Abstract
Pseudomonas aeruginosa, an important opportunistic pathogen, was isolated from environmental samples and compared to clinically derived strains. While P. aeruginosa was isolated readily from an experimental mushroom-growing unit, it was found only rarely in other environmental samples. A flagellin gene PCR-restriction fragment length polymorphism analysis of the isolates revealed that environmental and clinical P. aeruginosa strains are not readily distinguishable. The variation in the central regions of the flagellin genes of seven of the isolates was investigated further. The strains used included two strains with type a genes (998 bp), four strains with type b genes (1,258 bp), and one strain, K979, with a novel flagellin gene (2,199 bp). The route by which flagellin gene variation has occurred in P. aeruginosa is discussed.
Pseudomonas aeruginosa is a major cause of serious nosocomial infections, particularly in immunocompromised patients, and is the most common pathogen associated with cystic fibrosis (4), in which chronic lung colonization is a major factor in morbidity and mortality. P. aeruginosa has also been isolated from a number of environmental sources, including water (5, 12, 15), soil (19), plants, such as barley roots (7) and stored onions (21), and a gasoline-contaminated aquifer (3). However, the main habitat of P. aeruginosa remains controversial. Although P. aeruginosa is widely thought to be ubiquitous, the frequency of recovery of P. aeruginosa isolates is often very low (5, 15). It is also not clear whether environmental and clinical isolates of P. aeruginosa are different (12, 16). This fact has significant consequences for the proposed environmental release of P. aeruginosa as a plant growth promoter (6) and for bioremediation, including bioremediation that involves stimulation of natural Pseudomonas populations (23).
Recently, molecular typing methods have become useful for separating strains belonging to the same species. For example, the bacterial flagellin gene provides a new way to differentiate or subtype strains (27). In P. aeruginosa, two types of flagellin protein have been identified. These two types have been designated type a flagellin and type b flagellin, which can be distinguished on the basis of molecular size and reactions with type-specific polyclonal and monoclonal antibodies (1). Unlike Salmonella flagellins, the type a and b flagellins of P. aeruginosa do not exhibit phase variation; a single strain produces a single type of flagellin, and no switching between types a and b has been observed. Oligonucleotide primers for PCR amplification of the flagellin genes of P. aeruginosa have been developed. Restriction fragment length polymorphism (RFLP) analysis of PCR products has been used to separate 64 P. aeruginosa clinical isolates into 13 groups (29), and the usefulness of this method as a tool for strain separation was demonstrated when it was employed to provide evidence that a β-lactam-resistant strain had spread in a cystic fibrosis clinic (2).
In this paper we describe development of a method for isolating P. aeruginosa and characterization of strains obtained from a new experimental mushroom-growing unit and other sources in which a detailed analysis of flagellin gene sequences was performed. In addition, we discuss ways that flagellin gene variation may have occurred in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and culture.
The isolates used in this study and their sources are listed in Table 1. All of the clinical isolates were identified as P. aeruginosa by using the API-NE test (29). The following additional tests were performed on strains: ability to grow on nutrient agar at 42°C and production of soluble pigments on King’s B medium (10). To confirm the identity of strains, the exotoxin A (ETA) gene was amplified from a selection of strains by using primers ETA1 and ETA2 as described by Khan and Cerniglia (9). All of the strains were cultured on nutrient agar plates at 30°C for 24 h and were stored at 4°C. Liquid cultures were prepared by using Luria broth inoculated with strains and grown at 30°C and 150 rpm for 24 h.
TABLE 1.
P. aeruginosa isolates used in this study
| Isolate(s) | Source | Size of flagellin gene PCR product (kb) | RFLP group |
|---|---|---|---|
| 1, 2 | Soil | 1.25 | I |
| 3, 4, 5, 6, 7, 8 | Mushroom surfaces | 1.02 | V |
| 9, 10 | Casing | 1.02 | V |
| 11 | Casing | 1.25 | I |
| 12, 13, 14, 15, 16, 17, 18, 19, 20 | Casing | 1.02 | V |
| 21 | Casing | 1.02 | XVIII |
| 22, 23, 24, 25, 26 | Casing | 1.02 | V |
| 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 | Straw | 1.02 | V |
| 39 | Yard straw | 1.02 | V |
| 40 | Yard straw | 1.02 | VII |
| 41 | Yard straw | 1.02 | V |
| 42 | Floor water | 1.02 | V |
| 43 | Floor water | 1.25 | I |
| 44, 45, 46, 47 | Floor water | 1.02 | V |
| AQ28, AQ29, AQ30, AQ31, AQ32, AQ33 | Canadian aquifer (3)a | 1.02 | XV |
| AQ35, AQ36, AQ37, AQ38, AQ39, AQ40, AQ41 | Canadian aquifer (3) | 1.02 | XV |
| H150 | CF patientb | 1.02 | XIV |
| H191 | CF patient | 1.02 | XV |
| D278 | CF patient | 1.02 | XVI |
| H20, H115 | CF patients | 1.25 | XVII |
| 590, K701 | Clinical samples (29) | 1.02 | Various |
| 409, 593, C201, E4193 | Clinical samples (29) | 1.25 | Various |
| K979 | Clinical sample (29) | 2.0 | XIII |
The numbers in parentheses are reference numbers.
CF, cystic fibrosis.
Isolation of P. aeruginosa from environmental samples.
Samples were obtained from the mushroom-growing unit at Horticulture Research International, Wellesbourne, Warwickshire, United Kingdom, which opened in 1995, during August 1996 and were examined to determine whether P. aeruginosa was present. Samples of individual mushrooms (button stage), casing (a mushroom-growing medium), straw, and floor water were collected within the mushroom unit, and samples of straw were collected outside the unit, as were samples of the tap water supplied to the unit, unused casing, pasteurized compost, and chicken manure. Chicken manure and straw are components of the pasteurized compost that is used in the unit to grow mushrooms. Additional samples of casing and mushrooms were collected within the unit and straw samples were collected outside the unit in November 1996. Various soil samples from locations surrounding Horticulture Research International (Wellesbourne), including soil samples WQ, WQ1, and B1, were also analyzed. Water samples were obtained from the Coventry Canal, the River Avon (at Stratford upon Avon), and a pond site at Horticulture Research International (Wellesbourne).
Soil samples were initially screened for P. aeruginosa by suspending 1 g (wet weight) of each sample in 9 ml of 0.25× Ringer’s solution and vortexing the preparation for 30 s prior to plating 0.5 ml onto CN (Oxoid), CFC (Oxoid), PIA (Difco), or King’s B (10) medium. The plates were incubated for up to 48 h at a variety of temperatures (25, 30, 35, 37, and 42°C) in order to determine the optimum conditions. The method eventually used for casing, straw, pasteurized compost, chicken manure, and rescreening of some soil samples included a recovery step involving plating onto King’s B medium and incubation at 35°C for 4 h. Following incubation, each plate was divided into quarters, and each quarter was swabbed onto a CN plate (Oxoid), which was incubated overnight at 42°C. In order to determine the effectiveness of the recovery step, a casing sample and various soil samples were also screened without 4 h of incubation on King’s B medium at 35°C. The water and mushroom samples were treated differently prior to the recovery step; 0.5 ml of water was plated onto King’s B medium without dilution, and individual mushrooms, held by the stalk, were rolled directly onto King’s B medium. Colonies that were fluorescent and oxidase positive and exhibited growth on King’s B medium at 42°C were presumptively identified as P. aeruginosa colonies.
PCR amplification of the flagellin gene.
The central region of the P. aeruginosa flagellin gene was amplified by the method of Winstanley et al. (29). The conserved primers which were used, CW46 and CW45, bind at positions 146 and 2024 on the Pseudomonas putida Paw8 flagellin gene (28). After the PCR, aliquots (5 μl) of each reaction mixture were subjected to electrophoresis on a standard 0.7% (wt/vol) agarose gel containing 0.1 μg of ethidium bromide per ml to confirm the presence of an amplified product. Samples (5 μl) of the flagellin gene amplified products were digested in 15-μl (final volume) reaction mixtures with restriction enzymes MspI, HaeIII, CfoI, MboI, RsaI, and SalI. Digestion was performed by using the conditions recommended by the supplier (Life Technologies), and the digests were subjected to electrophoresis on 3% (wt/vol) MetaPhor agarose (Flowgen). The digested amplified products were electrophoresed alongside a PCR size marker (fragment sizes, 50, 150, 300, 500, 750, 1,000, 1,500, and 2,000 bp; R & D Systems).
Computer analysis of RFLP groups.
Levels of pairwise similarity between isolates were calculated with a metric that assigned equal weight to each fragment length for which at least one of the isolates produced a fragment; a score of 1 was assigned when both isolates produced the fragment, and a score of 0 was assigned when only one isolate produced the fragment. A hierarchical cluster analysis was performed by using the unweighted pair group with mathematical average algorithm of the GENSTAT 5 package, and a dendrogram was constructed.
DNA sequencing.
PCR products were purified by using a QIAquick PCR purification kit (Qiagen, Dorking, United Kingdom) according to the manufacturer’s instructions. Cycle sequencing was performed by using a Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems) with 0.2 μg of DNA per 200 bp of product and 10 ng of primer per reaction mixture. Samples were analyzed with an ABI automated sequencer (Applied Biosystems). The initial sequencing was performed by using primers CW45 and CW46. Internal primers were designed to determine the DNA sequence at intervals of 150 to 200 bp downstream from each previous reaction. The sequences obtained were compared to sequences obtained from databases and to each other by using the GAP, FASTA, and CLUSTALV packages of the Genetics Computer Group (University of Wisconsin). A DNA sequence composition and GCCG/CGGC motif analysis was performed by using Clone Manager for Windows 4.0 (S & E Software, State Line, Pa.).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the nucleotide sequences of the central regions of the flagellin genes determined in this study are X99098, X98280, X98281, X98461, X98462, X98463, X98464, and X98465.
RESULTS AND DISCUSSION
Isolation of P. aeruginosa.
During the first sampling period (August 1996), P. aeruginosa was isolated within the mushroom unit from casing, straw, floor water, and mushrooms and outside the unit from yard straw. No P. aeruginosa was detected in the tap water supplied to the unit or in the external samples of unused casing, pasteurized compost, or chicken manure. Strains were identified by PCR amplification of the ETA gene by using oligonucleotide primers ETA1 and ETA2, which gave a single 396-bp amplified product (ETA positive) for more than 150 isolates obtained from the mushroom-growing facility. Early in this work we found that using the selective medium alone was not sufficient to prevent isolation of many non-P. aeruginosa colonies. Although we found that a higher temperature could be used to select for P. aeruginosa, direct incubation at 42°C on a selective medium resulted in reduced levels of recovery. For example, the average number of CFU per plate obtained without a recovery period from the casing sample was 19, compared with an average of more than 200 CFU per plate when preincubation on King’s B medium at 35°C for 4 h was included. These values may not represent a genuine increase in the level of recovery as the method of swabbing cells from one plate to another could also lead to an increase in the number of CFU. However, if detection is more important than enumeration, then inclusion of a recovery step can provide greater reliability. Certainly there is a need for further assessment and development of methods for isolation of P. aeruginosa, since the methods commonly used have been designed for use with clinical samples rather than for use with environmental samples.
Three months after the first sampling period, P. aeruginosa was still detected within the mushroom unit in casing and yard straw samples, although the mushroom samples obtained at this time were negative. We could not prove that the yard straw was a source of P. aeruginosa within the unit. However, the methods employed in this study demonstrated that the casing, pasteurized compost, chicken manure, and water supplied to the unit were not sources of P. aeruginosa contamination. For compost and casing, pseudomonads were detected by culturing samples on PIA at 30°C, and after 2 days the levels were 107 to 108 CFU per g (dry weight); these pseudomonads were mainly Pseudomonas fluorescens and P. putida.
Application of the method, including the recovery step, to several soil samples and three water samples yielded only two ETA-positive P. aeruginosa isolates from soil and no isolates from water. Culturing on PIA revealed that the same soil samples contained 104 to 106 pseudomonad CFU per g (dry weight). These findings indicate that the experimental mushroom-growing unit, which has elevated temperature, humidity, and nutrient conditions, may offer an environment in which P. aeruginosa can thrive and thus should be added to the growing list of environmental hosts in which this organism can be found. Neither the exact reasons for the selective enhancement of P. aeruginosa in the mushroom unit nor the actual source of contamination is known. It is possible that employees in the unit are a source.
Flagellin gene RFLP analysis.
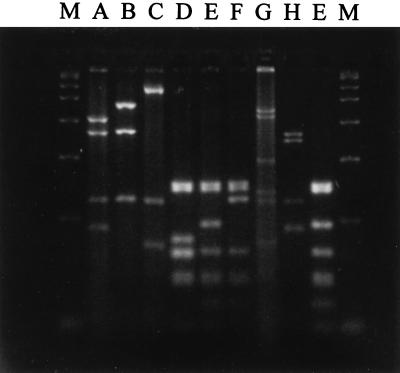
PCR amplification performed with oligonucleotide primers CW45 and CW46 gave a single amplified product with all of the ETA-positive strains of P. aeruginosa. Of the 45 mushroom unit ETA-positive strains tested, 43 had a 1.02-kb flagellin gene amplified product (type a flagellin) (29), while 2 had a 1.25-kb product (type b flagellin) (29). Both soil isolates had type b flagellins, whereas all of the aquifer isolates had type a flagellins. The results of the RFLP analysis of the flagellin gene products allowed us to place the two soil isolates, 45 mushroom unit isolates, 13 aquifer isolates, and five selected clinical isolates of P. aeruginosa into flagellin gene RFLP groups as described previously (29) (Table 1). Five of these groups, designated RFLP groups XIV, XV, XVI, XVII, and XVIII (Table 1), were not found in a previous study of clinical isolates of P. aeruginosa (29). Figure 1 shows all of the restriction patterns that have been observed to date when P. aeruginosa flagellin gene amplified products have been digested with restriction enzyme MboI. Although the majority of the new RFLP groups were the result of different combinations of previously described restriction patterns, one restriction fragment pattern that was not previously observed was obtained with MboI (Fig. 1, lane H) from the amplified flagellin genes of two clinical isolates (RFLP group XVII). A dendrogram showing the relationships among the 18 RFLP groups is shown in Fig. 2.
FIG. 1.
MboI-digested P. aeruginosa flagellin gene PCR products. The gel shows all of the patterns (patterns A through H) obtained with restriction enzyme MboI. Restriction pattern E is shown twice; it was derived once from a clinical isolate and once from a mushroom unit isolate. Lanes M contained a PCR marker (fragment sizes, 50, 150, 300, 500, 750, 1,000, 1,500, and 2,000 bp).
FIG. 2.
Relationships among P. aeruginosa flagellin gene RFLP groups. The dendrogram shows the relationships among the 18 RFLP groups (groups I through XVIII). RFLP groups II, III, IV, VI, VIII, IX, X, XI, XII, and XIII were not observed in this study but have been identified previously (29).
The majority of the mushroom unit isolates (41 isolates) were assigned to RFLP group V, and one isolate was assigned to RFLP group VII; both of these groups contain clinical isolates (29). A single mushroom unit isolate was assigned to RFLP group XVIII (Table 2), a group containing no clinical isolates. The genetic variation found within the mushroom isolates indicates that contamination of the unit involved more than one strain of P. aeruginosa. All but one of the mushroom unit isolates analyzed was placed in a flagellin gene RFLP group known to contain clinical isolates. The results of restriction of the flagellin gene amplified products failed to discriminate among the 13 aquifer isolates but did indicate that these isolates were different from the isolates found in soil and mushroom unit samples. Although we found that these isolates were indistinguishable from each other on the basis of flagellin gene RFLP analysis results, the aquifer isolates contain flagellin genes that are readily distinguishable from the flagellin genes of all but one clinical isolate that we analyzed. This suggests that the Canadian clinical isolates used by Foght et al. (3) differ considerably from the clinical isolates obtained in the United Kingdom or, more likely, that the molecular methods employed in the study of Foght et al. were not sufficiently sensitive to detect interstrain differences. These results highlight the fact that discrimination between potentially pathogenic and environmental isolates is not possible.
TABLE 2.
Levels of similarity of complete PCR product flagellin sequences of P. aeruginosa types a and b and P. aeruginosa K979 to each other and to the sequences of other bacteriaa
| Flagellin sequence | % Similarity to the flagellin sequence of:
|
||
|---|---|---|---|
| P. aeruginosa 590 (type a) | P. aeruginosa C201 (type b) | P. aeruginosa K979 | |
| P. aeruginosa 590 | 100 (100) | 58 (35) | 51 (25) |
| P. aeruginosa C201 | 59 (35) | 100 (100) | 44 (26) |
| P. aeruginosa K979 | 51 (25) | 44 (26) | 100 (100) |
| P. putida PRS2000b | 49 (56) | 47 (54) | 44 (43) |
| P. putida Paw8b | 52 (53) | 46 (56) | 40 (52) |
| Escherichia colic | 36 (26) | 30 (23) | 25 (20) |
| Salmonella rubisawd | 38 (26) | 35 (22) | 30 (20) |
| Salmonella typhimuriume | 37 (25) | (22) | 23 (21) |
| Campylobacter colif | 26 (20) | 22 (20) | 22 (21) |
| Campylobacter jejunif | 26 (20) | 24 (19) | 20 (20) |
| Bacillus subtilisg | 26 (21) | 21 (19) | 20 (21) |
Levels of similarity were determined by using the Genetics Computer Group program GAP. The values in parentheses are percentages of similarity for the central flagellin variable region, which were determined from sequences after the terminal conserved regions were deleted.
Data from reference 29.
Data from reference 17.
Data from reference 24.
Data from reference 8.
Data from reference 26.
Data from reference 11.
Flagellin gene sequence variation.
All seven strains used for flagellin gene sequencing were identified as ETA-positive P. aeruginosa strains. The estimated sizes of the PCR products representing most of the flagellin genes of seven P. aeruginosa strains were 1.02 kb (strains 590 and K701), 1.25 kb (strains 409, 593, C201, and E4193), and 2.0 kb (strain K979). The two type a sequences included in this study (the strain 590 and K701 sequences) aligned with more than 90% similarity with the previously published P. aeruginosa PAK sequence (22). The four type b flagellins were much larger, and their sequences aligned with the previously published type b sequences (20). The final strain, K979, produced an abnormally large central flagellin region that was 2,100 bp long. This sequence exhibited similarity to other flagellin sequences only in the N- and C-terminal regions. The overall levels of similarity of the P. aeruginosa type a and b and K979 flagellin sequences to each other and to previously published flagellin sequences are shown in Table 2.
The type a and b genes appear to be marginally more closely related to each other (57%) than to the K979 gene (43 to 52%). Comparisons of these flagellin genes to the genes of two P. putida flagellins indicated that the levels of similarity of the flagellin genes within P. aeruginosa are only slightly greater than their levels of similarity to the P. putida flagellin gene. Comparisons to other bacterial flagellin genes indicated that the levels of similarity were lower. If only the central variable regions (350-bp N-terminal truncation and 200-bp C-terminal truncation at either end) are compared, the levels of similarity of the P. aeruginosa sequences to each other and the sequences of P. putida and members of the Enterobacteriaceae are lower. However, central regions of the type a and b and K979 P. aeruginosa flagellin genes aligned with greatest similarity to P. putida flagellin genes rather than each other. Diversity in the flagellin central regions was expected since this area of the flagellin protein can vary greatly in amino acid sequence while the proteins remain more or less functionally equivalent (26). Although the flagellin genes identified in this study exhibit homology at their N- and C-terminal ends, it is not clear how the dramatic variation in the central region evolved.
Flagellin gene sequence composition analysis.
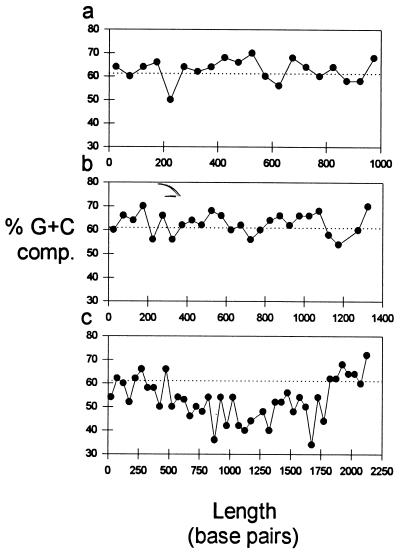
In an attempt to determine how the three different P. aeruginosa flagellin genes may have arisen, the G+C contents of the genes and the frequency of the P. aeruginosa GCCG/CGGC motif (25) were compared. The central regions of the type a and b flagellin genes had an average G+C content of 63%, which is equivalent to the G+C content of the P. aeruginosa chromosome (13, 14). The G+C contents determined for 50-bp segments of the type a and b flagellin genes were also found to be similar throughout the gene (Fig. 3). The P. aeruginosa K979 flagellin gene is more than twice as big as the type a gene. The average G+C content of this gene was 53%, but the G+C content along its length varied (Fig. 3). In the first 500 bp the G+C content was high and equivalent to typical values obtained for P. aeruginosa. After the first 500 bp the G+C content declined and was restored to the high value only from 1,800 bp to the end of the gene. In addition to a low G+C content (53%), this gene had a very low frequency (1 in 102) of the GCCG/CGGC motif. In the type a flagellin gene this motif occurred at a frequency of 1 in 32, which was equivalent to the frequency in the P. aeruginosa chromosomal genes in the GenBank database (25). This frequency was only slightly lower in type b genes, 1 in 42 bases. If the GCCG/CGGC motif occurred at random in a sequence, then it would be apparent once in every 254 bases, yet in P. aeruginosa it occurs once in every 32 bases. The reason for the frequent occurrence of this motif in the P. aeruginosa chromosome is not known, but its presence does allow it to be used as an indicator for determining P. aeruginosa sequences within DNA. Both the low G+C content of the K979 flagellin gene and the low frequency of the GCCG/CGGC motif indicate that this gene is not typical of the P. aeruginosa chromosome and differs considerably from the type a and b flagellin genes. This suggests that the K979 flagellin gene has acquired an additional section of DNA from outside the P. aeruginosa chromosome, and the low G+C content of the region between 500 and 1,800 bp may indicate that this region is the most likely area where recombination has occurred (Fig. 3). The predicted amino acid sequence revealed 25-amino-acid repeats at positions 172 to 197 and 268 to 293. This corresponds to base pair positions starting at positions 516 and 804, which may also indicate that a recombination event occurred. Recombination of large segments of DNA within and between bacterial strains offers the most likely explanation for P. aeruginosa flagellin gene variation. This scenario is similar to what is believed to occur in the genus Salmonella, in which the flagellin gene (fliC) is highly polymorphic, especially among Salmonella enterica strains with genes containing segments of DNA from diverse origins (18).
FIG. 3.
G+C contents of strain 590, which has a type a flagellin (a), strain 409, which has a type b flagellin (b), and strain K979 (c). G+C contents were calculated for 50-bp intervals and were plotted along the length of the PCR product. The average G+C content of P. aeruginosa is indicated by the dotted lines.
Overall, the flagellin gene PCR-RFLP method was used to separate P. aeruginosa strains into at least 18 groups. The results highlight the diversity present in this gene, which, as described in this study, may have come about through recombination events. The variability in this single gene makes it a particularly useful marker for strain detection and subspecies differentiation. However, the power of this method has also revealed that discrimination between potentially pathogenic strains and strains considered harmless is not possible. The fact that the majority of the P. aeruginosa isolates found within the experimental mushroom-growing unit were indistinguishable from clinical strains may also be of some concern to the food industry. Furthermore, our findings have considerable implications for the use of P. aeruginosa in the natural environment as bioremediation agents and plant growth promoters.
ACKNOWLEDGMENTS
The P. aeruginosa strains isolated from a gasoline-contaminated aquifer were gifts from J. M. Foght of the Department of Biological Sciences, University of Alberta, Edmonton, Alberta, Canada. We thank Angela Bardon of the University of Liverpool sequencing unit for operating the automated sequencer.
We acknowledge the financial support provided by the BBSRC, the Nuffield Foundation, and grant 044249/PMG/VW from The Wellcome Trust.
REFERENCES
- 1.Allison J S, Dawson M, Drake D, Montie T C. Electrophoretic separation and molecular weight characterization of Pseudomonas aeruginosa H-antigen flagellins. Infect Immun. 1985;49:770–774. doi: 10.1128/iai.49.3.770-774.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng K, Smyth R L, Govan J R W, Dohertt C, Winstanley C, Denning N, Heaf D P, Van Scane R, Hart C A. Spread of β-lactam resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet. 1996;348:639–642. doi: 10.1016/S0140-6736(96)05169-0. [DOI] [PubMed] [Google Scholar]
- 3.Foght J M, Westlake D W S, Johnson W M, Ridgway H F. Environmental gasoline-utilising isolates of Pseudomonas aeruginosa are taxonomically indistinguishable by chemotaxic and molecular methods. Microbiology. 1996;142:2333–2340. doi: 10.1099/00221287-142-9-2333. [DOI] [PubMed] [Google Scholar]
- 4.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobe S, Wingender J, Truper H G. Characterization of mucoid Pseudomonas aeruginosa strains isolated from technical water systems. J Appl Bacteriol. 1995;79:94–102. doi: 10.1111/j.1365-2672.1995.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 6.Höfte M, Seong K Y, Jurkevitch E, Verstraete W. Pyoverdin production by the plant growth beneficial Pseudomonas strain 7SNK2: ecological significance in soil. Plant Soil. 1991;130:249–257. [Google Scholar]
- 7.Iswandi A, Bossier P, Vandenabeele J, Verstraete W. Relation between soil microbial activity and the effect of seed inoculation with the rhizopseudomonad strain 7NSK2 on plant growth. Biol Fertil Soils. 1987;3:147–151. [Google Scholar]
- 8.Joys T M. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985;260:15758–15761. [PubMed] [Google Scholar]
- 9.Khan A A, Cerniglia C E. Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Appl Environ Microbiol. 1994;60:3739–3745. doi: 10.1128/aem.60.10.3739-3745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King E O, Ward M K, Rainey D E. Two simple media for the demonstratation of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 11.LaVallie E R, Stahl M L. Cloning of the flagellin gene from Bacillus subtilis and complementation studies of an in vitro-derived deletion mutation. J Bacteriol. 1989;171:3085–3094. doi: 10.1128/jb.171.6.3085-3094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitao J H, Alvim T, Sa-Correia L. Ribotyping of Pseudomonas aeruginosa isolates from patients and water springs and genome fingerprinting of variants concerning mucoidy. FEMS Immunol Med Microbiol. 1996;13:287–292. doi: 10.1111/j.1574-695X.1996.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 13.Palleroni N J. Family I. Pseudomonadaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 141–281. [Google Scholar]
- 14.Palleroni N J. Pseudomonas classification: a new case history in the taxonomy of Gram-negative bacteria. Antonie Lecumenhock. 1993;64:231–251. doi: 10.1007/BF00873084. [DOI] [PubMed] [Google Scholar]
- 15.Pellett S, Bigley D V, Grimes D J. Distribution of Pseudomonas aeruginosa in a riverine ecosystem. Appl Environ Microbiol. 1983;45:328–332. doi: 10.1128/aem.45.1.328-332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Römling U, Wingender J, Muller H, Tümmler B. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl Environ Microbiol. 1994;60:1734–1738. doi: 10.1128/aem.60.6.1734-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenhals G J, Whitfield C. Comparative analysis of flagellin sequences from Escherichia coli strains possessing serologically distinct flagellar filaments with a shared complex surface pattern. J Bacteriol. 1993;175:5395–5402. doi: 10.1128/jb.175.17.5395-5402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selander R, Li J, Boyd E F, Wang F, Nelson K. DNA sequence analysis of the genetic structure of populations of Salmonella enterica and Escherichia coli. In: Priest F G, editor. Bacterial diversity and systematics. New York, N.Y: Plenum Press; 1994. pp. 17–48. [Google Scholar]
- 19.Shirkot C K, Shirkot P, Gupta K G. Isolation from soil and growth characterisation of the tetramethylthiuram disulphide (TMTD) degrading strain of Pseudomonas aeruginosa. J Environ Sci Health. 1994;29:605–614. [Google Scholar]
- 20.Spangenberg C, Heuer T, Burger C, Tummler B. Genetic diversity of flagellins of Pseudomonas aeruginosa. FEBS Lett. 1996;396:213–217. doi: 10.1016/0014-5793(96)01099-x. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson D L. Spoilage of stored onion (Allium cepa L. var. cepa) by Pseudomonas aeruginosa (Schroeter) Migula in lowland Papua New Guinea. Trop Pest Manage. 1985;31:214–216. [Google Scholar]
- 22.Totten P A, Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990;172:7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyke M I, Gulley S L, Lee H, Trevors J T. Evaluation of microbial surfactants for recovery of hydrophobic pollutants from soil. J Ind Microbiol. 1993;11:163–170. [Google Scholar]
- 24.Wei L N, Joys T M. The nucleotide sequence of the H-1(r) gene of Salmonella rubislaw. Nucleic Acids Res. 1986;14:8227. doi: 10.1093/nar/14.20.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins B M. Gene transfer by bacterial conjugation: diversity of systems and functional specializations. Symp Soc Gen Microbiol. 1995;52:59–88. [Google Scholar]
- 26.Wilson D R, Beveridge T J. Bacterial flagellar filaments and their component flagellins. Can J Microbiol. 1993;39:451–472. doi: 10.1139/m93-066. [DOI] [PubMed] [Google Scholar]
- 27.Winstanley C, Morgan J A W. The bacterial flagellin gene as a biomarker for detection, population genetics, and epidemiological analysis. Microbiology. 1997;143:3071–3084. doi: 10.1099/00221287-143-10-3071. [DOI] [PubMed] [Google Scholar]
- 28.Winstanley C, Morgan J A W, Pickup R W, Saunders J R. Molecular cloning of two Pseudomonas flagellin genes and basal body structural genes. Microbiology. 1994;140:2019–2031. doi: 10.1099/13500872-140-8-2019. [DOI] [PubMed] [Google Scholar]
- 29.Winstanley C, Coulson M A, Wepner B, Morgan J A W, Hart C A. Flagellin gene and protein variation amongst clinical isolates of Pseudomonas aeruginosa. Microbiology. 1996;142:2145–2151. doi: 10.1099/13500872-142-8-2145. [DOI] [PubMed] [Google Scholar]