Abstract
PURPOSE
To investigate change in knee cartilage composition over 96 months in overweight and obese participants with constant weight compared to those with weight loss, and to assess how different weight loss regimens are associated with these changes.
METHODS
We studied right knees of 760 participants (age 62.6±9.0y; 465 females) with a baseline BMI>25kg/m2 from the Osteoarthritis Initiative with mild to moderate or with risk factors for knee osteoarthritis. Participants losing weight (>5% of baseline BMI over 72 months; N=380) were compared to controls with stable weight (SW, N=380). Participants losing weight were categorized based on weight loss method (diet and exercise, diet only, exercise only) and compared to those with stable weight. MRI at 3T was performed at baseline, 48- and 96-months. The association of weight loss and weight loss method with change in cartilage composition, measured with T2 mapping, was analyzed using mixed random effects models.
RESULTS
Compared to SW, weight loss was associated with a significantly slower increase in global (averaged over all compartments) cartilage T2 ((adjusted mean difference of change in T2 ms/year [95%CI] between the groups: 0.24 [0.20, 0.41] ms/year; P<0.001) and global deep layer cartilage T2 (0.35 [0.20, 0.42] ms/year; P<0.001), suggesting slower cartilage deterioration. Compared to the SW group, slower increases in global T2 were observed in the diet and diet and exercise groups, but not in the exercise only group (P=0.042, P=0.003 and P=0.85, respectively).
CONCLUSION
Our results suggest that weight loss may slow knee cartilage degeneration over 96 months, and that these potential benefits may differ by method of weight loss.
Keywords: Osteoarthritis, cartilage imaging, weight loss, magnetic resonance imaging, T2 relaxation time
Introduction
Obesity is one of the most common modifiable risk factors for osteoarthritis (OA) 1–3. Increased loading forces have previously been shown to cause increased cartilage degradation and previous studies have shown that metabolic factors may be associated with obesity and OA 4–8. For the detection of very early and potentially reversible cartilage changes, MRI-based compositional imaging, such as T2 relaxation time measurements, has previously shown to be useful due to its ability to quantify increases in water content of cartilage and abnormalities in collagen structure 9, 10.
A previous study found that knee cartilage T2 values increased significantly less over 48 months in participants losing a substantial amount of weight (>10% BMI loss) compared to those with moderate (5–10%) or no weight loss (<3%), suggesting that a protective effect of weight loss on knee cartilage in obese and overweight participants may depend on the amount of weight loss 11. Moreover, in a weight loss intervention trial it was shown that when comparing participants losing weight with diet and exercise, or diet or exercise only over 18 months, participants in the diet only group showed a greater reduction in knee compressive force and greater improvement in knee pain and function compared to participants in the exercise only group 12.
However, to the best of our knowledge, the association of weight change and different weight loss regimens with changes in knee cartilage biochemical composition along with structural integrity has never been analyzed over an observational period longer than 48 months.
The objectives of this longitudinal study were therefore: (i) to analyze the association of moderate or greater weight loss (>5% of baseline BMI) over 72 months with concurrent 96 months biochemical cartilage deterioration, as measured by T2 relaxation time MR imaging, and progression of structural changes of the knee joint, as assessed by the semi-quantitative WORMS score; and (ii) to explore how different weight loss regimens in obese and overweight participants impact cartilage T2 values, in comparison to participants without weight change.
Method
Participants
The Osteoarthritis Initiative (OAI; http://www.oai.ucsf.edu) is a prospective multi-center cohort study from which participants were selected for this analysis. The participants of the OAI are either healthy participants with risk factors for knee OA (incidence cohort) or with symptomatic knee OA with radiographic evidence of tibiofemoral OA (progression cohort). At all participating centers, this HIPAA-compliant study was approved by the local institutional review board and informed consent of each participant was obtained.
We studied OAI participants who were overweight or obese (BMI >=25) with complete BMI information at baseline, 12-, 24-, 48- and 72-months. Those with end-stage OA (KL>3) and with rheumatoid arthritis that developed during the study follow-up were excluded (Participant selection is illustrated in Figure 1).
Figure 1.
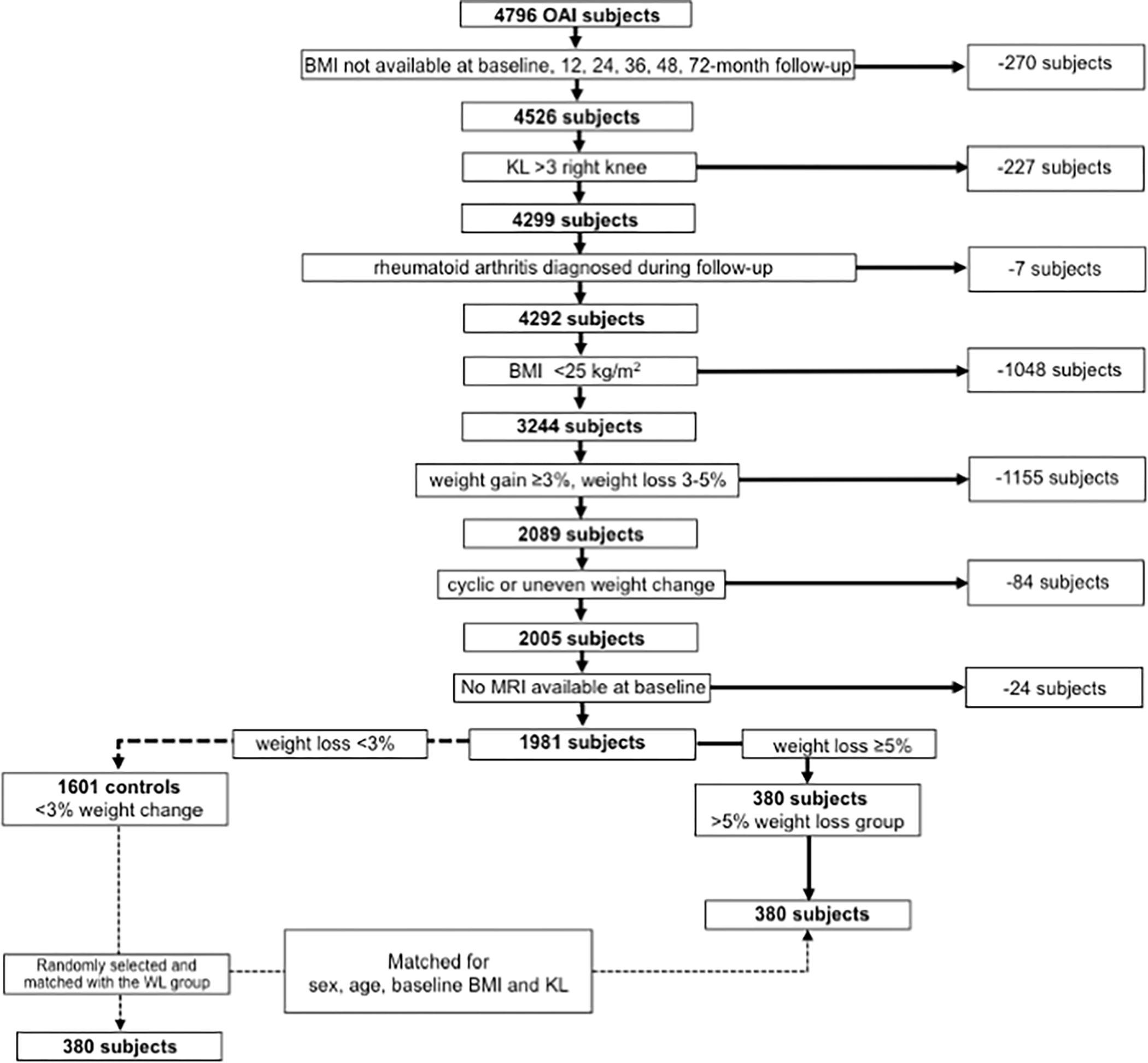
Patient selection from OAI database for the weight loss (WL) group.
In total, 3244 participants met the inclusion/exclusion criteria and were categorized according to weight change between baseline and 72-months. Those with weight gain (≥3% of baseline BMI) and with weight loss between 3–5% were excluded, to better define groups with weight loss and stable weight (N=1115). Also, those with ‘irregular’ weight change, cycling through weight gain and weight loss between the follow-up time points were excluded (N=84). For this determination, a linear regression model of the annual rate of change in BMI over 72 months was calculated and participants with a root mean square error of their weight change above the 95th percentile were categorized as participants with ‘irregular’ weight change, whereas participants with a root mean square error of their weight change below the 95th percentile were categorized as participants with ‘steady’ weight change 11. In the OAI, T2 maps were only obtained from the right knee. Therefore, participants with missing right knee MRI at baseline were excluded (N=24). This left 380 participants with weight loss >5% (weight loss group). From the remaining 1601 individuals with stable weight (stable weight group), 380 were randomly selected and frequency matched to the weight loss group in strata defined by baseline age (10 year strata from 45 to 65 and one 14 year strata from 65 to 79), sex (male/female), BMI (BMI in 2.5kg/m2 intervals) and KL grade (KL grade strata 0/1 and 2/3). MRI analysis was performed on all baseline MRIs of these matched 760 subjects, and on all available MRIs at 48-month and at 96-month follow-up for these subjects. The number of subjects with data for MRI analysis available for each follow-up time point were as follows: weight loss group: 48-month follow-up, N=269; 96-month follow-up, N=217; stable weight group: 48-month follow-up, N=266; 96-month follow-up, N=169. There was no significant difference found regarding the baseline subject charateristics age, BMI and KL grade as well as sex distribution between the participants that dropped out due to missing follow-up MRI scans at the 48-month (P≥0.34) and 96-month follow up (P≥0.27) as well as the participants that remained in the study until the end.
Weight loss questionnaire administered at 96-month follow-up
A weight loss questionnaire was administered to the participants at the 96-month follow-up. The questionnaire investigated how weight loss was achieved (i.e. increased physical activity, changes in diet, or a combination). Using the data from the questionnaire, we assessed the effect of the three different methods used for weight loss (diet only group (D group); exercise only group (E group); combination of diet and exercise group (D+E group)) on cartilage degeneration. Using the Katz comorbidity questionnaire, participants with cancer (e.g. throat, stomach, prostate cancer or leukemia), cardiac failure and/or other severe diseases (e.g. stroke, spine or hip fracture, severe infection) causing hospitalization that developed during the time period of the study, were excluded from these analyses 13.
MR Imaging
MR images were acquired using four identical 3.0T scanners (Siemens Magnetom Trio; Siemens Healthcare, Erlangen, Germany) and quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at four sites (University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; Memorial Hospital of Rhode Island, Pawtucket, RI and The Ohio State University, Columbus, OH). T2 relaxation time values were obtained using a sagittal two-dimensional (2D) multislice, multiecho (MSME) sequence with seven echo times (TEs; 10ms to 70ms) and a repetition time (TR) of 2700 ms. The following four sequences were obtained for the morphological analysis: (i) 2D intermediate-weighted fast spin echo (FSE) sequences with fat suppression in the sagittal plane; (ii) 2D proton density-weighted FSE sequences in the sagittal plane; (iii) 3D T1-weighted fast low-angle shot (FLASH) gradient-echo sequences, and (iv) 3D dual echo steady-state gradient-echo obtained in the sagittal plane, as described in the OAI MR protocol14.
Image Analysis
For the T2 analysis of the MR images an in-house, spline-based algorithm written in MATLAB (the Mathworks, Natick, Massachusetts) was used as previously described 15, 16. The cartilage of five compartments (patella (PAT), medial femoral condyle (MF), lateral femoral condyle (LF), medial tibia (MT), and lateral tibia (LT)) was semi-automatically segmented by two trained researchers (J.Z. and G.F.) using the first echo of the sagittal 2D MSME sequence and manually correcting the position of the points, in consensus and under supervision of an experienced radiologist (T.M.L.). The trochlea (TRO) was not segmented due to flow artifacts caused by the popliteal artery. T2 values of each compartment were calculated by using a mono-exponential decay model as the fitting function for the signal intensity using 6 echoes (TE 20–70 ms) after excluding the first echo in order to minimize errors and improve signal-to-noise ratio 15, 17. Mean T2 values were computed for each cartilage compartment, and the global T2 value for the overall knee joint was calculated from the mean of all compartments.
Laminar analysis algorithms automatically subdivided the cartilage of each compartment into a superficial layer (articular surface) and a deep layer (bone interface) of equal thickness 18. In addition, cartilage GLCM texture analysis was performed to evaluate the spatial distribution of cartilage T2 values within each cartilage compartment, reflecting heterogeneity of T2 values throughout the cartilage matrix, as a measure for cartilage matrix early degeneration 11, 19–22. Based on our previous work, two GLCM texture parameters were included in the analysis: contrast (contrast group) and variance (statistics group)11, 20, 23.
Morphological MR sequences from both groups were reviewed on a picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA) by two radiologists (B.J.S. and A.S.G. with both 5 years of experience, respectively), blinded to patient information, using the semi-quantitative modified WORMS grading system, as previously described 24, 25. In cases of disagreement, a consensus reading was performed with a third more experienced musculoskeletal radiologist (T.M.L. with 23 years of experience).
Statistical Analysis
Statistical analysis was performed with Stata/IC Version 13.1 software (StataCorp, College Station, TX) using a two-sided 0.05 level of significance. ANOVA was used to test mean differences for continuous measures and chi-square tests were used to test binary variables. Differences between the groups (weight loss group vs. stable weight group; stable weight group vs. D, D+E and E group) for baseline T2 and WORMS were calculated using a multivariable regression model, adjusting for age, sex, baseline BMI and KL score. Mixed-effects regression models adjusting for age, sex, baseline BMI, KL score were used to assess the differences in the annual rates of change of cartilage T2 and WORMS scores between participants with weight loss, and those with different weight loss methods, compared to the stable weight group. All models were checked for the following assumptions that were met (values are provided for the dependent variable baseline over all T2): We tested for nonlinearity and if nonlinearity was detected we used the non-linear models. We tested for nonlinearity by using an interaction between a quadratic term for time and the exposure variable (weight loss vs stable weight). If the quadratic term for time was significant, then we interpreted this as a quadratic relationship and a quadratic model was used. If the quadratic term was not significant, we used an interaction between time (linear) and the exposure group. Post estimation, the mixed models were used to quantify the differences in rates of change between groups. Normality of the dependent variables and the residuals (Shapiro Wilk test P > 0.05), homogeneity of variances (Levene’s test statistic=1.49, P= 0.16), absence of influential outliers in the data (max observed: Cook’s distance is 0.033 and leverage is 0.051), and absence of multicollinearity (none of predictor variable pairs have correlations above 0.2) were checked. We controlled for multiple measurements per participants by including the subject identification number as a random effect. The same mixed-effects regression models were repeated for the laminar as well as the exploratory texture analyses. For whole-joint analyses, average T2 values over all compartments (global knee cartilage T2) were used and therefore, no correction for multiple testing across the compartments had to be performed. The T2 analyses were repeated for the stable weight group in comparison to the D, D+E and E group, adjusting for age, sex, baseline BMI and baseline KL score.
Reproducibility
To calculate both of the intra- and inter-reader reproducibility for T2 measurements acquired for the present study, the reproducibility error was assessed by calculating the root mean square average of the single coefficients of variation (CV) on a percentage basis, as previously reported 26. Inter-reader reproducibility was assessed in 10 randomly selected participants between the two readers (J.Z. and G.F.) overall and for each of the five compartments segmented (PAT, MF, LF, MT, and LT). Averaged over all compartments, the inter-reader reproducibility for T2 measurements was 1.93%. The CVs for each compartment were 2.26% (range 1.12–2.52%) for PAT, 1.63% (range 1.48–1.94%) for MF, 1.59% (range 1.24–2.14%) for LF, 2.36% (range 2.01–2.63%) for MT, and 1.83% (range 1.56–2.32%) for LT. For intra-reader reproducibility, both readers repeated the T2 segmentations in the same 10 randomly selected participants with at least 14 days separating the readings. The intra-reader reproducibility for overall mean T2 measurements of J.S. and G.F. were 1.12% (range 0.93–2.28%) and 2.06% (range 1.05–2.31%), respectively.
In order to calculate the intra- and inter-reader reproducibility of the WORMS grading for the present study, each of the two readers (B.J.S. and A.S.G.) performed WORMS grading twice independently for 10 randomly selected participants, the two readings of each reader were at least 14 days apart. Intra-class correlation coefficients (ICCs) were calculated in order to compare the WORMS overall and to compare each WORMS subscore (meniscus, cartilage, BMEP) separately. The 95% confidence interval (CI) of the intra-reader agreement for overall WORMS grading as well as for the subscores meniscus, cartilage and BMEP ranged from 0.74 to 0.95. ICCs for inter-reader agreement were 0.83 (95%CI: 0.74–0.95) for overall WORMS. The 95%CI ranged from 0.74 to 0.94 for the subscores meniscus, cartilage and BMEP. Similar intra-reader and inter-reader agreements of WORMS gradings by our group have been published in previous studies 16, 25, 27.
Results
Subject Characteristics
Subject characteristics are presented in Table 1. When comparing participants with weight loss to those with stable weight, there were no differences found between the groups in mean baseline age and BMI as well as sex and KL distribution (P>0.18, respectively). After 96 months, weight loss participants had lost 3.52±1.83 kg/m2 on average, while the BMI change of the group with stable weight was 0.03 ± 0.86 kg/m2. When participants were distributed into categories of method of weight loss, participants in the D+E group (N=101, 58.8±7.7 years) were significantly younger than participants in the E or D group (E group: N=33, 62.2±8.9 years, P=0.005; D group: N=41, 62.7±8.2 years, P=0.045). Weight change trajectories are presented in Figure 2. However, there were no significant differences found between the weight loss method groups at baseline in the D+E, D or E group regarding WOMAC pain (D+E=2.3±2.6; D=2.1±3.1; E=2.0±2.8; P=0.52), WOMAC stiffness (D+E=1.4±1.5; D=1.6±1.4; E=1.3±1.5; P=0.77), WOMAC disability (D+E=7.7±10.6; D=7.5±10.4; E=6.7±9.0; P=0.60) and the SF-12 Mental Health (D+E=54.6±7.0; D=53.8±8.5; E=53.6±7.5; P=0.63). Moreover, there were no differences found when comparing the different weight loss groups concerning baseline BMI, sex, race and KL distribution or change in BMI/amount of weight loss (all P>0.18). In total, 11 participants that were free of OA at baseline (KL=0 or 1) progressed to KL 2 or higher over 96 months, these participants were in the stable weight group.
Table 1:
Subject demographics
| Stable overweight* N = 380 | >5% weight loss* N = 380 | |
|---|---|---|
|
| ||
| Baseline | ||
|
| ||
| Age [years ± SD] | 62.1 ± 8.6 | 63.0 ± 9.4 |
| Females [n (%)] | 233 (61.3%) | 232 (61.1%) |
| BMI [kg/m2 ± SD] | 29.9 ± 3.5 | 29.8 ± 3.6 |
| KL scores | ||
| K/L score 0 [n (%)] | 113 (29.7%) | 116 (30.5%) |
| K/L score 1 [n (%)] | 74 (19.5%) | 69 (18.2%) |
| K/L score 2 [n (%)] | 120 (31.6%) | 121 (31.8%) |
| K/L score 3 [n (%)] | 73 (19.2%) | 74 (19.5%) |
Participants in the two different groups are matched in terms of age, sex, baseline BMI and baseline KL score.
Pearson’s chi-squared test.
ANOVA.
Figure 2.
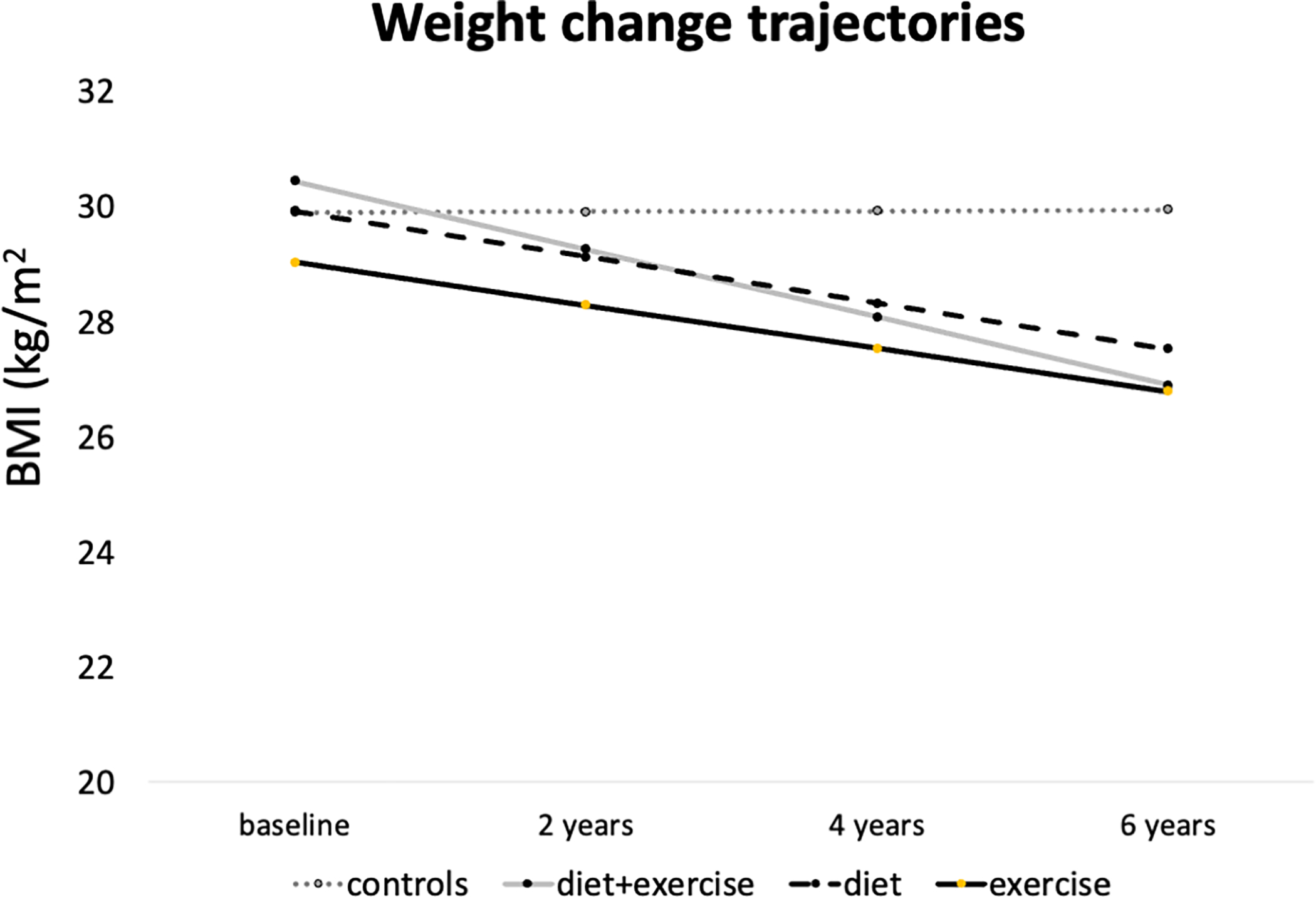
Trajectories of BMI for the control group as well as the different weight loss regimen groups (diet&exercise; diet only; exercise only).
Comparison of baseline and rates of change of cartilage T2 over 96 months between weight loss and stable weight groups
There were no significant differences at baseline in global cartilage T2 as well as in each compartment separately between participants with weight loss and those with stable weight (adjusted mean differences in baseline T2 between stable overweight group and 5% weight loss group [95% confidence interval (CI)]: global knee, 0.34 [−0.29, 0.37], P=0.13; patella, 0.16 [−0.16, 0.18], P=0.65; medial tibia, 0.55 [0.18, 0.61], P=0.19; lateral tibia, 0.50 [−0.37, 0.63], P=0.11; medial femur, 0.03 [−0.03, 0.05], P=0.76; lateral femur, 0.39 [−0.38, 0.57], P=0.17; Table 2). The rate of increase over 96 months of global T2 was significantly smaller in the weight loss group compared to the stable weight group, suggesting less cartilage degeneration over 96 months (adjusted mean difference of change in T2 ms/year [95%CI] between the stable overweight and the >5% weight loss group: 0.24 [0.20, 0.41] ms/year; P<0.001; Table 3). In the deep layer this effect was found for the global cartilage (Adjusted mean difference of change in T2 [95%CI]: global knee T2, 0.35 [0.20, 0.42], P<0.001) and in all compartments (patella: 0.15 [0.06, 0.19], P=0.03; medial femur, 0.33 [0.17, 0.40], P<0.001; lateral femur, 0.56 [0.42, 0.61], P<0.001; medial tibia, 0.42 [0.31, 0.48], P<0.001; lateral tibia, 0.24 [0.14, 0.42], P<0.001), whereas in the superficial layer the medial femur (0.21 [0.04, 0.45], P=0.01) and the medial tibia (0.48 [0.39, 0.75], P<0.001) showed a significantly lower rate of change in the weight loss group compared to the stable weight group, indicating decreased cartilage degeneration through weight loss over all compartments and especially in the medial compartment of the superficial layer. These results were supported by the texture analyses, which showed significantly less increase in contrast and variance averaged over all compartments, again compatible with less progression of cartilage degeneration in the weight loss group compared to the stable weight group (P<0.001; supplemental data).
Table 2:
Baseline T2 Parameters*
| T2 Parameters Baseline | Stable overweight group vs. (N=380) | >5% weight loss group (N=380) | P-value |
|---|---|---|---|
|
| |||
| Cartilage T2 | |||
| Global knee | 0.34 [−0.29, 0.37] | 0.13 | |
| PAT | 0.16 [−0.16, 0.18] | 0.65 | |
| MT | 0.55 [0.18, 0.61] | 0.19 | |
| LT | 0.50 [−0.37, 0.63] | 0.11 | |
| MF | 0.03 [−0.03, 0.05] | 0.76 | |
| LF | 0.39 [−0.38, 0.57] | 0.17 | |
|
| |||
| Deep layer T2 | |||
| Global knee | 0.36 [−0.06, 0.46] | 0.09 | |
| PAT | 0.06 [−0.10, 0.11] | 0.91 | |
| MT | 0.13 [−0.13, 037] | 0.15 | |
| LT | 0.45 [−0.03, 0.59] | 0.051 | |
| MF | 0.04 [−0.38, 0.12] | 0.93 | |
| LF | 0.33 [−0.22, 0.43] | 0.35 | |
|
| |||
| Superficial layer T2 | |||
| Global knee | 0.28 [0.21, 0.36] | 0.37 | |
| PAT | −0.04 [−0.10, 0.01] | 0.79 | |
| MT | 0.21 [0.11, 0.30] | 0.63 | |
| LT | 0.27 [0.19, 0.35] | 0.55 | |
| MF | 0.25 [0.15, 0.33] | 0.50 | |
| LF | 0.68 [0.59, 0.78] | 0.40 | |
Multivariable linear regression models adjusting for age, sex, baseline BMI and KL score. Adjusted mean differences [95% confidence interval] (ms).
PAT, patella; MT, medial tibia; LT, lateral tibia; MF, medial femur; LF, lateral femur
Table 3:
Comparison of rate of change of global and laminar T2 over 96-months
| T2 Parameters 96 months | Stable overweight vs. >5% weight loss Adjusted mean difference of change in T2 ms/year [95% CI] | P-value |
|---|---|---|
| Cartilage T2 | ||
|
| ||
| Global knee | 0.24 [0.20, 0.41] | <0.001 |
| PAT | 0.12 [0.06, 0.26] | 0.05 |
| MT | 0.45 [0.39, 0.59] | <0.001 |
| LT | 0.18 [0.13, 0.20] | 0.02 |
| MF | 0.19 [0.15, 0.30] | 0.03 |
| LF | 0.32 [0.24, 0.58] | 0.001 |
| Deep layer T2 | ||
| Global knee | 0.35 [0.20, 0.42] | <0.001 |
| PAT | 0.15 [0.06, 0.19] | 0.03 |
| MT | 0.42 [0.31, 0.48] | <0.001 |
| LT | 0.24 [0.14, 0.42] | <0.001 |
| MF | 0.33 [0.17, 0.40] | <0.001 |
| LF | 0.56 [0.42, 0.61] | <0.001 |
| Superficial layer T2 | ||
| Global knee | 0.04 [−0.13, 0.09] | 0.50 |
| PAT | 0.08 [0.06, 0.22] | 0.12 |
| MT | 0.48 [0.39, 0.75] | <0.001 |
| LT | 0.06 [0.02, 0.19] | 0.40 |
| MF | 0.21 [0.04, 0.45] | 0.01 |
| LF | 0.08 [0.05, 0.18] | 0.25 |
LF, lateral femur; LT, lateral tibia; MF, medial femur; MT, medial tibia; PAT, patella
The adjusted mean differences of associations of T2 relaxation times between the weight loss group and stable weight group over 96 months were assessed using multivariable regression models adjusting for age, sex, baseline BMI and baseline KL score. Significant results (P < 0.05) are bolded.
Comparison of rates of change of WORMS cartilage, meniscal and BMEP lesions over 96 months between weight loss and stable weight groups
At baseline there were no significant differences found between the stable weight and weight loss groups in cartilage, meniscal and BMEP WORMS scores (all P>0.05; supplemental data). Over 96 months, the weight loss group showed significantly lower rates of progression of the sum WORMS of both menisci together and the sum WORMS of the medial meniscus (Adjusted mean difference of rate of change/year [95% CI] between stable overweight and >5% weight loss group: WORMS meniscus lesions sum, 0.08 [0.02, 0.21], P=0.021 and WORMS medial meniscus lesions sum, 0.06 [0.02, 0.09], P=0.005; Table 4). There were no significant differences between weight loss and stable weight groups in the rate of change of the global knee BMEP score, the global knee cartilage score or the cartilage score for each compartment separately (P>0.05 for each comparison).
Table 4:
Comparison of rate of change of cartilage, meniscus and bone marrow edema pattern WORMS sum score over 96-months
| Rate of change of WORMS over 96 months | Stable overweight vs. >5% weight loss | P-value |
|---|---|---|
| Adjusted mean difference of rate of change/year [95% CI] | ||
|
| ||
| Cartilage lesions | ||
| Global knee | 0.10 [−0.06, 0.25] | 0.34 |
| PAT | 0.02 [0.00, 0.04] | 0.46 |
| T | 0.00 [−0.06, 0.05] | 0.93 |
| MT | 0.02 [−0.3, 0.04] | 0.42 |
| LT | 0.02 [−0.03, 0.04] | 0.46 |
| MF | 0.03 [−0.03, 0.04] | 0.28 |
| LF | 0.01 [−0.03, 0.04] | 0.74 |
| Meniscus lesions | ||
| Meniscus lesions sum | 0.08 [0.02, 0.21] | 0.021 |
| Medial meniscus lesions sum | 0.06 [0.02, 0.09] | 0.005 |
| Lateral meniscus lesions sum | 0.02 [0.00, 0.08] | 0.86 |
| BMEP lesions | ||
| BMEP lesions sum | 0.02 [0.00, 0.08] | 0.85 |
LF, lateral femur; LT, lateral tibia; MF, medial femur; MT, medial tibia; PAT, patella
The adjusted mean differences of associations of the rate of change of WORMS between the weight loss group and stable weight group over 96 months were assessed using multivariable regression models adjusting for age, sex, baseline BMI and baseline KL score. Significant results (P < 0.05) are bolded.
Rates of change in participants with different weight loss methods and participants with stable weight
At baseline there were no significant differences found in cartilage T2 between the participants with stable weight and the participants with different methods of weight loss (P>0.05, respectively; supplemental data). Over 96 months, the rates of increase in global cartilage T2 were lower in the D and D+E groups compared to the stable weight group (mean T2 (ms/year) [95% confidence interval (CI)]: stable weight group= 0.37 [0.26, 0.47] vs. D+E group 0.14 [0.09, 0.18], P=0.003; stable weight group vs. D group 0.15 [0.03, 0.46], P=0.04; Table 5), indicating less progression of cartilage degeneration in the diet groups compared to the group with stable weight. On the other hand, the E group (exercise only) showed no significant difference in cartilage T2 averaged over all compartments compared to the stable weight group (mean T2 (ms) [95% CI]: stable weight group vs. E group= 0.40 [0.24, 0.55], P=0.85). After excluding subjects with baseline KL = 3 and re-analyzing the datasets, there was no substantial change of the significance levels in change in global T2 and T2 of all compartments separately between the weight loss and the stable weight group (data not shown). Moreover, there were no significant differences between weight loss method groups compared to the stable weight group at baseline, or in the rate of change over 96 months, in the WORMS scores for cartilage, meniscus, or BMEP (P>0.05, data not shown).
Table 5:
Comparison of rate of change of T2 parameters for each weight loss method group compared to the stable weight group over 96 months
| Stable weight | Diet& Exercise | P-value | Diet | P-value | Exercise | P-value | |
|---|---|---|---|---|---|---|---|
| N=380 | N=101 | N=41 | N=33 | ||||
| T2 Parameters | Change in T2 ms/year 95% CI | Diet& Exercise vs. SW | Change in T2 ms/year 95% CI | Diet vs. SW | Change in T2 ms/year 95% CI | Exercise vs. SW | |
|
| |||||||
| Cartilage T2 | |||||||
| Global knee | 0.37 [0.26, 0.47] | 0.14 [0.09, 0.18] | 0.003 | 0.15 [0.03, 0.46] | 0.04 | 0.40 [0.24, 0.55] | 0.85 |
| PAT | 0.30 [0.21, 0.38] | 0.26 [0.02, 0.53] | 0.81 | 0.24 [0.18, 0.56] | 0.40 | 0.44 [0.03, 0.86] | 0.31 |
| MT | 0.55 [0.41, 0.69] | 0.06 [0.02, 0.32] | <0.001 | 0.07 [0.02, 0.36] | <0.001 | 0.42 [0.31, 0.75] | 0.13 |
| LT | 0.42 [0.29, 0.55] | 0.34 [0.11, 0.57] | 0.65 | 0.22 [−0.10, 0.53] | 0.44 | 0.52 [0.18, 0.86] | 0.12 |
| MF | 0.23 [0.17, 0.38] | 0.04 [0.02, 0.21] | 0.02 | 0.27 [0.05, 0.50] | 0.53 | 0.29 [0.07, 0.52] | 0.73 |
| LF | 0.53 [0.38, 0.69] | 0.25 [0.21, 0.41] | 0.02 | 0.51 [0.28, 0.73] | 0.14 | 0.53 [0.30, 0.76] | 0.78 |
| Deep layer T2 | |||||||
| Global knee | 0.40 [0.28, 0.52] | 0.08 [0.04, 0.12] | <0.001 | 0.09 [0.00, 0.18] | 0.001 | 0.34 [0.16, 0.53] | 0.21 |
| PAT | 0.20 [0.12, 0.28] | 0.13 [0.09, 0.39] | 0.38 | 0.09 [0.02, 0.42] | 0.22 | 0.24 [0.17, 0.64] | 0.85 |
| MT | 0.49 [0.34, 0.64] | 0.09 [0.02, 0.39] | <0.001 | 0.12 [0.05, 0.36] | <0.001 | 0.21 [0.07, 0.34] | 0.01 |
| LT | 0.38 [0.25, 0.51] | 0.33 [0.13, 0.52] | 0.13 | 0.06 [0.03, 0.28] | 0.01 | 0.33 [0.11, 0.55] | 0.34 |
| MF | 0.37 [0.18, 0.56] | 0.03 [0.01, 0.6] | 0.001 | 0.28 [0.01, 0.57] | 0.06 | 0.30 [0.02, 0.59] | 0.44 |
| LF | 0.63 [0.47, 0.80] | 0.16 [0.08, 0.31] | <0.001 | 0.13 [0.01, 0.34] | 0.002 | 0.55 [0.28, 0.80] | 0.18 |
| Superficial layer T2 | |||||||
| Global knee | 0.36 [0.29, 0.43] | 0.34 [0.13, 0.58] | 0.87 | 0.41 [0.10, 0.73] | 0.53 | 0.67 [0.38, 1.02] | 0.013 |
| PAT | 0.46 [0.35, 0.56] | 0.28 [0.04, 0.56] | 0.20 | 0.40 [0.08, 0.56] | 0.79 | 0.26 [0.04, 0.40] | 0.026 |
| MT | 0.61 [0.41, 0.80] | 0.13 [0.08, 0.25] | 0.001 | 0.17 [0.01, 0.72] | 0.02 | 0.63 [0.43, 0.92] | 0.96 |
| LT | 0.51 [0.41, 0.61] | 0.56 [0.24, 0.88] | 0.59 | 0.42 [0.02, 0.85] | 0.59 | 0.77 [0.31, 1.24] | 0.14 |
| MF | 0.18 [0.06, 0.63] | 0.09 [0.18, 0.37] | 0.27 | 0.12 [0.01, 0.41] | 0.07 | 0.33 [0.12, 1.50] | 0.023 |
| LF | 0.42 [0.34, 0.51] | 0.49 [0.21, 0.78] | 0.32 | 0.61 [0.22, 1.00] | 0.15 | 0.69 [0.27, 1.11] | 0.08 |
LF, lateral femur; LT, lateral tibia; MF, medial femur; MT, medial tibia; PAT, patella
The associations between different weight loss methods and rate of change in cartilage T2 over 96 months were assessed using multivariable regression models adjusting for age, sex, baseline BMI and baseline KL score. Significant results (P < 0.05) are bolded.
Discussion
In this study we analyzed the effects of weight loss on knee cartilage composition (T2 relaxation time) and structural deterioration of cartilage, menisci and bone marrow (WORMS) over 96 months. We found slower cartilage T2 increase in participants losing weight compared to those with stable weight, suggesting less progression of cartilage degeneration in participants losing weight, especially in the medial compartments. Moreover, we also found less progression of meniscal lesions, especially in the medial meniscus, over 96 months. This study also investigated the association of different weight loss regimens including diet, exercise and diet combined with exercise and knee joint cartilage composition and structural degeneration over 96 months. We found that individuals losing weight with diet and exercise as well as with diet only showed significantly less increase of cartilage T2 whereas those losing weight with exercise only showed no significant difference compared to those with stable weight in both layers over 96 months in the exercise only group.
Our findings of less cartilage degeneration, as measured with global T2 relaxation time, and T2 in the medial compartment of the knee cartilage, in individuals with weight loss compared to those without weight loss are in line with a previous study including participants with surgical and non-surgical weight loss, showing that reduced progression of cartilage thickness loss and quality deterioration in the medial compartment was associated with the amount of weight loss, measured using MR-based dGEMRIC measurements 28. Moreover, another study found an inverse linear relationship between weight change and medial cartilage volume loss, indicating that the greater the weight loss, the less the cartilage volume of the medial tibia decreased in average over 2.7 years 29. In contrast, weight gain was associated with increased cartilage volume loss in the medial tibia 29. However, all these studies had a relatively limited follow-up periods while our study has a follow-up period of 8 years and provides advanced MRI biomarkers including compositional cartilage assessment and WORMS as a semi-quantitative score for structural knee joint degeneration.
Interestingly, we have found that the evidence for a potential beneficial effect of weight loss on cartilage composition was seen in all knee compartments over 96 months. In the laminar analysis, the superficial layer showed less cartilage T2 increase in the medial femoral condyle and medial tibia in the weight loss group compared to those with stable weight. The lack of association of weight loss with morphological cartilage changes assessed using the subscore WORMS cartilage may be explained by the fact that differences in cartilage T2 relaxation time measurements may be detected before differences in morphological cartilage lesions may occur, as previous studies have shown that increased cartilage T2 values predicted longitudinal morphological degeneration in the cartilage, meniscus, and bone marrow in participants with risk factors for OA 15. However, the present study assessed WORMS outcomes over 8 years and it seems likely that any effects of weight loss on morphological progression would be detected in this time frame.
Our study also evaluated differences in compositional cartilage changes among groups reporting different weight loss methods over a duration of 96 months. A previous study assessing the associations between different types of weight loss (exercise, diet, diet and exercise) and structural knee changes over 18 months found no significant differences between the different groups in cartilage volume, thickness and percentage of denuded bone area 30, which may have been caused by the fairly short follow time of this previous study in comparison to our study, since a previous study has shown, that the weight loss interventions diet, exercise and diet plus exercise did not show consistent effects yet on serum levels of potential biomarkers of osteoarthritis, such as cartilage oligomeric matrix protein or transforming growth factor β1, after 18 months 31. Moreover, cartilage and meniscal lesions were not assessed semi-quantitatively in the previous study evaluating structural knee changes. In a further study, participants of both diet groups (diet and exercise group and diet only group) showed a significantly greater reduction of the inflammatory marker IL 6 compared to the exercise group, suggesting less inflammation and therefore less OA progression in the dietary groups 12. Moreover in the same study both dietary groups showed lower compressive forces compared to the exercise group — this comparison reached the level of significance in the diet group and showed a statistical trend in the diet and exercise group (P=0.05) 12. The reduction of inflammation and knee joint loading detected in the previous study in the dietary groups compared to the exercise group, may be consistent with our findings regarding the rate of progression of cartilage degeneration, showing significantly less T2 increase in the dietary groups compared to the stable weight group, whereas the exercise group showed no significant difference compared to the stable weight group in cartilage T2 change.
Our study has several limitations. Firstly, our study performed a retrospective analysis of weight loss in the OAI cohort, therefore several confounders could not be controlled for, e.g. exact amount of exercise or calorie uptake. Moreover, weight loss methods were self-reported only and no other data were available. Further investigations are needed of possible reasons for different effects of different weight loss methods, including different effects on lipids, blood sugar levels and metabolic status. Secondly, we can only speculate that any bias due to loss to follow-up will be similar in the two groups. The group sizes of participants with diet alone and exercise alone weight loss regimens were small in comparison to the diet plus exercise group and the amount of weight loss achieved was different between the groups. Although we adjusted for the amount of weight loss in total, this may be a potential confounder and needs to be considered when interpreting the data. Moreover, we did not account for the dependence between the different compartments of the same knee. It needs to be noted, that differences in change of T2 and WORMS were assessed in these analyses. Due to the large number of participants in total and fairly wide ranges of baseline parameters, the assessment of the level of significance of associations alone may lack of information and therefore it is important to consider these changes in cartilage composition and structural knee abnormalities in a clinical context.
In summary, our study showed that in individuals with risk factors or mild to moderate radiographic evidence for OA, moderate or greater weight loss (>5% of baseline BMI) was significantly associated with less progression over 96 months of compositional cartilage degeneration in all knee compartments as well as with less progression of meniscal lesions, compared to participants with stable weight. Participants who lost weight with diet and exercise and diet alone potentially showed less worsening of cartilage composition, while the participants in the exercise weight loss group did not show significant differences compared to the participants with stable weight over 96 months.
Supplementary Material
Acknowledgements
We would like to thank the participants and staff of the Coordinating Center of the OAI, as well as the UCSF QUIP-C group, for their invaluable assistance with patient selection, statistical analysis, and technical support.
Role of funding sources
The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2–2258, N01-AR-2–2259, N01-AR-2–2260, N01-AR-2–2261, and N01-AR-2–2262), with research conducted by the Osteoarthritis Initiative Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752).
Footnotes
Competing interest statement
None of the authors have any financial or other interests related to the manuscript submitted to Osteoarthritis and Cartilage that might constitute a potential conflict of interest.
References
- 1.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2010; 18: 24–33. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD, Breedveld F, Kvien TK. Controlling the obesity epidemic is important for maintaining musculoskeletal health. Ann Rheum Dis 2006; 65: 1401–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 2008; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutton CW. Osteoarthritis: the cause not result of joint failure? Ann Rheum Dis 1989; 48: 958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data W. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungmann PM, Kraus MS, Alizai H, Nardo L, Baum T, Nevitt MC, McCulloch CE, Joseph GB, Lynch JA, Link TM. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2013; 65: 1942–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, Carballido-Gamio J, Nevitt MC, Lynch J, McCulloch CE, Link TM. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res (Hoboken) 2013; 65: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull 2013; 105: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweet MB, Thonar EJ, Immelman AR, Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis 1977; 36: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, Gluer CC. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 2000; 18: 423–430. [DOI] [PubMed] [Google Scholar]
- 11.Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, Nevitt MC, McCulloch CE, Link TM. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2016; 24: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013; 310: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care 1996; 34: 73–84. [DOI] [PubMed] [Google Scholar]
- 14.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, Lynch JA, Nevitt MC, McCulloch CE, Majumdar S, Link TM. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2012; 20: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, Nevitt MC, Lynch J, McCulloch CE, Link TM. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging 2012; 35: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med 2010; 63: 181–193. [DOI] [PubMed] [Google Scholar]
- 18.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med 2010; 63: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med 2011; 65: 1184–1194. [DOI] [PubMed] [Google Scholar]
- 20.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, Lynch JA, McCulloch CE, Majumdar S, Link TM. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis Res Ther 2011; 13: R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haralick R, Shanmugam K, Dinstein I. Textural Features for Image Classification. IEEE Transactions on Systems, Man, and Cybernetics 1973; SMC-1: 610–618. [Google Scholar]
- 22.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology 2000; 214: 259–266. [DOI] [PubMed] [Google Scholar]
- 23.Yu A, Heilmeier U, Kretzschmar M, Joseph GB, Liu F, Liebl H, McCulloch CE, Nevitt MC, Lane NE, Link TM. Racial differences in biochemical knee cartilage composition between African-American and Caucasian-American women with 3 T MR-based T2 relaxation time measurements--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2015; 23: 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004; 12: 177–190. [DOI] [PubMed] [Google Scholar]
- 25.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, Nevitt MC, Lynch J, McCulloch CE, Link TM. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012; 64: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995; 5: 262–270. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, Link TM. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology 2011; 261: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, Sambrook PN, March L. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis 2012; 71: 26–32. [DOI] [PubMed] [Google Scholar]
- 29.Teichtahl AJ, Wluka AE, Tanamas SK, Wang Y, Strauss BJ, Proietto J, Dixon JB, Jones G, Forbes A, Cicuttini FM. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann Rheum Dis 2015; 74: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 30.Hunter DJ, Beavers DP, Eckstein F, Guermazi A, Loeser RF, Nicklas BJ, Mihalko SL, Miller GD, Lyles M, DeVita P, Legault C, Carr JJ, Williamson JD, Messier SP. The Intensive Diet and Exercise for Arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage 2015; 23: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua SD Jr., Messier SP, Legault C, Lenz ME, Thonar EJ, Loeser RF. Effect of an exercise and dietary intervention on serum biomarkers in overweight and obese adults with osteoarthritis of the knee. Osteoarthritis Cartilage 2008; 16: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


