Abstract

Tobacco-specific nitrosamine (TSNA) formation occurred during aerosol generation from select commercial cig-a-like e-cigarette products. To understand the drivers behind the potential formation of TSNAs in electronic cigarette (e-cigarette) aerosols and e-liquids, model e-liquid systems were generated in the lab to demonstrate that nitrite can react with nicotine and minor alkaloids to form TSNAs in e-liquids. In the presence of nitrite and nicotine, TSNA levels in e-liquids increased over time and the process was accelerated by elevated temperature. Additionally, TSNAs formed during aerosol generation when nitrite was present in the corresponding e-liquids. The commercial e-cigarette products that showed higher levels and formation of TSNAs were observed to contain nitrite and minor alkaloid impurities in the corresponding e-liquids. This study provides valuable information about drivers for TSNA formation in e-liquids and e-cigarette aerosols that may be applied to the evaluation and quality assurance of e-cigarette products.
1. Introduction
Tobacco-specific nitrosamines (TSNAs): N-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosoanatabine (NAT), and 4-nitrosoanabasine (NAB) are present in tobacco products and cigarette smoke.1,2 NNN and NNK are known carcinogens1,3−5 and are included on the Food and Drug Administration’s Abbreviated List of Harmful and Potentially Harmful Constituents (HPHCs) in tobacco and tobacco products.6 Studies have shown that TSNAs can form in tobacco during the tobacco curing process, which occurs via nitrosation of nicotine and minor alkaloids through the reaction with nitrite.2,7,8 TSNAs in cigarette smoke have been reported to occur both via direct transfer from tobacco filler, as well as from pyrosynthesis during the smoking process.9−11 TSNAs in moist smokeless tobacco (MST) can form during the processes of curing, fermentation, and product storage.2,12,13
In 2019, the FDA issued guidance for the premarket tobacco product application for Electronic Nicotine Delivery Systems (ENDS), specifying that levels of NNN and NNK should be measured in e-liquids and aerosols.14 ENDS, also referred to as electronic cigarettes (e-cigarettes), formulations typically contain tobacco-derived nicotine and may contain impurities of other tobacco constituents. Previous studies have reported that trace levels of TSNAs, minor alkaloids, and other HPHCs are present in e-cigarette e-liquids and aerosols.15−23 As there are fewer chemicals and lower potential for microbial activities in e-cigarette e-liquids,24−27 TSNA formation was anticipated to be less likely to occur in e-cigarette e-liquids and aerosols. Farsalinos et al. reported that TSNA levels in the aerosols were similar to the e-liquid content.16
However, when conducting a market survey in 2016, our preliminary internal studies showed that TSNA levels were higher in the aerosols than in the e-liquids for some brands of cig-a-like e-cigarette products, specifically some rechargeable e-cigarettes with disposable prefilled cartridges.19 These results indicated that TSNA formation within these products occurred during vaporization of the e-liquid to generate aerosols. The temperature at the center of the heating coil of cig-a-like e-cigarettes can reach 200–300 °C during the aerosol generation process,28−31 and this could potentially facilitate TSNA formation under necessary reaction conditions, such as the presence of the needed reactants. In addition, the presence of nitrite was detected in the corresponding e-liquids. The combination of nicotine and minor alkaloids with nitrite can lead to the formation of TSNAs in e-cigarette e-liquids and aerosols. To date, no literature has evaluated pathways for TSNA formation in e-cigarette e-liquid and aerosols. Consequently, the objective of this study was to investigate the effect of nitrite on the formation of TSNAs in e-cigarette e-liquids and aerosols to evaluate pathways for TSNA formation and to determine what other factors contribute to TSNA formation in the e-cigarette liquids and aerosols. As NNK and NNN are known carcinogens and present in some commercial e-cigarette products, we also performed a risk assessment to evaluate NNN and NNK exposures in these products.
2. Materials and Methods
2.1. Test Products
2.1.1. Reference E-Liquids
Two reference e-liquids were prepared in the lab to generate model systems for studying the impact of nitrite and minor alkaloids on TSNA formation in nicotine-based e-liquids and e-cigarette aerosols. The ingredients of propylene glycol (PG), glycerin (Gly), and nicotine used for preparing the e-liquids were all USP grade with purity greater than 99%, and the water was Type 1 (Milli-Q) water. E-liquid 1, composed of 82.5% (weight/weight) propylene glycol/glycerin (50:50), 15% water, and 2.5% nicotine, was used to study the TSNA formation in the e-liquids containing nicotine and in the corresponding aerosols. Depending on the purity of the tobacco-derived nicotine used for preparing e-liquids, different concentrations of minor alkaloid contaminations could be inherently present with different e-liquids. To offset any potential interferences from minor alkaloid contamination and nicotine effects, the e-liquid 2 model system, which was used to study the contribution of minor alkaloids on TSNA formation in e-liquids and aerosols, was prepared without any nicotine and was composed of 85% propylene glycol/glycerin (50:50) and 15% water. Empty MarkTen XL cartridges (Nu Mark, LLC, Richmond, VA) were filled with e-liquids for aerosol generation.
2.1.2. Commercial Cig-a-Like E-Cigarette Products
Eight top-selling commercial cig-a-like e-cigarette products (specifically, rechargeable e-cigarettes with disposable prefilled cartridges) that were available in the US market in 2017 were purchased for chemical analysis. In addition to others, these products included MarkTen XL e-cigarettes (Nu Mark LLC, an Altria company, Richmond, VA) with classic (3.5% nicotine by weight [NBW]) and menthol flavors (3.5% NBW). These purchased e-cigarette products, designated as products A through H, included a variety of flavors and nicotine concentrations ranging from 1.7 to 4.8% NBW. The main components in the e-liquids were analyzed using gas chromatography (GC) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD),44 and the percent compositions (w/w) of the major constituents in the e-liquids are provided in Table 1. All of these devices were low-power devices due to the size of the battery and the resistance of the coils being greater than 2.3 ohms (see the Supporting Information Table S1 for a summary of the physical properties of the e-cigarettes tested).
Table 1. Percent Composition by Weight (w/w) of the Major Constituents in the E-Liquids of the E-Cigarettes Testedc.
| product code | glycerin (%) | propylene glycol (%) | water (%) | nicotine (%) | menthol (%) | pH |
|---|---|---|---|---|---|---|
| A | 53.6 | 33.4 | 8.28 | 2.14 | ND | 8.43 |
| B | 85.2 | ND | 12.1 | 1.70 | ND | 8.78 |
| C | 54.2 | 35.0 | 6.62 | 2.17 | ND | 8.65 |
| Da | 33.2 | 49.6 | 10.1 | 3.34 | 3.10 | 9.48 |
| Eb | 56.1 | 23.8 | 14.7 | 3.36 | ND | 8.15 |
| F | 66.8 | 16.9 | 7.03 | 4.56 | ND | 7.11 |
| G | 59.0 | 24.2 | 6.15 | 4.43 | 0.87 | 7.44 |
| H | 59.2 | 24.1 | 6.94 | 4.33 | ND | 7.48 |
MarkTen XL menthol.
MarkTen XL classic.
ND-not detected.
2.1.3. E-Liquid from Commercial Cig-a-Like E-Cigarette Products
E-liquids from the commercial e-cigarette products were removed from the cartridges by centrifugation. Each cartridge was placed mouth-end down in a 25 mL ISOLUTE reservoir tube (P/N 120-1007-E, Biotage, Charlotte, NC), which was then placed in a centrifuge tube so that the cartridge would not contact the liquids removed from the cartridge, and centrifuged for 20 min at 3000 RPM.
2.1.4. Aerosol Collection
Aerosol collections were conducted under the environmental conditions specified in ISO 3402,15 with an operating temperature of 22.0 ± 2.0 °C and relative humidity of 60 ± 5%. The aerosol samples were collected on a Cerulean SM450 20 port linear puffing machine (Cerulean, Richmond, VA) using puffing regimen parameters of 55 mL puff volume, 5 s puff duration, and 30 s puff interval in a square wave profile. This aerosol generation and collection protocol is similar to Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA)-recommended method No. 8132 for machine aerosol generation, except for a 5 s puff duration instead of 3 s. A longer puff duration was chosen to increase aerosol generation because puff duration determines the time for which the coil is heated and is therefore directly related to the amount of aerosol that is generated.33 The empty MarkTen XL cartridges were filled with 900 mg of the reference e-liquid, which is the typical commercial loading for MarkTen XL cartridges, and device batteries were fully charged prior to aerosol collection. The aerosol was collected over 150 puffs unless specified otherwise or until battery exhaustion. To prevent cross-contamination from combustible tobacco products, all aerosol collections were conducted in a laboratory and on equipment that had only been used for the collection of e-cigarette aerosols. Additionally, blank collections were analyzed concurrently with the aerosol samples to confirm that background contamination had not occurred. The aerosols were collected on 44 mm fiberglass Cambridge filter pads (Part # 80202085, Borgwaldt, KC). The e-cigarette devices were weighed before and after aerosol collection to determine e-liquid consumption, and the pad holders were weighed before and after aerosol collection to obtain the aerosol mass collected. The liquid consumption was calculated by subtracting the e-cigarette device weight after puffing from the weight before puffing. Aerosol mass collected on the pads was calculated by determining the difference in pad holder weights before and after puffing.
2.2. Methods of Analysis
2.2.1. Analysis of TSNAs in E-Liquids and E-Cigarette Aerosols
The e-liquid and aerosol samples were analyzed for NNK (Chemical Abstracts Service (CAS) # 64091-91-4), NNN (CAS # 16543-55-8), NAB (CAS # 1133-64-8), and NAT (CAS # 71267-22-6) by Waters ACQUITY ultra-performance liquid chromatography (UPLC) with XEVO TQ-S micro tandem mass spectrometry (UPLC-MS/MS). The method was developed and adapted from the reference method “Determination of TSNAs in e-Cigarette Liquids and Aerosols by UPLC-MS/MS.”19 The column used was Waters ACQUITY UPLC BEH C18, 2.1 mm × 50 mm, 1.7 μm (Part # 186002550, Waters, Milford, MA), and the column temperature was set at 60 °C. Mobile phase A was Type I water, and mobile phase B was 0.1% acetic acid in methanol. The International Standards Organization (ISO) Guide 34-certified TSNA standard stock solutions were purchased from SPEX Certiprep (Metuchen, NJ), and the isotopically labeled internal standards for each corresponding TSNA were purchased from Toronto Research Chemicals (Ontario, Canada). The calibration standards ranged from 0.01 to 5 ng/mL for NNK, NAB, and NAT, and ranged from 0.02 to 10 ng/mL for NNN.
An aliquot of 0.5 g e-liquid and deuterated labeled internal standard solution was added to 10 mL of 100 mM ammonium acetate; the mixed solution was then preconcentrated (3:1) and cleaned up through solid-phase extraction (SPE). The SPE HyperSep Retain PEP Cartridges (Cat # 60107-203, Thermo Fisher Scientific, Waltham, MA) used were 60 mg in bed weight and 3 mL in column volume. The SPE cartridge was preconditioned with methanol and water, then 3 mL of extract was loaded onto the SPE cartridge. The loaded cartridge was then washed with Milli-Q water/methanol/ammonium hydroxide (65:4.5:0.5) and 0.01% formic acid, after which the cartridge was dried under vacuum, and finally, the analytes were eluted with 1 mL of methanol/water (70:30, v/v) with 0.1% acetic acid. The samples were then analyzed on a UPLC/MS/MS. The limit of quantification (LOQ) for the e-liquids was 0.2 ng/g of e-liquid for NNK, NAB, and NAT, and 0.4 ng/g of e-liquid for NNN based on the lowest calibration standard.
For e-cigarette aerosol samples, an internal standard solution was added to the pad with the collected aerosol and extracted with 10 mL of 100 mM ammonium acetate for 30 min. The samples were then prepared and analyzed in the same manner as the e-liquid samples. The LOQ for the aerosol samples was 0.2 ng/g of aerosol collected (0.002 ng/puff) for NNK, NAB, and NAT, and 0.4 ng/g of aerosol collected (0.004 ng/puff) for NNN, assuming 0.5 g or 50 puffs of aerosols were collected.
2.2.2. Analysis of Nitrite in E-Liquids
Nitrite (CAS # 14797-65-0) in e-liquids was analyzed using ion chromatography (IC). The method was developed and adapted from the IC method reported in the CORESTA technical report.34 The IC instrument consisted of a Thermo Scientific Dionex Ion Chromatography ICS 5000 and AERS 500 electrolytically regenerated suppressor, AS 19 analytical column (4 mm × 250 mm), and AG 19 guard column (4 mm × 50 mm; Sunnyvale, CA). The eluent consisted of potassium hydroxide at gradient concentration ranges of 10–55 mM and was generated with a potassium hydroxide eluent generator cartridge (Thermo Scientific). ISO Guide 34-certified nitrite stock solution (1000 ppm) was purchased from Thermo Scientific. The e-liquid sample (0.2 g) was added to 10 mL of Type I water and mixed well. The samples were then filtered and analyzed using IC with suppressed conductivity detection. The calibration range was 0.01–1 μg/mL. The LOQ for nitrite in e-liquid was 0.5 μg/g of e-liquid based on the lowest calibration standard.
2.2.3. Analysis of Minor Alkaloids in E-Liquids
E-liquids were analyzed for the minor alkaloids: nornicotine (CAS # 5746-86-1), anatabine (CAS # 2743-90-0), and anabasine (CAS # 13078-04-1) using a Waters ACQUITY UPLC with XEVO TQD mass spectrometer (Milford, MA). The method was developed and validated internally in our lab at Altria Client Services LLC. The column used was a Waters X-Bridge C18 2.1 mm × 50 mm (2.5 μm) column, and the column temperature was ambient. The mobile phase A was a mixture of water/1 M acetic acid/6 N ammonium hydroxide (900:10:13, v/v/v), adjusted to pH 10 with 6 N ammonium hydroxide or 1 M acetic acid. The mobile phase B was methanol. The minor alkaloid standards and the isotopically labeled deuterated internal standards were purchased from Toronto Research Chemicals (Ontario, Canada). An aliquot of 0.4 g of e-liquid samples was added to 10 mL of extraction solution (70:30, v/v, methanol:water) that contained the deuterated internal standards (2.0 μg/mL) and was mixed well. The extract was then analyzed on a UPLC/MS. The calibration ranges were 0.1–5 μg/mL for each minor alkaloid, and the LOQ was 2.5 μg/g of e-liquid for each minor alkaloid in the e-liquid.
2.3. Experimental Section
2.3.1. Experiments for TSNA Formation in E-Liquid Containing Nicotine and Nitrite
For an over time study, e-liquid 1 was fortified with three concentrations of nitrite (2, 10, and 50 μg/g) and stored in amber bottles under ambient conditions (22 °C) for 12 weeks. In addition, a control sample was evaluated, in which the unfortified e-liquid 1 was stored under identical conditions. An aliquot of liquid was removed and analyzed for TSNAs at various time points, including the initial time point, week 2, week 8, and week 12. Three replicates were analyzed for each sample.
For the temperature effect study, five sets of samples were prepared by transferring 0.2 g of the e-liquid 1 fortified with 2 μg/g nitrite into 20 mL headspace vials. Four sets of these samples were heated in a convection GC oven for 5 min at 80, 150, 200, and 250 °C, respectively, and the fifth sample set was held at ambient temperature (22 °C) to serve as a control sample. After heating, the samples were cooled down on the lab bench for 20 min and analyzed for TSNA content.
For TSNA formation in the aerosol study, the MarkTen XL cartridges filled with e-liquids 1 without fortification of nitrite and e-liquid 1 fortified with two levels of nitrite (2 and 10 μg/g) were vaped on a puffing machine, and 50 puffs of the aerosols were collected for TSNA analysis. In addition, the e-liquids used for filling the cartridges were also analyzed for TSNAs.
2.3.2. Experiments for TSNA Formation in E-Liquid Containing Minor Alkaloids and Nitrite
For sample preparation, the e-liquid 2 fortified with 2 μg/g of nitrite was then individually fortified with 10 μg/g nornicotine, 10 μg/g anabasine, and 10 μg/g anatabine, respectively. The levels of minor alkaloids and added nitrite were in the range of levels detected in e-liquids analyzed from select commercial cig-a-like e-cigarette products in the preliminary study.
For an over time study, the e-liquids that contained each respective minor alkaloid and nitrite were stored in amber bottles at room temperature (22 °C) and then analyzed for TSNAs at the initial time point, week 2, week 4, and week 8. In addition, a control sample was evaluated, in which unfortified e-liquid 2 was stored under identical conditions.
For the temperature effect study, the e-liquid 2 fortified with each minor alkaloid and nitrite was heated at 150 and 200 °C in a convection GC oven for 5 min. After cooling to room temperature on a lab bench for 20 min, the e-liquids were analyzed for TSNAs.
For TSNA in the aerosol study, MarkTen XL cartridges filled with e-liquid 2 fortified with nitrite and minor alkaloids were puffed for aerosol collections over three puff blocks, with 50 puffs in each puff block. TSNAs in the aerosol were analyzed, and the average levels of TSNAs in each puff block (or total 150 puffs) were normalized to ng per gram of aerosol mass. TSNAs were also analyzed in the e-liquids used for filling the cartridges.
3. Results and Discussion
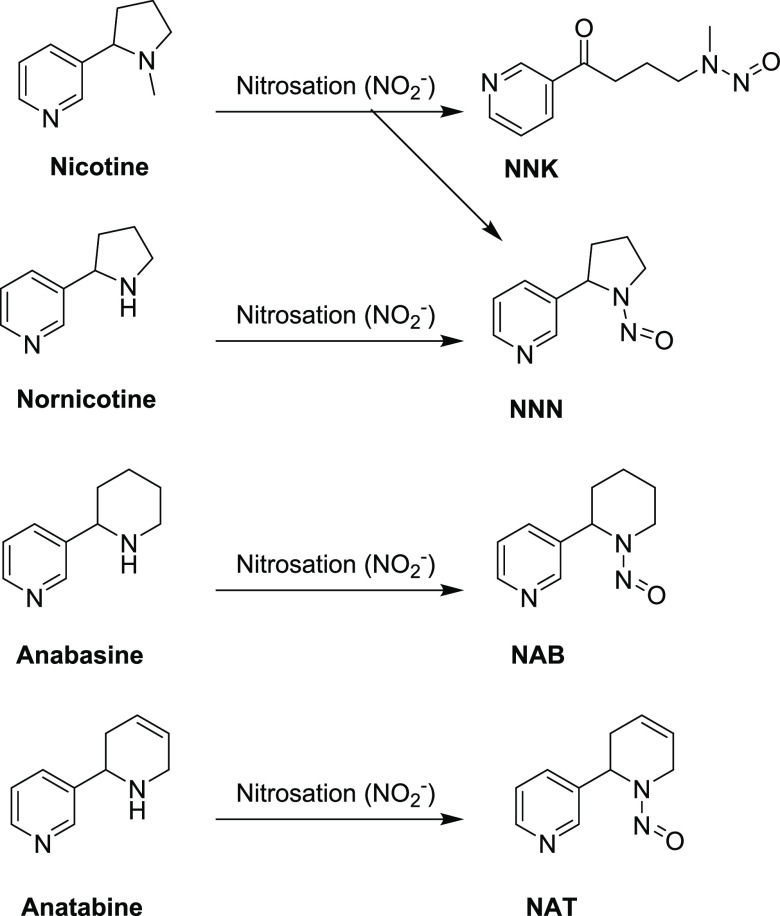
Hoffmann et al. reported that TSNAs are formed in tobacco through the nitrosation of nicotine and specific minor alkaloids that are inherent to the tobacco leaf/plant.8 Nicotine may react with nitrite to form NNK and NNN, nornicotine may react with nitrite to form NNN, anabasine may react with nitrite to form NAB, and anatabine may react with nitrite to form NAT. Figure 1 illustrates TSNA formation pathways. We based our hypothesis to study TSNA formation in e-liquids and e-cigarette aerosol on these pathways.
Figure 1.
TSNA formation pathway. NO2–- nitrite.
3.1. TSNA Formation in E-Liquid Containing Nicotine and Nitrite
E-liquids are typically made from tobacco-derived nicotine and may contain nitrite and other impurities such as minor alkaloids.15,17 As the TSNA formation pathway shows above, nicotine and nitrite are major precursors for TSNA formation. For this reason, we evaluated the contribution of nitrite to TSNA formation in the e-liquids containing nicotine and in the corresponding aerosols using the reference e-liquid 1 model system.
3.1.1. TSNA Formation in E-Liquid Containing Nicotine and Nitrite over time
The data for the control samples showed that NNK was either not detected or lower than the LOQ, and NNN was not detected over 12 weeks. The data for the samples fortified with different nitrite concentrations demonstrated that NNK and NNN concentrations increased linearly with time (Figure 2A,B). The slopes of the regression equations for NNK were greater than those of NNN when the same concentrations of nitrite were added, which indicated that the rate of NNK formation was higher than the rate of NNN formation in the e-liquids containing nicotine, and more NNK was formed compared to NNN in the nitrite fortified e-liquid 1. Figure 2A,B also demonstrated the reaction kinetics of the formation of NNK and NNN from nicotine and nitrite. The higher the concentration of added nitrite, the steeper the regression’s slope. The slopes represent the linear reaction rates that are dependent on the nitrite concentration, as the liquid used in this study contained excess nicotine (2.5% NBW). Based on the data in Figure 2A,B, the rate constant was 1.3 × 10–13 (nM/week) for NNK and 1.1 × 10–14 (nM/week) for NNN, calculated based on the publication by Caldwell et al.45
Figure 2.

Amount of NNK (A) and NNN (B) in e-liquid 1 fortified with different concentrations of nitrite and evaluated over 12 weeks (n = 3). Over the 12-week study, NNN was not detected in the samples fortified with 2 μg/g nitrite and is not shown on the chart. Error bars not seen in lines are due to small SDs.
A model e-liquid containing 97.5% PG/Gly (50:50, w/w) and 2.5% nicotine (without water) was also subjected to the same study as the model e-liquid 1 stored at room temperature (22 °C) over 12 weeks. The results showed that more NNN and NNK formed in the e-liquid without water compared to those formed in the model e-liquid 1 containing water at each time points over 12 weeks when nitrite was present (see the Supporting Information Table S2 for comparison of NNK and NNN formation in the model e-liquid 1 and e-liquid without water over 12 weeks when nitrite was present). This result is consistent with a report by Sun et al. that TSNA formation decreased as the water content increased during tobacco leave storage.46
3.1.2. Effect of Temperature on TSNA Formation in E-Liquids Containing Nicotine and Nitrite
TSNA levels in the e-liquid 1 fortified with nitrite at elevated temperatures are presented in Table 2. Initiation of NNK formation was observed at 150 °C, while detectable initiation of NNN formation was observed at 200 °C when samples were heated for 5 min. In addition, both the formation of NNK and NNN increased with increasing temperature. NAB and NAT were not detected (ND) in the liquids in Table 2, which was due to the lack of anabasine and anatabine precursors.
Table 2. Effect of Temperature on TSNA Formation in E-Liquids Containing Nitrite (n = 3)a.
| temperature (°C) | NNK (ng/g) | NNN (ng/g) | NAB (ng/g) | NAT (ng/g) |
|---|---|---|---|---|
| 22 (ambient temp.) | <2.5 | ND | ND | ND |
| 80 | <2.5 | ND | ND | ND |
| 150 | 7.77 ± 1.66 | ND | ND | ND |
| 200 | 1678 ± 14 | 35.4 ± 3.4 | ND | ND |
| 250 | 2286 ± 402 | 144.3 ± 15.1 | ND | ND |
ND-not detected.
3.1.3. TSNA Formation in Aerosol of E-Liquid Containing Nicotine and Nitrite
The aerosol mass collected on the pads and the liquid consumption estimated through device loss were comparable (See the Supporting Information Table S3 for a summary of the percent difference between aerosol mass collected and liquid consumption). TSNA levels in the aerosol were calculated on a ng/g-of-aerosol-mass-collected basis, as shown in Figure 3. TSNA in aerosol from the control sample (e-liquid 1 without fortification of nitrite) was not detected or was lower than LOQ, while NNK, NNN, and NAT were detected in the aerosol from the e-liquid fortified with nitrite. NAB levels in the aerosol were lower than the LOQ (0.2 ng/g) and were not included in Figure 3. Nitrosation of nicotine, as well as any nornicotine impurity in the e-liquid (for NNN only), contributed to the formation of NNK and NNN in the aerosol. The low levels of NAT (0.64 ng/g) in the aerosol could be the result of nitrosation of any anatabine impurity present in the e-liquid.
Figure 3.
TSNA in the aerosols of e-liquid 1 fortified with 0, 2, and 10 μg/g nitrite (n = 3). TSNA not detected or lower than the LOQ are not shown on the chart.
TSNA levels in the e-liquids and aerosols are compared in Table 3. The data show that the levels of TSNA in the aerosol are higher than those in the nitrite fortified e-liquid 1. These results indicated that additional TSNA formation occurred during the aerosolization process.
Table 3. Comparison of TSNA Levels in E-Liquid and Aerosol (n = 3 for E-Liquid, n = 4 for Aerosol)a.
| NNK (ng/g) |
NNN (ng/g) |
NAB (ng/g) |
NAT (ng/g) |
|||||
|---|---|---|---|---|---|---|---|---|
| nitrite added | liquid | aerosol | liquid | aerosol | liquid | aerosol | liquid | aerosol |
| 0 μg/g | ND | <0.37 | ND | <0.73 | ND | ND | ND | ND |
| 2 μg/g | 0.64 ± 0.06 | 12.0 ± 1.5 | ND | 1.6 ± 0.3 | ND | <0.37 | ND | <0.37 |
| 10 μg/g | 2.63 ± 0.02 | 53.8 ± 7.4 | ND | 3.4 ± 0.9 | ND | <0.39 | ND | 0.64 ± 0.16 |
ND-not detected.
3.2. TSNA Formation in E-Liquid Containing Minor Alkaloids and Nitrite
As the TSNA formation pathway shows (Figure 1), minor alkaloids (nornicotine, anabasine, and anatabine) are also precursors for TSNA formation when nitrite is present. They could also be potentially present as impurities in the tobacco-derived nicotine used in e-liquids, as discussed earlier. For this reason, we also studied the contribution of the minor alkaloids to TSNA formation in the e-liquids and in the corresponding aerosols using the reference e-liquid 2 model system. The e-liquid without nicotine was used to eliminate nicotine interference since nicotine could react with nitrite to form NNN as demonstrated in the TSNA Formation in E-Liquid Containing Nicotine and Nitrite section above.
3.2.1. TSNA Formation in E-Liquids Containing Minor Alkaloids and Nitrite over Time
The data showed that NNN, NNK, NAB, and NAT were not detected or were lower than LOQ in the control samples. In the e-liquid 2 fortified with nitrite and nornicotine, NNN was formed, and the levels increased over 8 weeks while NNK, NAB, and NAT were not detected. In the e-liquid 2 fortified with nitrite and anabasine, NAB was formed, and the levels increased over 8 weeks, while NNK, NNN, and NAT were not detected. In the e-liquid 2 fortified with nitrite and anatabine, NAT was formed, and the levels increased over 8 weeks, while NNK, NAB, and NNN were not detected. The amount of the respective TSNAs formed in the e-liquid containing nitrite and the minor alkaloids are shown in Figure 4. Higher levels of NNN and NAT were formed compared to NAB during the 8-week study, and the results indicated that the formation rate of NAB might be slower than that of NNN and NAT at room temperature condition (22 °C).
Figure 4.
TSNAs in e-liquid 2 with fortified minor alkaloids and nitrite, over 8 weeks (n = 3).
3.2.2. Effect of Temperature on TSNA Formation in E-Liquid Containing Minor Alkaloids and Nitrite
The data are listed in Table 4. NNN, NAB, and NAT were formed in the e-liquid 2 fortified with nitrite and nornicotine, anabasine, and anatabine, respectively. As heat is known to accelerate TSNA formation, higher amounts of each corresponding TSNA formed upon heating at 200 °C than at 150 °C. Note that for this set of experiments, NNK was not detected under most conditions, as we used the e-liquid 2 model system that did not contain nicotine.
Table 4. TSNA Levels in E-Liquid 2 Containing Minor Alkaloids and Nitrite at 150 and 200 °C (n = 3)a.
| NNK (ng/g) |
NNN (ng/g) |
NAB (ng/g) |
NAT (ng/g) |
|||||
|---|---|---|---|---|---|---|---|---|
| minor alkaloids contained | 150 (°C) | 200 (°C) | 150 (°C) | 200 (°C) | 150 (°C) | 200 (°C) | 150 (°C) | 200 (°C) |
| nornicotine | ND | <0.2 | 4.6 ± 1.6 | 49.2 ± 1.5 | ND | ND | ND | ND |
| anabasine | ND | ND | ND | ND | 0.37 ± 0.03 | 7.8 ± 3.9 | ND | ND |
| anatabine | ND | ND | ND | ND | ND | ND | 0.36 ± 0.07 | 8.4 ± 0.3 |
ND-not detected.
3.2.3. TSNA Formation in Aerosol of E-Liquid Containing Minor Alkaloids and Nitrite
The levels of TSNAs in the e-liquids and aerosol are compared in Figure 5. NNN and NAT levels were higher in the aerosol than those in their corresponding e-liquids. Trace levels of NAB (less than the LOQ of 0.2 ng/g) that formed in the aerosol are not shown in Figure 5. NNK was not formed in the aerosol due to the lack of the precursor nicotine. High levels of NNN were formed compared to NAB and NAT, which can be explained by the higher NNN formation rate that was demonstrated in the temperature study.
Figure 5.
Levels of TSNAs in e-liquid 2 fortified with 0.2 μg/g nitrite and 10 μg/g of each minor alkaloid, respectively, and the corresponding aerosols (n = 3 for e-liquid and n = 4 for aerosol).
3.3. Evaluation of Commercial Cig-A-Like E-Cigarette Products
Results from the above study with model e-liquid systems 1 and 2 demonstrated that the presence of nitrite and nicotine or minor alkaloids within e-liquids can facilitate the formation of TSNAs both in e-liquids and the aerosol of e-cigarette products. To corroborate these findings, we measured nitrite and minor alkaloid concentrations in e-liquids from eight commercially available cig-a-like e-cigarette products (products A through H) and correlated those findings to TSNA concentrations in the e-liquids and aerosols for the corresponding product. For evaluation of e-liquid in each product prior to the analysis, the e-liquids were removed from the cartridges through centrifugation, as detailed in the E-Liquid from Commercial Cig-a-Like E-Cigarette Products section above.
3.3.1. Nitrite and Minor Alkaloids in E-Liquid of Commercial Cig-A-Like E-Cigarette Products
Nitrite and minor alkaloids in the e-liquid of the commercial cig-a-like e-cigarette products were analyzed in three replicates, and the data are listed in Table 5. The nitrite levels were below the LOQ (0.5 μg/g) in the e-liquid of the majority of the products tested, with the exception of products G and H. Quantifiable nornicotine was present in five of the eight commercial e-cigarette products; quantifiable anabasine was present in the products E and H; and quantifiable anatabine was present in the products B, E, and H. The presence of nitrite and minor alkaloids in the e-liquid of commercial products was possibly due to the impurities within the nicotine derived from tobacco extracts, as tobacco normally contains both nitrite and minor alkaloids. Nitrite could also be present as a possible impurity from the e-liquid additives or ingredients, or from another source.
Table 5. Nitrite and Minor Alkaloids in the E-Liquid of Commercial Cig-A-Like E-Cigarettes (n = 3)c.
| product code | nitrite (μg/g) | nornicotine (μg/g) | anabasine (μg/g) | anatabine (μg/g) |
|---|---|---|---|---|
| A | <0.5 | 3.2 ± 1.0 | <2.5 | ND |
| B | <0.5 | 11.8 ± 2.5 | <2.5 | 2.6 ± 0.1 |
| C | <0.5 | <2.5 | ND | ND |
| Da | <0.5 | 4.0 ± 1.7 | ND | ND |
| Eb | <0.5 | 5.7 ± 0.3 | 8.3 ± 0.2 | 9.3 ± 0.1 |
| F | <0.5 | <2.5 | ND | ND |
| G | 0.8 ± 0.2 | <2.5 | ND | ND |
| H | 9.9 ± 0.2 | 8.9 ± 0.2 | 9.3 ± 0.2 | 2.5 ± 0.1 |
MarkTen XL menthol.
MarkTen XL classic.
ND-not detected.
3.3.2. TSNAs in Commercial Cig-A-Like E-Cigarette Products
The TSNAs detected in the e-liquids and the aerosols of each product A through H are listed in Tables 6 and 7. On average, TSNA levels in both the e-liquids and aerosols of commercial e-cigarette products B, G (NNK only), and H were much higher than the TSNA levels observed in the other products tested.
Table 6. TSNAs in the E-Liquid on a ng/g Basis for Commercial Cig-A-Like E-Cigarette Products A through H (n = 4)c.
| product code | NNK (ng/g) | NNN (ng/g) | NAB (ng/g) | NAT (ng/g) |
|---|---|---|---|---|
| A | <0.2 | ND | ND | ND |
| B | 3.37 | 31.3 | 9.59 | 23.2 |
| C | <0.2 | ND | ND | ND |
| Da | 0.52 | 0.48 | ND | ND |
| Eb | 1.81 | ND | <0.2 | <0.2 |
| F | 1.13 | ND | ND | 0.39 |
| G | 14.0 | 0.44 | ND | 0.25 |
| H | 27.2 | 12.8 | 3.42 | 1.27 |
MarkTen XL menthol.
MarkTen XL classic.
ND-not detected.
Table 7. TSNAs in the Aerosol of Commercial Cig-A-Like E-Cigarette Products A through H (n = 4)c.
| ng/g of aerosol mass collected |
ng/puff |
|||||||
|---|---|---|---|---|---|---|---|---|
| product code | NNK | NNN | NAB | NAT | NNK | NNN | NAB | NAT |
| A | 0.40 | ND | ND | ND | 0.0015 | ND | ND | ND |
| B | 4.46 | 30.3 | 8.30 | 21.2 | 0.021 | 0.15 | 0.040 | 0.10 |
| C | 0.90 | ND | ND | ND | 0.0045 | ND | ND | ND |
| Da | 2.55 | 3.03 | ND | ND | 0.011 | 0.013 | ND | ND |
| Eb | 1.98 | 0.65 | <0.14 | <0.14 | 0.0098 | 0.0032 | <0.0007 | <0.0007 |
| F | 2.28 | 0.79 | ND | 0.21 | 0.0083 | 0.003 | ND | 0.00080 |
| G | 13.3 | 3.45 | ND | <0.2 | 0.046 | 0.012 | ND | <0.0007 |
| H | 45.8 | 26.3 | 10.9 | 2.77 | 0.16 | 0.082 | 0.036 | 0.0090 |
MarkTen XL menthol.
MarkTen XL classic.
ND-not detected.
The comparison of TSNA levels in the e-liquid and aerosol of product B is shown in Figure 6A. The TSNA levels in the aerosol were comparable with those in the e-liquid in product B, and the absence of increased TSNA levels in the aerosol of product B was most likely due to the lack of quantifiable nitrite in its e-liquid (see Table 5).
Figure 6.
TSNA levels in the e-liquid and aerosol of commercial product B (A) and product H (B) (n = 4).
The comparison of TSNA levels in the e-liquid and the aerosol of commercial product H is shown in Figure 6B and demonstrates that the TSNA levels were significantly higher in the aerosol than in the e-liquid, indicating that TSNA formation occurred during vaporization and aerosol generation of commercial product H. The formation of TSNAs in the aerosol was most likely due to the high levels of nitrite and minor alkaloids observed in the commercial product H, which could lead to the formations of TSNA from the respective nicotine or minor alkaloid precursors (see Table 5).
Product G also contained measurable levels of NNK and NNN in the e-liquid and the levels increased during aerosol generation for NNN. The NNK values remained consistent between the e-liquid and the generated aerosol, indicating that the transfer process was primarily responsible for the measurable values in the aerosol, and little formation was occurring. The increase in NNN can be attributed to the presence of nitrite in the e-liquid, as discussed above for product H, where formation could occur from the reaction with nicotine or the trace levels of nornicotine present in the e-liquid.
As outlined in the pathway shown in Figure 1, nitrite and nicotine contribute to the formation of NNK and NNN; nornicotine and nitrite contribute to the formation of NNN; anabasine and nitrite contribute to the formation of NAB; and anatabine and nitrite contribute to the formation of NAT.
TSNA levels in the tested commercial product aerosols were much lower than in the cigarette smoke.27,35 All four TSNAs except NNK and NAB in one of the tested e-cigarette product aerosols were lower than TSNA yields in the smoke from 50 US commercial cigarettes reported in 2017 by Edwards et al.10 The data comparison is listed in Table 8.
Table 8. Comparison of TSNA in Commercial E-Cigarette Aerosol to Cigarette Smoked.
| product code | NNK (ng/e-cig or cig) | NNN (ng/e-cig or cig) | NAB (ng/e-cig or cig) | NAT (ng/e-cig or cig) |
|---|---|---|---|---|
| A | 0.16 | ND | ND | ND |
| B | 2.33 | 15.8 | 4.32 | 11.0 |
| C | 0.50 | ND | ND | ND |
| Da | 1.56 | 1.84 | ND | ND |
| Eb | 1.44 | 0.47 | <0.1 | <0.1 |
| F | 1.14 | 0.42 | ND | 0.106 |
| G | 6.41 | 1.66 | ND | <0.1 |
| H | 22.5 | 11.7 | 5.14 | 1.27 |
| cigarette ISO averagec | 55 | 85 | 11.7 | 80 |
| cigarette intense averagec | 122 | 189 | 24 | 186 |
| cigarette ISO rangec | 13–122.4 | 18.6–171.1 | 4.2–22 | 18.6–145.1 |
| cigarette intense smoke rangec | 39.9–245.9 | 32.8–323.3 | 6.5–41.4 | 44.3–291.7 |
MarkTen XL menthol.
MarkTen XL classic.
Data were from 50 commercial cigarettes from the report by Edwards et al.10
ND-not detected.
3.4. Risk Assessment
Quantitative risk assessment calculations were performed for NNN and NNK, which are classified as carcinogens by both the International Agency for Research on Cancer36,37 as well as the National Toxicology Program.38,39 The only published exposure limits for NNN and NNK were established by the California Office of Environmental Health Hazard Assessment in Proposition 65.40−42 No published exposure limits were available for NAB or NAT. Proposition 65 lists no significant risk levels (NSRLs) of 0.5 and 0.014 μg/day for NNN and NNK, respectively, which are defined as daily intake levels posing a 10–5 (i.e., 1 in 100,000) lifetime risk of cancer.41 These values provide risk assessors with the daily exposure levels of chemicals below which no additional significant toxicological risk may be incurred in a population.
The following is an example daily intake calculation that was performed using the ng/g NNK generated in the aerosol of product B (see Table 7). The average consumption of e-liquid from these commercial e-cigarette products is assumed to be approximately 1.8 g/day (or two cartridges per day).43 As stated in Section 3.1.3 above, it is also conservative to estimate that 1 g of aerosol is generated from 1 g of liquid, as aerosol generated is comparable to the weight loss of liquid.
Using the corresponding values for NNK, the daily exposure would be calculated to be
Repeating these calculations for both NNN and NNK measured in all eight commercial e-cigarette products, all of the estimated NNN exposure values calculated for the commercial e-cigarettes were below the NSRL, and all commercial products tested, with the exception of G and H, were also below the NSRL for NNK (see Table 9).
Table 9. Estimated Daily NNK and NNN Exposures for Commercial E-Cigarette Productsc.
| product code | NNK (ng/g aerosol) | calculated daily NNK exposure (μg/day) | NNN (ng/g aerosol) | calculated daily NNN exposure (μg/day) |
|---|---|---|---|---|
| A | 0.40 | 0.00072 | ND | ND |
| B | 0.46 | 0.00803 | 30.3 | 0.05454 |
| C | 0.90 | 0.00162 | ND | ND |
| Da | 2.55 | 0.00459 | 3.03 | 0.00545 |
| Eb | 1.98 | 0.00356 | 0.65 | 0.00117 |
| F | 2.28 | 0.00410 | 0.79 | 0.00142 |
| G | 13.3 | 0.02394 | 3.45 | 0.00621 |
| H | 45.8 | 0.08244 | 26.3 | 0.04734 |
MarkTen XL menthol.
MarkTen XL classic.
ND-not detected.
4. Conclusions
The evaluation of TSNA formation, as well as levels of nitrite and minor alkaloids observed in the commercial cig-a-like e-cigarette products, corroborated the findings generated from the model systems using the lab-prepared e-liquids and their corresponding aerosols. TSNA formation in the aerosols correlated with levels of nitrite and minor alkaloids in the corresponding e-liquid. No prior study analyzing nitrite in the e-liquid of commercial e-cigarette products has been reported in the literature to date, although studies have reported the presence of low levels of TSNAs and minor alkaloids in the e-liquid. This study provided evidence that nitrite was present in the e-liquid of some commercial cig-a-like e-cigarette products, and detectable levels of TSNAs were found in the aerosols generated from vaporizing the e-liquid. Commercial e-cigarette products that contained e-liquids with high levels of nitrite produced aerosols that had significantly higher TSNA levels compared to the corresponding e-liquid. The results also indicated that TSNA formation occurred during the aerosol generation process when nitrite was present in commercial e-cigarette products. Farsalinos16 reported that the TSNA levels present in the aerosol were similar to those in the e-liquid, which was most likely due to the absence of nitrite impurity in the e-liquids used in that study.
The results indicate on the occurrence of TSNA formation during aerosol generation of commercial e-cigarette products due to the presence of nitrite in the e-liquid, which is the first study to report that nitrite is present in some commercial e-cigarette products. This study provides valuable information about drivers for TSNA formation in e-liquids and e-cigarette aerosols that may be applied to the evaluation and quality assurance of e-cigarette products. To prevent TSNA formation in e-liquids either during shelf-life storage or in the aerosol formed during vaporization, e-liquids used in the products must be devoid of any nitrite impurity.
Glossary
Abbreviations
- CAS
Chemical Abstracts Service
- CORESTA
Cooperation Centre for Scientific Research Relative to Tobacco
- HPHCs
harmful and potentially harmful constituents
- IC
ion chromatography
- ISO
International Standards Organization
- LOQ
limit of quantification
- NAB
4-nitrosoanabasine
- NAT
N-nitrosoanatabine
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N-nitrosonornicotine
- NSRL
no significant risk level
- TSNAs
tobacco-specific nitrosamines
- UPLC-MS/MS
ultra-performance liquid chromatograph coupled to tandem mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.1c00417.
Influence of nitrite on the formation of TSNAs in electronic cigarette liquids and aerosols (PDF)
Author Present Address
† G.D.K. contributed equally. Juul Labs Inc, 1000 F St NW, Washington, DC 20004, USA
The authors declare no competing financial interest.
Supplementary Material
References
- Brunnemann K. D.; Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit. Rev. Toxicol. 1991, 21, 235–240. 10.3109/10408449109017910. [DOI] [PubMed] [Google Scholar]
- Brunnemann K. D.; Prokopczyk B.; Djordjevic M. V.; Hoffmann D. Formation and analysis of tobacco-specific N-nitrosamines. Crit. Rev. Toxicol. 1996, 26, 121–137. 10.3109/10408449609017926. [DOI] [PubMed] [Google Scholar]
- Hecht S. S.; Chen C. B.; Ohmori T.; Hoffmann D.. Comparative carcinogenicity in F344 rats of the tobacco-specific nitrosamines, N’-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone Cancer Res. 1980, 40 (2), 298–302.. [PubMed]
- Hoffmann D.; Castonguay A.; Rivenson A.; Hecht S. S. Comparative carcinogenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N’-nitrosonornicotine in Syrian golden hamsters. Cancer Res. 1981, 1981, 2386–2393. [PubMed] [Google Scholar]
- Hecht S. S. Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- U.S. FDA. Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke Under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act. Draft Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, 2012.
- deRoton C.; Wiernik A.; Wahlberg I.; Vidal B. Factors Influencing the Formation of Tobacco-Specific Nitrosamines in French Air-Cured Tobaccos in Trials and at the Farm Level. Beitr. Tabakforsch. Int./Contrib. Tob. Res. 2005, 21, 305–320. 10.2478/cttr-2013-0797. [DOI] [Google Scholar]
- Hoffmann D.; Brunnemann K. D.; Prokopczyk B.; Djordjevic M. V. Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health 1994, 41, 1–52. 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- Adams J. D.; Lee S. J.; Vinchkoski N.; Castonguay A.; Hoffmann D. On the formation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone during smoking. Cancer Lett. 1983, 17, 339–346. 10.1016/0304-3835(83)90173-8. [DOI] [PubMed] [Google Scholar]
- Edwards S. H.; Rossiter L. M.; Taylor K. M.; Holman M. R.; Zhang L.; Ding Y. S.; Watson C. H. Tobacco-Specific Nitrosamines in the Tobacco and Mainstream Smoke of U.S. Commercial Cigarettes. Chem. Res. Toxicol. 2017, 30, 540–551. 10.1021/acs.chemrestox.6b00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu S.; Borgerding M.; Reynolds J. Formation of Tobacco Specific Nitrosamines in Mainstream Cigarette Smoke; Part 1, FTC Smoking. Beitr. Tabakforsch. Int./Contrib. Tob. Res. 2008, 23, 19–31. 10.2478/cttr-2013-0845. [DOI] [Google Scholar]
- Djordjevic M. V.; Fan J.; Bush L. P.; Brunnemann K. D.; Hoffmann D. Effects of storage conditions on levels of tobacco-specific N-nitrosamines and N-nitrosamino acids in U.S. moist snuff. J. Agric. Food Chem. 1993, 41, 1790–1794. 10.1021/jf00034a051. [DOI] [Google Scholar]
- Fisher M. T.; Bennett C. B.; Hayes A.; Kargalioglu Y.; Knox B. L.; Xu D.; Muhammad-Kah R.; Gaworski C. L. Sources of and technical approaches for the abatement of tobacco specific nitrosamine formation in moist smokeless tobacco products. Food Chem. Toxicol. 2012, 50, 942–948. 10.1016/j.fct.2011.11.035. [DOI] [PubMed] [Google Scholar]
- U.S. FDA. Premarket Tobacco Product Applications for Electronic Nicotine Delivery Systems. Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, 2019.
- Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA) E-Cigarette Task ForceReference Report, Electronic Cigarettes: Assessment of Analytical Literature 2014. https://www.coresta.org/sites/default/files/technical_documents/main/ECIG-RefRep_Literature-Review_May2014.pdf (accessed June 21, 2018).
- Farsalinos K. E.; Gillman G.; Poulas K.; Voudris V. Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels. Int. J. Environ. Res. Public Health 2015, 12, 9046–9053. 10.3390/ijerph120809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E.; Gillman I. G.; Melvin M. S.; Paolantonio A. R.; Gardow W. J.; Humphries K. E.; Brown S. E.; Poulas K.; Voudris V. Nicotine Levels and Presence of Selected Tobacco-Derived Toxins in Tobacco Flavoured Electronic Cigarette Refill Liquids. Int. J. Environ. Res. Public Health 2015, 12, 3439–3452. 10.3390/ijerph120403439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora J. W.; Wilkinson C. T.; Sink K. M.; McKinney D. L.; Miller J. H. Nicotine-related impurities in e-cigarette cartridges and refill e-liquids. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 821–829. 10.1080/10826076.2016.1266500. [DOI] [Google Scholar]
- Jin X.C.; Avery K.C.; Flora J.W.; Wagner K.A.. Determinationof TSNAs ine-Cigarette Liquids and Aerosols by UPLC-MS/MS, In Posterat CORESTA Congress, 2016, SmokeScience/Product Technology Groups, STPOST 06: Berlin, https://sciences.altria.com/-/media/Project/Altria/Sciences/presentations/3/Flora-TSNA_20160816_FINAL.pdf 2016.
- Kim H. J.; Shin H. S. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1291, 48–55. 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- Margham J.; McAdam K.; Forster M.; Liu C.; Wright C.; Mariner D.; Proctor C. Supplement-Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- Moldoveanu S.; Zhu J.; Qian N. Analysis of Traces of Tobacco-Specific Nitrosamines (TSNAs) in USP Grade Nicotine, E-Liquids, and Particulate Phase Generated by the Electronic Smoking Devices. Beitr. Tabakforsch. Int./Contrib. Tob. Res. 2017, 27, 86–96. 10.1515/cttr-2017-0009. [DOI] [Google Scholar]
- Lisko J. G.; Tran H.; Stanfill S. B.; Blount B. C.; Watson C. H. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob. Res. 2015, 17, 1270–1278. 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tob. Control 2014, 23, ii11–ii17. 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora J. W.; Meruva N.; Huang C. B.; Wilkinson C. T.; Ballentine R.; Smith D. C.; Werley M. S.; McKinney W. J. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. 10.1016/j.yrtph.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Varlet V.; Farsalinos K.; Augsburger M.; Thomas A.; Etter J. F. Toxicity assessment of refill liquids for electronic cigarettes. Int. J. Environ. Res. Public Health 2015, 12, 4796–4815. 10.3390/ijerph120504796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L.; Knysak J.; Gawron M.; Kosmider L.; Sobczak A.; Kurek J.; Prokopowicz A.; Jablonska-Czapla M.; Rosik-Dulewska C.; Havel C.; Jacob P.; Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora J. W.; Wilkinson C. T.; Wilkinson J. W.; Lipowicz P. J.; Skapars J. A.; Anderson A.; Miller J. H. Method for the Determination of Carbonyl Compounds in E-Cigarette Aerosols. J. Chromatogr. Sci. 2017, 55, 142–148. 10.1093/chromsci/bmw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss O.; Bianchi I.; Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Shu S.; Guo Q.; Zhu Y. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos. Environ. 2016, 134, 61–69. 10.1016/j.atmosenv.2016.03.027. [DOI] [Google Scholar]
- International Standards Organization. ISO: 3402, Tobacco and Tobacco Products - Atmosphere for Conditioning and Testing, 1999.
- CooperationCentre for Scientific Research Relative to Tobacco (CORESTA). Recommended Method No 81. Routine analytical machine for e-cigarette aerosol generation and collection −definitions and standard conditions 2015, https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (accessed 2018).
- Wagner K. A.; Flora J. W.; Melvin M. S.; Avery K. C.; Ballentine R. M.; Brown A. P.; McKinney W. J. An evaluation of electronic cigarette formulations and aerosols for harmful and potentially harmful constituents (HPHCs) typically derived from combustion. Regul. Toxicol. Pharmacol. 2018, 95, 153–160. 10.1016/j.yrtph.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Jin C.; Wagner K.. Determination of Nitrite and Nitrate in Smokeless Tobacco Products by Ion Chromatography and Continuous Flow Analysis - 2016 Collaborative Study. Tobacco and Tobacco Products Analytes Sub-Group Technical Report, https://www.coresta.org/sites/default/files/technical_documents/main/TTPA-114-CTR_2016-CollStudy-NitriteNitrate_July2017.pdf2017.
- Konstantinou E.; Fotopoulou F.; Drosos A.; Dimakopoulou N.; Zagoriti Z.; Niarchos A.; Makrynioti D.; Kouretas D.; Farsalinos K.; Lagoumintzis G.; et al. Tobacco-specific nitrosamines: A literature review. Food Chem. Toxicol. 2018, 118, 198–203. 10.1016/j.fct.2018.05.008. [DOI] [PubMed] [Google Scholar]
- InternationalAgency for Research on Cancer (IARC) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1972-Present. (Multivolume work); World Health Organization, 1987. [Google Scholar]
- International Agency for Research on Cancer (IARC) Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, 1972-Present. (Multivolume Work); World Health Organization, 2007. [Google Scholar]
- National Toxicology Program 4-(N-Nitrosomethylamino)-1-(3-pyridyl)-1-butanone; NTP 11th Report on Carcinogens (64091-91-4) 2004–11.
- National Toxicology Program N-Nitrosonornicotine; NTP 12th Report on Carcinogens (16543-55-8), 2011–12. [PubMed]
- Officeof Environmental Health Hazard Assessment (OEHHA). CaliforniaEnvironmental Protection Agency, Reproductive and Cancer Hazard AssessmentSection, Expedited Cancer Potency Values and Proposed Regulatory Levels ForCertain Proposition 65 Carcinogens 1992.
- Officeof Environmental Health Hazard Assessment (OEHHA). CaliforniaEnvironmental Protection Agency, Reproductive and Cancer Hazard AssessmentSection, Expedited Cancer Potency Values and No Significant Risk Levels (NSRLs)for Six Proposition 65 Carcinogens: Carbazole, MeIQ, MeIQx, Methyl Carbamate,4-N-Nitrosomethylamino-1-(3-Pyridyl)-1-Butanone, Trimethyl Phosphate, https://oehha.ca.gov/media/downloads/proposition-65/chemicals/expedited2001.pdf2001.
- Office of EnvironmentalHealth Hazard Assessment (OEHHA). Proposition 65. In: NoSignificant Risk Levels (NSRLs) for Carcinogens and Maximum Allowable DoseLevels (MADLs) for Chemicals Causing Reproductive Toxicity, https://oehha.ca.gov/media/downloads/proposition-65/chemicals/safeharbor081513_0.pdf2013.
- Goniewicz M. L.; Gawron M.; Smith D. M.; Peng M.; Jacob P. 3rd; Benowitz N. L. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob. Res. 2017, 19, 160–167. 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORESTA. Recommended Method No. 84, Determination of Glycerin, Propylene Glycol, Water, and Nicotinein the Aerosol of E-cigarettes by Gas Chromatographic Analysis, 4th ed., https://www.coresta.org/determination-glycerin-propylene-glycol-water-and-nicotine-aerosol-e-cigarettes-gas-chromatographic2021.
- Caldwell W. S.; Greene J. M.; David R. P.; deBethizy J. D. The Nitrosation of Nicotine: A kinetic Study. Chem. Res. Toxicol. 1991, 4, 513–516. 10.1021/tx00023a003. [DOI] [PubMed] [Google Scholar]
- Sun W.; Shi H.; Zhou J.; Yang H.; Ji H.; Wang J.; Anne J.; Bai R.; Xu D.; Jiao Z.. Effect of moisture content, nitrate and nitrite on TSNA accumulation in burley tobacco after high temperature storage Coreasta Conference, https://www.coresta.org/sites/default/files/abstracts/2015_AP16_SunWenshu_0.pdf2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.