ABSTRACT
Background
Consolidated information on the effectiveness of COVID-19 booster vaccination in Europe are scarce.
Research design and methods
We assessed the effectiveness of a booster dose of an mRNA vaccine against any SARS-CoV-2 infection (symptomatic or asymptomatic) and severe COVID-19 (hospitalization or death) after over two months from administration among priority target groups (n = 18,524,568) during predominant circulation of the Delta variant in Italy (July–December 2021).
Results
Vaccine effectiveness (VE) against SARS-CoV-2 infection and, to a lesser extent, against severe COVID-19, among people ≥60 years and other high-risk groups (i.e. healthcare workers, residents in long-term-care facilities, and persons with comorbidities or immunocompromised), peaked in the time-interval 3–13 weeks (VE against infection = 67.2%, 95% confidence interval (CI): 62.5–71.3; VE against severe disease = 89.5%, 95% CI: 86.1–92.0) and then declined, waning 26 weeks after full primary vaccination (VE against infection = 12.2%, 95% CI: −4.7–26.4; VE against severe disease = 65.3%, 95% CI: 50.3–75.8). After 3–10 weeks from the administration of a booster dose, VE against infection and severe disease increased to 76.1% (95% CI: 70.4–80.7) and 93.0% (95% CI: 90.2–95.0), respectively.
Conclusions
These results support the ongoing vaccination campaign in Italy, where the administration of a booster dose four months after completion of primary vaccination is recommended.
KEYWORDS: SARS-CoV-2 infection, COVID-19, vaccine effectiveness, booster dose, delta variant, Italy
1. Introduction
In Italy, the COVID-19 vaccination campaign started on 27 December 2020 with priority given to health-care workers (HCW), subjects at increased risk of severe disease (i.e. elderly or otherwise high-risk populations), and essential non-healthcare workers (e.g. school personnel) [1]. The campaign was subsequently extended to the wider eligible population according to an age-based priority system. The primary vaccination series was completed using one, or a combination of two (heterologous vaccination), of the four authorized vaccines in Italy as of December 2021 (i.e. BNT162b2, Pfizer-BioNTech, Mainz, Germany/New York, United States (US); mRNA-1273, Moderna, Cambridge, United States (US); ChAdOx1-S, Oxford-AstraZeneca, Cambridge, United Kingdom (UK); and Ad26.COV2-S, Janssen-Cilag International NV, Beerse, Belgium).
On 27 September 2021, irrespective of the type and numbers of COVID-19 vaccine doses received for primary vaccination, the Italian Ministry of Health recommended a booster dose of an mRNA vaccine at least six months after the completion of the primary vaccination series to persons aged 60 years or above and other high-risk priority groups [1]. The recommendation was subsequently extended on 1 December to all persons aged 18–59 years and on 24 December 2021 to those aged 16–17 years. Finally, on 5 January 2022, it was extended to persons aged 12–15 years. On 24 November 2021, the recommended time-interval for administration of a booster dose was reduced from six to five months after completion of the primary series or diagnosis of infection, whichever came later. The interval was further reduced to four months on 10 January 2022.
In general, although some preprints and peer-reviewed articles have been made available, there is a lack of consolidated information on the effectiveness of COVID-19 booster vaccination in Europe [2–5]. This study aims to assess the effectiveness of a booster dose of an mRNA COVID-19 vaccine after over two months from administration among priority target groups at a time in which the Delta variant (B.1.617.2) was dominant in Italy (Delta phase: 19 July to 12 December 2021 [6]).
2. Methods
2.1. Data sources and selection of the study population
We linked data on vaccinated persons from the Italian National Vaccination Registry (held by the Ministry of Health) with data on notified laboratory-confirmed cases of SARS-CoV-2 infections from the National COVID-19 Integrated Surveillance System (coordinated by the Italian National Institute of Health), by using the individual tax code as key variable [7,8]. The National Vaccination registry includes information on demographic, professional, and clinical characteristics, including those giving priority access to vaccination, for all people who received at least one dose of a COVID-19 vaccine in medically attended facilities. The registry is expected to report dates and vaccine brand for all vaccine administrations, given it was not possible to privately purchase and self-administer COVID-19 vaccines. The National COVID-19 Surveillance System collects data on all notified laboratory-confirmed cases of SARS-CoV-2 infection, including date of testing positive and clinical outcomes (e.g. hospitalization and death). The system reports data for all cases who were laboratory-confirmed in medically attended facilities (pharmacies and private/public health centers) and does not include data of people who self-tested positive through at-home testing.
Data were extracted from both sources on 12 January 2022. We selected all 19,946,920 records of people belonging to the first group targeted for a COVID-19 vaccine booster dose (i.e. people aged ≥60 years and other high-risk priority groups: HCWs, residents in long-term-care facilities (LTCF), and persons with comorbidities or immunocompromised) who had received the first dose of a COVID-19 vaccine before 29 November 2021, thus allowing for at least 14 days of follow-up prior to the end of the Delta phase (on 12 December 2021) to ascertain a possible diagnosis of SARS-CoV-2 infection (Figure 1). For the analysis of severe COVID-19 outcomes (i.e. SARS-CoV-2 infection with subsequent hospitalization or death within 28 days) we considered only persons vaccinated with a first dose before 15 November 2021. In addition to at least 14 days of follow-up to ascertain a possible diagnosis of SARS-CoV-2 infection, this allowed four weeks of observation time post infection to detect the development of severe disease or death, accounting for 17 days of possible notification delays (Figure 1).
Figure 1.
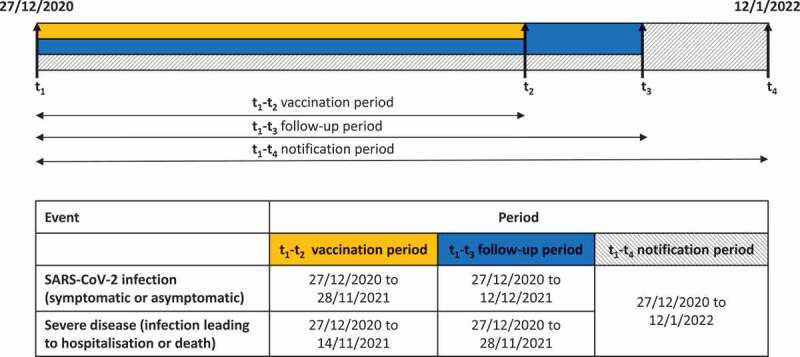
Timeline of the periods of selection and event ascertainment in the population under study, Italy, 27 December 2020 to 12 January 2022 (n = 18,524,568).
Persons who died before 19 July 2021 (starting date of the Delta phase) and those with missing demographic information (n = 21,136; 0.11%) were excluded (Figure 2). We also excluded cases of SARS-CoV-2 infection who tested positive before 19 July 2021 or prior to receiving their first vaccination dose (n = 1,220,583; 6.1%), and those with missing or inconsistent dates of occurrence of clinical outcomes (n = 880; 0.004%). Finally, we excluded vaccinated persons with inconsistent information about their vaccination schedule (n = 179,753; 0.90%), thus leaving a total of 18,524,568 vaccinated persons available for the analysis.
Figure 2.
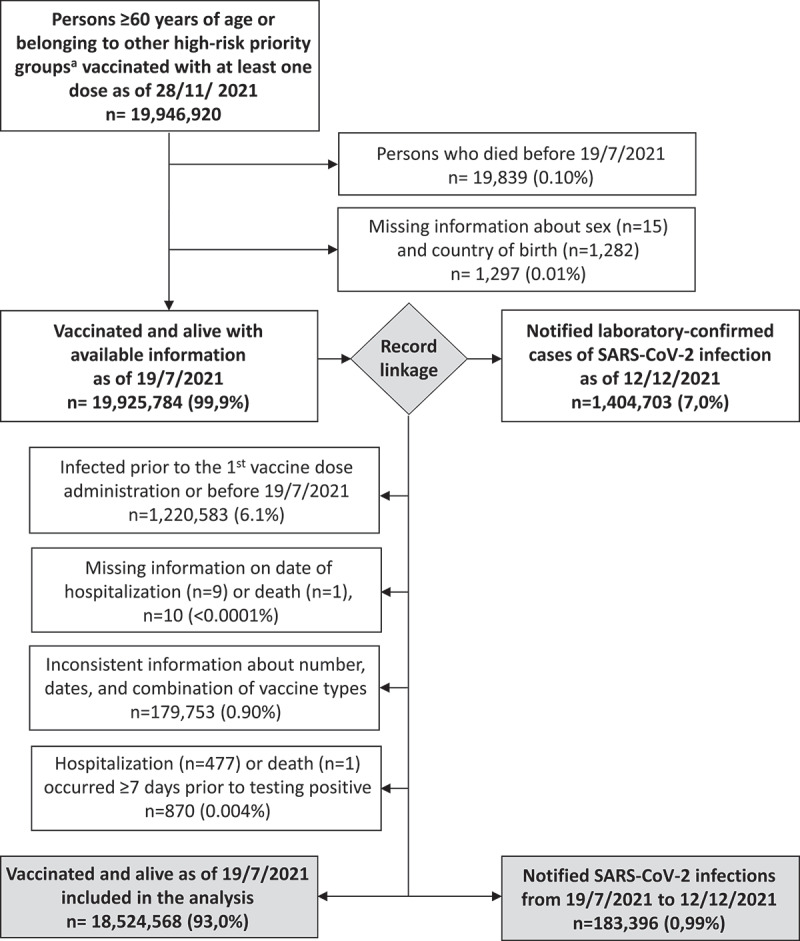
Selection of the population included in the analysis, Italy, 19 July to 28 November 2021 (n = 18,524,568).a i.e. healthcare workers, residents in long-term-care facilities, and persons with comorbidities or immunocompromised.
This study, based on routinely collected data, was not submitted for approval to an ethical committee because the dissemination of COVID-19 surveillance data was authorized by the Italian Presidency of the Council of Ministers on 27 February 2020 (Ordinance no. 640).
2.2. Statistical analysis
We analyzed all notified cases of SARS-CoV-2 infection (symptomatic or asymptomatic) who were laboratory-confirmed through a PCR test (97.6%) or, since 15 January 2021, through an antigenic test (2.4%), as per the current European Center for Disease Prevention and Control (ECDC) laboratory criteria for case definition [9]. Of these, cases who were hospitalized or died in the four weeks following infection with SARS-CoV-2 due to COVID-19 related causes were classified as severe [10].
We split individual data into weekly time intervals after each dose administration and selected all records in the timeframe between 19 July and 12 December 2021, during which the Delta variant was dominant in Italy [6]. We then used multilevel negative-binomial regression models with robust variance estimator to estimate the incidence rate ratios (IRR) of SARS-CoV-2 infection and of severe COVID-19 at different time-intervals since vaccination using the time-interval 4–10 days after the first dose as reference (assuming it as a proxy of exposure for the unvaccinated population [11]). We considered the time-intervals right-truncated at 13 weeks (approximately 3 months), 18 weeks (approximately 4 months), and 26 weeks (approximately 6 months) after completion of primary vaccination series to reflect the cutoff times more frequently recommended for the administration of a booster dose of vaccine worldwide. Time of follow-up ended on the date of SARS-CoV-2 infection for persons who experienced the study events, while it ended on the estimated date of death (see Supplementary document 1 for details about the estimation method) or was censored on 12 December 2021 and on 28 November 2021 for those who, at those dates, were alive and without a diagnosis of SARS-CoV-2 infection and severe COVID-19, respectively. Time of exposure, measured in days, was included as offset in the models.
The models were adjusted to account for possible confounding due to sex, age group (16–24 years, five-year age-groups from 25–29 to 80–84 years, and ≥85 years), country of birth (Italian-born and foreign-born), vaccine received for the first dose, priority group (HCW, LTCF residents, persons with comorbidities, immunocompromised persons, other priority groups, and none), and regional weekly incidence in the general population. Geographical region of vaccination was included into the models as random effect to account for clustering at the local level. The adjusted vaccine effectiveness (VE) was calculated as [(1-IRR)x100].
3. Results
3.1. Demographic and clinical characteristics of the study population
The demographic and clinical characteristics of vaccinated persons included in the study are presented in Table 1.
Table 1.
Demographic and clinical characteristics of persons included in the study by type of vaccine received as first dose, Italy, 19 July to 28 November 2021 (n = 18,524,568)
| |
BNT162b2a |
mRNA-1273b |
ChAdOx1-Sc |
Ad26.COV2-Sd |
Total (n = 31,762,584) |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Total | 12,264,712 | 66.2 | 1,814,836 | 9.8 | 4,185,148 | 22.6 | 259,872 | 1.4 | 18,524,568 | 100.0 |
| Sex | ||||||||||
| Female | 6,848,155 | 66.9 | 1,025,559 | 10.0 | 2,226,749 | 21.8 | 129,804 | 1.3 | 10,230,267 | 100.0 |
| Male | 5,416,557 | 65.3 | 789,277 | 9.5 | 1,958,399 | 23.6 | 130,068 | 1.6 | 8,294,301 | 100.0 |
| Age – median (IQR) | 60 | (69–81) | 61 | (70–81) | 64 | (70–75) | 62 | (65–70) | 61 | (69–78) |
| Age group | ||||||||||
| 16–39 years | 993,005 | 85.5 | 107,540 | 9.3 | 57,663 | 5.0 | 3,452 | 0.3 | 1,161,660 | 100.0 |
| 40–59 years | 1,935,070 | 85.7 | 237,270 | 10.5 | 78,127 | 3.5 | 6,748 | 0.3 | 2,257,215 | 100.0 |
| 60–79 years | 5,888,491 | 53.3 | 947,193 | 8.6 | 3,976,587 | 36.0 | 241,802 | 2.2 | 11,054,073 | 100.0 |
| ≥80 years | 3,448,146 | 85.1 | 522,833 | 12.9 | 72,771 | 1.8 | 7,870 | 0.2 | 4,051,620 | 100.0 |
| Country of birth | ||||||||||
| Italian-born | 11,674,958 | 66.2 | 1,723,900 | 9.8 | 4,015,139 | 22.8 | 225,308 | 1.3 | 17,639,305 | 100.0 |
| Foreign-born | 589,754 | 66.6 | 90,936 | 10.3 | 170,009 | 19.2 | 34,564 | 3.9 | 885,263 | 100.0 |
| Geographical macroareae | ||||||||||
| North-West | 3,188,808 | 62.4 | 510,607 | 10.0 | 1,307,158 | 25.6 | 101,390 | 2.0 | 5,107,963 | 100.0 |
| North-East | 2,295,668 | 65.1 | 305,621 | 8.7 | 857,753 | 24.3 | 64,803 | 1.8 | 3,523,845 | 100.0 |
| Center | 2,568,756 | 69.6 | 328,017 | 8.9 | 740,782 | 20.1 | 50,545 | 1.4 | 3,688,100 | 100.0 |
| South and Islands | 4,211,480 | 67.9 | 670,591 | 10.8 | 1,279,455 | 20.6 | 43,134 | 0.7 | 6,204,660 | 100.0 |
| Priority risk category | ||||||||||
| HCWs | 1,426,349 | 88.7 | 58,226 | 3.6 | 121,653 | 7.6 | 1,728 | 0.1 | 1,607,956 | 100.0 |
| LTCF residents | 243,974 | 86.3 | 34,944 | 12.4 | 3,383 | 1.2 | 325 | 0.1 | 282,626 | 100.0 |
| Persons with comorbiditiesf | 3,525,572 | 83.5 | 596,152 | 14.1 | 89,442 | 2.1 | 11,878 | 0.3 | 4,223,044 | 100.0 |
| Immunocompromisedf | 65,228 | 82.4 | 12,437 | 15.7 | 1,426 | 1.8 | 55 | 0.1 | 79,146 | 100.0 |
| Other risk categoriesg | 335,945 | 44.4 | 60,724 | 8.0 | 350,452 | 46.3 | 9,191 | 1.2 | 756,312 | 100.0 |
| Noneh | 6,667,644 | 57.6 | 1,052,353 | 9.1 | 3,618,792 | 31.3 | 236,695 | 2.0 | 11,575,484 | 100.0 |
HCW, healthcare workers; LTCF, long term care facility.
aBNT162b2, BioNTech-Pfizer, Mainz, Germany/New York, United States (US).
bmRNA-1273, Moderna, Cambridge, United States (US).
cChAdOx1-S, Oxford-AstraZeneca, Cambridge, United Kingdom (UK).
dAd26.COV2-S, Janssen-Cilag International NV, Beerse, Belgium.
eEurostat nomenclature of Italian territorial units for statistics (NUTS-1).
fConditions defining comorbidities giving priority access to vaccination and immunocompromise are listed in the supplement document 2.
gIncluding essential non-HCWs (e.g. school personnel) (n = 394,140; 52.1%), persons living with individuals at increased risk of severe COVID-19 (n = 154,308; 20.4%), and persons with risk exposure not specified (n = 207,864; 27.5%).
hage ≥60 years and no other risk factors.
We did not observe substantial differences in the distribution of first-dose vaccine brands by sex, country of birth, and geographical macroarea. In line with initial national vaccination policies, we observed a relatively higher utilization of the ChAdOx1-S Oxford-AstraZeneca vaccine in persons aged 60–79 years (n = 3,976,587; 36.0%), in persons who did not present any priority risk conditions (n = 3,618,792; 31.3%), and in those who were essential non-HCW workers (e.g. school personnel), persons living with individuals at increased risk of COVID-19, or with unspecified priority risk conditions (n = 350,452; 46.3%). As of 28 November 2021, 41,315 (0.22%) of the vaccinated persons included in the study had received a first vaccine dose within the previous 14 days, 265.563 (1.43%) were partially vaccinated (i.e. had received only a first vaccine dose by >14 days or a second dose by ≤14 days, possible only for vaccines with a two-dose primary schedule), 15,446,172 (83.4%) had completed the primary vaccination series (i.e. received a single-dose schedule or a second dose over 14 days before or a booster dose by ≤14 days), and 2,771,518 (15.0%) had completed primary vaccination and received a booster dose over 14 days before (93.6% with BNT162b2 Pfizer-BioNTech and 6.4% with mRNA-1273 Moderna) .
3.2. Vaccine effectiveness against SARS-CoV-2 infection and severe COVID-19 over time since vaccination
We observed an initial peak in VE against SARS-CoV-2 infection during the first three months (weeks 3–13) after completion of the primary vaccination series (VE = 67%, 95% confidence interval (CI): 63 to 71), followed by a progressive decline to 51% (95% CI: 44 to 58) during the fourth month (weeks 14–18), and to 12% (95% CI: −5 to 26) more than six months later (>26 weeks), when the protection induced by vaccine was estimated to be no longer significant (p = 0.147) (Table 2). Two weeks after booster-dose administration (weeks 3–10), VE against SARS-CoV-2 infection significantly increased to 76% (95% CI: 70 to 81) (p < 0.001). A similar trend in VE was observed in persons ≥80 years of age, those aged 60–79 years, HCWs, and other vaccine priority groups. In all these groups, VE was estimated to be in the range 74–79% two weeks after the booster-dose administration (3–10 weeks).
Table 2.
Vaccine effectiveness against SARS-CoV-2 infection and severe COVID-10 over time since vaccination by age group and priority risk category, Italy, 19 July to 12 December 2021 (n = 18,524,568)
| Any SARS-CoV-2 infectiona |
Severe COVID-19b |
||||||
| |
No. Cases |
Incidence per 100,000 PD |
Adjusted VEd(%) (95% CI) |
|
No.Cases |
Incidence per 100,000 PD |
Adjusted VEd(%) (95% CI) |
| Total | |||||||
| 4–10 days since 1st dose (reference) | 608 | 11.2 | ref. | 115 | 2.2 | ref. | |
| >2 wks. after 1st dose to ≤2 wks. after 2nd dosec | 7,451 | 6.7 | 29.3 (16.3 to 40.2) | 767 | 0.7 | 59.5 (49.4 to 67.6) | |
| 3–13 wks. after completion of primary series | 24,098 | 3.3 | 67.2 (62.5 to 71.3) | 1,406 | 0.2 | 89.5 (86.1 to 92.0) | |
| 14–18 wks. after completion of primary series | 25,561 | 4.9 | 51.4 (43.6 to 58.1) | 2,041 | 0.4 | 82.7 (76.5 to 87.3) | |
| 19–26 wks. after completion of primary series | 63,901 | 8.6 | 29.4 (15.5 to 40.9) | 4,366 | 0.7 | 75.9 (66.3 to 82.7) | |
| >26 wks. after completion of primary series to ≤2 wks. after booster dose | 56,691 | 12.5 | 12.2 (−4.7 to 26.4) | 3,912 | 1.1 | 65.3 (50.3 to 75.8) | |
| 3–10(8)e wks. after booster dose | 4,319 | 4.3 | 76.1 (70.4 to 80.7) | 171 | 0.4 | 93.0 (90.2 to 95.0) | |
| 60–79 years | |||||||
| 4–10 days since 1st dose (reference) | 446 | 10.7 | ref. | 89 | 2.2 | ref. | |
| >2 wks. after 1st dose to ≤2 wks. after 2nd dosec | 5,253 | 6.1 | 29.3 (11.1 to 43.8) | 463 | 0.6 | 65.3 (55.8 to 72.7) | |
| 3–13 wks. after completion of primary series | 17,118 | 3.0 | 68.1 (61.8 to 73.4) | 948 | 0.2 | 91.5 (88.6 to 93.7) | |
| 14–18 wks. after completion of primary series | 17,494 | 5.1 | 49.1 (38.2 to 58.0) | 1,099 | 0.3 | 85.0 (78.9 to 89.3) | |
| 19–26 wks. after completion of primary series | 40,901 | 10.1 | 21.8 (2.2 to 37.4) | 1,952 | 0.6 | 77.5 (66.9 to 84.8) | |
| >26 wks. after completion of primary series to ≤2 wks. after booster dose | 19,766 | 14.1 | 4.1 (−21.9 to 24.6) | 916 | 1.0 | 65.7 (49.0 to 76.9) | |
| 3–10(8)e wks. after booster dose | 1,219 | 4.3 | 75.7 (67.7 to 81.7) | 60 | 0.5 | 86.3 (79.0 to 91.1) | |
| ≥ 80 years | |||||||
| 4–10 days since 1st dose (reference) | 49 | 9.4 | ref. | 21 | 4.3 | ref. | |
| >2 wks. after 1st dose to ≤2 wks. after 2nd dosec | 792 | 7.1 | 30.1 (11.3 to 45.0) | 258 | 2.5 | 43.9 (20.8 to 60.2) | |
| 3–13 wks. after completion of primary series | 1,504 | 3.3 | 61.0 (50.4 to 69.3) | 340 | 0.8 | 80.7 (72.5 to 86.5) | |
| 14–18 wks. after completion of primary series | 3,302 | 3.5 | 54.2 (43.4 to 63.0) | 804 | 0.9 | 77.4 (69.3 to 83.3) | |
| 19–26 wks. after completion of primary series | 8,872 | 4.3 | 43.0 (29.0 to 54.2) | 2,163 | 1.1 | 70.4 (58.8 to 78.7) | |
| >26 wks. after completion of primary series to ≤2 wks. after booster dose | 15,650 | 9.0 | 18.5 (−5.0 to 36.8) | 2,698 | 1.9 | 58.7 (39.4 to 71.9) | |
| 3–10(8)e wks. after booster dose | 1,697 | 3.2 | 78.6 (71.3 to 84.8) | 96 | 0.4 | 94.2 (91.6 to 96.0) | |
| Healthcare workers | |||||||
| 4–10 days since 1st dose (reference) | 18 | 19.5 | ref. | 0 | 0.0 | ref. | |
| >2 wks. after 1st dose to ≤2 wks. after 2nd dosec | 304 | 13.0 | 31.8 (−3.8 to 55.2) | 8 | 0.4 | NC NC |
|
| 3–13 wks. after completion of primary series | 891 | 6.6 | 64.9 (42.6 to 78.6) | 12 | 0.1 | NC NC |
|
| 14–18 wks. after completion of primary series | 922 | 7.1 | 59.3 (28.1 to 76.9) | 15 | 0.1 | NC NC |
|
| 19–26 wks. after completion of primary series | 4,800 | 9.9 | 42.4 (2.0 to 66.2) | 84 | 0.2 | NC NC |
|
| >26 wks. after completion of primary series to ≤2 wks. after booster dose | 18,175 | 13.2 | 28.9 (−22.8 to 58.8) | 302 | 0.2 | NC NC |
|
| 3–10(8)e wks. after booster dose | 1,279 | 6.7 | 78.9 (62.4 to 88.2) | 5 | 0.1 | NC NC |
|
| High-riskpersonsf | |||||||
| 4–10 days since 1st dose (reference) | 141 | 14.5 | ref. | 19 | 2.0 | ref. | |
| >2 wks. after 1st dose to ≤2 wks. after 2nd dosec | 1,953 | 9.0 | 35.0 (21.6 to 46.0) | 215 | 1.0 | 56.5 (44.3 to 66.0) | |
| 3–13 wks. after completion of primary series | 7,398 | 4.1 | 68.7 (62.7 to 73.8) | 403 | 0.2 | 88.9 (84.6 to 92.0) | |
| 14–18 wks. after completion of primary series | 7,439 | 5.2 | 57.1 (46.7 to 65.5) | 600 | 0.4 | 81.4 (72.4 to 87.5) | |
| 19–26 wks. after completion of primary series | 17,299 | 8.6 | 37.5 (19.7 to 51.3) | 1,135 | 0.6 | 74.8 (64.8 to 81.9) | |
| >26 wks. after completion of primary series to ≤2 wks. after booster dose | 14,291 | 15.9 | 15.3 (−6.3 to 32.5) | 1,095 | 1.9 | 59.6 (38.5 to 73.4) | |
| 3–10(8)e wks. after booster dose | 1,230 | 5.8 | 73.9 (65.9 to 80.0) | 74 | 0.7 | 86.1 (77.6 to 91.3) | |
CI, confidence interval; NC, not calculable; PD, person days; wks., weeks.
aIncluding symptomatic and asymptomatic cases of SARS-CoV-2 infection.
bCOVID-19 cases who were hospitalized or died within 28 days from infection.
cincluding only persons who received vaccines with a two-doses primary schedule.
dVaccine effectiveness adjusted by sex, age group, country of birth, priority risk category, vaccine brand, and regional weekly incidence in the general population. Region of vaccination was included into the models as a random effect.
eThe observation period was up to 8 wks. after booster dose administration for the analysis of severe COVID-19.
fi.e. LTCF residents, and person with comorbidities, and immunocompromised persons.
Although to a much lesser extent, VE against severe COVID-19 also declined from 89% (95% CI: 86 to 92) during the first three months (weeks 3–13) after completion of the primary series to 65% (95% CI: 50 to 76) more than six months later (>26 weeks), increasing to 93% (95% CI: 90 to 95) two weeks after the booster-dose administration (weeks 3–8) (Table 2). This pattern was similar in persons aged ≥60 years and other high-risk groups, among whom VE against severe COVID-19 two weeks after booster-dose administration (3–8 weeks) was estimated to be in the range 86–94%.
4. Discussion
The Interim public health considerations for the provision of additional COVID-19 vaccine doses by the ECDC advocate for close monitoring of VE data and for more solid data to inform future policies on booster doses [12]. Current booster-dose uptake across the EU/EEA is extremely heterogeneous with coverage below 30% in several EU/EEA countries [13].
We found that, among people ≥60 years and high-risk groups, VE against SARS-CoV-2 infection and, to a lesser extent, against severe COVID-19 peaked in the time-interval 3–13 weeks and then declined, waning 26 weeks after full primary vaccination regardless of vaccine regimen. Two weeks after the administration of a booster dose, VE increased reaching levels similar to, and sometimes higher than, those estimated in the first three month following completion of primary vaccination.
Several studies have evaluated the effectiveness of a booster dose of COVID-19 vaccine against the Delta variant of SARS-CoV-2 [2–5,14–20], few of them conducted in European countries [2–5]. Although these studies were often based on different designs, study populations, outcome definitions, and reference groups, their results appear generally in line with our findings, showing waning of VE over time since completion of primary vaccination followed by an increase more than 7–14 days after the administration of a booster dose to levels that approximate the highest levels of effectiveness previously observed.
Our study has some limitations. We were unable to control for individual behavioral factors that may have modified the risk of infection. Specifically, persons who received a primary vaccination series of COVID-19 vaccine and those who additionally received a booster dose may have felt more protected compared to those vaccinated with only one dose, possibly resulting in an increased risk exposure and an underestimation of VE.
Second, we adopted a methodology using the time-interval 4–10 days after the first-dose administration as a proxy for the period spent without vaccine-induced protection rather than unvaccinated persons as reference to estimate VE. However, had we done the latter, we would likely have introduced a bias toward an overestimation of VE in the Italian context because since summer of 2021, access to social and working activities for unvaccinated persons is granted only to those testing negative for SARS-CoV-2 infection in the previous 48 hours. Due to this legislation, we observe a higher frequency of testing among unvaccinated people compared with those that are vaccinated, likely resulting in a higher probability of case detection in the former group. This methodological approach was already applied in other published studies on effectiveness of COVID-19 vaccines [21–24].
Finally, it is possible that highly susceptible persons were infected earlier than less susceptible ones and hence were excluded from the risk group used to estimate incidence after a booster dose, leading to overestimation of VE.
5. Conclusions
Our study confirms the effectiveness of an mRNA COVID-19 booster dose in preventing SARS-CoV2 infection and severe COVID-19 and support the ongoing booster vaccination campaign in Italy, where the administration of a booster dose four months after completion of primary vaccination series is currently recommended. Further analyses evaluating VE after a booster dose and its possible waning over time during the recent epidemic phase with predominance of the Omicron variant are needed and should be conducted as soon as sufficient follow-up data will be available.
Supplementary Material
Acknowledgments
We would like to thank Gianpaolo Scalia Tomba for his useful suggestions and:
The members of the Italian Integrated Surveillance of COVID-19 study group and of the COVID-19 Vaccines Registry group: Antonino Bella, Alberto Mateo Urdiales, Martina Del Manso, Massimo Fabiani, Matteo Spuri, Chiara Sacco, Stefano Boros, Maria Cristina Rota, Antonietta Filia, Marco Bressi, Maria Fenicia Vescio, Daniele Petrone, Marco Tallon, Corrado Di Benedetto, Paola Stefanelli, Serena Battilomo, Valeria Proietti, Flavia Riccardo, Patrizio Pezzotti.
The regional representatives of the Italian Integrated Surveillance of Covid-19: Antonia Petrucci (Abruzzo); Michele La Bianca (Basilicata); Anna Domenica Mignuoli (Calabria); Pietro Buono (Campania); Erika Massimiliani (Emilia-Romagna); Fabio Barbone (Friuli Venezia Giulia); Francesco Vairo (Lazio); Camilla Sticchi (Liguria); Danilo Cereda (Lombardia); Lucia Di Furia (Marche); Francesco Sforza (Molise); Annamaria Bassot (P.A. Bolzano); Pier Paolo Benetollo (P.A. Trento); Chiara Pasqualini (Piemonte); Lucia Bisceglia (Puglia); Maria Antonietta Palmas (Sardegna); Salvatore Scondotto (Sicilia); Emanuela Balocchini (Toscana); Anna Tosti (Umbria); Mauro Ruffier (Valle D’Aosta); Filippo Da Re (Veneto);
The Regional representatives of the Italian Covid-19 Vaccines Registry: Camillo Odio (Abruzzo); Michele Recine (Basilicata); Innocenza Ruberto (Calabria); Salvatore Ascione e Massimo Bisogno (Campania); Gandolfo Miserendino, Massimiliano Navacchia (Emilia-Romagna); Beatrice Del Frate, Emanuela Cau (Friuli Venezia Giulia); Diego Baiocchi, Danilo Fusco (Lazio); Domenico Gallo (Liguria); Maria Rosa Marchetti (Lombardia); Liana Spazzafumo (Marche); Raffaele Malatesta (Molise); Antonio Fanolla (P.A. Bolzano); Diego Conforti, Carlo Trentini (P.A. Trento); Antonino Ruggeri (Piemonte); Concetta Ladalardo, Nehludoff Albano (Puglia); Marco Corona, Paolo Lombardi (Sardegna); Massimo Iacono (Sicilia); Paolo Bruno Angori, Andrea Belardinelli (Toscana); Milena Solfiti (Umbria); Stefano Fioraso (Valle D’Aosta); Chiara Poma, Nadia Raccanello (Veneto).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics statement
This study, based on routinely collected data, was not submitted for approval to an ethical committee because the dissemination of COVID-19 surveillance data was authorized by the Italian Presidency of the Council of Ministers on 27 February 2020 (Ordinance no. 640). Because of the retrospective design and the large size of the population under study, in accordance with the Authorization n. 9 released by the Italian data protection authority on the 15th of December 2016, the individual informed consent was not requested for the conduction of this study.
Data sharing
Because of data sharing legal restrictions, the dataset including individual records cannot be made publicly available. However, aggregated data will be shared on reasonable request to the corresponding author (MF).
Author contributions
MF, MP, CM, SSA, AF, FDA, FR, AMU, FMI, and PPe designed the study. MS, MDM, MT, CS, MM, RDC, SBa, FV, MB, DP, and AB retrieved and prepared the data. MF, MP, MS, SSA, CS, VP, MM, and PPe carried out the analysis. MF, MP, CM, SSA, AF, FDA, FR, AMU, AS, PS, ATP, PPa, SBr, GR, FMI, MCR and PPe wrote the manuscript. All authors critically revised and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work.
Supplementary material
Supplemental data for this article can be accessed here
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Italian Ministry of Health . Piano vaccini anti-Covid-19. [cited 2022 Jan 31]. Available from: https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5452&area=nuovoCoronavirus&menu=vuoto
- 2.Veneti L, Bøås H, Bråthen Kristoffersen A, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 omicron BA.1 variant compared with the delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27(4): ii=2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is a published study conducted in Europe evaluating the effectiveness of a booster dose of a COVID-19 vaccine against hospitalization due to infection with the Delta variant of SARS-CoV-2.
- 3.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat Med. 2022. 10.1038/s41591-022-01699-1. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berec L, Šmíd M, Přibylová L, et al.Real-life protection provided by vaccination, booster doses and previous infection against covid-19 infection, hospitalisation or death over time in the Czech Republic: a whole country retrospective view. medRxiv. 2021. 2021.12.10.21267590 2021.12.10.21267590: Preprint 10.1101/2021.12.10.21267590 [DOI] [Google Scholar]; • Although not yet peer-reviewed, this is one of the few studies conducted in Europe that has evaluated the effectiveness of a booster dose of a COVID-19 vaccine against infection and severe disease due to the Delta variant
- 5.Holm Hansen C, Shelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv. 2021. 2021.12.20.21267966 2021.12.20.21267966: Preprint 10.1101/2021.12.20.21267966 [DOI] [Google Scholar]; • Although not yet peer-reviewed, this is one of the few studies conducted in Europe that has evaluated the effectiveness of a booster dose of a COVID-19 vaccine against infection due to the Delta variant
- 6.Istituto Superiore di Sanità (ISS) . Prevalenza e distribuzione delle varianti di SARS-CoV-2 di interesse per la sanità pubblica in Italia . Rapporto n. 16 del 19 gennaio 2022. Rome: ISS; 2022. [cited 2022 Jan 31]. Available from: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-19-gennaio-2022.pdf [Google Scholar]
- 7.Italian government, presidency of the council of ministers. data repository. [cited 2022 Jan 31]. Available from: https://www.governo.it/it/dipartimenti/commissario-straordinario-lemergenza-covid-19/15974
- 8.Riccardo F, Ajelli M, Andrianou XD, et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 march 2020. Euro Surveill. 2020;25(49):2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC) . Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. [cited 2022 January 31]. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition
- 10.World Health Organization (WHO) . International guidelines for certification and classification (coding) of COVID-19 as cause of death. [cited 2022 Jan 31]. Available from: https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf
- 11.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control (ECDC) . Interim public health considerations for the provision of additional COVID-19 vaccine doses. Stockholm: ECDC; 2021. [cited 2022 Jan 31]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Interim-public-health-considerations-for-the-provision-of-additional-COVID-19-vaccine-doses.pdf [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC) . Country overview report: week 4 2022. [cited 2022 Jan 31]. Available from: https://www.ecdc.europa.eu/en/covid-19/country-overviews; •• This web page reports weekly updates on epidemiological key indicators and vaccination coverage in EU/EAA countries
- 14.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barda N, Dagan N, Cohen, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patalon T, Gazit S, Pitzer VE, et al. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med. 2022;182(2):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to covid-19. N Engl J Med. 2021;385(26):2413–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence - 25 U.S. jurisdictions, April 4 - December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chodick G, Tene L, Patalon T, et al. Assessment of effectiveness of 1 dose of BNT162b2 Vaccine for SARS-CoV-2 infection 13 to 24 days after Immunization. JAMA Network Open. 2021;4(6):e2115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74(3):472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo-Urdiales A, Spila Alegiani S, Fabiani M, et al. Risk of SARS-CoV-2 infection and subsequent hospital admission and death at different time intervals since first dose of COVID-19 vaccine administration, Italy, 27 December 2020 to mid-April 2021. Euro Surveill. 2021;26(25):ii=2100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabiani M, Puopolo M, Morciano C, et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ. 2022;376:e069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


