Abstract
Exosomes are nanosized extracellular vesicles of endosomal origin that enclose a multitude of functional biomolecules. Exosomes have emerged as key players of intercellular communication in physiological and pathological conditions. In cancer, depending on the context, exosomes can oppose or potentiate the development of an aggressive tumor microenvironment, thereby impacting tumor progression and clinical outcome. Increasing evidence has established exosomes as important mediators of immune regulation in cancer, as they deliver a plethora of signals that can either support or restrain immunosuppression of lymphoid and myeloid cell populations in tumors. Here, we review the current knowledge related to exosome-mediated regulation of lymphoid (T lymphocytes, B lymphocytes and NK cells) and myeloid (macrophages, dendritic cells, monocytes, myeloid-derived suppressor cells and neutrophils) cell populations in cancer. We also discuss the translational potential of engineered exosomes as immunomodulatory agents for cancer therapy.
Keywords: Exosomes, cancer, immunotherapy, lymphoid cells, myeloid cells
Introduction
Exosomes are lipid bilayer-enclosed extracellular vesicles (EVs) ranging from 40 to 160 nm in diameter [1]. They are formed as intraluminal vesicles (ILVs) through the inward budding of endosomal membranes into multivesicular bodies (MVBs). After biogenesis, MVB-resident ILVs can follow the secretory pathway for release into the extracellular milieu as exosomes; or, as an alternative fate, they can merge with lysosomes for degradation [1–3]. Exosomes harbor multiple types of biomolecules, including nucleic acids (e.g. DNA, mRNA, miRNA, lncRNA) [4–7], lipids [8], metabolites [9, 10], proteins [11, 12] and carbohydrates [13]. Exosomes mediate intercellular communication through the transfer of their functional constituents to the target cells, or through engagement of membrane receptor-mediated signaling [14–16]. From a translational perspective, pre-clinical studies harnessing exosomes for cancer treatment have shown promising results [17–19]. Moreover, exosomes derived from body fluids and circulation can serve as non-invasive liquid biopsies, which allow for the early detection of pathologies, and for the assessment of patient prognosis and response to therapy [16, 20–22]. Of note, exosomes have been shown to harbor proteins that protect them from phagocytosis and complement-mediated lysis, mechanisms that may contribute to their stability [18, 23].
Microvesicles (MVs) are a class of EVs generated through direct budding from the plasma membrane. Their diameter ranges from 50 to 1,000 nm, and like exosomes, MVs were shown to mediate intercellular communication in physiological and pathological conditions [1, 2, 24].
The role of exosomes in shaping the immune landscape of tumors is an evolving area of research. An effective cycle of anti-tumor immune response begins with the release of antigens by cancer cells, and is followed by antigen-presenting cell (APC)-mediated processing and presentation of antigens to T lymphocytes. In turn, primed T lymphocytes infiltrate the tumor to perform their anti-tumor functions [25]. A broad spectrum of cell(s)-intrinsic and microenvironmental mechanisms orchestrate each stage of this cycle to determine whether a pro- or an anti-tumor immune response is mounted [25, 26]. Accordingly, exosome-derived signals can act to suppress or promote different aspects of immune responses in cancer [27–31].
Here, we discuss the current knowledge pertaining the contribution of exosomes in modulating lymphoid and myeloid cell functions in cancer. Moreover, we provide an overview on the role of engineered exosomes in cancer immunotherapy.
Exosomes regulate lymphoid cell functions in cancer
Exosomes modulate fundamental functional aspects of the lymphoid components of the tumor microenvironment (TME), and exert direct impact in immunosuppression, tumor progression and response to existing cancer therapies. In this section, we review the mechanisms of exosome-mediated regulation of T lymphocytes, B lymphocytes, and natural killer (NK) cell functions in cancer (Figure 1). A summary of these mechanisms is described in Table 1.
Figure 1: Exosome-mediated signaling modulate lymphoid cell functions in cancer.
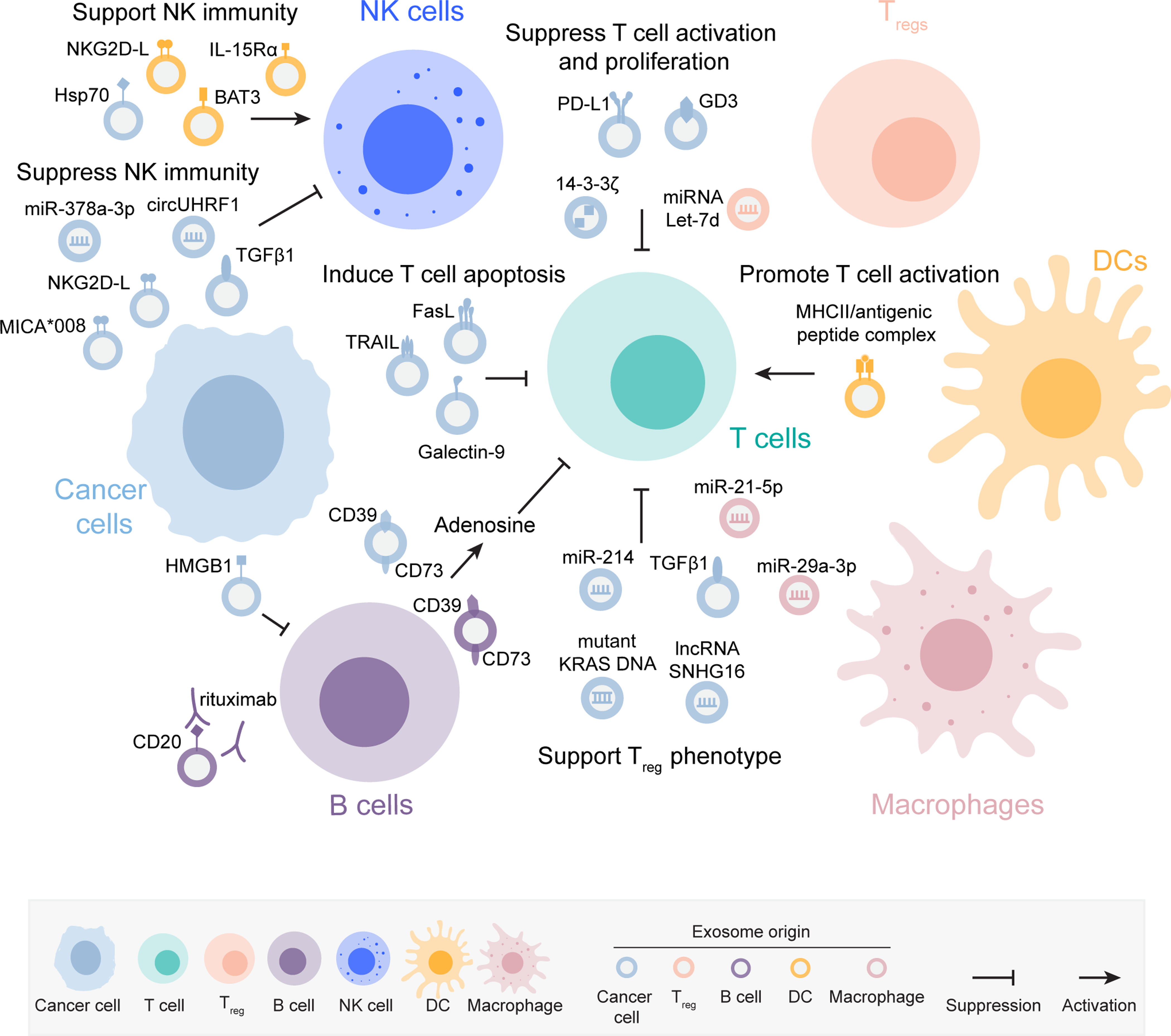
Exosomes derived from cancer cells and from various immune cell populations engage immunosuppressive signaling in recipient T cells, which impair T cell activation and proliferation, induce apoptosis and support a Treg phenotype. Antigen-presenting cell (APC)-derived exosomes harboring major histocompatibility complex II (MHCII)/antigenic peptide complex can present antigens to the target T cells and promote their activation. Exosomes derived from cancer cells suppress B cell functions. Exosomes from cancer cells and dendritic cells (DCs) signal to natural killer (NK) cells and can either promote or suppress NK cytotoxic function. The molecular cargoes in the exosomes mediating the immunomodulatory phenotypes in the lymphoid cells are indicated in the figure. The exosomes are color coded to represent the cell of origin, as described in the figure key.
Table 1:
Exosome-mediated regulation of lymphoid cell functions in cancer.
| Source | Functional molecule (s) | Target cell/molecule | Response in the target cell/molecule | Reference |
|---|---|---|---|---|
| Prostate cancer cells | FasL | CD8+ T cells | Exosomal FasL promoted apoptosis of CD8+ T cells. | [37] |
| Melanoma cells | FasL | T cells | MV-derived FasL induced apoptosis of Jurkat cells. | [40] |
| Melanoma and SCCHN | FasL | T cells | MVs induced apoptosis of CD8+ T cells and promoted Treg expansion. | [41] |
| Ovarian cancer | FasL | T cells | Membrane vesicles induced T cell apoptosis and suppressed TCR. | [42] |
| OSCC patient sera | FasL | T cells | MV-derived FasL induced T cell apoptosis and correlated with poor patient prognosis. | [43] |
| CRC cells and patient plasma | FasL and TRAIL | T cells | MV-derived FasL and TRAIL induced T cell apoptosis. | [46] |
| Metastatic melanoma | PD-L1 | CD8+ T cells | Exosomal PD-L1 impaired T cell functions, enhanced tumor growth and stratified patients into responders and non-responders to ICB therapy. | [16] |
| Plasma of HNSCC patients | PD-L1 | CD8+ T cells | Exosomal PD-L1 correlated with disease progression in HNSCC patients. Decreased CD8+ T cell activation. | [52] |
| GBM cancer cells and patient serum/plasma | PD-L1 | T cells | EV-resident PD-L1 suppressed T cell activation and proliferation. PD-L1 DNA in EVs from GBM patients correlated with tumor volume. | [53] |
| Breast cancer cells | PD-L1 | T cells | Exosomal PD-L1 impaired the activation and cancer killing potential of T cells, and enhanced tumor growth. | [54] |
| Prostate and melanoma cells | PD-L1 | CD8+ T cells | PD-L1 in exosomes suppressed T cell activity in the draining lymph node. | [55] |
| NSCLC patient serum | PD-L1 | - | Exosomal PD-L1 levels correlated with NSCLC disease progression. | [56] |
| Lung cancer cells and NSCLC patient plasma | PD-L1 | CD8+ T cells | Exosomal PD-L1 displayed immunosuppressive properties and correlated with PD-L1 levels of tumor tissues. | [57] |
| Cancer cells and melanoma patient-derived plasma | PD-L1 | T cells | Exosomal PD-L1 suppressed T cell functions and had predictive value in the response of melanoma patients to targeted and ICB therapies. | [22] |
| Plasma of gastric cancer patients | PD-L1 | T cells | 5-FU enhanced the levels of circulating exosomal PD-L1 in gastric cancer patients. In vitro, exosomal PD-L1 induced apoptosis of Jurkat cells and reduced activation of PBMCs. | [58] |
| NPC patient plasma of NPC-bearing mice | Galectin-9 | CD4+ T cells | Exosomal Galectin-9 induced apoptosis of EBV-specific CD4+ T cells upon binding to Tim-3. | [59] |
| NPC patient-derived serum and NPC cells | miRNAs | T cells | Exosomes inhibited T cell proliferation, induced a Treg phenotype and impaired TH1 and TH17 differentiation. | [60] |
| HCC cells | 14–3-3ζ | T cells | Exosomal 14–3-3ζ engaged an immunosuppressive phenotype in recipient T cells. | [61] |
| Ascites from ovarian cancer patients | Ganglioside GD3 | T cells | Exosomal GD3 decreased T cell activation. | [62] |
| Ascites from ovarian cancer patients | - | T cells | Exosomes from ascites suppressed T cell functions. | [63] |
| Plasma of melanoma patients | - | CD8+ T cells and NK cells | Enhanced apoptosis and suppressed proliferation and activation of CD8+ T cells, decreased NKG2D in NK cells. | [64] |
| Plasma of head and neck cancer patients | - | T cells and NK cells | Enhanced apoptosis of CD8+ T cells, suppressed the proliferation of CD4+ T cells and decreased NKG2D in NK cells. | [65] |
| Melanoma cancer cells | miRNAs | CD4+ T cells | Exosomes reduced the levels of BCL-2, BCL-xL and MCL-1 and enhanced apoptosis of CD4+ T cells. | [66] |
| Melanoma | TNF | T cells | Exosomes enhanced ROS levels in T lymphocytes. | [67] |
| Malignant effusion | TGFβ1 | Treg | Exosomal TGFβ1 supported maintenance of Treg number and suppressive function. | [38] |
| Lung cancer cells | miR-214 | Treg | MV-derived miR-214 suppressed PTEN and promoted Treg expansion. | [68] |
| NSCLC cells or patient serum | mutant KRAS DNA | CD4+ T cells | Exosomal mutant KRAS DNA promoted the conversion of naïve CD4+ T cells into Treg-like cells. | [69] |
| Breast cancer | lncRNA SNHG16 | T cells | Exosomes induced CD73+γδ1 Tregs. | [70] |
| Regulatory T cells | Let-7d | TH1 cells | Exosomal Let-7d decreased TH1 cells proliferation and IFNγ secretion. | [71] |
| Macrophages | miR-29a-3p and miR-21–5p | CD4+ T cells | Exosomal miR-29a-3p and miR-21–5p suppressed STAT3 and regulated Treg/TH17 ratio. | [14] |
| Various cancer cells | CD39 and CD73 | ATP/T cells | Exosomes mediated hydrolysis of ATP and generated adenosine. | [72] |
| B cells | CD39 and CD73 | ATP/T cells | B-cell-derived EVs hydrolyzed ATP into adenosine and restrained post-chemotherapeutic CD8+ T cell responses. | [73] |
| B cells | MHC class II/antigenic peptide complex | T cells | The exosomes from mouse and human B cells induced antigen-specific MHC class II T cell responses. | [77] |
| Engineered mast cells | HLA-DR1-HA | T cells | Exosomes harboring MHC/antigenic peptide complex activated T cells weakly in comparison to DCs incubated with the exosomes. | [78] |
| DCs | MHCI, MHCII, CD86, tumor peptide | T cells | DC-derived exosomes pulsed with tumor antigens primed cytotoxic T cells to mediate anti-tumor responses. | [79] |
| HCC cells and patients | HMGB1 | B cells | HMGB1 in exosomes promoted expansion of Bregs via TLR2/4-MAPK signaling pathway. | [85] |
| Esophageal cancer | - | B cells | MVs induced the conversion of naïve B cells into Bregs, which in turn suppressed the proliferation of CD8+ T cells. | [86] |
| Melanoma and thymoma cells infected with mycoplasma | - | B cells | Exosomes enhanced the activation and expansion of inhibitory B cells. | [87] |
| PDAC cells and patient plasma | Tumor-associated antigens | Immunoglobulins | Exosomes operated as decoys against complement-mediated cytotoxicity. | [88] |
| B-cell lymphoma | CD20 | anti-CD20 antibody | Exosomal CD20 bound to rituximab and protected B-cell lymphoma from antibody attack. | [89] |
| Serum from melanoma patients on neoadjuvant ICB trial | CD20 and CD27 | - | B cell-related exosomes CD20+ and CD27+ were increased in responders compared to non-responders to ICB therapy. | [92] |
| Acute myeloid leukemia patients and PDX | - | NK and CD8+ T cells | Enhanced apoptosis of CD8+ T cells and decreased NKG2D levels in NK cells. | [94] |
| B lymphoblastoid, mesothelioma, and prostate, cancer cells |
TGFβ1 and NKG2D ligands | NK and CD8+ T cells | Exosomes reduced NKG2D in NK and CD8+ T cells in a MICA and TGFβ1-dependent manner. | [95] |
| Cervical cancer cells | MICA*008 | NK cells | Exosomal MICA*008 decreased surface levels of NKG2D. | [96] |
| Mammary carcinoma cells | - | NK cells | Murine and human cancer-derived exosomes suppressed NK functions. | [97] |
| HCC | circUHRF1 | NK cells | Exosomes induced NK cell exhaustion and may be linked to resistance to anti-PD-1 therapy. | [98] |
| Irradiated U87 cells | miR-378a-3p | NK cells | Exosomes impaired NK cytotoxicity through down-regulation of granzyme B. | [99] |
| Pancreatic and colon cancer cells | Hsp70 | NK cells | Exosomal Hsp70 enhanced NK activation, migration and cytolytic activity. | [100] |
| DCs and 293T cells | BAT3 | NK cells | Exosomal BAT3 enhanced TNFα and IFNγ secretion from NK cells. | [101] |
| Irradiated melanoma cells | - | NK cells | Exosomes from irradiated melanoma cells enhanced the infiltration of NK cells producing IFNγ into tumors and delayed tumor growth in an NK-dependent manner. | [102] |
| DCs | IL-15Rα and NKG2D ligands | NK cells | DC-derived exosomes enhanced NK cell proliferation and activation via IL-15R and NKG2D. | [103] |
T lymphocytes
T cells mediate key immune responses in the context of infection, cancer and autoimmune diseases. Cytotoxic CD8+ T lymphocytes and CD4+ T helper 1 (TH1) cells are major players of anti-tumor immune responses [32, 33]. On the other hand, regulatory T cells (Tregs) are an immunosuppressive subset of CD4+ T cells in the tumor microenvironment [34]. Therefore, mechanisms that favor the recruitment and expansion of cytotoxic CD8+ T lymphocytes and TH1 cells over Tregs support an efficient anti-tumor immune response. T helper 17 (TH17) cells have a dual role in cancer, as they can either accelerate tumor progression through induction of angiogenesis and immunosuppression, or foster anti-tumor immunity through recruitment and activation of immune cells [35].
Several T cell-intrinsic and -extrinsic factors can promote dysfunction and exhaustion of CD4+ and CD8+ T lymphocytes in cancer. These factors include persistent T cell receptor (TCR) engagement, immune-checkpoint signals, cytokine milieu, nutrient and oxygen availability [26, 36]. Exosomes are often an overlooked component contributing to T cell dysfunction in cancer. However, a growing body of evidence has demonstrated that exosomes derived from cancer and from TME cells can engage an array of functional responses in T cells, which include inducing apoptosis, suppressing proliferation and activation, and fostering a Treg phenotype [16, 27, 37, 38].
The interaction between Fas ligand (FasL) with Fas receptor initiates T cell apoptosis through activation of a caspase cascade [39]. FasL was identified in MVs and exosomes derived from different sources, including melanoma [40, 41], prostate cancer [37], squamous cell carcinoma of the head and neck (SCCHN) [41], ovarian cancer [42], oral squamous cell carcinoma (OSCC) [43], and activated T cells [41, 44, 45]. Strikingly, exosomes and MVs harboring FasL have been implicated in inducing apoptosis of T lymphocytes [37, 40–43]. In addition to FasL, MVs isolated from colorectal cancer (CRC) cells and patient plasma were decorated with TNF-related apoptosis-inducing ligand (TRAIL), and induced T cell apoptosis in a FasL- and TRAIL-dependent manner [46]. In addition to promoting T cell apoptosis, FasL in serum-derived membrane vesicles correlated with tumor burden and nodal involvement in OSCC patients, thus suggesting a potential role as a biomarker [43]. The T cell apoptosis induced by FasL-containing tumor derived-MVs could be prevented upon pre-treatment with IRX-2, an immunotherapeutic agent that contains physiological amounts of various cytokines [47]. Additionally, IRX-2 prevented the MV-induced up-regulation of Fas receptor, abrogated caspase 8 activation, and promoted nuclear translocation of nuclear factor kappa B (NF-κB) in T cells [48]. The cytoprotection conferred by IRX-2 was dependent on phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) axis and on protein synthesis, as the use of an Akt inhibitor or cycloheximide abrogated the anti-apoptotic effects of IRX-2 in T lymphocytes [47, 48].
Another mechanism of immune evasion in cancer is initiated by the interaction between the programmed death-ligand 1 (PD-L1), found in cancer cells and in TME cell populations, with the programmed cell death protein 1 (PD-1) receptor, expressed on activated T cells [49, 50]. Notably, exosomal PD-L1 has emerged as an additional mechanism of tolerance in cancer [31, 51]. In vitro, exosomal PD-L1 dramatically suppressed T cell activation and proliferation [16, 52, 53]; decreased the levels of TH1 cytokines and granzyme B [16]; inhibited cell killing and the activation of extracellular signal-regulated kinase (ERK) and NF-κB in T cells [54]. Moreover, exosomes can transfer the PD-L1 protein to cancer and TME cells and convert them from PD-L1-negative to PD-L1-positive, suggesting a mechanism whereby exosomes can amplify immunosuppression in the tumor microenvironment through horizontal transfer of PD-L1 protein to cells [54]. In vivo, exosomal PD-L1 enhanced B16-F10 tumor growth and suppressed anti-tumor immunity systemically [16]. Moreover, in the TRAMP-C2 and MC-38 tumor models, exosomal PD-L1 enhanced tumor progression and suppressed the activity of CD8+ T cells in the draining lymph nodes, a phenotype that was rescued upon blockage of exosome secretion or upon PD-L1 deletion [55]. Exosomal PD-L1 also accelerated the growth of orthotopic 4T1 tumors, and the blockage of exosome secretion with GW4869 or Rab27a knockout (KO) had a synergistic effect with anti-PD-L1 treatment to suppress 4T1 tumor growth [54]. In addition to fostering tumor progression and immune tolerance, exosomal PD-L1 has been shown to provide prognostic value to several types of cancer. In head and neck squamous cell carcinoma (HNSCC), the levels of PD-L1 in plasma-derived exosomes were significantly higher in patients with active disease in comparison to patients with no evidence of disease after therapy [52]. In glioblastoma (GBM) patients, the levels of PD-L1 DNA in serum and plasma-derived EVs correlated with tumor volume [53]. In non-small cell lung cancer (NSCLC), the levels of PD-L1 in serum-derived exosomes were higher in patients with progressed disease features, including advanced tumor stage, larger tumor size, positive lymph node status and metastasis, thereby suggesting that exosomal PD-L1 can be used to monitor NSCLC progression [56]. In addition, the abundance of PD-L1 in exosomes isolated from plasma of NSCLC patients correlated with PD-L1 positivity in tumor tissues [57]. In metastatic melanoma patients, changes in the levels of circulating exosomal PD-L1 at early stages of anti-PD1 therapy could stratify patients into clinical responders from non-responders [16]. Moreover, the levels of exosomal PD-L1 inversely correlated with the response of melanoma patients to immune and targeted therapies [22]. A recent study found that the use of 5-fluorouracil (5-FU) may influence the levels of exosomal PD-L1 in circulation. In stage III–IV gastric cancer patients undergoing repeated cycles of 5-FU treatment, the levels of exosomal PD-L1 increased in comparison to baseline [58].
In addition to FasL and PD-L1, exosomes isolated from cancer patients and from cancer cells induced immunosuppressive traits in the target T cells through several mechanisms. Exosomes isolated from plasma of Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma (NPC) patients or mice transplanted with NPC tumors, harbored Galectin-9. Upon binding to the receptor T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), exosomal Galectin-9 promoted apoptosis of EBV-specific CD4+ T cells. The neutralization of Galectin-9 or Tim-3 reversed the exosome-induced apoptosis [59]. Exosomes derived from NPC cells and patients inhibited T cell proliferation, induced a Treg phenotype and hindered TH1 and TH17 differentiation. Specifically, the NPC exosomes reduced the levels of interleukin-2 (IL-2), interferon gamma (IFNγ) and interleukin-17 (IL-17) and increased the levels of interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) released from CD4+ and CD8+ T cells [60]. The exosome-mediated transfer of 14–3-3ζ (or 14–3-3 protein zeta) from hepatocellular carcinoma (HCC) cells to T cells elicited immunosuppression, as demonstrated by impaired T cell activation and proliferation, and enhanced exhaustion and Treg phenotype [61]. Exosomes from ascites fluid of ovarian cancer patients harbored the ganglioside GD3 at their surface, which contributed to the suppression of T cell activation. This finding was validated via addition of GD3 in liposomes, which also inhibited T cell activation; and through the use of GD3 blocking antibody or sialidase treatment, which ameliorated the exosome-induced T cell phenotype [62]. In addition, exosomes isolated from ascites fluid of ovarian cancer patients inhibited TCR-dependent activation of T cells, a phenotype that was transient and reversible upon exosomes removal [63]. Exosomes isolated from plasma of melanoma patients suppressed activation and proliferation, and enhanced apoptosis of CD8+ T cells. The melanoma-derived exosomes had increased levels of the immunosuppressive proteins FasL and TRAIL and decreased levels of the immunostimulatory proteins OX40 ligand (OX40L), OX40 and CD40 ligand (CD40L) [64]. Exosomes isolated from plasma of head and neck cancer patients with active disease enhanced apoptosis of CD8+ T cells and suppressed the proliferation of CD4+ T cells. The precise mechanism of action of the exosomes was not determined; however, the levels of several immunosuppressive proteins were increased, namely cyclooxygenase-2 (COX-2), transforming growth factor beta 1 (TGFβ1), PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and TRAIL [65]. B16-F10-derived exosomes enhanced tumor growth and apoptosis of CD4+ T cells. The exosomes increased the levels of Fas on T cell surface and enhanced caspase activation. Moreover, miRNAs that suppress the levels of anti-apoptotic proteins may be responsible for the effect of B16-F10-derived exosomes on CD4+ T cells [66]. Furthermore, melanoma-derived exosomes harboring tumor necrosis factor (TNF) transmitted redox signaling to recipient T lymphocytes [67].
In cancer, several reports have shown that EVs can foster a Treg phenotype. Surface-bound TGFβ1 in exosomes isolated from malignant effusions of cancer patients supported tolerance through the maintenance of Treg number and suppressive function [38]. Tumor-derived MVs induced the expansion of CD4+ CD25+ FoxP3+ Tregs, as opposed to MVs derived from DCs, which were not implicated in promoting the Treg phenotype [41]. Cancer cell-derived MVs transferred miR-214 to CD4+ T cells to suppress phosphatase and tensin homolog (PTEN) expression and promote Treg expansion. Notably, the miR-214-induced CD4+ CD25+ FoxP3+ Tregs increased the levels of secreted IL-10 and promoted tumor growth in vivo [68]. Exosomes isolated from mutant KRAS NSCLC cell lines and patient serum promoted the conversion of naïve CD4+ T cells into Tregs. The transfection of naïve T cells with mutant KRAS cDNA mirrored the phenotype, suggesting a role for exosome-mediated transfer of mutant KRAS DNA in driving the conversion. Accordingly, tumor tissues from mutant KRAS patients were enriched in FoxP3+ Tregs in comparison to the WT KRAS counterparts [69]. Breast cancer-derived exosomes induced CD73+γδ1 Treg cells through exosome-mediated delivery of the lncRNA SNHG16. In the recipient cells, lncRNA SNHG16 competitively bound to miR-16–5p, enabling the activation of TGFβ1/SMAD5 pathway and promoting the expression of CD73 [70].
Exosomes derived from TME cells also contribute to the suppression of T cell functions. The exosome-mediated transfer of the miRNA Let-7d from Tregs to TH1 cells decreased TH1 proliferation and IFNγ secretion [71]. In epithelial ovarian cancer (EOC) patients, the Treg/TH17 ratio was higher in tumors and in metastatic tissues in comparison to benign tumors and peritoneum. Notably, the imbalance in the Treg/TH17 ratio occurred through exosome-mediated transfer of miR-29a-3p and miR-21–5p from macrophages to CD4+ T cells, which suppressed signal transducer and activator of transcription 3 (STAT3) signaling [14].
Exosomes can suppress T cell function not only directly, but also indirectly through the processing of intermediates that elicit tolerance. In fact, cancer-derived exosomes harboring CD39 and CD73 mediated the hydrolysis of extracellular adenosine triphosphate (ATP) to generate adenosine, which in turn suppressed T cell functions [72]. Chemotherapeutic treatments trigger tumor cell death and release vast amounts of ATP to the extracellular milieu. CD19+ B cell-derived EVs hydrolyzed extracellular ATP via CD39 and CD73 into adenosine, which then suppressed CD8+ T cell responses in the post-chemotherapy setting. Notably, serum-derived CD19+ EVs were increased in tumor-bearing mice and in cancer patients, and had an inverse correlation with improved patient prognosis post-chemotherapy. Hypoxia-inducible factor-1α (HIF-1α) supported the release of CD19+ EVs from B cells through increase of Rab27a expression. In vivo, the silencing of Rab27a in B cells improved the efficacy of chemotherapy and increased the infiltration of CD8+ T cells [73, 74]. Of note, adenosine can also induce a tolerogenic phenotype in dendritic cells (DCs) [75, 76]. Therefore, the exosome-mediated conversion of ATP into adenosine likely foster immunosuppression in distinct cell populations of the tumor microenvironment.
Exosomes can also transmit activating signals to T cells. Indeed, APC-derived exosomes carrying antigenic peptide/major histocompatibility complex (MHC) and costimulatory proteins were shown to directly present antigens to T cells and induce their activation. However, the potency of exosome-induced T cell activation was weaker than the one elicited by APCs [77–79]. Of note, exosomes can also mediate indirect antigen presentation through the transfer of antigenic peptide/MHC complexes to APCs [78, 80–82]. Mechanisms of direct and indirect antigen presentation mediated by EVs have been previously reviewed [27, 83].
B lymphocytes
B cells have multiple roles in the context of cancer immunity. They can produce immunoglobulins, present antigens, provide costimulatory signals and release cytokines. Importantly, the TME contain functionally heterogeneous B cell populations, which can either support or suppress anti-tumor immunity [84].
HCC-derived exosomes promoted the expansion of the TIM-1+ regulatory B cell (Breg) population, which expressed IL-10, and suppressed CD8+ T cell proliferation, tumor necrosis factor-α (TNF-α) and IFNγ production. Mechanistically, exosome-bound high mobility group box-1 (HMGB1) engaged the toll-like receptor 2/4 (TLR2/4) and mitogen-activated protein kinase (MAPK) signaling in B cells to drive the Breg phenotype. This mechanism can contribute to immune tolerance in HCC patients, as the accumulation of Bregs in HCC tumors correlated with poor clinical outcome [85]. MVs isolated from esophageal cancer cells induced the conversion of naïve B cells into TGFβ-producing Bregs, which in turn suppressed the proliferation of CD8+ T cells [86].
Mycoplasma-infected cancer cells released exosomes that modulated B cell functions. Specifically, the exosomes enhanced the activation and expansion of inhibitory B cells, which suppressed T cell proliferation and impaired TCR signaling. This finding suggests a potential exosome-mediated mechanism implicated in mycoplasma-dependent immunosuppression [87].
A study has shown that pancreatic ductal adenocarcinoma (PDAC)-derived exosomes harbor tumor antigens able to induce autoantibodies and to bind to circulating immunoglobulins. Moreover, the PDAC-derived exosomes inhibited PDAC serum-mediated complement-dependent cytotoxicity against cancer cells. Taken together, the authors suggest that PDAC-derived exosomes can function as decoys to oppose complement-mediated cytotoxicity towards cancer cells [88].
A treatment modality in the setting of B-cell lymphoma corresponds to the administration of the anti-CD20 antibody rituximab. Exosomes have been shown to function as decoys that impair rituximab therapy. Specifically, B-cell lymphomas released exosomes containing CD20, which bound to the therapeutic anti-CD20 antibodies and protected the cancer cells from targeting and consequent cell death [89].
Recent studies revealed that B cells and tertiary lymphoid structures are associated with improved clinical outcome in metastatic melanoma, sarcoma and renal cell carcinoma patients undergoing immune checkpoint blockade (ICB) therapy [90–92]. In this setting, exosomes can be used to distinguish responders from non-responders to ICB therapy through the evaluation of B cell markers at exosome surface. Indeed, B cell-related exosomes positive for CD20 and CD27, were significantly increased in responders in comparison to non-responders to ICB therapy [92].
NK cells
NK cells are an innate lymphoid cell population with cytolytic function towards microbial agents and tumors [93].
Several reports have shown that exosomes can reduce the levels of the activating receptor natural killer group 2 member D (NKG2D) in NK cells. Indeed, NKG2D levels were decreased in NK cells treated with exosomes from melanoma [64], head and neck cancer patients [65], and acute myeloid leukemia (AML) patients and patient-derived xenografts (PDX) [94]. Shedding light into the potential mechanisms of exosome-mediated downregulation of NKG2D, a study has shown that cancer exosomes harbored TGFβ1 and several NKG2D ligands (MICA, MICB, ULBP-1, ULBP-2, or ULBP-3). The exosome-mediated reduction of NKG2D in NK cells was partially rescued upon MICA neutralization, and almost abolished upon TGFβ1 neutralization, suggesting a dominant role for TGFβ1 in this mechanism [95]. Similarly, HeLa-derived exosomes carried the full-length membrane-bound MICA*008 protein, and the treatment of NK cells with exosomal MICA*008 decreased surface levels of NKG2D [96].
Exosomes derived from mammary carcinoma enhanced tumor growth and inhibited NK cytotoxic activity, release of perforin, expression of cyclin D3 and activation of Jak3 pathway, suggesting that cancer-derived exosomes can favor tumor growth through impairment of NK functions [97]. HCC-derived exosomes promoted NK cell exhaustion through circular ubiquitin-like with PHD and ring finger domain 1 (circUHRF1) RNA, which suppressed miR-449c-5p and induced TIM-3 expression in NK cells. Notably, in HCC patients, higher circUHRF1 levels were associated with resistance to anti-PD1 therapy, and in subcutaneously implanted HCCLM3 tumors, the knockdown of circUHRF1 enhanced the sensitivity to anti-PD-1 therapy [98]. Exosomal miR-378a-3p from irradiated U87 cancer cells impaired NK cytotoxicity through down-regulation of granzyme B. In line with this finding, the expression of miR-378a-3p in exosomes derived from GBM and cervix cancer patients treated with radiotherapy inversely correlated with the serum levels of granzyme B [99].
Exosomes have also been shown to potentiate NK cell functions. Cancer-derived exosomes enhanced NK activation, migration and cytolytic activity in a heat shock protein 70 (Hsp70)-dependent manner. Mechanistically, exosomal Hsp70 elicited apoptosis of cancer cells through NK release of granzyme B [100]. DC and 293T-derived exosomes harboring the protein HLA-B-Associated Transcript-3 (BAT3) bound to NKp30 receptor in NK cells and enhanced the secretion of TNF-α and IFNγ. The dependency of exosomal BAT3 in mediating this phenotype was confirmed through overexpression and silencing experiments [101]. The intratumoral injection of exosomes from irradiated melanoma cells delayed B16F10GP tumor growth and enhanced the infiltration of NK cells producing IFNγ. The depletion of NK cells abrogated the exosome-mediated anti-tumor phenotype, as opposed to the depletion of CD8+ T cells, which had no effect [102]. DC-derived exosomes from murine and human origin enhanced NK cell proliferation and activation through IL-15Rα and NKG2D, respectively. Accordingly, in a phase I clinical trial testing DC-derived exosomes as cancer vaccine, a subset of melanoma patients restored the number and the NKG2D-dependent functions of NK cells [103].
Exosomes regulate myeloid cell functions in cancer
In the cancer setting, exosomes modulate key aspects of myeloid cell functions. In this section, we describe exosome-mediated mechanisms implicated in the functional reprogramming of tumor-associated macrophages (TAMs), DCs, monocytes, myeloid-derived suppressor cells (MDSCs), and neutrophils (Figure 2). A summary of these mechanisms can be found in Table 2.
Figure 2: Exosome-mediated signaling modulate myeloid cell functions in cancer.
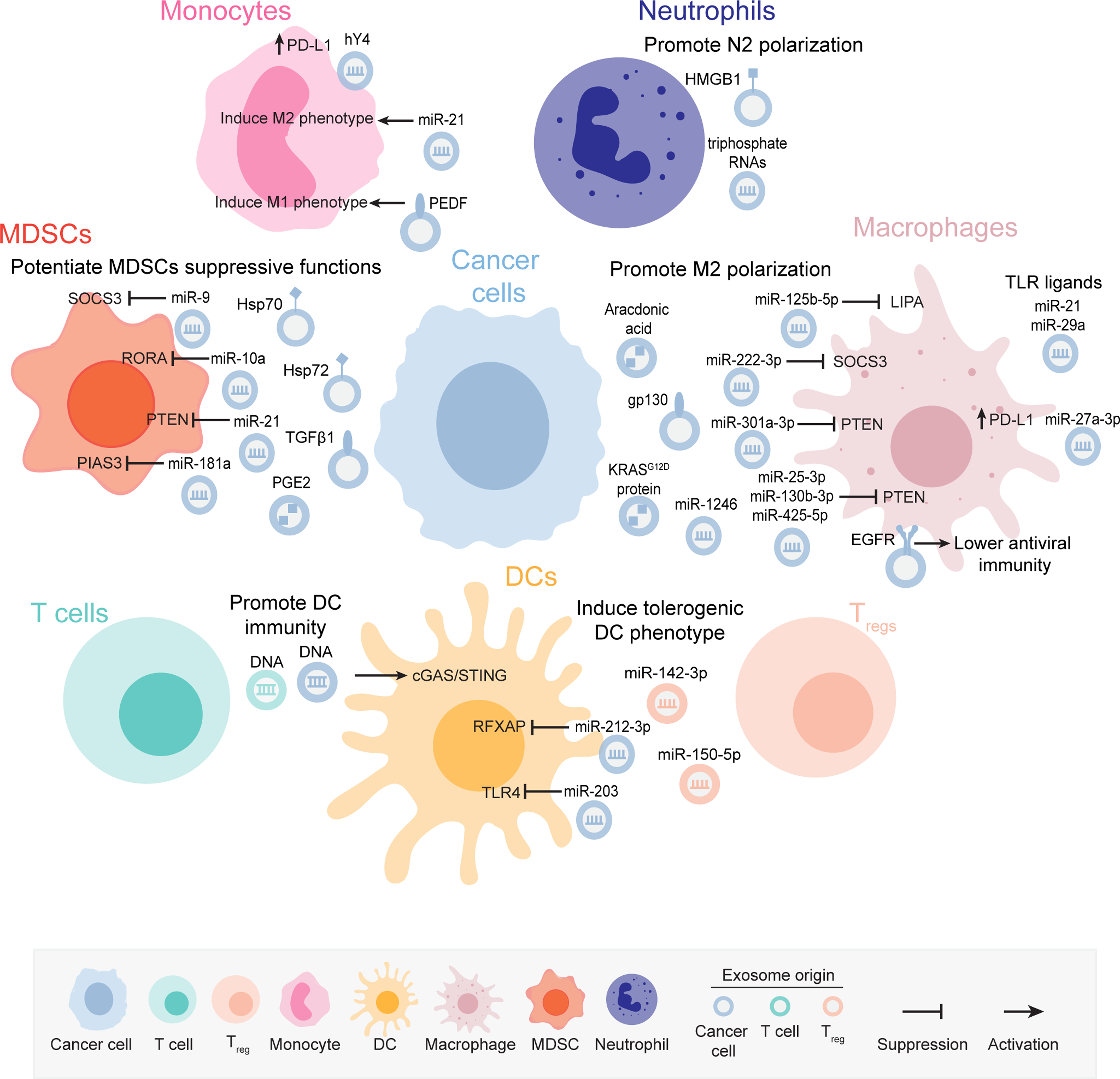
Cancer cell-derived and immune cells-derived exosomes mediate the reprogramming of several myeloid cell populations towards a tumor-promoting and immunosuppressive phenotype through the delivery of nucleic acids and proteins or via engagement of receptor-mediated signaling. Certain exosomal cargoes also trigger anti-tumor immune responses via myeloid cell targeting. The molecular cargoes in the exosomes mediating the immunomodulatory phenotypes in the myeloid cells are indicated in the figure. The representation of the exosomes is color coded to depict the cell of origin, which is shown in the figure key.
Table 2:
Exosome-mediated regulation of myeloid cell functions in cancer.
| Source | Functional molecule (s) | Target cell | Response in the target cell | Reference |
|---|---|---|---|---|
| EOC cells | miR-222–3p | Macrophages | Exosomal miR-222–3p suppressed SOCS3, activated STAT3, induced M2 polarization and enhanced tumor progression. | [106] |
| p53-mutant cancer cells | miR-1246 | Macrophages | Exosomal miR-1246 reprogramed macrophages to a tumor-promoting state. | [107] |
| Melanoma cells | miR-125b-5p | Macrophages | Exosomal miR-125b-5p targeted LIPA in macrophages and promoted survival. | [108] |
| Hypoxic pancreatic cancer cells | miR-301a-3p | Macrophages | Exosomal miR-301a-3p induced M2 phenotype through PTEN/PI3K signaling. | [109] |
| Pancreatic cancer cells | Arachidonic acid | Macrophages | Exosomes shuttled arachidonic acid and promoted M2 phenotype. | [110] |
| Pancreatic cancer cells undergoing ferroptosis | KRASG12D protein | Macrophages | Exosomes promoted M2 polarization through STAT3-dependent fatty acid oxidation. | [111] |
| Metastatic osteosarcoma cells | - | Macrophages | Exosomes induced M2 polarization and impaired phagocytosis, efferocytosis, and macrophage-dependent tumor cell killing. | [112] |
| Lung cancer cells | - | Macrophages | Exosomes induced M2 polarization and enhanced macrophage oxygen consumption rate. | [113] |
| CRC cells | miR-25–3p, miR-130b-3p and miR-425–5p | Macrophages | Exosomes induced M2 polarization through suppression of PTEN and activation of PI3K/Akt signaling and contributed to the establishment of liver metastasis. | [114] |
| Breast cancer cells | gp130 | Macrophages | Exosomal gp130 promoted activation of STAT3 signaling, enhanced the levels of pro-tumorigenic cytokines and the survival of BMDMs. | [115] |
| Liver cancer cells undergoing ER stress | - | Macrophages | Exosomes promoted the secretion of IL‑6, MCP‑1, IL‑10 and TNFα in macrophages through STAT3 signaling. | [118] |
| Breast cancer cells undergoing ER stress | miR-27a-3p | Macrophages | Exosomes promoted immune evasion through increase in PD-L1 expression in macrophages. | [119] |
| Breast cancer cells | - | Macrophages | Exosomes promoted activation of NF-κB pathway in macrophages and enhanced the levels of IL-6, TNFα, GCSF and CCL2 in a TLR2-dependent manner. | [120] |
| Gastric cancer cells | - | Macrophages | Exosomes induced the activation of NF-κB and the expression of the pro-inflammatory factors IL-6, TNFα, and CCL2 in macrophages to promote tumor progression. | [121] |
| Lung cancer cells | miR-21 and miR-29a | Macrophages | Exosomal miR-21 and miR-29a favored a pro-metastatic inflammatory response by serving as ligands of TLR receptors in macrophages, promoting NF-κB activation and increasing the secretion of IL-6 and TNFα. | [122] |
| Lung cancer cells | EGFR | Macrophages | Activated EGFR in exosomes lowered the host innate antiviral immunity through MEKK2/IRF3 axis. | [15] |
| Pancreatic cancer cells | miR-212–3p | DCs | Exosomal miR-212–3p targeted RFXAP, decreased MHC II levels and induced immune tolerance in DCs. | [124] |
| Pancreatic cancer cells | miR-203 | DCs | Exosomal miR-203 decreased the expression levels of TLR4 and the secretion of TNFα and IL-12 in DCs. | [125] |
| Breast cancer cells | - | DCs | Exosomes suppressed the differentiation of myeloid precursor cells into DCs in an IL-6-dependent manner. | [126] |
| LLC and 4T1 cells | - | DCs | Inhibited DC maturation. | [127] |
| Regulatory T cells | miR-150–5p and miR-142–3p | DCs | Induced tolerogenic phenotype in DCs. | [128] |
| Breast cancer cells | DNA | DCs | Breast cancer cells treated with Topocan released exosomes containing DNA, which activated the cGAS/STING pathway in DCs. | [129] |
| Breast cancer cells | DNA | DCs | Exosomal DNA primed DCs to elicit anti-tumor responses through cGAS/STING pathway. | [130] |
| T cells | DNA | DCs | EV-derived DNA engaged cGAS/STING pathway in DCs. | [131] |
| Pancreatic cancer | - | Monocytes | Exosomes decreased HLA-DR expression in monocytes, and induced arginase and ROS. | [136] |
| Snail-Expressing Cancer Cells | miR-21 | Monocytes | Exosomal miR-21 induced M2 polarization and tumorigenesis. | [137] |
| Chronic lymphocytic leukemia | noncoding Y RNA hY4 | Monocytes | Exosomal hY4 enhanced the expression of PD-L1 and the release of cytokines in a TLR7-dependent manner. | [138] |
| GBM stem cells | - | Monocytes | Exosomes skewed monocytes toward M2 phenotype and enhanced PD-L1 expression. | [139] |
| Gastric cancer cells | - | Monocytes | Exosomes mediated monocytes differentiation into PD1+ TAMs with M2 phenotype, which suppressed CD8+ T cell functions. | [140] |
| Non-metastatic melanoma cells | PEDF | Monocytes | Exosomal PEDF promoted patrolling monocytes differentiation into TRAIL+ M1 macrophages with cancer killing potential. | [141] |
| Thymoma, mammary carcinoma, and colon carcinoma cells |
Hsp72 | MDSCs | Exosomal Hsp72 elicited immunosuppressive signaling in MDSCs via TLR2/STAT3 axis. | [143] |
| Renal cancer cells | HSP70 | MDSCs | Exosomes promoted MDSC proliferation and activation via TLR2 signaling to promote tumor growth and immunosuppression. | [144] |
| Breast cancer cells | miR-9 and miR-181a | MDSCs | Exosomes activated JAK/STAT signaling in eMDSCs through the targeting of SOCS3 and PIAS3. | [145] |
| Breast tumors | TGFβ1 and PGE2 | MDSCs | Exosomal TGFβ1 and PGE2 promoted MDSCs accumulation and accelerated tumor growth. | [146] |
| Hypoxic glioma cancer cells | miR-10a and miR-21 | MDSCs | Exosomes promoted MDSCs expansion and activation through miR-10a/Rora/IκBα/NF-κB and miR-21/Pten/ PI3K/AKT pathways. | [147] |
| OSCC cells | miR-21 | MDSCs and γδ T cells | Hypoxic exosomes inhibited γδ T cells functions through MDSCs. | [148] |
| Gastric cancer cells | HMGB1 | Neutrophils | Exosomal HMGB1 induced autophagy and pro-tumor activation of neutrophils via TLR4/NF-κB signaling | [151] |
| CRC stem cells | tri-phosphate RNAs | Neutrophils | Exosomes enhanced the expression of IL-1β via NF-κB signaling to promote tumorigenesis. The depletion of neutrophils abrogated CRCSC-induced tumorigenesis. | [152] |
| Breast cancer cells | - | Neutrophils | Exosomes enhanced the formation of NETs in vitro, and accelerated the formation of thrombosis in vivo. | [153] |
Macrophages
Macrophages are cells from the mononuclear phagocytic system involved in the clearance of cells, pathogens and substances through phagocytosis. In addition, macrophages secrete a plethora of immunomodulatory cytokines and mediate key functions of innate and adaptive immune responses. In cancer, macrophages display exceptional functional plasticity and are traditionally classified as tumor-restraining M1, and tumor-promoting M2 population [104, 105].
Several studies reported that cancer cell-derived exosomes mediate the polarization of macrophages towards the tumor-promoting M2 phenotype. Exosomes isolated from EOC cells and patient serum were enriched with miR-222–3p, which was transferred to macrophages to induce M2 polarization. Mechanistically, miR-222–3p enhanced STAT3 activation through targeting of suppressor of cytokine signaling 3 (SOCS3). In vivo, miR-222–3p induced the M2 phenotype, promoted tumor growth, and increased the density of CD31+ microvessels and LYVE-1+ lymphatic vessels. Accordingly, the levels of miR-222–3p in serum-derived exosomes from EOC patients were higher than healthy individuals [106]. p53-mutant cancer cells reprogrammed macrophages to a tumor-supporting anti-inflammatory state through exosome-mediated transfer of miR-1246. In macrophages, miR-1246 enhanced the secretion of anti-inflammatory cytokines and epithelial to mesenchymal transition (EMT)-promoting factors. In vivo, cancer cells co-transplanted with the mutant p53-reprogrammed macrophages or with macrophages transfected with miR-1246 enhanced tumor growth and metastasis. In CRC patients, mutant p53 positively correlated with TAMs and with miR-1246 expression [107]. Melanoma-derived exosomes transferred miR-125b-5p to macrophages and suppressed the expression of lysosomal acid lipase A (LIPA). In turn, this contributed to tumor-promoting properties and enhanced survival of macrophages [108]. Hypoxic pancreatic cancer cells shuttled miR-301a-3p to macrophages through exosomes. In macrophages, miR-301a-3p promoted M2 polarization via PTEN/PI3K signaling. The M2 polarized macrophages enhanced the metastatic ability of pancreatic cancer cells, both in vitro and in vivo [109]. Exosomes from the human PDAC cells AsPC-1 induced macrophage polarization towards the immunosuppressive M2 phenotype. Moreover AsPC-1 exosomes contained high levels of arachidonic acid, which can regulate inflammatory responses upon conversion to prostaglandin. Indeed, macrophages treated with AsPC-1-derived exosomes enhanced the secretion of prostaglandin 2, and of several other factors implicated in tumor progression, including vascular endothelial growth factor A (VEGFA), IL-6, IL-1β, monocyte chemoattractant protein-1 (MCP-1), TNF-α and matrix metalloproteinase-9 (MMP-9) [110]. Pancreatic cancer cells undergoing autophagy-dependent ferroptosis released exosomes containing oncogenic KRASG12D protein, which was transferred to macrophages to promote M2 polarization through STAT3-dependent fatty acid oxidation. The blockage of KRASG12D release or uptake abrogated the macrophage-mediated pancreatic tumor growth in vivo. In pancreatic cancer patients, high KRASG12D in macrophages correlated with decreased survival [111]. Exosomes released by metastatic osteosarcoma cells, but not from non-metastatic osteosarcoma cells, induced M2 polarization and impaired phagocytosis, efferocytosis, and macrophage-dependent tumor cell killing [112]. Exosomes derived from lung cancer cells promoted M2 polarization and remodeling of macrophage metabolism, as demonstrated by enhanced oxygen consumption rate [113]. The exosome-mediated transfer of miR-25–3p, miR-130b-3p and miR-425–5p from CRC cells to macrophages induced M2 polarization through suppression of PTEN. In turn, the M2 macrophages induced EMT and angiogenesis, and enhanced the metastatic dissemination of cancer cells to the liver. Accordingly, in CRC patients, the expression levels of miR-25–3p, miR-130b-3p and miR-425–5p in serum-derived exosomes correlated with tumor progression and metastasis [114].
Exosomes released from breast cancer cells transferred gp130 to bone marrow-derived macrophages (BMDMs), which in turn promoted the activation of STAT3 signaling, enhanced the levels of pro-tumorigenic cytokines and the survival of BMDMs. The inhibition of gp130 or blockage of exosome uptake in BMDMs rescued the exosome-mediated phenotype, thus confirming that exosomal gp130 is involved in the polarization of BMDMs to a tumor-supporting phenotype [115].
Endoplasmic reticulum (ER) stress can impede the development of anti-tumor immune responses through reprogramming of several immune cell populations [116, 117]. In macrophages, exosomes derived from liver cancer cells undergoing ER stress promoted the secretion of IL‑6, MCP‑1, IL‑10 and TNF-α through STAT3 signaling [118]. Another study has shown that exosomal miR-27a-3p released from breast cancer cells undergoing ER stress promoted immune evasion through up-regulation of PD-L1 expression in macrophages [119]. These two studies may indicate that exosomes can contribute to ER stress-mediated reprogramming of macrophages.
Cancer cell-derived exosomes have been shown to activate NF-κB signaling in macrophages [120–122]. Breast cancer-derived exosomes promoted the activation of NF-κB and enhanced the levels of IL-6, TNF-α, granulocyte colony-stimulating factor (GCSF) and C-C motif chemokine ligand (CCL2) in macrophages; a phenotype dependent on TLR2 and MyD88, but not on TLR4 or TLR3/7/8/9. In addition, palmitoylated proteins at the exosome surface also contributed to the exosome-mediated NF-κB activation [120]. Exosomes derived from gastric cancer cells induced the activation of NF-κB and the expression of the pro-inflammatory factors IL-6, TNF-α, and CCL2 in macrophages. In turn, the exosome-reprogrammed macrophages enhanced the invasive, migratory and proliferative capabilities of cancer cells in vitro [121].
In macrophages, exosomal miRNAs were shown to function as agonists of TLR receptors to elicit a pro-metastatic inflammatory response. Specifically, miR-21 and miR-29a in exosomes from cancer cells operated as ligands of TLR receptors in macrophages, promoting NF-κB activation and increasing the secretion of IL-6 and TNF-α. This mechanism enhanced the formation of lung multiplicities in vivo, using a model of tail vein injection of Lewis lung cancer (LLC) cells in mice [122].
Lung cancer cells deployed exosomes to lower the host innate antiviral immunity. This occurred through the transfer of activated epidermal growth factor receptor (EGFR) from exosomes to the host macrophages. Using the LLC model combined with viral infection, the authors observed an increased viral load and impaired innate immunity upon exosome administration, which was dependent on EGFR and mitogen-activated protein kinase kinase kinase 2 (MEKK2). Mechanistically, MEKK2 phosphorylated interferon regulatory factor 3 (IRF3), mediated IRF3 poly-ubiquitination and inhibited IRF3 dimerization, nuclear translocation and transcriptional activity in the setting of viral infection [15].
DCs
DCs are regarded as specialized antigen-presenting cells mediating pivotal functions in innate and adaptive immune responses. In cancer, an array of signals can impair the differentiation and maturation of DCs, thereby favoring the emergence of DCs with tolerogenic potential [123].
Exosomes derived from cancer cells can contribute to the emergence of dysfunctional DCs. miR-212–3p was transferred from pancreatic cancer cells to DCs through exosomes. In DCs, miR-212–3p targeted the regulatory factor X-associated protein (RFXAP) and decreased MHCII expression, contributing to immune tolerance. In situ hybridization and immunohistochemistry (IHC) analyses of PDAC patient tissues revealed a negative correlation between miR-212–3p and RFXAP [124]. The exosome-mediated transfer of miR-203 from pancreatic cancer cells to DCs decreased the expression of TLR4 and reduced the secretion of TNF-α and interleukin-12 (IL-12) [125]. Exosomes derived from murine and human breast cancer cells suppressed DC differentiation. In vivo, the exosomes from TS/A murine mammary adenocarcinoma cells targeted myeloid precursor cells in the bone marrow. Mechanistically, TS/A exosomes enhanced the levels of IL-6 and activation of Stat3 signaling in CD11b+ cells. The involvement of IL-6 in the exosome-mediated impairment of DC differentiation was confirmed using bone marrow cells from IL-6 KO mice. In that setting, the tumor exosomes only partially inhibited DC differentiation, and the treatment with recombinant IL-6 restored the exosome-dependent inhibition of DC differentiation [126]. Exosomes released by LLC and 4T1 cancer cells inhibited differentiation, induced apoptosis, and enhanced the expression of PD-L1 in DCs. Moreover, the exosomes inhibited DC migration to lymph nodes, decreased TH1 differentiation and induced a Treg phenotype. The treatment with anti-PD-L1 partially rescued the CD4+ T cell functions suppressed by the exosome-treated DCs [127]. In addition to cancer cells, Tregs have been shown to release exosomes that impair DC functions. The levels of miR-150–5p and miR-142–3p were elevated in DCs upon interaction with Tregs or treatment with their shed exosomes. In turn, this induced a tolerogenic DC phenotype, as measured by increased IL-10 and reduced IL-6 levels [128].
Exosomes have also been shown to support DC immunity through activation of cyclic GMP–AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway [129–131]. The cytosolic DNA-sensing pathway cGAS/STING is a crucial defense mechanism against infections, which promotes the transcription of type I interferons and the activation of NF-κB [132]. In cancer, the role cGAS/STING pathway is context-dependent, as reports have shown that this pathway can either promote or suppress anti-tumor immunity [133, 134]. Breast cancer cells treated with Topocan shed exosomes containing DNA, which promoted DC activation through engagement of cGAS/STING pathway [129]. Exosomes derived from irradiated mouse breast cancer cells transferred double-stranded DNA (dsDNA) to DCs and enhanced the levels of costimulatory molecules and STING-mediated activation of IFN-I in DCs. In vivo, the exosome-mediated transfer of dsDNA engaged CD8+ T cell responses, and had a protective role in tumor development [130]. Accordingly, T cell-derived EVs transferred genomic and mitochondrial DNA to DCs, which engaged the cGAS/STING pathway and promoted expression of IRF3-dependent interferon regulated genes in DCs. Notably, this EV-dependent DNA priming, mediated resistance of DCs to subsequent viral infections [131].
Monocytes
Monocytes are an innate immune cell population able to differentiate into tumor-associated macrophages and dendritic cells, promote angiogenesis, remodel the extracellular matrix, kill tumor cells, and recruit lymphocytes. Notably, this versatile cell population can either suppress or promote anti-tumor immunity depending on the context [135].
Cancer cell-derived exosomes have been shown to foster immunosuppressive functions of monocytes. In pancreatic cancer patients, immunosuppressive monocytes (defined as CD14+HLA-DRlo/neg) in circulation were increased in comparison to controls. Notably, pancreatic cancer-derived exosomes mediated the downregulation of HLA-DR in monocytes, induced arginase expression and ROS [136]. The overexpression of the EMT transcriptional factor Snail in HNSCC cells enhanced the production of miR-21, which was released in exosomes. Exosomal miR-21 was transferred to monocytes, where it suppressed the expression of M1 and increased the levels of M2 macrophage markers. In vivo, the knockdown of miR-21 in cancer cells decreased tumor growth, M2 infiltration and angiogenesis in subcutaneous tumors. In HNSCC patient samples, a high expression of miR-21 correlated with increased SNAI1 and with M2 polarization, as measured by the marker MRC1 [137]. Exosomes isolated from plasma of chronic lymphocytic leukemia (CLL) patients were enriched with the noncoding Y RNA hY4 in comparison to exosomes from healthy donors. The treatment of monocytes with hY4 induced expression of PD-L1, as well as the release of CCL2, C-C motif chemokine ligand 4 (CCL4), and IL-6. This monocyte reprogramming occurred in a TLR7-dependent manner, and the use of chloroquine ameliorated the effects induced by hY4 and inhibited CLL development in vivo [138]. Exosomes from glioblastoma-derived stem cells (GSC) also induced an immunosuppressive phenotype by promoting monocyte differentiation into M2 macrophages and enhancing the levels of PD-L1 [139]. Similarly, exosomes from gastric cancer mediated monocytes differentiation into PD1+ TAMs with M2 characteristics in vitro and in vivo. Notably, the PD1+ macrophages correlated with poor prognosis in gastric cancer, and suppressed CD8+ T cell functions, as measured by decreased proliferation, IFNγ and perforin levels [140].
Exosomes can also signal to monocytes to elicit anti-tumor immunity. In fact, pigment epithelium-derived factor (PEDF) on the surface of exosomes from non-metastatic melanoma cells promoted activation of an innate immune response that prevented the formation of melanoma metastasis in the lungs. The mechanism was mediated through Nr4a1 induction in monocytes, which mediated patrolling monocytes expansion, recruitment, and differentiation into TRAIL+ macrophages with M1 features, able to kill and phagocyte cancer cells [141].
MDSCs
MDSCs are a population of immature myeloid cells with strong immunosuppressive ability in the tumor microenvironment [142].
Cancer cell-derived exosomes have been shown to potentiate the immunosuppressive nature of MDSCs though distinct mechanisms. Exosomal Hsp72 enhanced MDSCs suppressive functions through engagement of TLR2 and activation STAT3 signaling [143]. Likewise, renal cancer cell-derived exosomes harboring HSP70 promoted MDSC proliferation and activation through engagement of TLR2 signaling. Notably, the MDSCs primed by the renal cancer cell-derived exosomes enhanced renal tumor growth and promoted immunosuppression [144]. Exosomes released from breast cancer cells supported the expansion of early-stage MDSCs (eMDSCs) through the transfer of miR-9 and miR-181a. Mechanistically, miR-9 and miR-181a activated the janus kinase (JAK)/signal transducer and activator of transcription (STAT) axis in eMDSCs through the targeting of SOCS3 and protein inhibitor of activated STAT3 (PIAS3), respectively. The amplification of immature eMDSCs suppressed murine and human T cell immunity. In 4T1 tumors, the enhanced infiltration of eMDSCs supported tumor growth and immune escape [145]. Exosomes derived from murine breast tumors induced the accumulation of MDSCs, accelerated tumor growth and decreased survival. Mechanistically, exosomal TGFβ1 and prostaglandin E2 (PGE2) were implicated in this phenotype, as their neutralization attenuated the exosome-mediated induction and accumulation MDSCs, as well as the enhanced tumor growth [146]. Hypoxic glioma exosomes promoted MDSCs expansion and activation through miR-10a/Rora/IκBα/NF-κB and miR-21/Pten/ PI3K/AKT axis. In vivo, orthotopic tumors generated from miR-10a or miR-21 knockout cells had less MDSC infiltration than the ones generated from control cells [147]. In line with this finding, exosomes shed by hypoxic OSCC enhanced the suppressive effect of MDSCs on γδ T cells through miR-21/PTEN/PD-L1 axis in MDSCs. Accordingly, the simultaneous targeting of miR-21 and PD-L1 in tumor-bearing mice delayed tumor growth, decreased MDSCs and favored γδ T cell infiltration into tumors [148].
Neutrophils
Neutrophils are regarded as the first line of defense during inflammation and infections and are the most abundant leukocyte population in circulation. Notably, neutrophils can infiltrate into tumors and be instructed by cancer and TME cells to either promote or restrain tumor progression. The functional plasticity of neutrophils in tumors prompted their classification into tumor-restraining N1 population, and tumor-promoting N2 population [149, 150].
Gastric cancer cell-derived and tissue-derived exosomes harboring HMGB1 skewed neutrophils toward a protumor N2 phenotype and induced autophagy through activation of TLR4/NF-κB signaling. Notably, HMGB1 expression was also increased in gastric tumor tissues and was associated with poor prognosis in gastric cancer patients [151].
In the context of CRC, RNA contained in exosomes released by tumor stem-like cells educated neutrophils towards a pro-tumorigenic state. Colorectal cancer stem cells (CRCSCs)-derived exosomes localized in the bone marrow and sustained the survival of neutrophils through delivery of tri-phosphate RNAs that enhanced the expression levels of IL-1β via a pattern recognition-NF-κB axis. Then, CRCSCs secrete CXCL1 and CXCL2 to recruit the exosome-primed neutrophils and promote tumorigenesis. The depletion of neutrophils using a Ly6G antibody abrogated CRCSC-induced tumorigenesis [152].
Cancer-derived exosomes may contribute to cancer-associated thrombosis through neutrophil extracellular traps (NETs). 4T1-bearing mice had higher number of circulating neutrophils and increased levels of plasma DNA and myeloperoxidase. Using models of venous and arterial thrombosis, the authors observed an accelerated thrombus formation in tumor-bearing mice in comparison to tumor-free control animals. In vitro, 4T1-derived exosomes enhanced the formation of NETs, and in vivo, 4T1-derived exosomes administered intravenously in G-CSF-treated mice accelerated the formation of venous thrombosis [153].
Exosomes as therapeutic agents for cancer immunotherapy
As discussed in the previous sections, exosomes derived from several cell types harbor functional molecules able to elicit immune responses in cancer. Due to their biocompatibility, long circulatory half-life and amenability to modification, exosomes have emerged as promising therapeutic delivery systems [154–157]. They have been successfully used as delivery vehicles for nucleic acids, proteins, antibodies, nanobodies, compounds and chemotherapeutic drugs, and can be produced in large-scale using good manufacturing practices [18, 154, 158–168]. Likely due to the fact that exosomes are naturally generated in living organisms, their administration to mice and humans displayed low toxicity [169–172]. Moreover, exosomes are efficiently internalized by recipient cells through several mechanisms, including clathrin-dependent endocytosis and clathrin-independent pathways, namely phagocytosis, macropinocytosis, lipid raft–mediated uptake, caveolin-mediated internalization, direct membrane fusion, and receptor-mediated entry [1, 173]. Altogether, these features make exosomes an attractive delivery system for cancer immunotherapy. Indeed, the concept of exploiting exosomes to engage anti-tumor immune responses is starting to emerge, as preclinical and clinical studies have shown the feasibility to engineer exosomes that deliver tumor-associated antigens (TAAs) or engage immunostimulatory pathways in lymphoid and myeloid cells.
Exosomes were engineered to deliver activating signals to T lymphocytes. A platform called synthetic multivalent antibodies retargeted exosome (SMART-Exo) was designed to redirect and activate T lymphocytes toward cancer cells. SMART-Exo displaying anti-CD3 together with anti-EGFR [174] or anti-HER2 antibodies [161] enabled simultaneous activation and redirection of T cells toward EGFR- or HER2-expressing breast cancer cells, respectively. The SMART-Exo therapy promoted anti-tumor immune responses both in vitro and in vivo [161, 174] (Figure 3a). Leukemia cells were engineered to produce exosomes carrying the B7 costimulatory proteins: B7–1 (CD80), B7–2 (CD86), or both. The exosomes carrying both leukemia cell-associated antigens and the B7 costimulatory signals enhanced T cell proliferation, TH1 cytokines secretion, fostered cytotoxic responses, and generated protective immunity in mice challenged with L1210 cells [175] (Figure 3b).
Figure 3: Engineered exosomes for cancer immunotherapy.
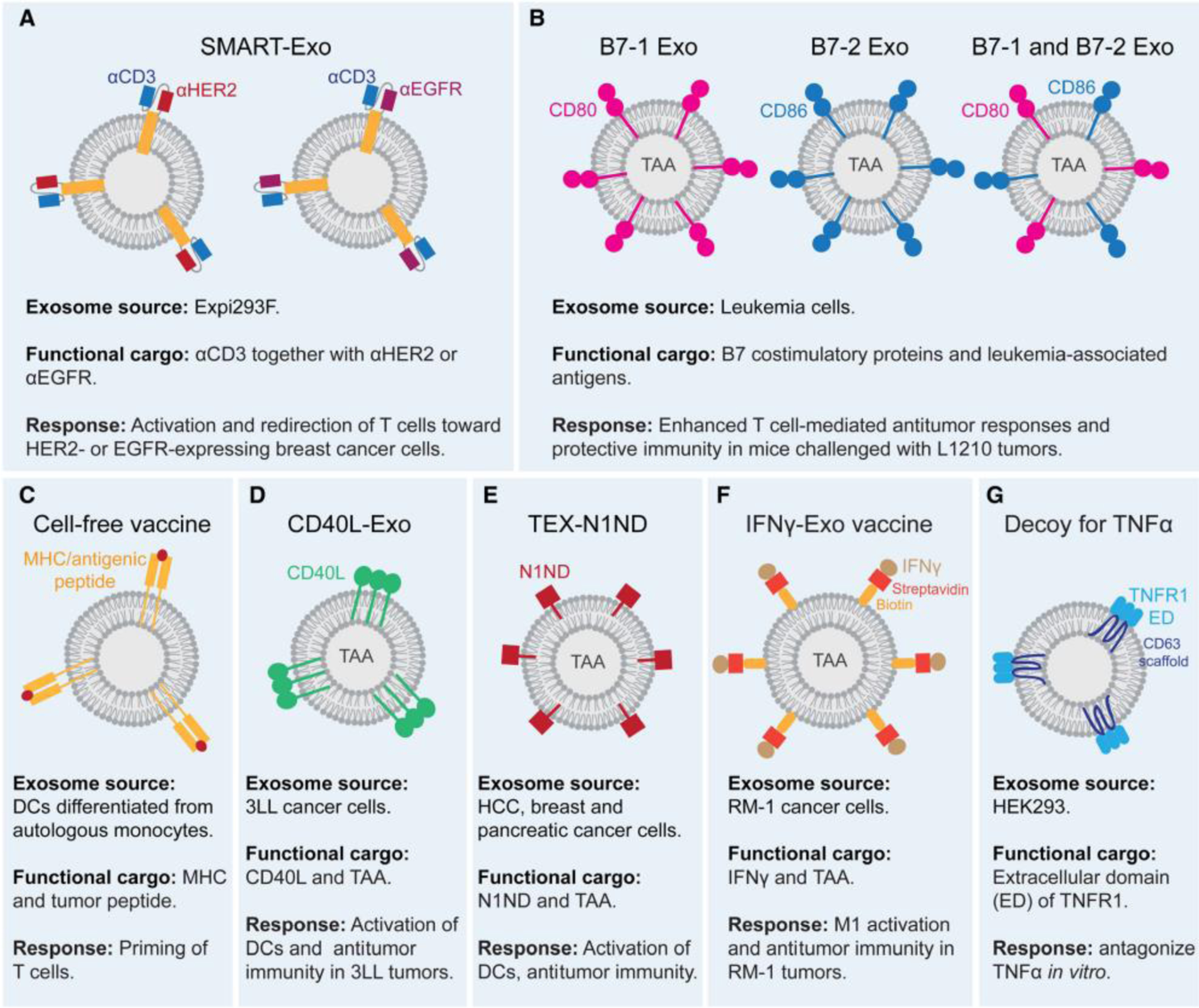
(a) Engineered exosomes displaying anti-CD3 together with anti-human epidermal growth factor receptor 2 (HER2) or anti-epidermal growth factor receptor (EGFR) activate and retarget T cells toward HER2+ and EGFR+ breast cancer cells. (b) Leukemia-derived exosomes containing tumor-associated antigens (TAAs) and harboring the B7 costimulatory proteins confer protective immunity. (c) Exosome-based cell-free vaccine from autologous monocytes isolated from advanced cancer patients. Monocytes are differentiated into dendritic cells (DCs) and loaded with tumor-associated antigens. Then, DCs are used to produce exosomes for patient administration. (d) Engineered 3LL-derived exosomes harboring CD40 ligand (CD40L) promote DC activation, T cell priming, and induce anti-tumor immunity. (e) Engineered cancer cell-derived exosomes harboring TAA and N1ND enhance DC activation and promote anti-tumor immunity. (f) A vaccine for prostate cancer treatment based on streptavidin-tagged interferon gamma (IFNγ) immobilized at the membrane of biotinylated cancer-derived exosomes. The engineered exosomes induce macrophage polarization towards the M1 phenotype and promote anti-tumor immunity. (g) Exosomes containing the binding domain of tumor necrosis factor receptor 1 (TNFR1) were engineered to antagonize tumor necrosis factor alpha (TNF-α).
Tumor peptide-pulsed DC-derived exosomes were used as cell-free vaccines to treat cancer and showed encouraging results in the preclinical setting [79]. In the clinical setting however, concluded phase I and II clinical trials exploiting exosome-based cell-free vaccines in advanced cancer patients showed limited therapeutic benefit, albeit excellent safety and tolerability were observed [170–172, 176] (Figure 3c). More information on the therapeutic use of DC-derived exosomes in cancer can be found in published reviews on the topic [83, 177, 178].
The activation of the CD40 receptor by CD40 ligand (CD40L) promotes DC differentiation, maturation and enhances the secretion of immunostimulatory cytokines [179]. 3LL Lewis lung cells were engineered to produce exosomes harboring CD40L (CD40L‐EXO). The CD40L‐EXO promoted the activation of bone marrow-derived DCs in vitro. Splenocytes isolated from mice immunized with CD40L‐EXO showed enhanced secretion of TH1 cytokines, and increased cytotoxic activity against 3LL cells, but not towards B16 cells. In mice immunized with CD40L-EXO and challenged with 3LL tumors, the tumor burden was decreased and the survival prolonged in comparison to controls. Moreover, CD40L‐EXO also elicited anti-tumor responses in established 3LL tumors [180] (Figure 3d).
Aiming at improving the efficacy of DC-based vaccines, a study exploited cancer cell-derived exosomes to prime DCs through the simultaneous delivery of TAA and of a TLR4 adjuvant signal. For that, the N-terminus domain of HMGN1 (N1ND) was covalently bound to exosomes. DCs pulsed with the engineered exosomes (called DCTEX-N1ND) displayed increased activation and enhanced the cancer killing potential of T cells. In subcutaneous HCC, pancreatic and breast tumor models, DCTEX-N1ND delayed tumor growth and induced anti-tumor immunity. In orthotopic HCC tumors, DCTEX-N1ND decreased tumor mass, increased survival, and abolished metastasis to the lungs. Importantly, DCTEX-N1ND amplified effector and memory T cell populations, generating a robust and persistent anti-tumor immunity [19] (Figure 3e).
A protein anchoring approach based on the immobilization of streptavidin‐tagged IFNγ at the membrane of biotinylated exosomes was used to develop an exosome-based vaccine for prostate cancer treatment. The basis for this approach consists in simultaneously promoting antigen presentation (by exosomal TAAs) and in enhancing maturation of APCs (by exosomal IFNγ). The vaccine promoted M1 macrophage polarization and enhanced their ability to engulf pro-tumorigenic and immunosuppressive exosomes shed by cancer cells. In subcutaneous RM-1 tumors, the administration of the exosome-based vaccine delayed tumor growth and prolonged survival. The vaccine also induced a remodeling in the populations of circulating immune cells, as demonstrated by the increase in IFNγ+ CD8+ T cells and the decrease in Tregs. Finally, the exosome-based vaccine had a synergistic effect with a tumor cell-based vaccine to induce anti-tumor immune responses [181] (Figure 3f).
Exosomes were also engineered to antagonize TNF-α-mediated inflammation by acting as decoys that display the binding domain of TNF receptor-1 on their surface. The efficacy of the engineered exosomes in antagonizing TNF-α was demonstrated in vitro [182] (Figure 3g).
From a translational perspective, exosomes can be engineered not only to elicit anti-tumor immune responses as exemplified above, but also to suppress/normalize the exacerbated activation of the immune system, which commonly occur in the setting of autoimmune diseases.
Concluding remarks and future directions
As discussed in this review, exosomes provide signals that interfere in each stage of the cancer immunity cycle. They can modulate the various lymphoid and myeloid cell populations of the TME to support or restrain anti-tumor immunity, and can be harnessed for therapeutic purposes in cancer immunotherapy. Despite the advances in basic biology and translational science achieved in the field, several aspects of exosome-mediated immunoregulation in cancer are thus far unexplored, and when addressed, they have the potential to provide valuable functional insights to the field, to advance the development of novel cancer therapies, and to pinpoint additional biomarker candidates for cancer diagnosis, prognosis and assessment of therapy response.
Thus far, the field has majorly focused on how exosomes derived from a specific type of cancer cell modulate a specific subset of lymphoid or myeloid cell population. Nevertheless, a systematic evaluation of how exosomes derived from different types of cancer cells and TME sub-populations affect the immune landscape of tumors is lacking. In fact, studies that determine the functional contribution of exosomes from cancer-associated fibroblasts, endothelial cells, pericytes, and from the distinct subsets of immune cells in cancer deserve further attention. The advent of technologies allowing for the study of the immune landscape of tumors at a single cell level can help to solve this puzzle. In fact, these technologies can provide a comprehensive map on how exosomes from a specific source signal to the various populations of the immune microenvironment, thus guiding the field to uncover aspects of exosome-mediated regulation of the immune system previously overlooked. Collectively, breakthroughs in these areas will not only allow for the mapping of complex circuits of exosome-mediated signaling in tumor immunity, but also open new avenues for translational intervention.
Much of the work available in the field was performed with exosomes generated in vitro, and the development of systems allowing for the in vivo study of exosome-mediated regulation of the immune system is an open field of research that needs further attention.
From a biomarker perspective, several studies have demonstrated the value in exploiting exosome-resident immunomodulatory molecules derived from patients to monitor disease status and therapy response. This is an extremely exciting field of research that can be further developed and potentially impact cancer diagnosis and decision making in the clinical setting.
Finally, the therapeutic application of exosomes to elicit anti-tumor immunity has the potential to offer novel options for cancer treatment. So far, exosomes have been exploited to develop therapies that engage T lymphocyte- and APC-mediated immunity. Nevertheless, exosomes can also be engineered and harnessed to engage anti-tumor immune responses in other cell populations of the tumor microenvironment.
The field of exosome-mediated immunoregulation in cancer is an evolving area of research that holds great potential to decipher additional mechanisms contributing to cancer aggressiveness. Targeting these mechanisms may provide new avenues to treat cancer patients.
Acknowledgements
We thank Dr. Antonios Chronopoulos and Dr. Didem Ağaç Çobanoğlu for proofreading the manuscript. F.G.K. funding as an Odyssey Fellow is supported by the Odyssey Program and Theodore N. Law for Scientific Achievement at The University of Texas MD Anderson Cancer Center. R.K. laboratory is supported by research funds from The University of Texas MD Anderson Cancer Center. The exosome-related research in the Kalluri laboratory is funded by NCI RO1 CA213233, NCI RO1 CA195733, and NCI CA231465.
Abbreviations:
- Akt
protein kinase B
- AML
acute myeloid leukemia
- APC
antigen-presenting cell
- ATP
adenosine triphosphate
- BAT3
HLA-B-Associated Transcript-3
- BMDM
bone marrow-derived macrophage
- Breg
regulatory B cell
- CCL2
C-C motif chemokine ligand 2
- CCL4
C-C motif chemokine ligand 4
- CD40L
CD40 ligand
- cGAS
cyclic GMP–AMP synthase
- circUHRF1
circular ubiquitin-like with PHD and ring finger domain 1
- CLL
chronic lymphocytic leukemia
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- CRCSC
colorectal cancer stem cell
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DC
dendritic cell
- dsDNA
double-stranded DNA
- EBV
Epstein-Barr virus
- EGFR
epidermal growth factor receptor
- eMDSC
early-stage MDSC
- EMT
epithelial-to-mesenchymal transition
- EOC
epithelial ovarian cancer
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- EVs
extracellular vesicles
- FasL
Fas ligand
- GBM
glioblastoma
- GCSF
granulocyte colony-stimulating factor
- GSC
glioblastoma stem cell
- HIF-1α
hypoxia-inducible factor-1α
- HCC
hepatocellular carcinoma
- HMGB1
high mobility group box-1
- HNSCC
head and neck squamous cell carcinoma
- Hsp70
heat shock protein 70
- ICB
immune checkpoint blockade
- IFNγ
interferon gamma
- IHC
immunohistochemistry
- IL-1β
interleukin-1β
- IL-2
interleukin-2
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-12
interleukin-12
- IL-17
interleukin-17
- ILVs
intraluminal vesicles
- IRF3
interferon regulatory factor 3
- JAK
janus kinase
- KO
knockout
- LIPA
lysosomal acid lipase A
- LLC
Lewis lung cancer
- MCP-1
monocyte chemoattractant protein-1
- MDSC
myeloid-derived suppressor cell
- MEKK2
mitogen-activated protein kinase kinase kinase 2
- MHC
major histocompatibility complex
- MMP-9
matrix metalloproteinase-9
- MVBs
multivesicular bodies
- MVs
microvesicles
- NETs
neutrophil extracellular traps
- NF-κB
nuclear factor kappa B
- NK
natural killer
- NKG2D
natural killer group 2 member D
- NPC
nasopharyngeal carcinoma
- NSCLC
non-small cell lung cancer
- OSCC
oral squamous cell carcinoma
- OX40L
OX40 ligand
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PDAC
pancreatic ductal adenocarcinoma
- PDX
patient-derived xenografts
- PEDF
pigment epithelium-derived factor
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- PIAS3
protein inhibitor of activated STAT3
- PTEN
phosphatase and tensin homolog
- RFXAP
regulatory factor X-associated protein
- ROS
reactive oxygen species
- SCCHN
squamous cell carcinoma of the head and neck
- SMART-Exo
synthetic multivalent antibodies retargeted exosome
- SOCS3
suppressor of cytokine signaling 3
- STAT
signal transducer and activator of transcription
- STAT3
signal transducer and activator of transcription 3
- STING
stimulator of interferon genes
- TAA
tumor-associated antigen
- TAM
tumor-associated macrophage
- TCR
T cell receptor
- TGFβ1
transforming growth factor beta 1
- TH1
T helper 1
- TH17
T helper 17
- Tim-3
T-cell immunoglobulin and mucin-domain containing-3
- TLR
toll-like receptor
- TME
tumor microenvironment
- TNF
tumor necrosis factor
- TNF-α
tumor necrosis factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
- Treg
regulatory T cell
- VEGFA
vascular endothelial growth factor A
- 5-FU
5-fluorouracil
Footnotes
Conflict of interest
MDACC and R.K. hold patents in the area of exosome biology that are licensed to Codiak Biosciences, Inc. MDACC and R.K. are stock equity holders in Codiak Biosciences, Inc. R.K. is a consultant and scientific adviser for Codiak Biosciences, Inc. F.G.K. has no conflict of interest to declare.
References
- 1.Kalluri R. & LeBleu VS (2020) The biology, function, and biomedical applications of exosomes, Science. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Niel G, D’Angelo G. & Raposo G. (2018) Shedding light on the cell biology of extracellular vesicles, Nat Rev Mol Cell Biol. 19, 213–228. [DOI] [PubMed] [Google Scholar]
- 3.McAndrews KM & Kalluri R. (2019) Mechanisms associated with biogenesis of exosomes in cancer, Mol Cancer. 18, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A. & Kalluri R. (2014) Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer, J Biol Chem. 289, 3869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ & Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat Cell Biol. 9, 654–9. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Chen X, Yi J, Liu Y, Li D, Wang J, Hou D, Jiang X, Zhang J, Wang J, Zen K, Yang F, Zhang CY & Zhang Y. (2016) Identification and Characterization of 293T Cell-Derived Exosomes by Profiling the Protein, mRNA and MicroRNA Components, PLoS One. 11, e0163043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, Xia K, Chang Y, Liu J. & Yuan W. (2018) Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment, Mol Cancer. 17, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N. & Khvorova A. (2016) High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources, J Extracell Vesicles. 5, 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altadill T, Campoy I, Lanau L, Gill K, Rigau M, Gil-Moreno A, Reventos J, Byers S, Colas E. & Cheema AK (2016) Enabling Metabolomics Based Biomarker Discovery Studies Using Molecular Phenotyping of Exosome-Like Vesicles, PLoS One. 11, e0151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig N, Gillespie DG, Reichert TE, Jackson EK & Whiteside TL (2020) Purine Metabolites in Tumor-Derived Exosomes May Facilitate Immune Escape of Head and Neck Squamous Cell Carcinoma, Cancers (Basel). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK & Meckes DG Jr. (2016) Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers, Oncotarget. 7, 86999–87015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M. & Thery C. (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes, Proc Natl Acad Sci U S A. 113, E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams C, Royo F, Aizpurua-Olaizola O, Pazos R, Boons GJ, Reichardt NC & Falcon-Perez JM (2018) Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives, J Extracell Vesicles. 7, 1442985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y. & Wang X. (2018) Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer, Cancer Immunol Res. 6, 1578–1592. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Wang L, Dai T, Jin K, Zhang Z, Wang S, Xie F, Fang P, Yang B, Huang H, van Dam H, Zhou F. & Zhang L. (2018) Tumor-derived exosomes antagonize innate antiviral immunity, Nat Immunol. 19, 233–245. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X. & Guo W. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response, Nature. 560, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, Ge S, Li J, Ning T, Deng T, Fan Q, Li H, Sun W, Ying G. & Ba Y. (2018) Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA, Cancer Sci. 109, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ & Kalluri R. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature. 546, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo B, Qi H, Lu Z, Chen L, Sun B, Yang R, Zhang Y, Liu Z, Gao X, You A, Wu L, Jing R, Zhou Q. & Yin H. (2020) Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice, Nat Commun. 11, 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D. & Kalluri R. (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer, Nature. 523, 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Kojima K, Mobley JA & West AB (2019) Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease, EBioMedicine. 45, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros MP, Arnould L, Garrido C, Aubin F. & Gobbo J. (2020) Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients, J Extracell Vesicles. 9, 1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton A, Harris CL, Court J, Mason MD & Morgan BP (2003) Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59, Eur J Immunol. 33, 522–31. [DOI] [PubMed] [Google Scholar]
- 24.Tricarico C, Clancy J. & D’Souza-Schorey C. (2017) Biology and biogenesis of shed microvesicles, Small GTPases. 8, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen DS & Mellman I. (2013) Oncology meets immunology: the cancer-immunity cycle, Immunity. 39, 1–10. [DOI] [PubMed] [Google Scholar]
- 26.Speiser DE, Ho PC & Verdeil G. (2016) Regulatory circuits of T cell function in cancer, Nat Rev Immunol. 16, 599–611. [DOI] [PubMed] [Google Scholar]
- 27.Robbins PD & Morelli AE (2014) Regulation of immune responses by extracellular vesicles, Nat Rev Immunol. 14, 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor DD & Gercel-Taylor C. (2011) Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments, Semin Immunopathol. 33, 441–54. [DOI] [PubMed] [Google Scholar]
- 29.Whiteside TL (2016) Exosomes and tumor-mediated immune suppression, J Clin Invest. 126, 1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurywchak P, Tavormina J. & Kalluri R. (2018) The emerging roles of exosomes in the modulation of immune responses in cancer, Genome Med. 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daassi D, Mahoney KM & Freeman GJ (2020) The importance of exosomal PDL1 in tumour immune evasion, Nat Rev Immunol. 20, 209–215. [DOI] [PubMed] [Google Scholar]
- 32.Maher J. & Davies ET (2004) Targeting cytotoxic T lymphocytes for cancer immunotherapy, Br J Cancer. 91, 817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borst J, Ahrends T, Babala N, Melief CJM & Kastenmuller W. (2018) CD4(+) T cell help in cancer immunology and immunotherapy, Nat Rev Immunol. 18, 635–647. [DOI] [PubMed] [Google Scholar]
- 34.Togashi Y, Shitara K. & Nishikawa H. (2019) Regulatory T cells in cancer immunosuppression - implications for anticancer therapy, Nat Rev Clin Oncol. 16, 356–371. [DOI] [PubMed] [Google Scholar]
- 35.Guery L. & Hugues S. (2015) Th17 Cell Plasticity and Functions in Cancer Immunity, Biomed Res Int. 2015, 314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thommen DS & Schumacher TN (2018) T Cell Dysfunction in Cancer, Cancer Cell. 33, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL & Min WP (2005) Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis, Blood Cells Mol Dis. 35, 169–73. [DOI] [PubMed] [Google Scholar]
- 38.Wada J, Onishi H, Suzuki H, Yamasaki A, Nagai S, Morisaki T. & Katano M. (2010) Surface-bound TGF-beta1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions, Anticancer Res. 30, 3747–57. [PubMed] [Google Scholar]
- 39.O’Brien DI, Nally K, Kelly RG, O’Connor TM, Shanahan F. & O’Connell J. (2005) Targeting the Fas/Fas ligand pathway in cancer, Expert Opin Ther Targets. 9, 1031–44. [DOI] [PubMed] [Google Scholar]
- 40.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G. & Fais S. (2002) Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles, J Exp Med. 195, 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ & Whiteside TL (2009) Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes, J Immunol. 183, 3720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J. & Whiteside TL (2003) T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors, Clin Cancer Res. 9, 5113–9. [PubMed] [Google Scholar]
- 43.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S. & Whiteside TL (2005) Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes, Clin Cancer Res. 11, 1010–20. [PubMed] [Google Scholar]
- 44.Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila-Flores A, Merida I. & Izquierdo M. (2011) Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes, Cell Death Differ. 18, 1161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X. & Wang J. (2012) Activated T cell exosomes promote tumor invasion via Fas signaling pathway, J Immunol. 188, 5954–61. [DOI] [PubMed] [Google Scholar]
- 46.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G. & Rivoltini L. (2005) Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape, Gastroenterology. 128, 1796–804. [DOI] [PubMed] [Google Scholar]
- 47.Czystowska M, Han J, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW, Signorelli K. & Whiteside TL (2009) IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death, Cell Death Differ. 16, 708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czystowska M, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW & Whiteside TL (2011) Mechanisms of T-cell protection from death by IRX-2: a new immunotherapeutic, Cancer Immunol Immunother. 60, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR & Honjo T. (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation, J Exp Med. 192, 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okazaki T. & Honjo T. (2007) PD-1 and PD-1 ligands: from discovery to clinical application, Int Immunol. 19, 813–24. [DOI] [PubMed] [Google Scholar]
- 51.Xie F, Xu M, Lu J, Mao L. & Wang S. (2019) The role of exosomal PD-L1 in tumor progression and immunotherapy, Mol Cancer. 18, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE & Whiteside TL (2018) Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients, Clin Cancer Res. 24, 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C, Rooj AK, Krasemann S, Carter BS, Chen CC, Steed T, Treiber J, Rodig S, Yang K, Nakano I, Lee H, Weissleder R, Breakefield XO, Godlewski J, Westphal M, Lamszus K, Freeman GJ, Bronisz A, Lawler SE & Chiocca EA (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles, Sci Adv. 4, eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, Cha JH, Hou J, Hsu JL, Sun L. & Hung MC (2018) Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth, Cell Res. 28, 862–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L. & Blelloch R. (2019) Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory, Cell. 177, 414–427 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Li C, Zhi C, Liang W, Wang X, Chen X, Lv T, Shen Q, Song Y, Lin D. & Liu H. (2019) Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients, J Transl Med. 17, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, Sung YH, Pack CG, Jung MK, Han B, Kim K, Kim WS, Nam SJ, Choi CM, Yun M, Lee JC & Rho JK (2019) Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer, Exp Mol Med. 51, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang M, Fan Y, Che X, Hou K, Zhang C, Li C, Wen T, Wang S, Cheng Y, Liu Y. & Qu X. (2020) 5-FU-Induced Upregulation of Exosomal PD-L1 Causes Immunosuppression in Advanced Gastric Cancer Patients, Front Oncol. 10, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, Adhikary D, Mautner J. & Busson P. (2009) Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells, Blood. 113, 1957–66. [DOI] [PubMed] [Google Scholar]
- 60.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX & Li J. (2014) Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma, Oncotarget. 5, 5439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, Sun B. & Lu X. (2018) 14–3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes, Cell Death Dis. 9, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shenoy GN, Loyall J, Berenson CS, Kelleher RJ Jr., Iyer V, Balu-Iyer SV, Odunsi K. & Bankert RB (2018) Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments, J Immunol. 201, 3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shenoy GN, Loyall J, Maguire O, Iyer V, Kelleher RJ Jr., Minderman H, Wallace PK, Odunsi K, Balu-Iyer SV & Bankert RB (2018) Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses, Cancer Immunol Res. 6, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma P, Diergaarde B, Ferrone S, Kirkwood JM & Whiteside TL (2020) Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells, Sci Rep. 10, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S. & Whiteside TL (2017) Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer, Clin Cancer Res. 23, 4843–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Yang Y, Wang W, Zhang Y, Chen Z, Hao C. & Zhang J. (2018) Melanoma-released exosomes directly activate the mitochondrial apoptotic pathway of CD4(+) T cells through their microRNA cargo, Exp Cell Res. 371, 364–371. [DOI] [PubMed] [Google Scholar]
- 67.Soderberg A, Barral AM, Soderstrom M, Sander B. & Rosen A. (2007) Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes, Free Radic Biol Med. 43, 90–9. [DOI] [PubMed] [Google Scholar]
- 68.Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, Wang Z, Chen X, Zhang W, Yokoyama S, Wang C, Li L, Li L, Hou D, Dong L, Xu T, Hiroi T, Yang F, Ji H, Zhang J, Zen K. & Zhang CY (2014) Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth, Cell Res. 24, 1164–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalvala A, Wallet P, Yang L, Wang C, Li H, Nam A, Nathan A, Mambetsariev I, Poroyko V, Gao H, Chu P, Sattler M, Bild A, Manuel ER, Lee PP, Jolly MK, Kulkarni P. & Salgia R. (2019) Phenotypic Switching of Naive T Cells to Immune-Suppressive Treg-Like Cells by Mutant KRAS, J Clin Med. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ni C, Fang QQ, Chen WZ, Jiang JX, Jiang Z, Ye J, Zhang T, Yang L, Meng FB, Xia WJ, Zhong M. & Huang J. (2020) Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+gammadelta1 Treg cells, Signal Transduct Target Ther. 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC & Wilson MS (2014) MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells, Immunity. 41, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clayton A, Al-Taei S, Webber J, Mason MD & Tabi Z. (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production, J Immunol. 187, 676–83. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu C, Chen Y, Zhou W, Lin A, Yu L, Zhang W, Xue Z, Wang J. & Cai Z. (2019) Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8(+) T Cell Responses, Immunity. 50, 738–750 e7. [DOI] [PubMed] [Google Scholar]
- 74.LeBleu VS & Kalluri R. (2019) Exosomes Exercise Inhibition of Anti-Tumor Immunity during Chemotherapy, Immunity. 50, 547–549. [DOI] [PubMed] [Google Scholar]
- 75.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I. & Dikov MM (2008) Adenosine receptors in regulation of dendritic cell differentiation and function, Blood. 112, 1822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Challier J, Bruniquel D, Sewell AK & Laugel B. (2013) Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity, Immunology. 138, 402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ & Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles, J Exp Med. 183, 1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P. & Bonnerot C. (2002) Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells, Int Immunol. 14, 713–22. [DOI] [PubMed] [Google Scholar]
- 79.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G. & Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat Med. 4, 594–600. [DOI] [PubMed] [Google Scholar]
- 80.Thery C, Duban L, Segura E, Veron P, Lantz O. & Amigorena S. (2002) Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes, Nat Immunol. 3, 1156–62. [DOI] [PubMed] [Google Scholar]
- 81.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E. & Zitvogel L. (2004) Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells, J Immunol. 172, 2126–36. [DOI] [PubMed] [Google Scholar]
- 82.Wakim LM & Bevan MJ (2011) Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection, Nature. 471, 629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G. & Zitvogel L. (2016) Dendritic cell-derived exosomes for cancer therapy, J Clin Invest. 126, 1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsou P, Katayama H, Ostrin EJ & Hanash SM (2016) The Emerging Role of B Cells in Tumor Immunity, Cancer Res. 76, 5597–5601. [DOI] [PubMed] [Google Scholar]
- 85.Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L, Wang G, Deng Y, Yang Y. & Chen G. (2018) Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion, J Immunother Cancer. 6, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y, An J, Huang S, He J. & Zhang J. (2015) Esophageal cancer-derived microvesicles induce regulatory B cells, Cell Biochem Funct. 33, 308–13. [DOI] [PubMed] [Google Scholar]
- 87.Yang C, Chalasani G, Ng YH & Robbins PD (2012) Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells, PLoS One. 7, e36138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Capello M, Vykoukal JV, Katayama H, Bantis LE, Wang H, Kundnani DL, Aguilar-Bonavides C, Aguilar M, Tripathi SC, Dhillon DS, Momin AA, Peters H, Katz MH, Alvarez H, Bernard V, Ferri-Borgogno S, Brand R, Adler DG, Firpo MA, Mulvihill SJ, Molldrem JJ, Feng Z, Taguchi A, Maitra A. & Hanash SM (2019) Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity, Nat Commun. 10, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L. & Wulf GG (2011) Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3, Proc Natl Acad Sci U S A. 108, 15336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM & Jonsson G. (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma, Nature. 577, 561–565. [DOI] [PubMed] [Google Scholar]
- 91.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA & Fridman WH (2020) B cells are associated with survival and immunotherapy response in sarcoma, Nature. 577, 556–560. [DOI] [PubMed] [Google Scholar]
- 92.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L. & Wargo JA (2020) B cells and tertiary lymphoid structures promote immunotherapy response, Nature. 577, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morvan MG & Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks, Nat Rev Cancer. 16, 7–19. [DOI] [PubMed] [Google Scholar]
- 94.Hong CS, Danet-Desnoyers G, Shan X, Sharma P, Whiteside TL & Boyiadzis M. (2019) Human acute myeloid leukemia blast-derived exosomes in patient-derived xenograft mice mediate immune suppression, Exp Hematol. 76, 60–66 e2. [DOI] [PubMed] [Google Scholar]
- 95.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD & Tabi Z. (2008) Human tumor-derived exosomes down-modulate NKG2D expression, J Immunol. 180, 7249–58. [DOI] [PubMed] [Google Scholar]
- 96.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M. & Reyburn HT (2010) Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes, Cancer Res. 70, 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE & Zhang HG (2006) Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function, J Immunol. 176, 1375–85. [DOI] [PubMed] [Google Scholar]
- 98.Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, Cai JB & Ke AW (2020) Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma, Mol Cancer. 19, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Briand J, Garnier D, Nadaradjane A, Clement-Colmou K, Potiron V, Supiot S, Bougras-Cartron G, Frenel JS, Heymann D, Vallette FM & Cartron PF (2020) Radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits natural killer cells cytotoxicity, Epigenomics. 12, 397–408. [DOI] [PubMed] [Google Scholar]
- 100.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA & Multhoff G. (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells, Cancer Res. 65, 5238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A. & Pogge von Strandmann E. (2008) Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function, PLoS One. 3, e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jella KK, Nasti TH, Li Z, Lawson DH, Switchenko JM, Ahmed R, Dynan WS & Khan MK (2020) Exosome-Containing Preparations From Postirradiated Mouse Melanoma Cells Delay Melanoma Growth In Vivo by a Natural Killer Cell-Dependent Mechanism, Int J Radiat Oncol Biol Phys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L. & Chaput N. (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha, PLoS One. 4, e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang M, McKay D, Pollard JW & Lewis CE (2018) Diverse Functions of Macrophages in Different Tumor Microenvironments, Cancer Res. 78, 5492–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou J, Tang Z, Gao S, Li C, Feng Y. & Zhou X. (2020) Tumor-Associated Macrophages: Recent Insights and Therapies, Front Oncol. 10, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, Chen X. & Wang X. (2016) Epithelial ovarian cancer-secreted exosomal miR-222–3p induces polarization of tumor-associated macrophages, Oncotarget. 7, 43076–43087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG & Harris CC (2018) Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246, Nat Commun. 9, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerloff D, Lutzkendorf J, Moritz RKC, Wersig T, Mader K, Muller LP & Sunderkotter C. (2020) Melanoma-Derived Exosomal miR-125b-5p Educates Tumor Associated Macrophages (TAMs) by Targeting Lysosomal Acid Lipase A (LIPA), Cancers (Basel). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L. & Qiu Z. (2018) Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis, Cancer Res. 78, 4586–4598. [DOI] [PubMed] [Google Scholar]
- 110.Linton SS, Abraham T, Liao J, Clawson GA, Butler PJ, Fox T, Kester M. & Matters GL (2018) Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages, PLoS One. 13, e0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J. & Tang D. (2020) Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein, Autophagy, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolf-Dennen K, Gordon N. & Kleinerman ES (2020) Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions, Oncoimmunology. 9, 1747677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pritchard A, Tousif S, Wang Y, Hough K, Khan S, Strenkowski J, Chacko BK, Darley-Usmar VM & Deshane JS (2020) Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization, Cells. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang D, Wang X, Si M, Yang J, Sun S, Wu H, Cui S, Qu X. & Yu X. (2020) Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages, Cancer Lett. 474, 36–52. [DOI] [PubMed] [Google Scholar]
- 115.Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, Wen SW, Wiegmans AP & Moller A. (2018) Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling, Front Immunol. 9, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cubillos-Ruiz JR, Bettigole SE & Glimcher LH (2017) Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer, Cell. 168, 692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li A, Song NJ, Riesenberg BP & Li Z. (2019) The Emerging Roles of Endoplasmic Reticulum Stress in Balancing Immunity and Tolerance in Health and Diseases: Mechanisms and Opportunities, Front Immunol. 10, 3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He C, Hua W, Liu J, Fan L, Wang H. & Sun G. (2020) Exosomes derived from endoplasmic reticulum-stressed liver cancer cells enhance the expression of cytokines in macrophages via the STAT3 signaling pathway, Oncol Lett. 20, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yao X, Tu Y, Xu Y, Guo Y, Yao F. & Zhang X. (2020) Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages, J Cell Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M. & Wang SE (2014) Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB, Sci Rep. 4, 5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun Y, Pan Z, Qian H. & Xu W. (2016) Exosomes derived from gastric cancer cells activate NF-kappaB pathway in macrophages to promote cancer progression, Tumour Biol. 37, 12169–12180. [DOI] [PubMed] [Google Scholar]
- 122.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D. & Croce CM (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response, Proc Natl Acad Sci U S A. 109, E2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF & Sancho D. (2020) Dendritic cells in cancer immunology and immunotherapy, Nat Rev Immunol. 20, 7–24. [DOI] [PubMed] [Google Scholar]
- 124.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, Xiang J, Wu Z, Jiang G. & Cao L. (2015) Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212–3p, Oncotarget. 6, 29877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou M, Chen J, Zhou L, Chen W, Ding G. & Cao L. (2014) Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203, Cell Immunol. 292, 65–9. [DOI] [PubMed] [Google Scholar]
- 126.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C. & Zhang HG (2007) Tumor exosomes inhibit differentiation of bone marrow dendritic cells, J Immunol. 178, 6867–75. [DOI] [PubMed] [Google Scholar]
- 127.Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, Pan J. & Qi C. (2018) Tumor exosomes block dendritic cells maturation to decrease the T cell immune response, Immunol Lett. 199, 36–43. [DOI] [PubMed] [Google Scholar]
- 128.Tung SL, Boardman DA, Sen M, Letizia M, Peng Q, Cianci N, Dioni L, Carlin LM, Lechler R, Bollati V, Lombardi G. & Smyth LA (2018) Regulatory T cell-derived extracellular vesicles modify dendritic cell function, Sci Rep. 8, 6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, Akira S, Matsuda T. & Kawai T. (2017) DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity, J Immunol. 198, 1649–1659. [DOI] [PubMed] [Google Scholar]
- 130.Diamond JM, Vanpouille-Box C, Spada S, Rudqvist NP, Chapman JR, Ueberheide BM, Pilones KA, Sarfraz Y, Formenti SC & Demaria S. (2018) Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs, Cancer Immunol Res. 6, 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torralba D, Baixauli F, Villarroya-Beltri C, Fernandez-Delgado I, Latorre-Pellicer A, Acin-Perez R, Martin-Cofreces NB, Jaso-Tamame AL, Iborra S, Jorge I, Gonzalez-Aseguinolaza G, Garaude J, Vicente-Manzanares M, Enriquez JA, Mittelbrunn M. & Sanchez-Madrid F. (2018) Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts, Nat Commun. 9, 2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Motwani M, Pesiridis S. & Fitzgerald KA (2019) DNA sensing by the cGAS-STING pathway in health and disease, Nat Rev Genet. 20, 657–674. [DOI] [PubMed] [Google Scholar]
- 133.Zhu Y, An X, Zhang X, Qiao Y, Zheng T. & Li X. (2019) STING: a master regulator in the cancer-immunity cycle, Mol Cancer. 18, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwon J. & Bakhoum SF (2020) The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer, Cancer Discov. 10, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olingy CE, Dinh HQ & Hedrick CC (2019) Monocyte heterogeneity and functions in cancer, J Leukoc Biol. 106, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Javeed N, Gustafson MP, Dutta SK, Lin Y, Bamlet WR, Oberg AL, Petersen GM, Chari ST, Dietz AB & Mukhopadhyay D. (2017) Immunosuppressive CD14(+)HLA-DR(lo/neg) monocytes are elevated in pancreatic cancer and “primed” by tumor-derived exosomes, Oncoimmunology. 6, e1252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsieh CH, Tai SK & Yang MH (2018) Snail-overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes, Neoplasia. 20, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haderk F, Schulz R, Iskar M, Cid LL, Worst T, Willmund KV, Schulz A, Warnken U, Seiler J, Benner A, Nessling M, Zenz T, Gobel M, Durig J, Diederichs S, Paggetti J, Moussay E, Stilgenbauer S, Zapatka M, Lichter P. & Seiffert M. (2017) Tumor-derived exosomes modulate PD-L1 expression in monocytes, Sci Immunol. 2. [DOI] [PubMed] [Google Scholar]
- 139.Gabrusiewicz K, Li X, Wei J, Hashimoto Y, Marisetty AL, Ott M, Wang F, Hawke D, Yu J, Healy LM, Hossain A, Akers JC, Maiti SN, Yamashita S, Shimizu Y, Dunner K, Zal MA, Burks JK, Gumin J, Nwajei F, Rezavanian A, Zhou S, Rao G, Sawaya R, Fuller GN, Huse JT, Antel JP, Li S, Cooper L, Sulman EP, Chen C, Geula C, Kalluri R, Zal T. & Heimberger AB (2018) Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes, Oncoimmunology. 7, e1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z. & Li Y. (2018) Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression, Oncogenesis. 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J, Miller SD, Ifergan I, Lee Y, Osman I, Thaxton CS & Volpert OV (2017) Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche, Nat Commun. 8, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar V, Patel S, Tcyganov E. & Gabrilovich DI (2016) The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment, Trends Immunol. 37, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C. & Ghiringhelli F. (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells, J Clin Invest. 120, 457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao Y, Xu H, Li N, Wang H, Ma L, Chen S, Liu J, Zheng Y. & Zhang Y. (2020) Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression, Cell Commun Signal. 18, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, Guo X. & Yu J. (2020) Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer, Oncogene. [DOI] [PubMed] [Google Scholar]
- 146.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J. & Zhang HG (2009) Induction of myeloid-derived suppressor cells by tumor exosomes, Int J Cancer. 124, 2621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H. & Li G. (2018) Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways, Oncogene. 37, 4239–4259. [DOI] [PubMed] [Google Scholar]
- 148.Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, Wang S, Jiang J, Lang J. & Zhu G. (2019) Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral gammadelta T cell equilibrium via tumor-derived exosomes, Oncogene. 38, 2830–2843. [DOI] [PubMed] [Google Scholar]
- 149.Uribe-Querol E. & Rosales C. (2015) Neutrophils in Cancer: Two Sides of the Same Coin, J Immunol Res. 2015, 983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coffelt SB, Wellenstein MD & de Visser KE (2016) Neutrophils in cancer: neutral no more, Nat Rev Cancer. 16, 431–46. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X, Shi H, Yuan X, Jiang P, Qian H. & Xu W. (2018) Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration, Mol Cancer. 17, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hwang WL, Lan HY, Cheng WC, Huang SC & Yang MH (2019) Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer, J Hematol Oncol. 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, Werneck CC, Sielski MS, Vicente CP & Monteiro RQ (2017) Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis, Sci Rep. 7, 6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Syn NL, Wang L, Chow EK, Lim CT & Goh BC (2017) Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges, Trends Biotechnol. 35, 665–676. [DOI] [PubMed] [Google Scholar]
- 155.Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S. & Bultema JJ (2016) Designer exosomes as next-generation cancer immunotherapy, Nanomedicine. 12, 163–9. [DOI] [PubMed] [Google Scholar]
- 156.Kibria G, Ramos EK, Wan Y, Gius DR & Liu H. (2018) Exosomes as a Drug Delivery System in Cancer Therapy: Potential and Challenges, Mol Pharm. 15, 3625–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xunian Z. & Kalluri R. (2020) Biology and therapeutic potential of mesenchymal stem cell-derived exosomes, Cancer Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao L, Gu C, Gan Y, Shao L, Chen H. & Zhu H. (2020) Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis, J Control Release. 318, 1–15. [DOI] [PubMed] [Google Scholar]
- 159.Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Heo WD & Choi C. (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module, Nat Commun. 7, 12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P. & Schiffelers RM (2016) Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting, J Extracell Vesicles. 5, 31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, Epstein AL, Lenz HJ & Zhang Y. (2020) Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy, Mol Ther. 28, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aqil F, Munagala R, Jeyabalan J, Agrawal AK & Gupta R. (2017) Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin, AAPS J. 19, 1691–1702. [DOI] [PubMed] [Google Scholar]
- 163.Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B. & Xiao Z. (2020) Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer, J Nanobiotechnology. 18, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J. & Nie G. (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials. 35, 2383–90. [DOI] [PubMed] [Google Scholar]
- 165.Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, Qiang L, Li G, Han Z, Yuan Y. & Gao S. (2019) Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy, J Nanobiotechnology. 17, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim SM, Yang Y, Oh SJ, Hong Y, Seo M. & Jang M. (2017) Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting, J Control Release. 266, 8–16. [DOI] [PubMed] [Google Scholar]
- 167.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA & Yang X. (2019) Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy, Nat Commun. 10, 3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS & Kalluri R. (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer, JCI Insight. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, Chalmers J, Schmittgen TD & Phelps MA (2017) Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells, J Extracell Vesicles. 6, 1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB & Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, J Transl Med. 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E. & Zitvogel L. (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial, J Transl Med. 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H. & Li G. (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer, Mol Ther. 16, 782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mulcahy LA, Pink RC & Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake, J Extracell Vesicles. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ & Zhang Y. (2018) Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity, J Am Chem Soc. 140, 16413–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu W, Huang F, Ning L, Hao J, Wan J. & Hao S. (2020) Enhanced immunogenicity of leukemia-derived exosomes via transfection with lentiviral vectors encoding costimulatory molecules, Cell Oncol (Dordr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L. & Chaput N. (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology. 5, e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N. & Zitvogel L. (2014) Dendritic cell-derived exosomes as immunotherapies in the fight against cancer, J Immunol. 193, 1006–11. [DOI] [PubMed] [Google Scholar]
- 178.Tian H. & Li W. (2017) Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges, Ann Transl Med. 5, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y. & Noelle RJ (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system, Immunol Rev. 229, 152–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J, Wang L, Lin Z, Tao L. & Chen M. (2014) More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells, Mol Med Rep. 9, 125–31. [DOI] [PubMed] [Google Scholar]
- 181.Shi X, Sun J, Li H, Lin H, Xie W, Li J. & Tan W. (2020) Antitumor efficacy of interferon-gamma-modified exosomal vaccine in prostate cancer, Prostate. 80, 811–823. [DOI] [PubMed] [Google Scholar]
- 182.Duong N, Curley K, Brown A, Campanelli A, Do MA, Levy D, Tantry A, Marriott G. & Lu B. (2019) Decoy exosomes as a novel biologic reagent to antagonize inflammation, Int J Nanomedicine. 14, 3413–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]


