ABSTRACT
Adverse reactions after vaccination with COVID-19 mRNA vaccines are common; however, the association between adverse reactions and humoral responses is uncertain. To determine whether humoral immune responses after BNT162b2 vaccine administration were associated with local and systemic adverse reactions, we conducted a prospective observational cohort study in a single tertiary referral center. Healthcare workers who received the first dose of BNT162b2 vaccine were recruited. SARS-CoV-2 anti-spike IgG antibody titers were measured three weeks after the second dose and information about adverse reactions after vaccination was collected. Among the 887 participants, 641 (72.3%) were women. The median age was 38 (range, 22–74) years. All but one showed anti-spike IgG levels well above the cutoff, with a median level of 13,600 arbitrary units/mL. Overall, 800 (92.2%) participants reported some reactions after the first dose and 822 (96.3%) after the second dose. Significantly more participants reported systemic reactions after the second dose than after the first dose (P < .01), and 625 (73.6%) reported that reactions were stronger after the second dose. Factors positively associated with elevation of anti-spike IgG levels were history of asthma (24% higher if present, P = .01) and stronger reactions after the second dose (19% higher if experienced, P = .02). The majority of participants showed good humoral responses and reported some adverse reactions after vaccination. Anti-spike IgG levels were significantly higher if adverse reactions after the second dose were stronger than those after the first dose. These findings may help inform current and future vaccine recipients.
KEYWORDS: COVID-19, mRNA vaccine, systemic adverse reactions, humoral response, anti-Spike IgG antibody
Introduction
Since the first case of pneumonia in late 2019,1 coronavirus disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally. Pharmaceutical and non-pharmaceutical interventions have been used to decrease morbidity and mortality from COVID-19; but the control of the pandemic would be difficult without herd immunity through vaccination.
A number of vaccines against SARS-CoV-2 have been developed. Among these, the BNT162b2 mRNA vaccine is one of the most commonly inoculated vaccines in the world.2 Its efficacy against symptomatic and confirmed COVID-19 was 95.0% in the phase three trial.3 It has already prevented symptomatic COVID-19 in a real-world setting.4-9
Humoral immunity, especially neutralizing antibodies, plays a central role against SARS-CoV-2.10,11 Early reports suggest that the levels of neutralizing antibodies and anti-spike IgG antibodies correlate with protection.12-14 In a study where anti-spike IgG antibody levels after vaccination with virus vector vaccines (ChAdOx1) were analyzed to determine the association with protection against SARS-CoV-2, the levels of anti-spike IgG above 4,446 arbitrary unit (AU)/mL and 40,923 AU/mL were estimated to be associated with 50% and 80% vaccine efficacy, respectively.12
Multiple factors, such as age, sex, ethnicity, and prior SARS-CoV-2 infection, have been reported to be associated with anti-spike IgG levels.15,16 Cellular immunity works cooperatively with humoral immunity to induce a sufficient immune response to control SARS-CoV-2 replication and has been implicated as a marker for past infection or severity of COVID-19.17,18 However, the role of cellular immunity and its association with humoral responses after vaccination remains to be elucidated.
Local and systemic adverse reactions after vaccination with mRNA are frequent, and approximately two-thirds of the recipients reported local and/or systemic reactions after BNT162b2 vaccines in different studies.3,19 While they are largely mild, they are considered a major cause of SARS-CoV-2 vaccine hesitancy.19 However, some argue that these reactions might reflect the successful induction of an effective immune response.20 In other vaccines, including those for hepatitis B virus, pneumococcus, Haemophilus influenzae, poliovirus, prophylactic paracetamol treatment before vaccination have been associated with fewer recipients with fever and lower levels of antibody after vaccination, which suggests that early reactions after vaccination are indeed associated with higher levels of antibody.21,22 To date, data on mRNA vaccines for SARS-CoV-2 are still scarce. A few small studies reported that local and systemic reactions after vaccination with BNT162b2 were not associated with humoral response after vaccination.23-26 In contrast, correlations between adverse reactions after vaccination and higher antibody levels were suggested in other recent studies;27,28 therefore, it still remains uncertain.
In this study, we aimed to investigate 1) factors associated with humoral immune response, with special interest in local and systemic adverse reactions, and 2) factors associated with adverse reactions after BNT162b2 vaccine administration among healthcare workers in a single healthcare system in Japan.
Methods
Study design and participant recruitment
We conducted a prospective observational study at the University of Tokyo Hospital, Tokyo, Japan. In Japan, SARS-CoV-2 vaccination with the BNT162b2 mRNA vaccine for healthcare workers became available in February 2021. Healthcare workers who opted to receive the first dose of BNT162b2 mRNA vaccine at the University of Tokyo Hospital from March 12 to 31 March 2021, were invited to participate in the study. Participants were recruited via e-mail, poster advertising, and direct recruitment at the vaccination site. They were included if they indicated their willingness to participate before the first vaccination. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the University of Tokyo Hospital (approval number: 2020353NI). Written consent was obtained from each participant at the start of the study.
Study timeline and data collection
Blood samples and clinical information of the study participants were collected before the first and second doses and 3 weeks after the second dose (Figure 1). We measured anti-spike IgG antibody levels using a chemiluminescent microparticle immunoassay (SARS-CoV-2 IgG II Quant assay, Abbott Architect, U.S.) 3 weeks after the second dose.29,30 The positive cutoff antibody level was defined as 50.0 AU/mL according to the manufacturer’s instructions. The test sensitivity and specificity were estimated at 98.1% and 99.6%, respectively, according to the U.S. Food and Drug Administration.31 We measured anti-nucleocapsid IgG antibody levels to assess the participants basic serologic status to distinguish between people who were already infected with SARS-CoV-2 and those who were not (using iFlash-SARS-CoV-2 IgG, YHLO Biotechnology Company). The positive cutoff index was defined as 10.0 according to the manual provided by the manufacturer. In addition, we measured the counts and levels of the following before the first and second dose: complete blood cell counts, renal function, liver function, electrolytes, lipids, C-reactive protein, D-dimer, prothrombin time, and activated partial thromboplastin time (APTT). The T-cell response in the peripheral blood was evaluated using an interferon gamma release assay. We used the QuantiFERON SARS-CoV-2 Research Use Only (RUO), including blood collection tubes of the SARS-CoV-2 Starter Set and Control Set (Qiagen, Hilden, Germany). Briefly, whole blood was incubated with SARS-CoV-2 antigens in the tubes, and the IFN-γ concentration in plasma was measured by enzyme-linked immunosorbent assay according to the manufacturer’s instructions. With a cutoff of .15 IU/mL, the test sensitivity and specificity were determined as 98.3% and the specificity as 100%, respectively, according to the manufacturer’s instructions.32 Individual IFN-γ concentrations were calculated by subtraction from the baseline concentration.
Figure 1.
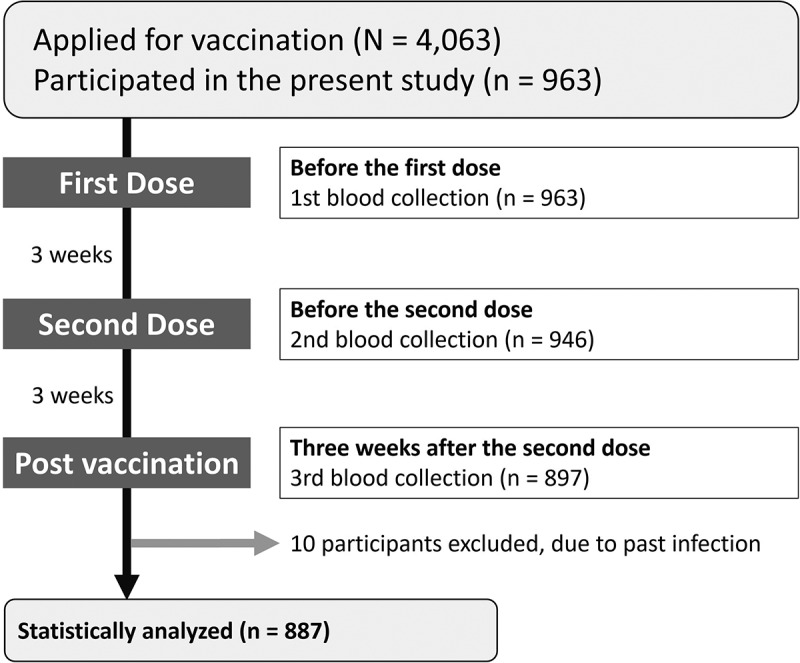
Study flow.
The flowchart of participants included in the analysis of this study.
The following characteristics were collected via an online questionnaire (Google Forms platform) at the first sample collection: participants’ age, race, sex, height, weight, job category, comorbidities, smoking and drinking status, and exposure to outpatients or inpatients. The self-reported information on the history of COVID-19 or close contact with confirmed COVID-19 patients was collected at each visit for blood sample collection. In addition, information on adverse reactions after the first and second doses of vaccination was collected. Adverse reactions included administration site pain, administration site swelling, administration site redness, fever, chills, fatigue, headache, vomiting, diarrhea, generalized muscle pain, joint pain, and other symptoms (free text comments). We defined pain, swelling, and redness of the administration site as local reactions, and other adverse reactions as systemic reactions. We specifically asked the participants if the reactions after the second dose were weaker, the same, or stronger than the reactions after the first dose.
Statistical analysis
Only information from participants who completed all three blood tests was used for the analysis. The Brunner–Munzel test or Kruskal–Wallis test was used for comparisons of continuous variables. Fisher’s exact test and the chi-square test were used for other categorical variables. We performed multiple regression analysis and a sensitivity analysis to assess the factors associated with log-transformed levels of anti-spike IgG antibody (eMethods and eTable S1 in the Supplement). We explored the factors associated with self-reported adverse reactions using multiple logistic regression analysis in the same manner. We performed all statistical analyses using R 4.0.333 with “lawstat”34 and “tidyverse”35 packages. All tests were two-tailed, and a P value <.05 was considered statistically significant.
Results
Characteristics of the study participants
Between March 12 and 31, 2021, 4,063 employees received at least one dose of BNT162b2 vaccine. Overall, 963 employees participated in the study. After excluding 66 participants who did not undergo blood tests at all the three time points and 10 participants who responded that they had been diagnosed with COVID-19, 887 participants were analyzed (Figure 1 and Table 1). Eight hundred and thirty-seven (94.4%) completed the three questionnaires. The median age of the participants was 38 (range, 22–74) years and female participants (n = 641, 72.3%) were slightly younger than male participants (38.0 vs. 39.0 years, P = .06). The majority of participants reported no underlying medical problems. Only 21 (2.4%) participants reported active smoking, and 75 (8.5%) reported past smoking. Four hundred and eighty-six (54.8%) participants reported social drinking and 173 (19.5%) reported daily alcohol consumption.
Table 1.
Characteristics of the study participants with three completed blood collections
| Characteristics | Total (n = 887) |
Male (n = 246) |
Female (n = 641) |
P value |
|---|---|---|---|---|
| Answered web questionnaire | ||||
| After the first dose (n, %) | 868 (97.9) | 239 (97.2) | 629 (98.2) | |
| After the second dose (n, %) | 854 (96.3) | 237(96.4) | 617 (96.0) | |
| Completed (n, %) | 837 (94.4) | 230 (93.6) | 607 (94.6) | |
| Age (years, median [range]) | 38 [22-74] | 39.5 [23-65] | 38 [22-74] | 03 |
| 29≦ (n, %) | 185 (20.8) | 27 (12.5) | 158 (24.0) | |
| 30–39 (n, %) | 290 (32.8) | 96 (38.7) | 194 (30.5) | |
| 40–49 (n, %) | 246 (27.9) | 78 (30.2) | 168 (27.0) | |
| 50–59 (n, %) | 133 (15.1) | 33 (13.7) | 100 (15.6) | |
| ≧60 (n, %) | 33 (3.5) | 12 (4.8) | 21 (2.9) | |
| BMI (kg/m2, median [range]) | 21.2 [14.9–41.5] | 22.5 [16.5–38.7] | 20.6 [14.9–41.5] | <.01 |
| Job category | <.01 | |||
| Nurse (n, %) | 340 (35.5) | 12 (4.9) | 328 (51.3) | |
| Physician (n, %) | 226 (23.6) | 144 (58.5) | 82 (12.8) | |
| Other medical staff (n, %) | 180 (18.8) | 62 (25.2) | 118 (18.5) | |
| Non-medical staff (n, %) | 139 (14.5) | 28 (11.4) | 111 (17.4) | |
| Comorbidity | ||||
| Allergic disease except for asthma (n, %) | 83 (9.4) | 26 (10.6) | 57 (8.9) | 44 |
| Asthma (n, %) | 64 (7.2) | 14 (5.7) | 50 (7.8) | 31 |
| Hypertension (n, %) | 27 (3.0) | 12 (4.8) | 15 (2.3) | 07 |
| Dyslipidemia (n, %) | 22 (2.4) | 11 (4.5) | 11 (1.7) | 03 |
| Diabetes mellitus (n, %) | 9 (1.0) | 2 (.8) | 7 (1.1) | 1.00 |
| History of smoking | <.01 | |||
| Never smoker (n, %) | 791 (89.1) | 205 (83.3) | 586 (91.4) | |
| Past smoker (n, %) | 75 (8.5) | 37 (15.0) | 38 (6.9) | |
| Active smoker (n, %) | 21 (2.4) | 4 (1.6) | 17 (2.7) | |
| Alcohol consumption | <.01 | |||
| No consumption (n, %) | 228 (25.7) | 44 (17.9) | 184 (28.7) | |
| Social drinking (n, %) | 486 (54.8) | 128 (52.0) | 358 (55.9) | |
| Daily consumption (n, %) | 173 (19.5) | 74 (30.1) | 99 (15.4) | |
| Exposure to inpatients (n, %) | ||||
| Baseline (n = 897) | 486 (54.8) | 143 (58.1) | 343 (53.5) | 25 |
| After the first dose (n = 558) | 304 (55.3) | 81 (55.5) | 223 (55.2) | 1.00 |
| After the second dose (n = 540) | 288 (54.1) | 83 (57.7) | 205 (52.8) | 37 |
| Exposure to outpatients (n, %) | ||||
| Baseline (n = 897) | 381 (43.0) | 151 (61.4) | 230 (35.9) | <.01 |
| After the first dose (n = 558) | 234 (42.5) | 79 (54.1) | 155 (38.3) | <.01 |
| After the second dose (n = 540) | 228 (42.9) | 83 (57.6) | 145 (37.4) | <.01 |
Abbreviation: BMI, body mass index.
Healthcare workers were classified as medical and non-medical staff. Medical staff included nurses, physicians, pharmacists, clinical laboratory technicians, radiologists, and medical students. Non-medical staff included administrative workers, medical clerks, and other staff without direct contact with the patients.
Basic serologic status of participant anti-nucleocapsid antibody
Of 887 participants, only 9 showed positive results for anti-nucleocapsid IgG above the cutoff index (10.0) 3 weeks after the second dose. Of note, the recent study evaluating the same kit suggested some asymptomatic individuals might show false positive results.36 The median level was .06, which was far below the cutoff line.
Humoral response to vaccination
Of 887 participants, all but one participant who was taking immunosuppressants showed increased levels of anti-spike IgG above the cutoff level (50.0 AU/mL) 3 weeks after the second dose. The median level was 13,600 (interquartile range [IQR], 8,840–20,200) AU/mL (Figure 2).
Figure 2.
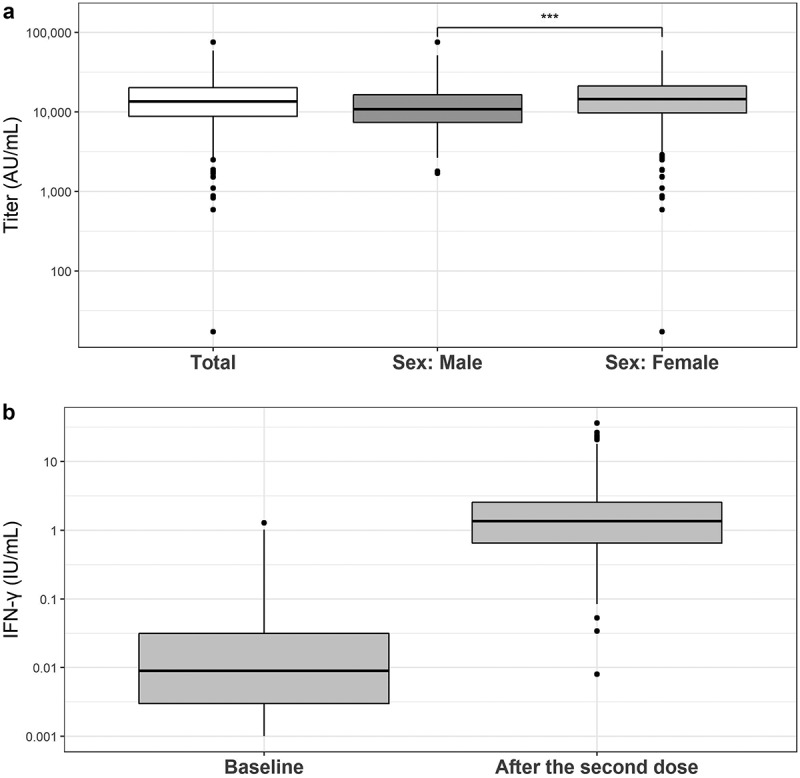
Level of anti-spike IgG and IFN-γ three weeks after the second dose of the BNT162b2 vaccine.
Both box plots show the median, quartiles, and outliers. a) Anti-spikeIgG levels after the second dose. IgG levels in female were higher than levelsin male (***P < .001). b) Levels of IFN-γ at baseline and threeweeks after the second dose.
Cellular response to vaccination
We measured the IFN-γ response to specific T cell antigens to evaluate cellular immunity. Compared with the baseline level before vaccination, we observed elevated IFN-γ levels in 99.2% of participants. The median levels were .003 (IQR, −.005–.017) AU/ml at baseline and 1.386 (IQR, .666–2.561) AU/mL 3 weeks after the second dose (Figure 2).
Self-Reported adverse reactions
Overall, 800 (92.2%) participants reported some reactions after the first dose (Table 2, Supplementary figure 1). Female participants were more likely to report reactions than male participants (93.9% vs. 88.0%, P < .01). Seven hundred and fifty-five (87.0%) and 332 (38.2%) participants reported local and systemic reactions, respectively.
Table 2.
Adverse reactions occurring in the participants after the first and second doses of the BNT162b2 vaccine
| Adverse reactions | After the first dose (n = 868) | After the second dose (n = 854) | P value |
|---|---|---|---|
| Any (n, %) | 800 (92.2) | 822 (96.3) | 03 |
| Focal reactions (n, %) | 755 (87.0) | 738 (86.4) | <.01 |
| Systemic reactions (n, %) | 332 (38.2) | 665 (77.9) | <.01 |
| Administration site pain (n, %) | 746 (85.9) | 720 (84.3) | <.01 |
| Administration site pain (short)* (n, %) | 475 (54.7) | 394 (45.9) | <.01 |
| Administration site pain (long)** (n, %) | 271 (31.2) | 326 (38.2) | <.01 |
| Administration site swelling (n, %) | 119 (13.7) | 205 (24.0) | <.01 |
| Administration site redness (n, %) | 44 (5.1) | 115 (13.5) | <.01 |
| Fever (n, %) | 23 (2.6) | 396 (46.4) | <.01 |
| Fatigue (n, %) | 170 (19.6) | 542 (63.5) | <.01 |
| Headache (n, %) | 97 (11.2) | 333 (39.0) | <.01 |
| Chill (n, %) | 13 (1.5) | 236 (27.7) | 04 |
| Emesis (n, %) | 3 (.3) | 21 (2.5) | 05 |
| Diarrhea (n, %) | 16 (1.8) | 27 (3.2) | <.01 |
| Generalized muscle pain (n, %) | 130 (15.0) | 229 (26.8) | <.01 |
| Joint pain (n, %) | 24 (2.8) | 226 (26.5) | <.01 |
| Took antipyretic (n, %) | 24 (2.8) | 291 (34.1) | <.01 |
| Other symptoms (n, %) | 60 (6.9) | 84 (9.8) | <.01 |
| Axillary lymphadenopathy (n, %) | 9 (1.0) | 25 (2.9) | <.01 |
| Dizziness (n, %) | 6 (.7) | 6 (.7) | <.01 |
| Skin rash (n, %) | 7 (.8) | 7 (.8) | <.01 |
| Drowsiness (n, %) | 6 (.7) | 9 (1.0) | <.01 |
| Itchiness (%) | 9 (1.0) | 10 (1.2) | 07 |
Data were analyzed by two-tailed Chi-square test or Fisher’s exact test, as appropriate. *Administration site pain that disappeared on the day after injection is defined was “Administration site pain (short)”.
**Administration site pain that remained at least two days after injection was defined as “Administration site pain (long)”.
After the second dose, 822 participants (96.3%) reported some reactions. There was no sex difference in the proportion of participants who reported reactions. Seven hundred thirty-eight (86.4%) and 668 (77.9%) participants reported local and systemic reactions, respectively. Significantly more participants reported systemic reactions after the second dose than after the first dose (77.9% vs. 38.2%, P < .01). About three-quarters (n = 625, 73.6%) of participants reported that the reactions were stronger after the second dose than after the first dose whereas 97 (11.4%) participants reported stronger reactions after the first dose. One-hundred and fourteen (13.4%) participants reported that the reactions after the first and second doses were about the same. None of the participants experienced anaphylaxis.
Factors associated with the levels of anti-spike IgG antibody
The results of the univariate analysis are shown in eTable S2 in the supplement. Multiple regression analysis revealed that participant characteristics, history of natural infection, close contact with confirmed COVID-19 patients, exposure to inpatients, and self-reported reactions were significantly associated with anti-spike IgG antibody levels (Table 3). Compared to the participants who reported that the reactions were about the same after the first and second doses, those who experienced stronger reactions after the second dose than after the first dose had a statistically significant higher anti-spike IgG antibody levels by 19% (P = .02) whereas those who experienced stronger reactions after the first dose did not (P = .31) (Figure 3). Other factors positively associated with the antibody level were history of asthma (24% higher if present, P = .01) and lymphocyte counts (significant but minimal effect, P = .02). Similarly, the factors negatively associated with the antibody level were increased age (11% lower every 10 years, P < .01), and history of daily alcohol consumption (11% lower if present, P = .04). Notably, the IFN-γ response to the specific T cell antigen was not associated with this level.
Table 3.
Multiple regression analysis of factors associated with the levels of anti-spike IgG antibody after the BNT162b2 vaccine administration
| Explanatory variables | Partial regression coefficient* | 95% Confidence interval* | P value |
|---|---|---|---|
| Age | 988 | (.982, .993) | <.01 |
| Female sex | 1.150 | (.982, 1.348) | 08 |
| Nurse | 0.970 | (.868, 1.084) | 59 |
| Physician | 1.034 | (.919, 1.163) | 58 |
| Asthma | 1.243 | (1.055, 1.466) | <.01 |
| Hypertension | 0.863 | (.678, 1.098) | 23 |
| Never smoking | 0.964 | (.833, 1.117) | 63 |
| Active smoking | 0.795 | (.579, 1.092) | 16 |
| Daily alcohol consumption | 894 | (.803, .994) | 04 |
| Exposure to inpatients | 1.044 | (.951, 1.147) | 37 |
| Focal adverse reactions only (after the second dose) | 0.970 | (.718, 1.309) | 84 |
| Systemic adverse reactions (after the second dose) | 1.236 | (.911, 1.677) | 17 |
| The comparison of adverse reactions after the first and the second dose | |||
| Stronger adverse reactions after the first dose than that after the second dose | 1.092 | (.921, 1.294) | 31 |
| Stronger adverse reactions after the second dose than that after the first dose | 1.191 | (1.031, 1.376) | 02 |
| Creatinine (after the first dose, mg/dL) | 1.220 | (.792, 1.88) | 37 |
| Blood urea nitrogen (after the first dose, mg/dL) | 0.999 | (.986, 1.012) | 83 |
| Sodium (after the first dose, mEq/L) | 0.99 | (.962, 1.018) | 48 |
| Chloride (after the first dose, mEq/L) | 1.000 | (.996, 1.0480) | 09 |
| Aspartate aminotransferase (after the first dose, IU/L) | 1.002 | (.996, 1.008) | 54 |
| LDL-cholesterol (after the first dose, mg/dL) | 1.000 | (.999, 1.002) | 72 |
| Triglyceride (after the first dose, mg/dL) | 1.000 | (.999, 1.001) | 99 |
| Lymphocyte (after the first dose,/μL) | 1.000 | (1.000, 1.000) | 02 |
| Hemoglobin (after the first dose, g/dL) | 0.965 | (.924, 1.007) | 10 |
| Eosinophil (after the first dose, %) | 1.000 | (.999, 1.000) | 21 |
| Glucose (after the first dose, mg/dL) | 0.998 | (.996, 1.001) | 16 |
| Hemoglobin A1C (after the first dose, %) | 1.016 | (.905, 1.141) | 79 |
| Activated partial thromboplastin time (after the first dose, sec) | 1.012 | (.997, 1.028) | 11 |
| Prothrombin time (after the first dose, international normalized ratio) | 1.124 | (.527, 2.399) | 76 |
Factors significantly associated with anti-spike IgG levels (P value <.05) are highlighted in bold.
*Partial regression coefficient and 95% Confidence Interval were calculated from 10 raised to the power of the value of the original.
Figure 3.
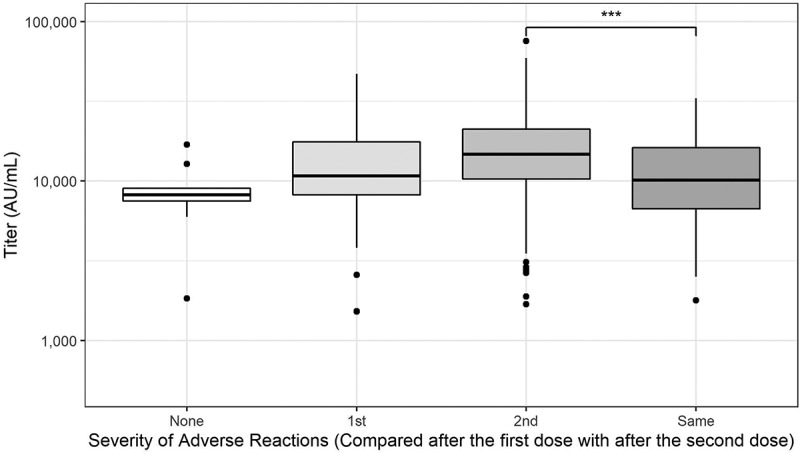
Severity of adverse reactions (compared with those post first and second doses).
Box plots of anti-spike IgG titers after second vaccination showing themedian, quartiles, and outliers. The following four groups were included: “None,”“1st,” “2nd,” and “Same” representing participants whohad no reactions after either dose, stronger reactions after 1stdose, stronger reactions after 2nd dose, and same level of reactionsafter the 1st and 2nd doses, respectively. IgG levels in participantswho had stronger reactions after 2nd dose were higher than levels inparticipants who had same level of reactions after the 1st and 2nddoses (***P < .001).
In sensitivity analysis, strong adverse reactions after the second dose were significant in all stratified groups except for stratified with the history of asthma (eTable S1).
Factors associated with self-reported adverse reactions
Multivariate logistic regression analysis was performed to assess the factors associated with 1) any reactions, reactions only at the local site, and any systemic reactions after the first dose; and 2) reactions only at the local site, any systemic reactions, fever, fatigue, and headache after the second dose. Because over 95% of the participants reported some adverse reactions after the second dose, factors associated with any adverse reactions were not examined.
After the first dose (Table 4)
Table 4.
Multiple regression analysis of factors associated with adverse reactions after the first and second dose of BNT162b2 vaccine
| Explanatory variable |
Odds ratio |
95% Confidence interval |
P value |
|---|---|---|---|
| After the first dose | |||
| Any adverse reactions | |||
| Age | 96 | (.94, .99) | <.01 |
| Female sex | 2.01 | (.86, 4.72) | 11 |
| Physician | 0.84 | (.44, 1.65) | 61 |
| Creatinine (mg/dL) | 1.08 | (.09, 15.21) | 95 |
| No alcohol consumption | 2.05 | (1.15, 3.62) | 01 |
| Focal adverse reactions only | |||
| Asthma | 55 | (.30, .98) | 046 |
| Platelet (×104/μL) | 1.03 | (1.01, 1.06) | <.01 |
| Albumin (g/dL) | 1.56 | (.92, 2.65) | 10 |
| Systemic adverse reactions | |||
| Female sex | 1.41 | (.86, 1.17) | 18 |
| Nurse | 0.92 | (.64, 1.32) | 66 |
| Physician | 0.71 | (.46, 1.32) | 11 |
| Allergic diseases | 1.68 | (1.01, 2.80) | 046 |
| Asthma | 2.07 | (1.13, 3.84) | 02 |
| Dyslipidemia | 0.39 | (.11, 1.10) | 10 |
| Hemoglobin (g/dL) | 1.00 | (.86, 1.17) | 98 |
| Platelet (×104/μL) | 96 | (.93, .99) | 04 |
| Prothrombin time (international normalized ratio) |
6.40 |
(.54, 76.81) |
14 |
|
After the second dose |
|
|
|
| Focal adverse reactions only | |||
| Physician | 1.44 | (.94, 2.17) | 09 |
| Exposure to inpatients | 0.70 | (.48, 1.03) | 07 |
| Systemic adverse reactions (after the first dose) | 0.70 | (.32, 1.56) | 37 |
| Focal adverse reactions only (after the first dose) | 1.02 | (.54, 2.05) | 96 |
| Fatigue (after the first dose) | 0.63 | (.30, 1.27) | 20 |
| Triglyceride (after the first dose, mg/dL) | 1.00 | (1.00, 1.00) | 13 |
| Systemic adverse reactions | |||
| Female sex | 1.09 | (.69, 1.71) | 70 |
| Physician | 0.76 | (.49, 1.21) | 24 |
| Systemic adverse reactions (after the first dose) | 1.61 | (.76, 3.36) | 21 |
| Focal adverse reactions only (after the first dose) | 1.16 | (.61, 2.11) | 64 |
| Pain (long, after the first dose) | 1.33 | (.88, 2.03) | 18 |
| Fatigue (after the first dose) | 1.59 | (.81, 3.20) | 19 |
| Headache (after the first dose) | 1.06 | (.51, 2.34) | 89 |
| LDL-cholesterol (after the first dose, mg/dL) | 1.00 | (.99, 1.00) | 11 |
| Triglyceride (after the first dose, mg/dL) | 1.00 | (1.00, 1.00) | 22 |
Factors significantly associated with adverse reactions (P value <.05) are highlighted in bold.
Age (odds ratio [OR], .96; 95% confidence interval [95% CI], .94–.99; P < .01), and no alcohol consumption (OR, 1.99; 95% CI, 1.10–3.54; P = .02) were associated with any reactions. Platelet counts (OR for every 10,000/μL, 1.03; 95% CI, 1.01–1.06; P < .01) and history of asthma (OR, .55; 95% CI, .30–.99; P = .048) were associated with reactions only at the local sites. Platelet counts (OR for every 10,000/μL, .97; 95% CI, .94–.99; P = .01) and history of asthma (OR, 2.09; 95% CI, 1.14–3.87; P = .02) were also associated with systemic reactions as well as history of allergic diseases (OR, 1.68; 95% CI, 1.01–2.80; P = .05).
After the second dose (Table 4, eTable S3 in the supplement)
There were no factors associated with reactions at the local site or any systemic reactions.
Discussion
In this study, we examined anti-spike IgG antibody levels, adverse reactions, and their relationship among 887 healthcare workers who received the BNT162b2 mRNA vaccine. As in previous studies,37,38 the level of anti-spike IgG was elevated in almost all the participants after the second dose. Local and systemic adverse reactions were reported in 86.4% and 77.9% of participants after the second dose, respectively, which are higher than those reported in previous studies,3,19 but the majority of the participants in this study were younger than 60 years old. Moreover, healthcare workers might be more attentive to adverse reactions than non-healthcare workers. The incidence of these uncommon reactions may have been underestimated.
We identified several factors associated with higher antibody levels. A history of asthma remained a factor significantly associated with higher anti-spike IgG antibody levels, whereas increased age and daily alcohol consumption were associated with lower antibody levels. This result contrasts with the observations that asthma was associated with declined humoral immunity in children after vaccination with other vaccines (i.e., measles, mumps, and rubella (MMR), and hepatitis B virus).39-41 There are few reports about humoral immunity against SARS-CoV-2 in adults with asthma so far and further investigations are needed to confirm our findings. Aging and alcohol abuse are known to increase the incidence of various infectious diseases.42,43 Together with the recent study,44 our findings highlight the increased risk of people with increased age and daily alcohol consumption even after vaccination.
Participants reporting stronger reactions after the second dose than that after the first showed significantly higher levels of anti-spike IgG antibody. Adverse reactions are unwelcome, but their presence might reflect a stronger immune response, which is suggested in previous reports.21,22 Recently, two previous studies reported the association between adverse reactions and immune response after vaccination with SARS-CoV-2 mRNA vaccines.27,28 Our finding further supports this association. In view of anticipated repeated vaccination with mRNA,45 our finding might also serve as an encouraging message (that reactions are not welcome but may be a good sign) to the general public, if communicated appropriately.
Although cellular response was observed in almost all the participants after the second dose as elevation of IFN-γ level, it showed little correlation with antibody levels. A similar study using the BNT162b2 vaccine also showed a similar result.46 As another recent study showed that antigen-specific CD8+ T cells in peripheral blood increased rapidly after vaccination,47 the IFN-γ level measured 3 weeks after the second dose may not reflect the peak level after vaccination. However, our findings indicate that cellular response is well maintained at least 3 weeks after the second dose. The detailed kinetics and characteristics of the cellular responses in our participants are now under analysis.
The predictive factors for different adverse reactions after mRNA vaccination have been insufficiently studied. We found that factors differed depending on the types of adverse reactions, as well as the timing after the doses. Previously, older people showed less reactions19 and women had a doubled risk for moderate systemic reactions compared to men.48 Consistent with these studies, our younger and female participants were more likely to report adverse reactions after the first dose. Interestingly, a history of asthma and alcohol consumption were also significantly associated with adverse reactions after the first dose and with antibody levels. These shared predictors for both anti-spike IgG antibody levels and adverse reactions may further support the hypothesis that at least some adverse reactions result from an immune response and therefore may serve as a surrogate for antibody response.
Consistent with other studies,3,19 systemic adverse reactions, such as fever, fatigue, and headache, were twice as common after the second dose as after the first dose. This observation is important because these reactions can be a major reason for absenteeism and may recur after additional doses. Although a history of confirmed COVID-19 was the only factor predictive of any systemic reactions, participants who had experienced fever, fatigue, and headache after the first dose were two to three times more likely to experience the same symptoms. Headache was more common among female participants, and less so among participants with daily alcohol consumption. Although further research is needed to confirm our findings, our findings would be informative for people who are to receive or who have received the BNT162b2 vaccine.
This study has limitations. First, all adverse reactions after vaccination and backgrounds of participants were self-reported; therefore, we could not confirm their presence objectively. Furthermore, the severity, extent, and details of the reactions were not evaluated and therefore recall bias might have occurred. In addition, we did not evaluate if there was a correlation between severity of adverse reactions after the first dose and anti-spike IgG antibody levels after the first dose. Second, this was a single-center study using the BNT162b2 vaccine involving healthcare workers. It remains uncertain whether our findings can be generalized to other populations and other COVID-19 mRNA vaccines. Third, although we observed a significant difference in anti-spike IgG antibody levels between those with and without the factors, the clinical significance of this difference in levels, particularly its potential for protection, remains to be studied. Fourth, we only evaluated the association between adverse reactions and antibody levels 3 weeks after the second dose, and baseline anti-spike antibody levels before the vaccination were not measured although there were a few participants who had reported the history of COVID-19. Future studies are needed to evaluate the effects of adverse reactions on antibody response in the long term, as well as with additional doses. Fifth, although we found that some factors, LDL-cholesterol and creatinine were associated with some adverse reactions (eTable S3), the clinical significance of these associations warrants further investigation. Sixth, we excluded participants with confirmed self-reported COVID-19 infections; however, asymptomatic COVID-19 infections during the study might have been missed, which could have affected the immune response.
In conclusion, we found that the majority of participants showed good humoral response and a variety of non-critical adverse reactions with the BNT162b2 vaccine. The observed association between different adverse reactions and increased anti-spike IgG antibody levels might be helpful information for those who are to receive or who have received the BNT162b2 vaccine. Future studies are needed to confirm our findings and evaluate the effects of adverse reactions and cellular responses on antibody levels in the long term.
Supplementary Material
Acknowledgements
We thank all the colleagues who participated in this study.
We would like to thank Editage (www.editage.com) for English language editing.
Funding Statement
This work was supported by The University of Tokyo, Promoting practical use of measures against coronavirus disease 2019 (COVID-19).
Disclosure statement
Dr. Okamoto reports personal fees from Astrazeneca, Astellas Pharm, Sanyo Chemical, and Ono Pharmaceutical outside of the submitted work. Dr. Harada reports personal fees from BD, personal fees from Meiji, personal fees from Shionogi, personal fees from Sumitomo Dainippon Pharma, grants and personal fees from MSD, personal fees from Astellas, personal fees from Beckman Coulter Diagnostics, personal fees from FUJIFILM Toyama Chemical, personal fees from NISSUI PHARMACEUTICAL, outside the submitted work.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2048559.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–10. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser HR, Esteban O-O, Diana B, et al. Coronavirus pandemic (COVID-19) 2021. Aug 16. https://ourworldindata.org/coronavirus
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang L, Hijano DR, Gaur AH, Geiger TL, Neufeld EJ, Hoffman JM, Hayden RT.. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. Jama. 2021;325(24):2500–02. doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD, et al. Bnt162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R.. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. Jama. 2021;325(24):2457–65. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertollini R, Chemaitelly H, Yassine HM, Al-Thani MH, Al-Khal A, Abu-Raddad LJ. Associations of vaccination and of prior infection with positive PCR test results for SARS-CoV-2 in airline passengers arriving in Qatar. JAMA. 2021;326(2):185–88. doi: 10.1001/jama.2021.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–96. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582–89. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. 2021:21258528. doi: 10.1101/2021.03.17.21253847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A, et al. Bnt162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–77. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 14.Donal TS, Adam CH, and Javier G-J, et al. Two doses of SARS-CoV-2 vaccination induce more robust immune responses to emerging SARS-CoV-2 variants of concern than does natural infection. Res Sq. 2021:rs.3.rs-226857/v2. doi: 10.21203/rs.3.rs-226857/v2. [DOI] [Google Scholar]
- 15.Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, Conti L, De Virgilio A, De Marco F, Di Domenico EG, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. doi: 10.1016/j.eclinm.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabal KA, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance. 2021;26(6):2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moderbacher CR, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, et al. Antigen-Specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, Hippenstiel S, Dingeldey M, Kruse B, Fauchere F, et al. SARS-CoV-2-Reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–74. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 19.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201–02. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 20.Sprent J, King C. COVID-19 vaccine side effects: the positives about feeling bad. Sci Immunol. 2021;6(60):eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doedée AM, Boland GJ, Pennings JL, de Klerk A, Berbers GAM, van der Klis FRM, de Melker HE, van Loveren H, Janssen R, et al. Effects of prophylactic and therapeutic paracetamol treatment during vaccination on hepatitis B antibody levels in adults: two open-label, randomized controlled trials. PLoS One. 2014;9(6):e98175. doi: 10.1371/journal.pone.0098175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prymula R, Siegrist C-A, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374(9698):1339–50. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 23.Hwang YH, Song KH, Choi Y, Go S, Choi S-J, Jung J, Kang CK, Choe PG, Kim N-J, Park WB, et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36:1486–91. kjim.2021.210. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi M, Higa Y, and Esaki A, et al. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? medRxiv. 2021;21258444. doi: 10.1101/2021.06.08.21258444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda K, Amano M, Uemura Y, Tsuchiya K, Matsushima T, Noda K, Shimizu Y, Fujiwara A, Takamatsu Y, Ichikawa Y, et al. Correlates of neutralizing/sars-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. medRxiv. 2021:21261237. doi: 10.1101/2021.07.27.21261237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coggins SAA, Laing ED, Olsen CH, Goguet E, Moser M, Jackson-Thompson BM, Samuels EC, Pollett SD, Tribble DR, Davies J, et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. medRxiv. 2021:21259544. doi: 10.1101/2021.06.25.21259544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rechavi Y, Shashar M, Lellouche J, Yana M, Yakubovich D, Sharon N. Occurrence of BNT162b2 vaccine adverse reactions is associated with enhanced SARS-CoV-2 IgG antibody response. Vaccines (Basel). 2021;9(9). doi: 10.3390/vaccines9090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debes AK, Xiao S, Colantuoni E, Egbert ER, Caturegli P, Gadala A, Milstone AM. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern Med. 2021;181(12):1660–62. doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott . Abbott ARCHITECT SARS-CoV-2 IgG II Quant Reagent Instructions for Use 2021. 2021. Aug 16. https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2-
- 30.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, Xu J, SoRelle JA, Alexis C, Fankhauser K, et al. Clinical evaluation of the Abbott alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J Clin Microbiol. 2021;59(7):e00388–21. doi: 10.1128/JCM.00388-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U. S. Food and Drug Administration . EUA authorized serology test performance 2022. 2022. Jan 5. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 32.Aiello A, Najafi Fard S, Petruccioli E, Petrone L, Vanini V, Farroni C, Cuzzi G, Navarra A, Gualano G, Mosti S, et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int J Infect Dis. 2021;106:338–47. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core team . R: the R project for statistical computing 2021. 2021. Aug 16. https://www.r-project.org/
- 34.Gastwirth JL, Gel YR, Hui WL, et al. Lawstat: tools for biostatistics, public policy, and law: Comprehensive R Archive Network (CRAN); 2021. Sep 4. https://cran.r-project.org/web/packages/lawstat/index.html
- 35.Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, et al. Welcome to the tidyverse. J Open Source Software. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 36.Mitani A, Horie T, Yokoyama R, Nakano Y, Hamada K, Inoue Y, Saito M, Ishii T, Sunohara M, Takahashi R, et al. Interpretations of SARS-CoV-2 IgM and IgG antibody titers in the seroepidemiological study of asymptomatic healthy volunteers. J Infect Chemother. 2022. ;28(2):266–72. doi: 10.1016/j.jiac.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalkanen P, Kolehmainen P, Häkkinen HK, Huttunen M, Tähtinen PA, Lundberg R, Maljanen S, Reinholm A, Tauriainen S, Pakkanen SH, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zani A, Caccuri F, Messali S, Bonfanti C, Caruso A. Serosurvey in BNT162b2 vaccine-elicited neutralizing antibodies against authentic B.1, B.1.1.7, B.1.351, B.1.525 and P.1 SARS-CoV-2 variants. Emerg Microbes Infect. 2021;10(1):1241–43. doi: 10.1080/22221751.2021.1940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol. 2014;134(2):247–59. doi: 10.1016/j.jaci.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmermann CA, Osuna CE, Steuerwald U, Weihe P, Poulsen LK, Grandjean P. Asthma and allergy in children with and without prior measles, mumps, and rubella vaccination. Pediatr Allergy Immunol. 2015;26(8):742–49. doi: 10.1111/pai.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bednarek A, Bodajko-Grochowska A, Klepacz R, Szczekala K, Zarzycka D, Emeryk A. Clinical form of asthma and vaccine immunityin preschoolers. Postepy Dermatol Alergol. 2021;38(1):123–30. doi: 10.5114/ada.2021.104287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romeo J, Wärnberg J, Nova E, Díaz LE, Gómez-Martinez S, Marcos A. Moderate alcohol consumption and the immune system: a review. Br J Nutr. 2007;98(Suppl 1):S111–5. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- 43.Barr T, Helms C, Grant K, Messaoudi I. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:242–51. doi: 10.1016/j.pnpbp.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, Kaneda A, Matsushita K, Hanaoka H, Nakada T-A, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021:j.cmi.2021.07.042. doi: 10.1016/j.cmi.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfizer/BioNtech . Pfizer and BioNtech provide update on booster program in light of the delta-variant 2021; [accessed 2021 Jul 8; 2021 Sep 2]. https://cdn.pfizer.com/pfizercom/2021-07/Delta_Variant_Study_Press_Statement_Final_7.8.21.pdf?IPpR1xZjlwvaUMQ9sRn2FkePcBiRPGqw
- 46.Van Praet JT, Vandecasteele S, De Roo A, De Vriese AS, Reynders M. Humoral and cellular immunogenicity of the BNT162b2 messenger RNA Coronavirus disease 2019 vaccine in nursing home residents. Clin Infect Dis. 2021;73:ciab300. doi: 10.1093/cid/ciab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, Csernalabics B, Lang-Meli J, Janowska I, Staniek J, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–73. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monforte A, Tavelli A, Perrone PM, Za A, Razzini K, Tomasoni D, Bordoni V, Romanò L, Orfeo N, Marchetti G, et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: data from 3,078 health care workers. EClinicalMedicine. 2021;36:100914. doi: 10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


