Abstract
5-Hydroxymethylfurfural (HMF) is a valuable platform chemical derived from biomass and lots of research focuses on the synthesis of HMF from fructose and glucose. Herein, conversion of bio-carbohydrates to 5-hydroxymethylfurfural (HMF) was studied in the three-component deep eutectic solvent (DES) system, which was composed of choline chloride (ChCl), boric acid and substrates such as fructose, glucose and sucrose. Bio-carbohydrates handled under typical reaction conditions gave satisfactory conversion (44% for fructose and 31% for glucose) and yield of HMF (35% for fructose and 21% for glucose) in 1 h. Moreover, owing to the benefits of DES, the initial substrate content could be higher and the reaction temperature could be reduced, thus side reactions were effectively avoided and the selectivity of HMF was better (ranging from 79% to 100% for fructose and from 65% to 100% for glucose). We believe this method could provide a promising alternative for conversion of bio-carbohydrates to HMF and a better utilization of biomass.
The conversion process of fructose and glucose in the three-component DES system. Substrates such as fructose, glucose and sucrose treated with this DES system could convert to HMF in a satisfactory yield and selectivity.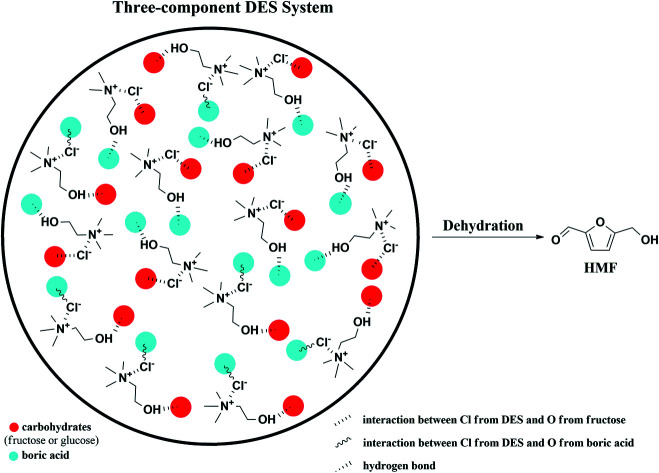
Introduction
The existing and daily-growing environmental problems and energy concerns have enhanced our need to seek renewable resources to lessen the reliance on fossil-based fuels whose reservation is decreasing day by day. Under this circumstance, biomass feedstock, with renewability, easy-accessibility, commercial efficiency and many other advantages, has come into sight to fulfil our requirements.1,2 Carbohydrates, which are the most common biomass feedstock, can be converted to different high value-added platform chemicals like furfural, levulinic acid, 5-hydroxymethylfurfural (HMF) and others.3,4 In 2004 the United States Department of Energy (U. S. DoE) pronounced 15 kinds of the most promising biomass derivatives5 and HMF was listed among them. HMF, as one of the most valuable biomass-derived chemicals, can be obtained through acid-catalyzed dehydration reactions of carbohydrates. There are lots of choices for the multi-utilization of HMF, especially in the production of 2,5-furandicarboxylic acid (FDCA),6 which is a key role in the production of the new bio-based polyester polyethylene furanoate (PEF).7,8 In addition, varieties of intermediate chemicals with certain value such as 2,5-dimethylfuran (DMF), 2,5-diformylfuran (DFF), 2,5-bis-(hydroxymethylfuran) (BHMF) can be synthesized from HMF through different catalytic ways.9–11 Recently, it was discovered that HMF even could impact some biological process such as fermentation and anaerobic digestion.12–14 Consequently, HMF is very attractive and promising, which makes the research about synthesis of HMF to be a hot topic in recent decades.
Commonly, HMF can be obtained from fructose and glucose through catalytic conversion.15 In terms of solvents, a lot of researches have been developed so far. Among these solvent systems, ionic liquids (ILs) were widely used as solvents owing to its unique properties such as negligible vapor pressure and thermal stability.16 ILs were first used as solvents for the dehydration process of fructose by Clément and Claude.17 Then Zhao et al. discussed the catalytic effect of different metal chlorides in ILs for the dehydration conversion of monosaccharides to HMF.18 Besides, a wide range of solvents, including aqueous media,19 melting mixtures solvents,20 biphasic systems21 and organic solvents22 have also been studied. Specifically, Hu et al. described the successful conversion of fructose, glucose, sucrose and other sugars to HMF in IL [EMIM]BF4 using SnCl4 as catalyst.23 P. H. Tran and his colleagues demonstrated the synthesis of HMF from fructose and glucose using the combination of biphasic phase and ionic liquids as reaction media.24
However, ILs are not widely used on large scale in production considering the high costs and inconvenience to prepare.25 Deep eutectic solvents (DESs) have emerged to overcome these limitations. DES is a type of mixture solvent composed of hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA), which has lower melting point than either of the individual component.26,27 Furthermore, one requires only the mixing of components, without any synthetic or purification steps. Generally, DESs share many physicochemical properties with ILs, such as low volatility and high stability.28 Considering these advantages, some specific DESs system have been utilized as solvents in the conversion of fructose and glucose to HMF.29–31 More importantly, in 2009 König's group found fructose was able to form DES when mixed with choline chloride (ChCl) – a quaternary ammonium salt with many advantages such as low price, relatively low toxicity and good degradability,32–34 thus ChCl was a suitable choice as HBA in the catalytic DES system.
Catalysts applied in the conversion process are also essential. When dehydration of fructose was first carried out in ILs by Clément and Claude, Amberlyst-15 was chosen to be the catalyst, resulted in 50% yield of HMF. Afterwards, Zhao et al. used metal chlorides instead, excellent yield up to 83% were obtained, however the selectivity was poor. Other catalysts, such as NHC/metal complex,35 mineral acid catalyst,31,36 solid acid catalyst,37–39 have been intended for the conversion of bio-carbohydrates to HMF as well. Yoshida et al. pointed out that appropriate acidity, especially the medium-strong acid could maintain optimal balance between catalytic activity, yield of product and selectivity.40
Herein, we introduced ChCl as HBA into the conversion process of fructose and glucose, boric acid was chosen to be HBD as well as catalyst (Fig. 1). The polyhydroxy structure of fructose and glucose could enhance the acidity of boric acid, thus better improved the catalytic efficiency.41 The three-component DES system was formed, which showed reactivity at lower temperature (90–120 °C) compared to ordinary methods.42–44 The influence of the substrates initial content, reaction temperature and reaction time were studied. In addition, the catalytic scope of other carbohydrates substrates such as sucrose, xylose and microcrystalline cellulose were also studied.
Fig. 1. Reaction studied in this work. Conversion of fructose and glucose to HMF.
Results and discussion
The influence of initial substrates content
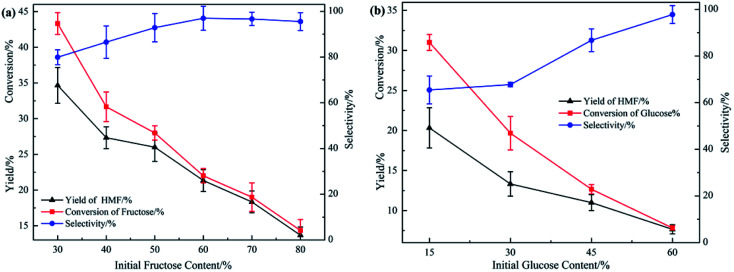
For the purpose of studying the influence of initial substrates content, a series of reactions were conducted at different fructose content ranging from 30 wt% to 80 wt% and at different glucose content ranging from 15 wt% to 60 wt%. Other reaction conditions were: ChCl/boric acid: 1/1(mol mol−1), temperature: 100 °C, reaction time: 1 h.
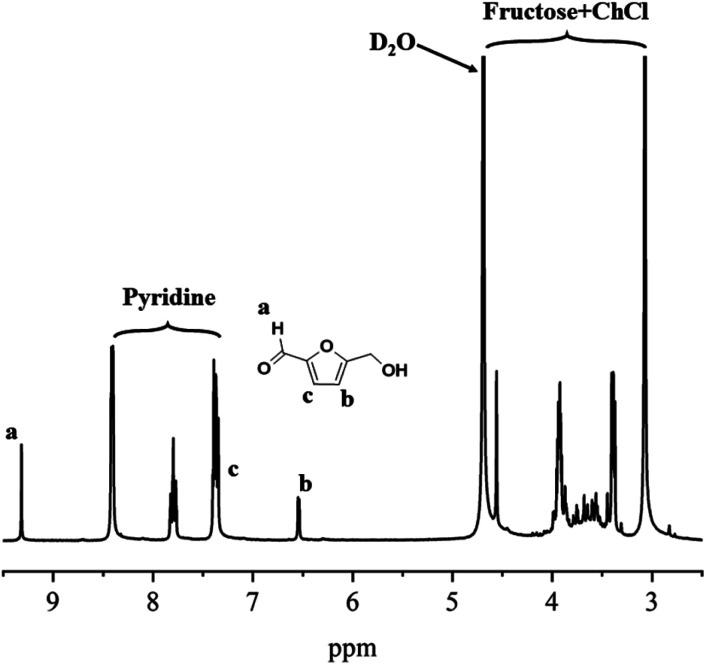
As shown in Fig. 2, with the increasing of initial substrates content, the conversion of substrates and yield of HMF both decreased a lot. For fructose, conversion and yield of HMF decreased from 42% to 13% and 32% to 13% correspondingly, and these results for glucose decreased from 30% to 8% and 15% to 8%, respectively. While the selectivity both gradually increased to about 100%. This was maybe because of the dealing capacity of boric acid: as the substrates content increased, the concentration of boric acid relatively decreased at the same time, leading to the increase in dealing pressure of boric acid, in other words, each boric acid molecule would “deal with” more substrates. The fructose or glucose involved in the reaction consumed at a lower speed, so the conversion of substrates and yield of HMF both decreased. The difference in selectivity was mainly caused by carbonization of HMF. When the conversion of substrates stayed at high level, the carbonization was severe due to the active groups of HMF,45 which led to a decrease in the yield of HMF, thus the selectivity was poor. From 1H NMR spectra showed in Fig. 4, another advantage observed of this DES system was that there was no detection of other side products and no observation of further decomposition of HMF to levulinic acid (LA) and formic acid. For glucose the system was still effective (Fig. 2(b), S1(b) and (c)†), but yield of HMF was relatively lower than that from fructose. In common, the conversion of glucose to HMF involves two steps: isomerization of glucose to fructose, and dehydration of fructose to HMF, the isomerization is catalyzed by Lewis acid. Although boric acid is a Lewis acid, it was still challenging for boric acid to catalyze the isomerization process owing to its weak acidity, thus the overall yield and conversion was comparatively lower than that obtained from fructose.
Fig. 2. Yield of HMF, conversion of substrates and selectivity obtained at different (a) fructose content, ranging from 30 wt% to 80 wt%. (b) Glucose content, ranging from 15 wt% to 60 wt%.
Fig. 4. 1H NMR spectra obtained with 50% fructose content.
The influence of reaction temperature
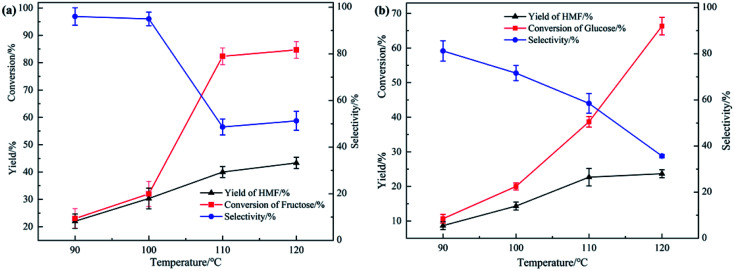
Usually, fructose and glucose, especially the latter, would convert to HMF at 160 °C or higher. In order to understand the impact of reaction temperature, a series of experiments were operated at temperature between 90 °C to 120 °C using fructose and glucose as substrates, respectively. If the content of substrates was too low, the yield was high but the selectivity was poor; if the content of substrates was too high, the selectivity was high while the yield was poor. Considering the balance between the yield and selectivity, we chose 50 wt% content for fructose and 30 wt% content for glucose.
As shown in Fig. 3, with the increase of reaction temperature, yield of HMF from fructose gradually increased from 20% to 44% and conversion increased from 20% to 82% at the same time, while the selectivity decreased from 100% to 51%; for glucose, yield of HMF and conversion increased from 8% to 23% and 10.5% to 66%, respectively, but selectivity decreased a lot. This situation maybe because as the temperature of the reaction system increased, the reaction rate increased correspondingly, which increased the consumption rate of substrates involved in the reaction, resulting in a continuous increase in the conversion and yield. However, owing to the high temperature, more serious carbonization of HMF was the main reason of the lower selectivity. Notably, when the reaction temperature increased from 110 °C to 120 °C for glucose conversion, yield of HMF increased by only 3%, but the selectivity decreased by nearly 20%, indicating that HMF carbonized more seriously at high temperature, which made it difficult to improve the yield of HMF. Results of the 1H NMR spectra (Fig. S1(a)†) showed that even though the temperature reached 120 °C, there was still no yield of other by-products.
Fig. 3. Yield of HMF, conversion of substrates and selectivity obtained at different reaction temperature ranging from 90 °C to 120 °C. (a) Fructose content: 50 wt%. (b) Glucose content: 30 wt%.
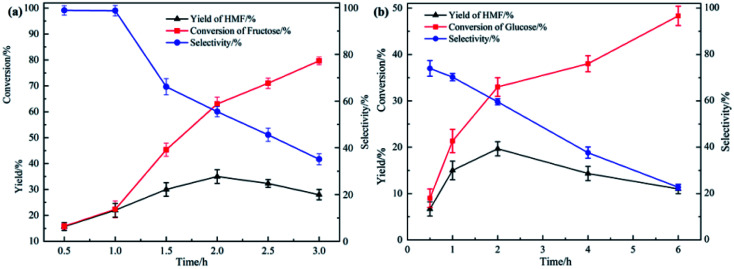
The influence of reaction time
A series of experiments were performed to investigate the effect of the reaction time on the fructose and glucose dehydration reaction (Fig. 5). Likewise, we chose 100 °C, 50 wt% for fructose and 30 wt% for glucose as reaction conditions. For both fructose and glucose, firstly as the reaction time increased, conversion and yield of HMF increased. While as the reaction continued, yield of HMF did not increase with the further consumption of substrates, and selectivity decreased all along. The growth in yield was because the amount of monosaccharides participating in the reaction gradually depleted to generate HMF as the reaction proceeded. Yet, since the long time at relatively high temperature, carbonization of HMF became more serious, yield of HMF did not increase along with the conversion of substrates afterwards, which led to the selectivity reduced dramatically.
Fig. 5. Yield of HMF, conversion of substrates and selectivity obtained at after different reaction time from (a) 0.5 h to 3 h. Fructose content: 50 wt%. (b) 0.5 h to 6 h. Glucose content: 30 wt%.
Conversion of other substrates
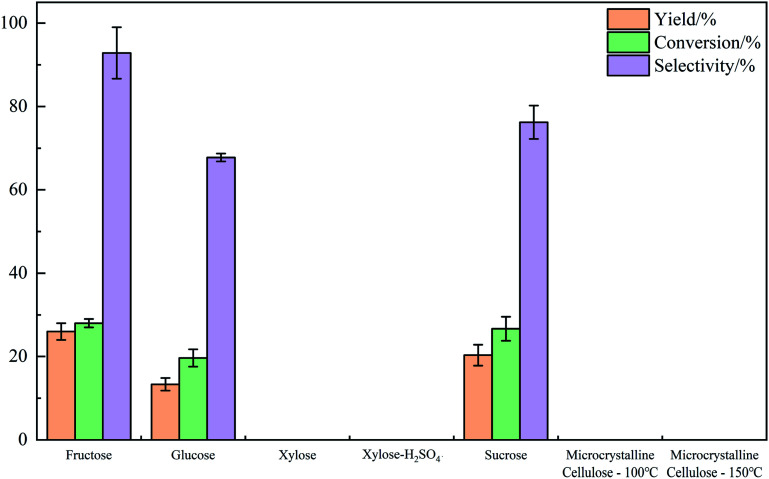
In order to find out the catalytic scope of this system, other substrates including sucrose, xylose and microcrystalline cellulose were also tested. Reaction conditions: ChCl/boric acid: 1/1(mol mol−1); temperature: 100 °C, except for microcrystalline cellulose at 100 °C and 150 °C; time: 1 h; substrates content: 30 wt%. For xylose, H2SO4 was applied as alternative catalyst additionally.
As can be seen from Fig. 6, the DES system showed the highest catalytic efficiency for the conversion of fructose to HMF. For glucose, the overall efficiency was relatively lower because the isomerization of glucose to fructose was difficult for boric acid to catalyze. For sucrose, the catalytic effect of this system was even a bit better than that of glucose. It was due to the structural particularity of sucrose. A sucrose molecule could be hydrolyzed to a fructose molecule and a glucose molecule, thus with the hydrolysis of sucrose during the reaction, fructose and glucose existed in the system at the same time, and the reaction activity was better than that when glucose was used as substrate. For another monosaccharide xylose, the DES showed no obvious catalytic activity, because no product was detected under our experiment conditions; moreover, when H2SO4 with stronger acidity was applied as catalyst, there was still no yield of product, which meant acidity of catalyst was not the reason. This maybe because of the different reaction mechanism – since the product of xylose dehydration is furfural, xylose could hardly be converted in the DES system. For microcrystalline cellulose, 150 °C was chosen additionally because it hardly reacted when carried out at 100 °C. However, no degradation product was found after the raising of temperature, which might due to the special semi-crystalline structure of microcrystalline cellulose. This structure inhibited interaction bond from forming between ChCl or boric acid in DES and microcrystalline cellulose, thus it cannot be degraded.
Fig. 6. Yield of HMF, conversion and selectivity obtained with different substrate.
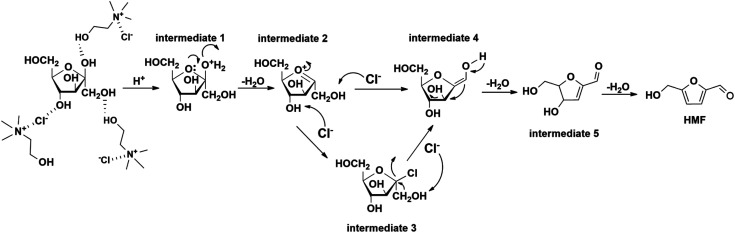
Mechanism for conversion of fructose to HMF
One possible mechanism for catalytic conversion of fructose to HMF in the three -component DES system was proposed, as shown in Fig. 7. First, ChCl and fructose interacted with each other through interaction between Cl from DES and O from fructose and hydrogen bond just like described before, then fructose converted to intermediate 1 catalyzed by boric acid. The chloride ion derived from ChCl could serve as nucleophile. Under this circumstance, oxonium ions which formed as intermediates 2 and 3 were easy to convert into intermediates 4 and 5, then further dehydration resulted in the production of HMF.46,47 Besides, when too high temperature or too long reaction time were applied, the decrease in yield of HMF and no existence of LA or formic acid observed in 1H-HMR spectra meant HMF might directly undergo carbonization in DES system without further decomposition.
Fig. 7. Possible reaction mechanism for the conversion of fructose to HMF in the three-component DES catalytic system.
Conclusions
We studied the conversion of bio-carbohydrates to HMF using a three-component DES system, which was composed of ChCl, boric acid and the substrates used. Boric acid acted as not only HBD but also the catalyst for the conversion process. For fructose, 82% conversion of fructose and 44% yield of HMF were obtained at 120 °C in 1 h at 50 wt% content of fructose; it was also effective for glucose, but yield of HMF was relatively lower (up to 23%) because of the weak acidity of boric acid. Another advantage of this DES system was that the selectivity of HMF was high. There was no generation of other side products and no further decomposition of HMF to LA or formic acid except the carbonization of HMF. Furthermore, this system showed catalytic activity for other bio-carbohydrates such as sucrose. This study should provide a promising alternative for conversion of carbohydrates to HMF.
Experimental
Materials
Commercially available ChCl and boric acid were purchased from Aladdin. Fructose, glucose, sucrose, xylose, microcrystalline cellulose and pyridine were purchased from Sigma-Aldrich. ChCl was dried at 120 °C in the oven before usage and kept in the desiccator with color changing silica gel. Other materials were used without further purification.
General procedure for the preparation of pre-DES
Suitable amount of each DES component (ChCl/boric acid: 1/1(mol mol−1)) was weighed in a round-bottom flask. The resulting mixture was heated for 30 min at 80 °C with stirring until the formation of a limpid liquid phase. The liquid obtained was dried under reduced pressure at 60 °C for 1 h and kept in a desiccator with calcium chloride.
General procedure for the dehydration of monosaccharides
For a typical reaction, 0.863 g (30 wt%) fructose was added in a Schlenk bottle with 2.013 g (10 mmol) of the pre-DES (the mixture of ChCl and boric acid). The mixture was put at 25–30 °C to stir for about 30 min until achieving complete dissolution and the three-component DES was formed. Meanwhile a stream of nitrogen was introduced into the solution to eliminate oxygen and prevent furan ring from oxidation. After that, the resulting solution was heated to the appropriate temperature and kept stirring for a certain time. Results under different conditions such as different initial substrates content, reaction temperature and reaction time were evaluated.
Characterization of products
After a certain time of reaction, the resulting product was weighted quantitatively and dissolved in D2O and pyridine was chosen as the internal standard. After stirring well-distributed, the mixture was filtered with a filter head (Φ0.22 μm) to eliminate some insoluble matter. 1H NMR spectra were obtained on a Bruker 300 MHz spectrometer for the analysis of the conversion of bio-carbohydrates and yield of HMF. Yield of HMF was calculated according to the peak located at 9.45 ppm (s, H, H–C O), conversion of fructose was calculated according to the peak located at 3.94 ppm originated from the hydrogen atoms on the methylene (s, 2H, –CH2–OH), conversion of glucose was calculated according to the peak located at 5.1 ppm originated from the hydrogen atoms on the methyne (d, H, –O–CH–OH). The yield of HMF, conversion of carbohydrate, and selectivity of HMF were calculated with following formulas.
 |
1 |
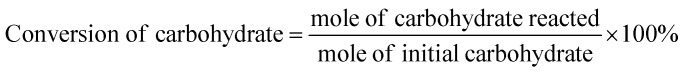 |
2 |
 |
3 |
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We gratefully thank the funding from the National Natural Science Foundation of China (No 51973212), the Department of Science and Technology of Jilin Province (No 20210203119SF and 20210203173SF) and the Open Research Fund of Shanghai Key Laboratory of Green Chemistry and Chemical Processes, East China Normal University.
Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01688e
References
- McKendry P. Bioresour. Technol. 2002;83(1):37–46. doi: 10.1016/s0960-8524(01)00118-3. [DOI] [PubMed] [Google Scholar]
- Alonso D. M. Bond J. Q. Dumesic J. A. Green Chem. 2010;12(9):1493–1513. [Google Scholar]
- Roman-Leshkov Y. Chheda J. N. Dumesic J. A. Science. 2006;312:1933–1937. doi: 10.1126/science.1126337. [DOI] [PubMed] [Google Scholar]
- Gallezot P. Chem. Soc. Rev. 2012;41(4):1538–1558. doi: 10.1039/c1cs15147a. [DOI] [PubMed] [Google Scholar]
- Werpy T. Petersen G. Aden A. Bozell J. J. Jones S. Nato Adv. Sci. Institutes. 2004;1:21–61. [Google Scholar]
- Zhang Z. Zhen J. Liu B. Lv K. Deng K. Green Chem. 2015;17(2):1308–1317. [Google Scholar]
- Jiang M. Liu Q. Zhang Q. Ye C. Zhou G. J. Polym. Sci., Part A: Polym. Chem. 2012;50(5):1026–1036. [Google Scholar]
- Wang G. Jiang M. Zhang Q. Wang R. Qu X. Zhou G. Polym. Degrad. Stab. 2018;153:272–280. [Google Scholar]
- Teddy B. Sebastien N. Huat P. P. Ignacio M.-C. Vries J. G. Heeres H. J. Angew. Chem., Int. Ed. 2011;50(50):7083. [Google Scholar]
- Cukalovic A. Stevens C. V. Green Chem. 2010;12(7):1201–1206. [Google Scholar]
- Chen M.-Y. Chen C.-B. Zada B. Fu Y. Green Chem. 2016;18(13):3858–3866. [Google Scholar]
- Tan Z. Liu Y. Liu H. Yang C. Niu Q. Cheng J. J. J. Environ. Chem. Eng. 2021;9(5):106104. [Google Scholar]
- Tan Z. Li X. Yang C. Liu H. Cheng J. J. Chem. Eng. J. 2021:424. [Google Scholar]
- Liu Y. Geng Y. X. Zhou Q. Yuan W. Q. J. Chem. Technol. Biotechnol. 2018;93(3):849–854. [Google Scholar]
- Dashtban M. Gilbert A. Fatehi P. RSC Adv. 2014;4(4):2037–2050. [Google Scholar]
- Yin Y. Ma C. Li W. Luo S. Liu Y. Wu X. Wu Z. Liu S. Ind. Crops Prod. 2021:160. [Google Scholar]
- Lansalot-Matras C. Moreau C. Catal. Commun. 2003;4(10):517–520. [Google Scholar]
- Zhao H. Holladay J. E. Brown H. Zhang Z. C. Science. 2007;316:1597–1600. doi: 10.1126/science.1141199. [DOI] [PubMed] [Google Scholar]
- Hansen T. S. Woodley J. M. Riisager A. Carbohydr. Res. 2009;344(18):2568–2572. doi: 10.1016/j.carres.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Ruß C. König B. Green Chem. 2012;14(11):2969. [Google Scholar]
- Yan L. Ma R. Wei H. Li L. Zou B. Xu Y. Bioresour. Technol. 2019;279:84–91. doi: 10.1016/j.biortech.2019.01.120. [DOI] [PubMed] [Google Scholar]
- Shi S. Wu Y. Liu P. Zhang M. Zhang Z. Gao L. Xiao G. Catal. Lett. 2021:16. [Google Scholar]
- Hu S. Zhang Z. Song J. Zhou Y. Han B. Green Chem. 2009;11(11):1746–1749. [Google Scholar]
- Tran P. H. Phan H. B. Thi Nguyen Q. B. Luong C. M. Tran K. N. Mol. Catal. 2021;503:111428. [Google Scholar]
- Korner S. Albert J. Held C. Front. Chem. 2019;7:661. doi: 10.3389/fchem.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A. P. Capper G. Davies D. L. Rasheed R. K. Tambyrajah V. Chem. Commun. 2003;12(1):70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Tang B. Zhang H. Row K. H. J. Sep. Sci. 2015;38(6):1053–1064. doi: 10.1002/jssc.201401347. [DOI] [PubMed] [Google Scholar]
- Li X. Row K. H. J. Sep. Sci. 2016;39(18):3505–3520. doi: 10.1002/jssc.201600633. [DOI] [PubMed] [Google Scholar]
- Marullo S. Rizzo C. D'Anna F. ACS Sustainable Chem. Eng. 2019;7(15):13359–13368. [Google Scholar]
- Liu F. Barrault J. De Oliveira Vigier K. Jerome F. ChemSusChem. 2012;5(7):1223–1226. doi: 10.1002/cssc.201200186. [DOI] [PubMed] [Google Scholar]
- Zuo M. Le K. Li Z. Jiang Y. Zeng X. Tang X. Sun Y. Lin L. Ind. Crops Prod. 2017;99:1–6. [Google Scholar]
- Ilgen F. Ott D. Kralisch D. Reil C. Palmberger A. Konig B. Green Chem. 2009;11(12):1948–1954. [Google Scholar]
- Juneidi I. Hayyan M. Mohd Ali O. Environ. Sci. Pollut. Res. 2016;23(8):7648–7659. doi: 10.1007/s11356-015-6003-4. [DOI] [PubMed] [Google Scholar]
- Radosevic K. Bubalo M. C. Srcek V. G. Grgas D. Dragicevic T. L. Redovnikovic I. R. Ecotoxicol. Environ. Saf. 2015;112:46–53. doi: 10.1016/j.ecoenv.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Yong G. Zhang Y. Ying J. Y. Angew. Chem., Int. Ed. 2008;47(48):9345–9348. doi: 10.1002/anie.200803207. [DOI] [PubMed] [Google Scholar]
- Sweygers N. Alewaters N. Dewil R. Appels L. Sci. Rep. 2018;8(1):7719. doi: 10.1038/s41598-018-26107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N. Zhang M. Pan X. Zhang J. Gao L. Xiao G. Catal. Lett. 2020;151(7):1984–1992. [Google Scholar]
- Jori P. K. Jadhav V. H. Catal. Lett. 2021:482–490. [Google Scholar]
- Ramesh A. Rajesh D. Shanthi K. Bhargav P. B. Nguyen-Le M.-T. Biomass Bioenergy. 2021:154. [Google Scholar]
- Yoshida H. Asghari F. S. Ind. Eng. Chem. Res. 2006;45:2163–2173. [Google Scholar]
- Pettersson L. Andersson I. Acta Chem. Scand. 1973;27:1019–1028. [Google Scholar]
- Souzanchi S. Nazari L. Venkateswara Rao K. T. Yuan Z. Tan Z. Charles Xu C. J. Ind. Eng. Chem. 2021;101:214–226. [Google Scholar]
- Chheda J. N. Román-Leshkov Y. Dumesic J. A. Green Chem. 2007;9(4):342–350. [Google Scholar]
- Pagán-Torres Y. J. Wang T. Gallo J. M. R. Shanks B. H. Dumesic J. A. ACS Catal. 2012;2(6):930–934. [Google Scholar]
- van Zandvoort I. Wang Y. Rasrendra C. B. van Eck E. R. Bruijnincx P. C. Heeres H. J. Weckhuysen B. M. ChemSusChem. 2013;6(9):1745–1758. doi: 10.1002/cssc.201300332. [DOI] [PubMed] [Google Scholar]
- Amarasekara A. S. Williams L. D. Ebede C. C. Carbohydr. Res. 2008;343(18):3021–3024. doi: 10.1016/j.carres.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S. Dutta S. Saha B. Green Chem. 2011;13(10):2859. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.