yadav T, Srivastava N, Mishra G, et al. Recombinant vaccines for COVID-19. Hum Vaccin Immunother. 2020. Nov 24;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pourkarim F, Pourtaghi‐Anvarian S, Molnupiravir: RH. A new candidate for COVID‐19 treatment. Pharmacol Res Perspect. 2022. Feb;10(1):e00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painter GR, Natchus MG, Cohen O, et al. Developing A direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol. 2021. Oct;50:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article contains detailed information on the development of Molnupiravir historically and how it was adapted for the treatment of COVID-19 disease. Additionally, it discusses the safety and efficacy of Molnupiravir and summarises Phase 1 study results
- 8.Frediansyah A, Tiwari R, Sharun K, et al. Antivirals for COVID-19: a critical review. Clin Epidemiol Global Health. 2020. Jul;9:90–98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antiviral Therapy | Coronavirus Disease COVID-19 [Internet] . COVID-19 treatment guidelines. [cited 2021. Nov 15]. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/summary-recommendations; • This is an excellent resource that critically reviews other antivirals used to treat COVID-19 disease and sheds light on the benefits and limitations of other medications used.
- 10.Oral COVID-19 antiviral, Paxlovid, approved by UK regulator . Medicines and healthcare products regulatory agency. GOU.UK: 2021. [cited 2022 Jan 16. Available at: https://www.gov.uk/government/news/oral-covid-19-antiviral-paxlovid-approved-by-uk-regulator [Google Scholar]
- 11.Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of Phase 2/3 EPIC-HR study. Pfizer Inc; 2021. [cited 2022 Jan 16. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate [Google Scholar]
- 12.Tomashek KM, Dodd LE, Dodd LE. Remdesivir for the treatment of covid-19–final report. N Engl J Med. 2020;383(19):1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotrovimab Dosing & Administration | Emergency Use Authorization (EUA) [Internet] . www.sotrovimab.com. [cited 2022 Mar 3]. Available from: https://www.sotrovimab.com/hcp/dosing-and-administration
- 14.MHRA approves Xevudy (sotrovimab), a COVID-19 treatment found to cut hospitalisation and death by 79% . GOV.UK: Medicines and Healthcare products Regulatory Agency; 2021. [cited 2022 Jan 16. Available from: https://www.gov.uk/government/news/mhra-approves-xevudy-sotrovimab-a-covid-19-treatment-found-to-cut-hospitalisation-and-death-by-79 [Google Scholar]
- 15.Kulabaş N, Yeşil T, Küçükgüzel I. Evaluation of molnupiravir analogues as novel coronavirus (SARS-CoV-2) RNA-dependent RNA polymerase (RdRp) inhibitors – an in silico docking and admet simulation study. Journal of Research in Pharmacy [Internet]. 2021;25(6): 967–981. Available from: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1560909 [Google Scholar]
- 16.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N Engl J Med. 2022. Jan 26;386(10):995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TGA eBS - Product and Consumer Medicine Information Licence [Internet] . www.ebs.tga.gov.au. cited 2022 Mar 3. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2022-PI-01047-1&d=20220303172310101; • This is a very useful resource that contains detailed information on Molnupiravir’s dosage, adverse effects, interactions, cautions and contraindications.
- 18.Painter WP, Holman W, Bush JA, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021. Mar 1;65(5):e02428–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Use of Molnupiravir in Pregnancy. UK Teratology Information Service . 2021. [cited 2022 Mar 1]. Available from: https://www.medicinesinpregnancy.org/bumps/monographs/USE-OF-MOLNUPIRAVIR-IN-PREGNANCY
- 20.Use of molnupiravir for the treatment of COVID-19 . European Medicines Agency. 2022. cited 2022 Mar 1]. Available from: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-assessment-report_en.pdf; • This article by the European Medicines Agency highlights the indications for Molnupiravir use, in addition to the benefits and limitations of its use.
- 21.Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother. 2021. Dec;76(12):3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer WA, Eron JJ, Holman W, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Science Translational Medicine. 2021. Jan 1;14(628): eabl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merck . Merck and Ridgeback’s investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate COVID-19 in positive interim analysis of Phase 3 study. Merck. 2021. Oct.; •• This article reveals the recent phase 3 interim analysis results, showing the risk of hospitalisation and death reducing significantly by 50%. This will be very significant in decisions to utilise and incorporate Molnupiravir in future COVID-19 disease treatment.
- 24.Commissioner O of the . Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults [Internet]. FDA, 2021. [cited 26 Dec 2021]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
- 25.Molnupiravir: le ministère des Solidarités et de la Santé prend acte de la décision de la Haute Autorité de Santé (HAS) . Ministère des Solidarités et de la Santé. 2021. [cited 2022 Mar 1. Available from: https://solidarites-sante.gouv.fr/actualites/presse/communiques-de-presse/article/molnupiravir-le-ministere-des-solidarites-et-de-la-sante-prend-acte-de-la


