Abstract
BACKGROUND:
Neurodegenerative diseases increase in incidence with age. Prior studies using differing populations and gait paradigms have reported various parameters changing with age, some of which correlate with falls and mortality. Here we use three different paradigms to evaluate gait and balance in healthy non-fallers.
RESEARCH QUESTION:
What objective gait and balance parameters are correlated with aging.
METHODS:
Healthy subjects aged 21–79 years without histories of falls, lower extremity orthopedic procedures or chronic pain were included. Subjects walked on a 20×4 foot pressure sensor mat (Zeno Walkway, Protokinetics, Havertown, PA) under three different gait paradigms, (i) steady-state gait, (ii) dual-task while texting on a cellular phone and (iii) tandem gait. Data was collected and analyzed using PKMAS software (Protokinetics). Linear regression analysis, stepwise multivariate analysis, and grouped analysis of gait parameters was performed using SPSS 22 (IBM).
RESULTS:
Seventy-five subjects were enrolled. Grouped analysis and linear regression analysis showed differing significance in parameters tested. Step-wise multivariate analysis of all 31 parameters assessed from three different gait paradigms, showed weak but significant correlations in age with (i) stride-to-stride variability in (i) integrated-pressure of footsteps and (ii) stride-length during steady-state gait, (iii) mean stride-length on dual-task, and (iv) mean step-width on tandem gait (R2 =0.382, t=2.26, p=0.026).
SIGNIFICANCE:
In a population of healthy subjects without prior history of falls or medical illness that should affect gait, there were weak but significant age-related changes in objective measures of steady state gait and balance. Future prospective longitudinal data will help predict the relevance of this in relation to falls in the elderly.
Search terms: gait, balance, aging, tandem gait, dual-task
INTRODUCTION
As people age, gait and balance change, leading to falls with associated morbidity and mortality [1], and an estimated 19 billion dollars in annual healthcare costs in the US alone [2]. Quality of life is significantly affected by the development of fear of falling [3]. Gait changes can be early indicators of neurodegenerative disorders such as the hypokinetic-rigid gait of Parkinson’s disease [4], or neurologic manifestations of medical diseases such as sensory ataxia from neuropathy due to diabetes. Vestibular dysfunction [5] and visual contrast sensitivity [6] in the elderly can also contribute to overall gait impairment. Gait and balance impairments may also be preclinical manifestations of underlying disorders including cardiovascular disease [7] and dementia [8, 9].
Before we can understand how gait changes in association with age related disease processes, it is important to understand objectively whether normal aging changes gait. There have been a number of large studies in groups of elderly subjects, and smaller studies in younger populations of subjects or comparing different age groups, that provide evidence for objectively definable changes in gait with age. Some of these include increase in stride width and decrease in gait speed [10–12], increased variability in step-length and step-width [13], stride-width [14], stride-time and swing-time, stance-time and single-support-time [15] with advancing age. Increased stride-time variability has also been correlated with increased fall risk in elderly subjects [16]. In younger individuals, the dual-task of texting on a cellular phone while walking has been reported to decrease gait velocity and increase lateral deviation [17]. In older adults, gait changes during varying dual-tasks have been associated with decreased gait velocity [18, 19], and decreased swing-time with increased swing-time variability [20]. Decreased gait velocity in elderly subjects has not only been reported to correlate with an increased risk for falls [21], but also a predictor of cognitive decline [22] and increased all-cause mortality [23]. Some of these studies used grouped analysis of different age groups [14, 15, 17, 19] or correlation coefficients [11, 13], others used regression analysis over the entire age group they studied [10, 12]. Not all studies looked at the same parameters or performed gait assessments using the same instrumentation or walking tasks. A large study combining multiple consortiums has attempted to overcome some of these issues but only looked at an older population (age>60) of subjects [15].
An alternative hypothesis has also been proposed, suggesting that any significant gait change in the elderly is a pre-manifestation of an underlying neurological disease, not caused by “normal aging”. In support of this argument, approximately 20% of elderly individuals over age 85 had no gait dysfunction based on questionnaire and subsequent visual examination [24]. Another study defined a more “robust normal” group of subjects from a “conventional normal” group, and found a number of gait measures, including stride-velocity [25], were better in the “robust normal” group.
To our knowledge no single study has looked at all aspects of gait kinematics objectively, in a healthy population, over a wide continuum of age ranges, including assessments of dual-task and balance on the same population at the same point in time. Our current study was designed to bridge this gap. We evaluated three different conditions in a population of healthy, non-falling adults between the ages of 18 and 80; (i) steady-state gait to establish baseline gait, (ii) gait during dual-tasking using a commonplace task of texting while walking, to see whether increased cognitive load affected elderly subjects gait more than younger subjects, and (iii) tandem gait as a measure of balance.
METHODS
Subjects
Subjects age 21–79 without prior history of falls were prospectively recruited at the University of Arkansas for Medical Sciences using flyers after obtaining IRB approval (UAMS IRB #203471). Those with history of prior orthopedic procedures on the back or lower extremities and chronic pain syndromes were excluded. All subjects completed a questionnaire that included past medical history to exclude any gait-related disease states.
Gait analysis
Subjects walked on a 20’x4’ pressure sensor impregnated mat, Zeno Walkway (ProtoKinetics, Havertown, PA), and data was collected and analyzed using Protokinetic Movement Analysis Software (PKMAS, ProtoKinetics) [26, 27]. Three paradigms were analyzed: (1) steady-state walk: subjects walked eight full lengths of the mat, walking off at both ends, (2) dual-task: subjects walked 8 lengths while simultaneously typing on a cellular phone, and (3) tandem walked (heel-toe) the length of the mat. For steady-state and dual-task gait, the first and last steps on the mat were excluded to minimize acceleration and deceleration effects. Objective gait variables were analyzed for steady-state and dual-task gait (see supplementary materials for detailed definitions). For tandem walking, we defined two additional parameters not in PKMAS. Path-width (the distance between the two most lateral footsteps over the entire 20’ tandem walk) and step-width (the distance between consecutive steps) (Figure 3A). In our scatter plots we show the mean value (filled symbols) and stride-to-stride variability (unfilled symbols) of each individuals’ trial for each gait parameter assessed in each of the three different gait paradigms (left side, filled symbols). Stride-to-stride variability was calculated as the percent coefficient of variation (%CV = standard deviation/mean), to allow for more accurate comparison of gait parameters with differing absolute values.
Statistical analysis
Analysis was performed using SPSS version 24 (IBM). Normality was assessed using the Schapiro-Wilk test. Linear regression analysis was performed on all variables as a function of age followed by step-wise multivariate analysis to determine the variables most influenced by age. One-way ANOVA (parametric) or Mann-Whitney (non-parametric) tests were applied for comparison between age groups. Dual-task gait changes were calculated as the ratio of dual-task/steady-state gait (texting/baseline) for each subject for each gait parameter. Using age, the predicted values for the four significant parameters in our multivariate model was calculated from the equations for their univariate linear regression fits. The residuals between the actual and predicted values were used to determine “outliers” (1–2 standard deviations from the fit), and statistical significance between age groups calculated by chi-square.
RESULTS
Seventy-five subjects (mean age 46.9 ± 17.1 years; 56% female and 90% right-handed) were enrolled and analyzed. Older subjects (median-split at age 50) had a greater percentage of hypertension, hyperlipidemia, and diabetes, while two younger subjects had neuropathy (Table 1).
Table 1:
Subject demographics
| All subjects (n=75) | Age <50 (n=37) | Age ≥50 (n=38) | |
|---|---|---|---|
| Average age (years) | 46.9 +/− 17.1 | 31.2 +/− 6.7 | 62.3 +/− 7.1 |
| Percent Female | 56% | 56.8% | 55.3% |
| Right handed | 90% | 89.2% | 92.1% |
| Medical Conditions | |||
| None | 26.7% | 43.2% | 10.5% |
| Hypertension | 22.7% | 0.0% | 44.7% |
| Hypercholesterolemia | 17.3% | 2.7% | 31.6% |
| Migraines | 16.0% | 13.5% | 18.4% |
| Depression or Anxiety | 9.3% | 8.1% | 10.5% |
| Asthma | 9.3% | 10.8% | 7.9% |
| Diabetes | 6.7% | 2.7% | 10.5% |
| Gastroesophageal Reflux | 5.3% | 5.4% | 5.3% |
| Seasonal Allergies | 5.3% | 8.1% | 2.6% |
| Heart Disease | 4.0% | 0.0% | 7.9% |
| Neuropathy | 2.7% | 5.4% | 0.0% |
Steady-state gait
Linear regression analysis of the mean value of gait parameters for each individual, as a function of their age, showed weak but significant correlation for mean integrated-pressure (R2=0.06, p=0.042), stance% (R2=0.05, p=0.046), swing% (R2=0.05, p=0.042), and single support % (SS%; R2=0.06, p=0.042) (Figure 1A, H, I, J; filled diamonds). Linear regression analysis of the stride-to-stride variability in gait parameters, calculated as the percent coefficient of variation in any given parameter for each subject (%CV) showed weak but significant correlation for all variables except stride width and foot area (Figure 1; unfilled diamonds). On step-wise multivariate analysis of the significant variables, %CV integrated-pressure (t = 3.82, p<0.001) and %CV stride-length (t=3.33, p=0.002) remained significant in the equation with an R2 of 0.29.
Figure 1. Steady state gait.
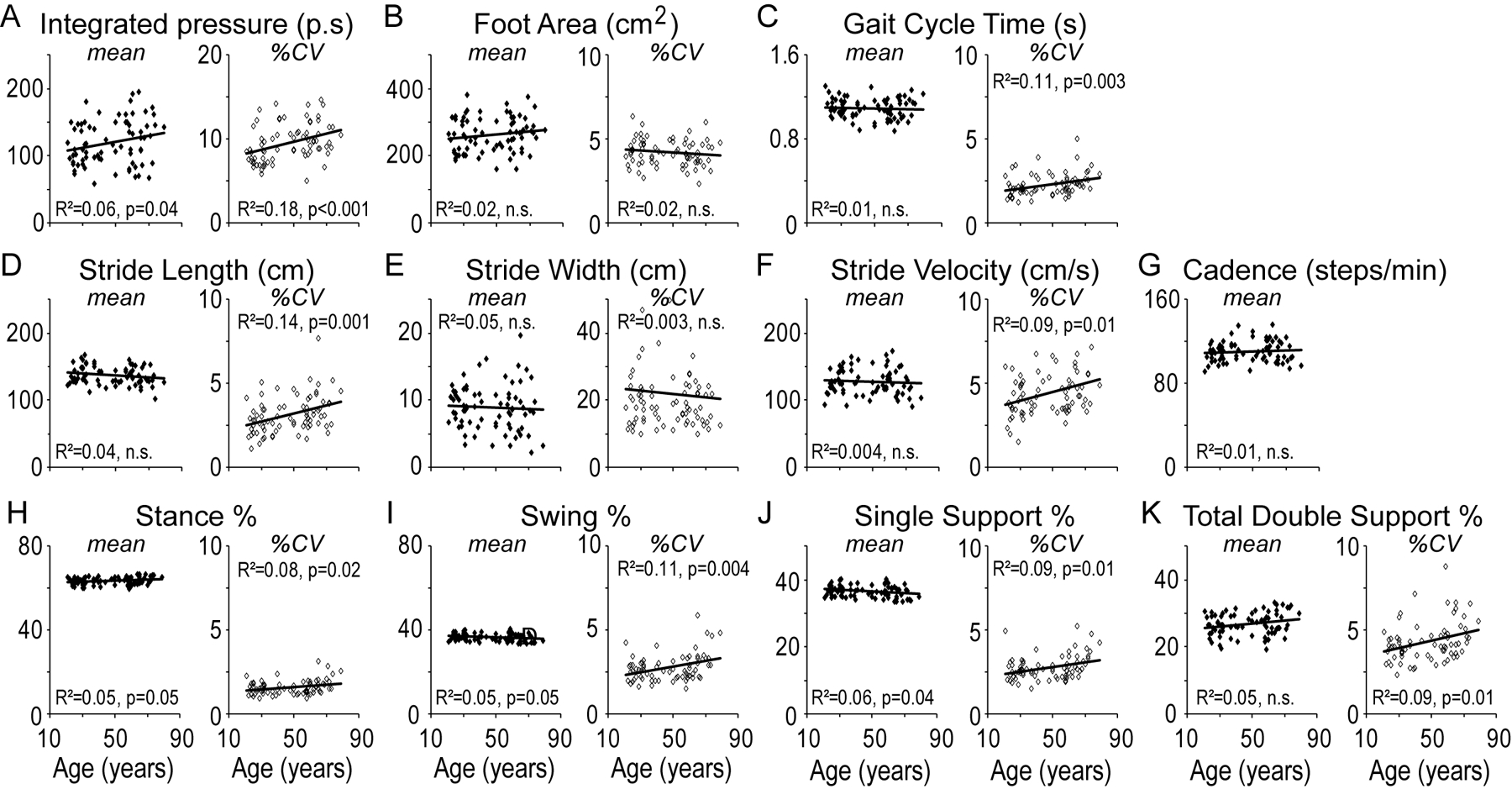
Mean (left panel, filled diamonds) and stride-to-stride variability (%CV; right panel, unfilled diamonds) in objective gait measures as a function of age shown for the parameters of (A) Integrated-pressure, (B) Foot Area, (C) Gait Cycle Time, (D) Stride-length, (E) Stride Width, (F) Stride Velocity, (G) Cadence, (H) Stance Percent, (I) Swing Percent, (J) Single Support Percent and (K) Total Double Support Percent. Statistical values are reported for ANOVA.
In order to compare our results to previous studies that used split age groups, we also split subjects using a median age of 50. In this analysis there was no statistically significant difference in the mean values of the gait variables (Supplementary Figure 1). The %CV was significantly higher in the older age group for integrated-pressure (U=373, p<0.001; Mann-Whitney), gait-cycle-time (U=506, p=0.037; Mann-Whitney), stride-length (U=434, p=0.004; Mann-Whitney), and total double support % (DS%; U=491, p=0.025; Mann-Whitney)(Supplementary Figure 1A, C, D, and K respectively).
Gait during dual-task condition
In order to determine if gait was variably affected with aging on addition of a task that distracted from walking, all subjects performed the steady-state gait task (baseline) while typing/reading on a cellular phone (texting). For each subject we calculated the ratio texting/baseline for the means and %CV of each gait parameter. Linear regression analysis of the ratio (texting/baseline) as a function of age, showed weak but significant correlations for mean stride-length (Figure 2D), stride-velocity (Figure 2F), stance% (Figure 2H), swing% (Figure 2I), SS% (Figure 2J), DS% (Figure 2K), and %CV in swing% (Figure 2I). On step-wise multivariate analysis, mean stride-length remained the only significant variable (R2 = 0.13, t=-3.231, p =0.002).
Figure 2. Gait during dual-task (texting on a cellular phone).
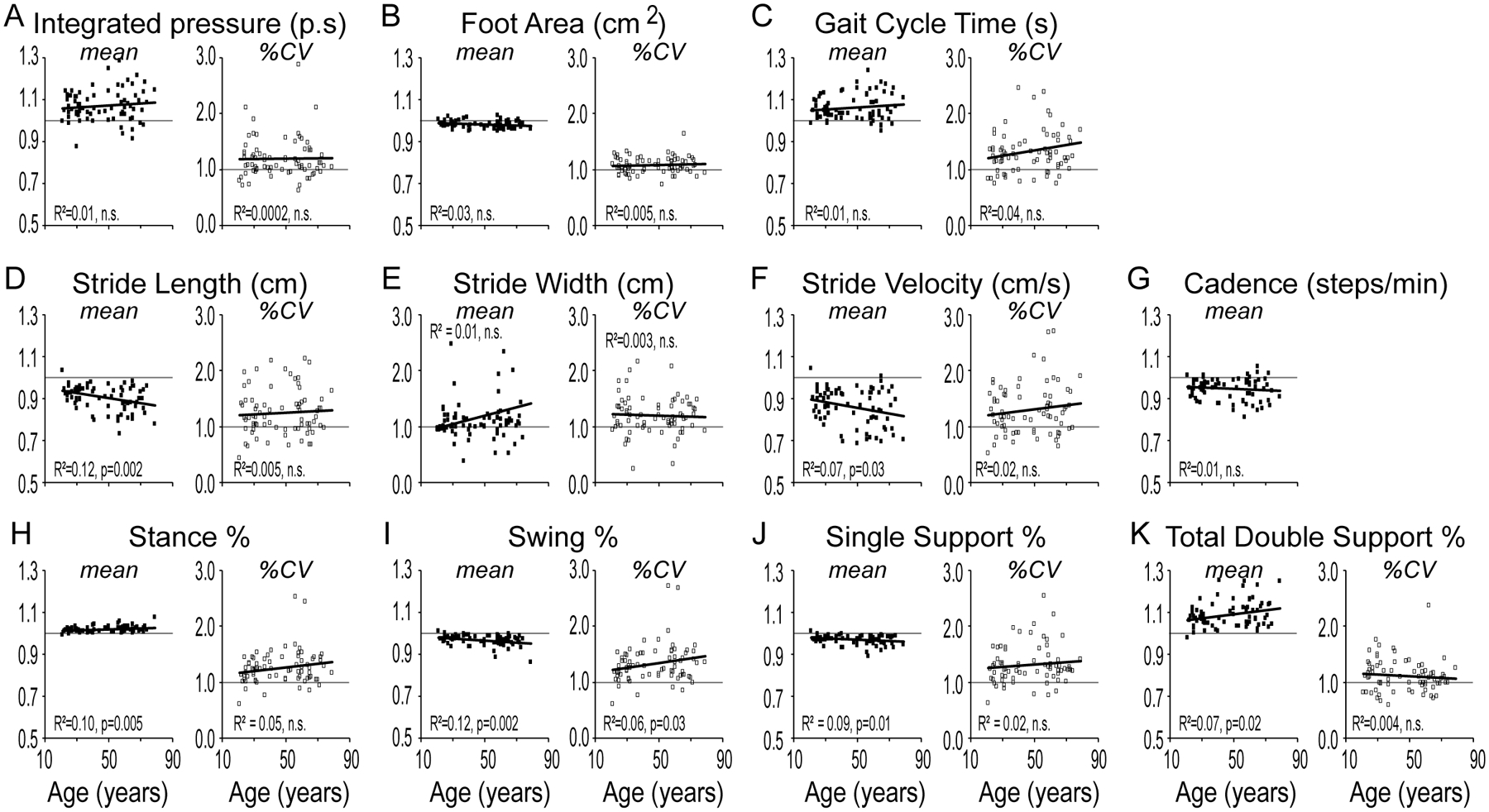
Mean (left panel, filled squares) and stride-to-stride variability (%CV; right panel, unfilled squares) in objective gait measures (texting/baseline) as a function of age shown for the parameters of (A) Integrated-pressure, (B) Foot Area, (C) Gait Cycle Time, (D) Stride-length, (E) Stride Width, (F) Stride Velocity, (G) Cadence, (H) Stance Percent, (I) Swing Percent, (J) Single Support Percent and (K) Total Double Support Percent. Statistical values are reported for ANOVA.
On age group analysis, mean stride-length (p=0.005; ANOVA), mean swing% (p=0.007; ANOVA) and stance% (p=0.013; ANOVA), and DS% (p=0.048; Mann-Whitney) were significantly higher in the older age group while dual-tasking (Supplementary Figure 2A). There was no statistically significant difference in %CV between the two groups (Supplementary Figure 2B).
Balance on tandem gait
In order to determine the effect of aging on balance, we employed the tandem gait task (heel-toe walking). Linear regression analysis showed weak but statistically significant correlation for mean step-width (Figure 3F), SS% (Figure 3I), DS% (Figure 3J), and %CV in integrated-pressure (Figure 3B), step-length (Figure 3D), stride-velocity (Figure 3H) and SS% (Figure 3I). On step-wise multivariate analysis, mean step-width (t=2.66, p=0.01) and %CV in integrated-pressure (t=2.78, p=0.007) remained significant variables with an R2 of 0.23.
Figure 3. Tandem gait.
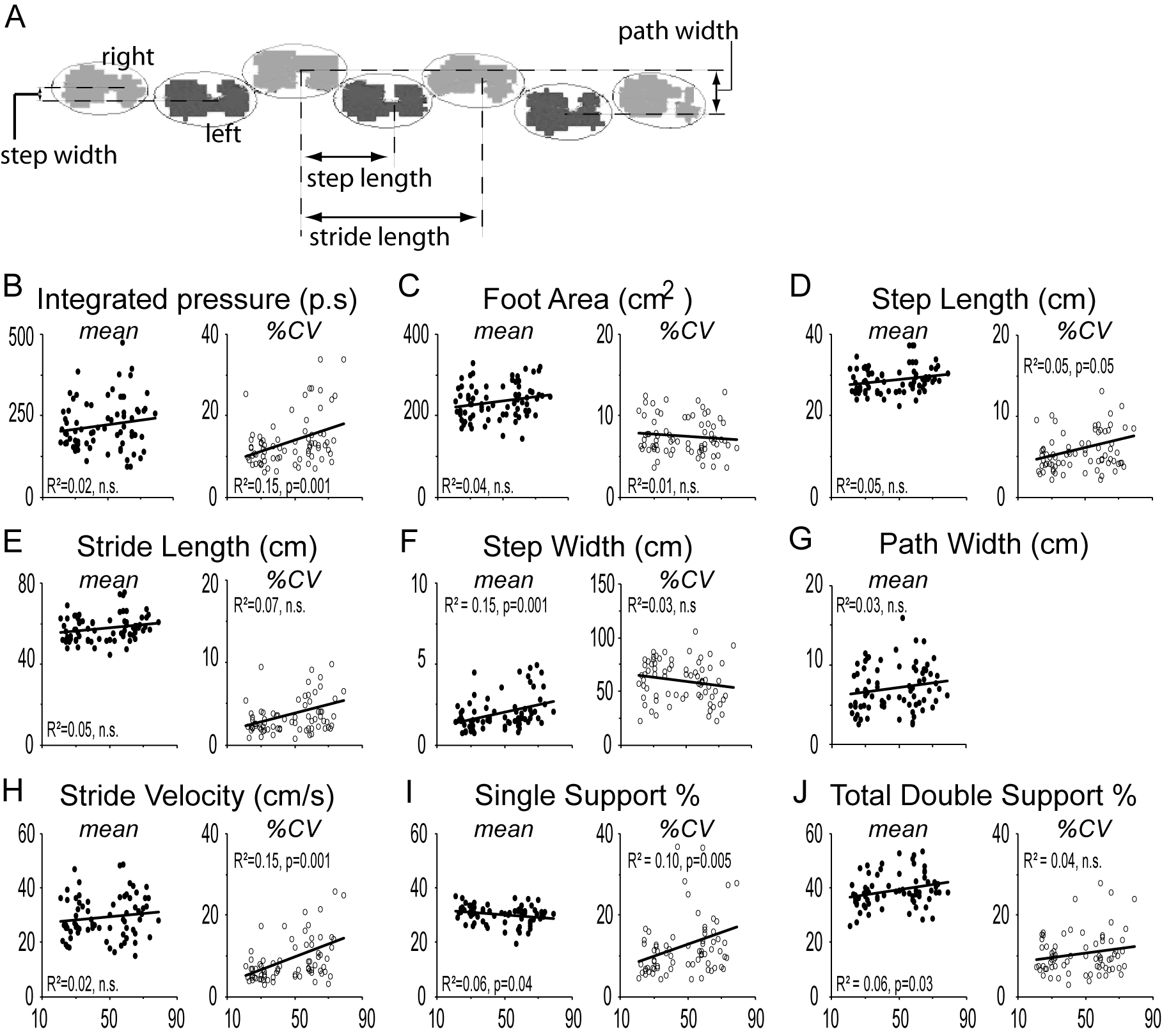
(A) Definition of step-width as the distance between the centers of consecutive steps and the path width as the distance between the centers of the two most extreme lateral steps along the entire path walked. Mean (left panel, filled circles) and stride-to-stride variability (%CV; right panel, unfilled circles) in objective gait measures (texting/baseline) as a function of age shown for the parameters of (B) Integrated-pressure, (C) Foot Area, (D) Step-length, (E) Stride-length, (F) Step-width, (G) Path Width, (H) Stride Velocity, (I) Single Support Percent and (J) Total Double Support Percent. Statistical values are reported for ANOVA.
On grouped analysis, mean step-length (p=0.039; ANOVA), stride-length (p=0.043; ANOVA), step-width (U=504, p=0.035; Mann-Whitney), and DS% (p=0.028; ANOVA) were significantly higher in the older group while mean SS% (p=0.034; ANOVA) was lower in the older group (Figure 3). There was also significantly higher %CV in the older aged group in the parameters of integrated-pressure (U=453, p=0.008; Mann-Whitney), step-length (U=506, p=0.036; Mann-Whitney), stride-length (U=448, p=0.007; Mann-Whitney), and SS% (U=498, p=0.03; Mann-Whitney) (Supplementary Figure 3A, D, E and I respectively).
Combined analysis
Step-wise multivariate regression analysis was performed using all the significant gait parameters from all three paradigms., This analysis showed significance in %CV in integrated-pressure and stride-length during steady-state gait, mean stride-length during dual-task, and mean step-width on tandem gait (R2 of 0.382, t=2.26, p=0.026). Calculating residuals from the predicted values of each of these parameters based on their linear regression fits we found that the number of subjects that were >2 standard deviations (SD) away was approaching significance amongst the elderly subjects (age >50 from the fit of dual-task mean stride-length (p=0.113) and tandem mean step-width (p=0.095) compared to the younger group (Supplementary Table 1). 50% of the elderly subjects were >1 SD from the fit for dual-task stride-length compared to 13.5% of younger subjects (p<0.001).
DISCUSSION
In this study we evaluated healthy subjects (age range 21–79) without a history of falls or clear confounding medical issues that would affect their gait, in order to determine the effect of aging on objective spatiotemporal gait parameters. The major strengths of our study include the wide age range of subjects, the relatively large subject population compared to most studies, the use of novel objective measures of gait change, and the combined use of three different gait paradigms. The three paradigms included self-paced steady-state gait, a dual-task paradigm of texting while walking (to assess the effects of cognitive load on gait) and tandem gait (to assess balance). From these three paradigms we defined 59 parameters for each individual, and using step-wise multiple regression analysis, found four parameters that were significantly correlated with advancing age: (i) variability in integrated-pressure and (ii) variability in stride-length during steady-state gait, (iii) mean stride-length on dual-task, and (iv) mean step-width on tandem-gait.
Prior studies have looked at one or more of our paradigms, exploring for the most part changes in spatiotemporal gait parameters individually. Our results differ from prior studies in regards to changes in mean gait parameters in steady state gait with age. While we found no difference in mean stride-length or stride-velocity with age using either linear regression analysis (Figure 1D,F) or grouped means with split groups (Supplementary Figure 1D,F), prior studies in elderly subjects (age >60) [10, 12, 13, 15], and wider age distributions (age > 22) [11, 28] showed decreased mean velocity (or stride velocity) and stride (or step) length with age. On univariate analysis we did see increased variability in stride-velocity with aging, which could be a pre-manifestation of decreased mean stride velocity.
Our results showed increased variability in stride-length as one of the main four features that change with age in a multi-step regression model. Prior studies have shown mixed results; elderly populations showed increased stride-length variability with age [13, 15], but wider age range populations didn’t [28]. In our study, based on regression analysis, a 14% change in stride-length variability could be accounted for by age alone, while a previous study (that included subjects with known neurologic disease) showed a 25% change [13]. Determining a range of stride-length variability change with aging is important as it is also effected in pathologic states such as Parkinson’s disease [29, 30].
In our cohort, when attention was divided between walking and performing a cognitive task (dual-task), we found a decreased mean stride-length with aging, which was one the four significant variables in our model. Other studies have also reported a decrease in step-length [31] in addition to stride (or mean) velocity [17, 19] during dual tasking, which we found also in our univariate models but not in the final combined model. Cognitive dysfunction and slower gait speed have also been reported to have a bidirectional relationship, with a decline in gait speed predicting a decline in cognitive function [22]. Additionally, relative stride-velocity impairment while dual-tasking has been associated with a 2–3 fold risk of progression to dementia [9]. We also found the largest scatter in dual task stride-length amongst the four parameters in our model with 50% of our elderly subjects (age >50) >1 SD removed from the linear regression fit. A dual-task paradigm in assessment of gait changes should therefore be included in all protocols.
Some of the differences seen in our cohort compared to previous studies, could be from our selection of a healthier population of elderly subjects, as we excluded subjects with history of prior falls, chronic pain syndromes or orthopedic intervention involving the back or lower limbs. Additionally none of our older subjects had major neurologic disease, which was not the case in some studies (see [13] for example). Consistent with this idea, Oh-Park and colleagues defined a “conventional normal” and “robust normal” group and found less age related decline in stride velocity, amongst other variables, in their “robust normal” group [25]. It has also been suggested that gait changes in the elderly is a pre-manifestation of an underlying neurologic disorder [24]. While we cannot directly comment on this hypothesis, in our cohort, the number of elderly subjects (age >50) that were >2 SD removed from the fit of dual-task mean stride-length (p=0.113) and tandem mean step-width (p=0.095) was larger than the younger cohort, a highly significant 50% of elderly subjects were >1 SD from the fit for dual-task stride-length (see Supplementary Table 1). This increased scatter with age, could be contributing to a larger change found in parameters with age, and some of these “outliers” may actually have undiagnosed early stage neurologic diseases. Future identification and more detailed longitudinal neurologic examination of these “outliers” would help differentiate these possibilities.
We also assessed changes in foot strike parameters with age, and found that increased variability in the pressure applied while walking was one of the core features in our combined model. Foot strike variability is important to consider in future assessments of aging as we recently reported increased variability in PD patients with falls and freezing of gait [29].
In a similar study design to ours, analyzing turns in place of dual-task, Verlinden and colleagues grouped 30 gait measures into 7 “factors” [32]. In their model, gait variability, and tandem gait were 2/3 factors showing the strongest association with age, consistent with our findings of increased steady-state stride-length variability and mean tandem step-width. As tandem gait can assess multiple balance pathways (cerebellar, vestibular, sensorimotor) it is an important assessment to include when defining age-related gait changes. Whether wider step-width in the “outliers” in our elderly population predicts future falls, as has been reported for decreased gait velocity [21], requires longitudinal study designs of non-fallers.
The limitations of our study include our inability to make clear inferences into the evolution of gait regulatory pathways with aging as we didn’t perform a complete neurologic exam (including for neuropathy, visual or vestibular dysfunction) on our patients. Other studies can help guide us. Brach and colleagues showed that impairment in cognitive tasks, finger tapping, sensory function, strength, mood and leg pain were related to stance-time variability (most prominently in slow walkers), but not step-length or step-width variability [33]. The variability we found in stride-length should therefore not have been affected by these domains. In 1732 community dwelling elders, without neurologic or orthopedic disease, only 10% and 17% had strength or proprioceptive impairments respectively [34]. In our cohort, only two younger aged subjects reported neuropathy and the four older subjects with diabetes had a narrower step-width on tandem within their age group, arguing against an undiagnosed diabetic neuropathy. The mean tandem path width was not effected by age in our cohort, arguing against prominent vestibular dysfunction (Figure 3G). It was reported that impaired contrast sensitivity, not visual acuity, correlates with decreased stride-length [6] and mean steady-state stride-length was not a parameter in our final model. The recently proposed guidelines establishing a standard protocol for gait studies in elderly populations also did not include testing subjects’ cognition, sensory function, vestibular function or vision in the core assessments [15].
Our overall findings suggest that as one ages, there is more variability in the pressure applied while stepping and the length of each stride. Stride-length is shorter when attention is distracted, which could either be due to competition between different pathways or as a compensatory strategy to maintain balance. Additionally, a wider base is required in order to maintain balance on tandem as one ages. Future studies on healthy individuals, with a wide age distribution, using the consortium approach to study larger populations from different ethnic and cultural backgrounds, performed with a complete neurologic assessment and longitudinal follow-up will allow us to determine if these changes expand beyond the primarily Caucasian population we studied and whether the changes we see correlate with the development of future falls or predict future development of neurologic disease.
Supplementary Material
Highlights:
Older subjects had increased variability in stride length during steady state gait
Older subjects had increased variability in integrated foot pressure during steady state gait
Older subjects had decreased mean stride length with a multi-modal dual-task
Older subjects had increased mean step width on tandem walking
ACKNOWLEDGEMENTS
This work was supported in part by the University of Arkansas Clinician Scientist Program and the Center for Translational Neuroscience (GM110702). We also appreciate the mentorship and advice of Dr. Edgar Garcia-Rill and Dr. Charlotte Hobbs. Most importantly we thank the subjects who generously provided their time to participate in this research.
Disclosures
The study has no sponsors.
Dr. Virmani received salary support from the University of Arkansas for Medical Sciences’ Clinician Scientist Program.
Jesal Shah has nothing to disclose in relation to this manuscript
Dr. Gupta has nothing to disclose in relation to this manuscript
Dr. Larson-Prior has nothing to disclose in relation to this manuscript
Footnotes
This work has not been accepted for prior publication.
The authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research. The corresponding author guarantees the accuracy of the references. The corresponding author had full access to all the data and has the right to publish any and all data.
All authors have agreed to conditions noted on the Authorship and Contributorship Form.
REFERENCES
- [1].Verghese J, Ambrose AF, Lipton RB, Wang C. Neurological gait abnormalities and risk of falls in older adults. J Neurol. 2010;257:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jorstad EC, Hauer K, Becker C, Lamb SE, ProFa NEG. Measuring the psychological outcomes of falling: a systematic review. Journal of the American Geriatrics Society. 2005;53:501–10. [DOI] [PubMed] [Google Scholar]
- [4].Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain : a journal of neurology. 1996;119 (Pt 2):551–68. [DOI] [PubMed] [Google Scholar]
- [5].Fife TD, Baloh RW. Disequilibrium of unknown cause in older people. Ann Neurol. 1993;34:694–702. [DOI] [PubMed] [Google Scholar]
- [6].Duggan E, Donoghue O, Kenny RA, Cronin H, Loughman J, Finucane C. Time to Refocus Assessment of Vision in Older Adults? Contrast Sensitivity but Not Visual Acuity Is Associated With Gait in Older Adults. J Gerontol A Biol Sci Med Sci. 2017. [DOI] [PubMed] [Google Scholar]
- [7].Bloem BR, Gussekloo J, Lagaay AM, Remarque EJ, Haan J, Westendorp RG. Idiopathic senile gait disorders are signs of subclinical disease. Journal of the American Geriatrics Society. 2000;48:1098–101. [DOI] [PubMed] [Google Scholar]
- [8].Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–8. [DOI] [PubMed] [Google Scholar]
- [9].Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, Borrie MJ, Hachinski VC, Wells J, et al. Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leiper CI, Craik RL. Relationships between physical activity and temporal-distance characteristics of walking in elderly women. Phys Ther. 1991;71:791–803. [DOI] [PubMed] [Google Scholar]
- [11].Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25:292–9. [DOI] [PubMed] [Google Scholar]
- [12].Lusardi MM, Pellecchia GL, Schulman M. Functional Performance in Community Living Older Adults. Journal of Geriatric Physical Therapy. 2003;26:14–22. [Google Scholar]
- [13].Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability--a population-based study of older people. Age Ageing. 2010;39:191–7. [DOI] [PubMed] [Google Scholar]
- [14].Beauchet O, Allali G, Annweiler C, Bridenbaugh S, Assal F, Kressig RW, et al. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55:702–6. [DOI] [PubMed] [Google Scholar]
- [15].Beauchet O, Allali G, Sekhon H, Verghese J, Guilain S, Steinmetz JP, et al. Guidelines for Assessment of Gait and Reference Values for Spatiotemporal Gait Parameters in Older Adults: The Biomathics and Canadian Gait Consortiums Initiative. Front Hum Neurosci. 2017;11:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–6. [DOI] [PubMed] [Google Scholar]
- [17].Lamberg EM, Muratori LM. Cell phones change the way we walk. Gait & posture. 2012;35:688–90. [DOI] [PubMed] [Google Scholar]
- [18].Smith E, Cusack T, Blake C. The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait & posture. 2016;44:250–8. [DOI] [PubMed] [Google Scholar]
- [19].Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait & posture. 2007;26:113–9. [DOI] [PubMed] [Google Scholar]
- [20].Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: Results from the Baltimore Longitudinal Study of Aging. Age Ageing. 2017;46:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu B, Hu X, Zhang Q, Fan Y, Li J, Zou R, et al. Usual walking speed and all-cause mortality risk in older people: A systematic review and meta-analysis. Gait & posture. 2016;44:172–7. [DOI] [PubMed] [Google Scholar]
- [24].Bloem BR, Haan J, Lagaay AM, van Beek W, Wintzen AR, Roos RA. Investigation of gait in elderly subjects over 88 years of age. J Geriatr Psychiatry Neurol. 1992;5:78–84. [DOI] [PubMed] [Google Scholar]
- [25].Oh-Park M, Holtzer R, Xue X, Verghese J. Conventional and robust quantitative gait norms in community-dwelling older adults. Journal of the American Geriatrics Society. 2010;58:1512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Egerton T, Thingstad P, Helbostad JL. Comparison of programs for determining temporal-spatial gait variables from instrumented walkway data: PKmas versus GAITRite. BMC Res Notes. 2014;7:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lynall RC, Zukowski LA, Plummer P, Mihalik JP. Reliability and validity of the protokinetics movement analysis software in measuring center of pressure during walking. Gait & posture. 2017;52:308–11. [DOI] [PubMed] [Google Scholar]
- [28].Gabell A, Nayak US. The effect of age on variability in gait. Journal of gerontology. 1984;39:662–6. [DOI] [PubMed] [Google Scholar]
- [29].Shah J, Pillai L, Williams DK, Doerhoff SM, Larson-Prior L, Garcia-Rill E, et al. Increased foot strike variability in Parkinson’s disease patients with freezing of gait. Parkinsonism Relat Disord. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2003;149:187–94. [DOI] [PubMed] [Google Scholar]
- [31].Kao PC, Higginson CI, Seymour K, Kamerdze M, Higginson JS. Walking stability during cell phone use in healthy adults. Gait & posture. 2015;41:947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Verlinden VJA, van der Geest JN, Hoogendam YY, Hofman A, Breteler MMB, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait & posture. 2013;37:500–5. [DOI] [PubMed] [Google Scholar]
- [33].Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait & posture. 2008;27:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Beauchet O, Launay CP, Fantino B, Allali G, Annweiler C. Respective and combined effects of impairments in sensorimotor systems and cognition on gait performance: a population-based cross-sectional study. PLoS One. 2015;10:e0125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


