Abstract
Objective
To assess the feasibility of Fractional exhaled Nitric Oxide (FeNO) as a simple, non-invasive, cost-effective and portable biomarker and decision support tool for risk stratification of COVID-19 patients.
Methods
We conducted a single-center prospective cohort study of COVID-19 patients whose FeNO levels were measured upon ward admission by the Vivatmo-me handheld device. Demographics, COVID-19 symptoms, and relevant hospitalization details were retrieved from the hospital databases. The patients were divided into those discharged to recover at home and those who died during hospitalization or required admission to an intensive care unit, internal medicine ward, or dedicated facility (severe outcomes group).
Results
Fifty-six patients were enrolled. The only significant demographic difference between the severe outcomes patients (n = 14) and the home discharge patients (n = 42) was age (64.21 ± 13.97 vs. 53.98 ± 15.57 years, respectively, P = .04). The admission FeNO measurement was significantly lower in the former group compared with the latter group (15.86 ± 14.74 vs. 25.77 ± 13.79, parts per billion [PPB], respectively, P = .008). Time to severe outcome among patients with FeNO measurements ≤11.8 PPB was significantly shorter compared with patients whose FeNO measured >11.8 PPB (19.25 ± 2.96 vs. 24.41 ± 1.09 days, respectively, 95% confidence interval [CI] 1.06 to 4.25). An admission FeNO ≤11.8 PPB was a significant risk factor for severe outcomes (odds ratio = 12.8, 95% CI: 2.78 to 58.88, P = .001), with a receiver operating characteristics curve of 0.752.
Conclusions
FeNO measurements by the Vivatmo-me handheld device can serve as a biomarker and COVID-19 support tool for medical teams. These easy-to-use, portable, and noninvasive devices may serve as valuable ED bedside tools during a pandemic.
Keywords: COVID-19, FeNO, Prognosis, ACE2, Scoring
Abbreviations: FeNO, Fractional exhaled Nitric Oxide; COVID-19, Coronavirus Disease 2019; ICU, Intensive Care Unit; SRAS-CoV-2, Severe Acute Respiratory Syndrome CoronaVirus 2; iNOS, Inducible Nitric Oxide Synthase; ACE2, Angiotensin-Converting Enzyme 2; ED, Emergency Department; TLVMC, Tel-Aviv Medical Center; PPB, Parts Per Billion
1. Introduction
Since December 2019, the world has experienced an outbreak of coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is grossly estimated that in a little more than 1 year COVID-19 has infected hundreds of millions and caused over 3 million deaths, with current estimations of daily new cases in the hundreds of thousands worldwide. While the majority of patients will experience a mild form of the disease, as many as 5% of infected patients will sustain a life-threatening disease, such as acute respiratory distress syndrome and cardiovascular failure [1,2] with complication rates reaching up to 21.6% among hospitalized patients [3]. The high infectivity of SARS-CoV-2 and the significant associated morbidity and mortality, even among monitored inpatients, led to medical systems being overwhelmed by the pandemic worldwide.4 [4].
Risk stratification of new COVID-19 cases and optimization of the utilization of existing medical systems in a limited resources environment are often mentioned among the strategies to cope with the threat posed by COVID-19. These measures include the need to improve patient and medical resource prioritization, reduce in-hospital team and patient exposure to SARS-CoV-2 and other nosocomial infections, together with the identification and early discharge of mild cases with favorable outcomes to home care. Several clinical characteristics have been associated with poor prognosis of COVID-19 patients [[5], [6], [7]]. Early discrimination between mild COVID-19 cases from moderate and severe cases that require hospitalization and monitoring in internal wards or intensive care units (ICUs), however, remains an unmet challenge [8]. This discrimination is crucial for clinical decision-making and resource allocation in clinical dilemmas, such as who should be quarantined, hospitalized in internal wards, or sent to an ICU. Therefore, the availability of an accessible, easy-to-use, and affordable prognostic measure for COVID-19 patients is essential.
Fractional exhaled Nitric Oxide (FeNO) is an easily achievable, noninvasive measurement of exhaled air that is to known to be associated with respiratory dynamics in the clinical setting. For instance, it was shown to be effective in monitoring asthma exacerbations and in assessing the clinical course of various respiratory viral infections [[9], [10], [11], [12], [13]]. FeNO is produced in airway epithelial and inflammatory cells mainly by the enzyme inducible nitric oxide synthase (iNOS). Angiotensin-converting enzyme 2 (ACE2) was shown to be involved in airway NO production via downstream effects on iNOS [[14], [15], [16], [17]], and to be a significant airway and vascular regulator [18,19]. Much like its counterpart SARS-CoV-1, SARS-CoV-2 was shown to invade respiratory epithelial cells via the ACE2 receptor. Interestingly, ACE2 was shown to be downregulated during a SARS-CoV-1 infection [20]. It is therefore logical to assume the existence of a negative correlation between SARS-CoV-2 infection burden and ACE2 airway expression, representing a correlation that could affect downstream airway NO production and therefore FeNO measurements. Thus, FeNO may serve as an indirect predictor of COVID-19 disease burden, allowing risk stratification and medical decision-making regarding COVID-19 patients. The purpose of this study was to assess the potential use of FeNO as a prognostic biomarker of outcome severity and its application as a tool for risk stratification for supporting management decision-making of COVID-19 patients at admission to the emergency department (ED).
2. Materials and methods
2.1. Study design
This was a single-center, prospective cohort study whose aim was to assess the applicability of admission FeNO levels as a biomarker for hospitalization outcome of COVID-19 patients.
2.2. Study population
Adult patients hospitalized between December 2020 and March 2021 in dedicated COVID-19 wards in the Tel Aviv Medical Center (TLVMC), a major tertiary hospital in central Israel, were prospectively enrolled in this study. Inclusion criteria were a positive oropharyngeal and nasopharyngeal swab SARS-CoV-2 PCR test and the ability to perform the FeNO test. Patients unable or unwilling to sign an informed consent or to undergo the FeNO measurement procedure were excluded from the study. This study was approved by the TLVMC institutional ethics committee (IEC, 0355-20-TLV).
2.3. Study procedure and data collection
Following enrolment, the study patients were given a short explanation of the exhalation procedure and requested to perform the FeNO measurements. In order to optimize the chances for successful measurement, each participant was requested to perform 3 FeNO measurements, which were later averaged to represent the enrolment FeNO. They were then requested to respond to a questionnaire on demographics (age, sex, and comorbidities), COVID-19 symptoms (fever, fatigue, cough, myalgia, nasal congestion, sore throat, diarrhea, and dyspnea), and onset dates. Further clinical data were obtained from electronic medical files, including vital signs (temperature, heart rate, blood pressure, and O2 saturation), laboratory evaluations (complete blood counts, coagulation tests, biochemistry tests, inflammatory markers, such as C-reactive protein [CRP], troponin, and venous blood gas analysis) on admission and before discharge, length of stay, treatment, and clinical outcomes (e.g., home discharge, transfer to an internal medicine ward, or transfer to the ICU, or death).
Follow-up telephone calls to the study participants were made 14 and 28 days after hospital discharge. They were queried about the above-cited COVID-19 symptoms and further need of medical attention. Hospital readmission and mortality during the 28 days post-discharge were assessed via the TLVMC electronic medical files.
The cohort of patients was divided into 2 groups comprised of those who were discharged home with no required additional medical attention (the “home discharge” group), and those with subsequent complications/severe outcomes, including death, admission to the ICU, mortality, or transfer to a non-COVID-19 internal medicine ward or a dedicated COVID-19 medical ward for continuous medical treatment (the “severe outcome” group).
2.4. FeNO measurement
Tests were executed by means of the Bosch's handheld Vivatmo-me device (Bosch Healthcare Solutions, Waiblingen, Germany) for FeNO measurements [21]. The Vivatmo-me is a handheld device utilizing a single-use mouthpiece and a chemical field-effect transistor, which allows the measurement of FeNO in the range of 5–300 parts per billion (PPB) with an accuracy of ±5 PPB for values < 50 PPB [22], thus conforming to the technical standards of the American Thoracic Society [23] and the European Respiratory Society (ERS) [24]. The Vivatmo-me device measures the FeNO level by installation of a single-use chemical field-effect transistor. Following a short self-calibration by the device, the subject is requested to exhale through the single-use mouthpiece in a steady flow for approximately 5 s. At the end of the measurement, the FeNO result is instantly displayed on the device's LED screen. In order to minimize possible cross-contamination s, the study personnel were properly protected with full personal protective equipment (PPE), and the Vivatmo-me device was thoroughly sanitized between uses together with single-use mouthpiece replacements.
2.5. Statistical analysis
Statistical analyses were performed with IBM SPSS, and graphic representation with GraphPad Prism software. Given the small sample size of the severe outcomes group, all of the continuous variables were analyzed by means of a non-parametric approach which included the Mann-Whitney U test. Categorical variables were tested with Pearson's χ2 test for contingency tables or Fisher Exact test, as appropriate. Correlation analyses were performed with Spearman rho (ρ) tests. Survival analysis was by Kaplan-Meier survival plots with group comparisons assessed by the log-rank test. A univariate binary logistic model was used for evaluation of the association between FeNO and severe outcomes. The discriminative capability of the model was assessed by a receiver operating characteristics (ROC) curve. All statistical tests and/or confidence intervals were performed at α = 0.05 (2-sided). All P-values were rounded off to 2 decimal places.
3. Results
3.1. Study population
Fifty-six patients (mean age±standard deviation [SD] 56.5 ± 17.2 years, 51.8% females) were enrolled in the study. Table 1 describes their basic demographic characteristics. Only the variable of age was significantly different between the 2 groups, with the home-discharged patients being younger than the severe outcome patients (54 ± 15.6 vs. 64.2 ± 14 years, respectively, P = .04).
Table 1.
Cohort characteristics.
| Variables | Entire cohort (n = 56) | Home discharge (n = 42) | Severe outcome (n = 14) | P-value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 56.5 (17.2) | 54 (15.6) | 64.21 (14) | 0.04 | |
| Sex, No. (%) |
Women | 29 (51.8) | 24 (57.1) | 5 (35.7) | 0.17 |
| Men | 27 (48.2) | 18 (42.9) | 9 (64.3) | ||
| Comorbidities, No. (%) | Cardiovascular | 11 (19.6) | 7 (16.7) | 4 (28.6) | 0.44 |
| Hypertension | 20 (35.7) | 13 (31) | 7 (50) | 0.2 | |
| Diabetes mellitus | 11 (19.6) | 7 (16.7) | 4 (28.6) | 0.43 | |
| Chronic respiratory disease | 6 (10.7) | 4 (9.5) | 2 (14.3) | 0.63 | |
| Malignancies | 7 (12.5) | 6 (14.3) | 1 (7.1) | 0.67 | |
| Immune deficiency | 3 (5.4) | 2 (4.8) | 1 (7.1) | 1 | |
| Asthma | 8 (14.3) | 7 (16.7) | 1 (7.1) | 0.66 | |
| Smoking | 1 (1.8) | 0 (0) | 1 (7.1) | 0.25 | |
Bold indicates significant.
3.2. COVID-19 hospitalization and outcomes
Table 2 details the vital signs, laboratory values, and x-ray imaging findings at presentation to the ED for suspected COVID-19 infection. Both study groups had similar symptoms (data not shown), with the exception of the complaint of fatigue which was more prevalent among patients in the severe outcome group compared to the home discharge group (78.6% vs. 47.6%, respectively, P = .04). Time from onset of COVID-19 symptoms to presentation was comparable between the groups (8.7 ± 5.4 vs. 10.2 ± 4.6 days, P = .1). Vital signs at admission (heart rate, blood pressure, and body weight) were similar, however, body temperature was significantly higher and the O2 saturation measurement was significantly lower in the severe outcomes group compared with the home discharge group (37.97 ± 0.97 vs. 37.35 ± 0.81 °C, P = .04 and 89.79 ± 5.18 vs. 92.76 ± 7.23 O2%, P = .03, respectively). Laboratory values, including acute-phase reactants (hemoglobin, white blood cell count, platelets, INE and CRP) were similar for the 2 groups. Most (n = 52, 92.9%) of the participants underwent admission chest x-ray imaging and the findings were similar for both groups, with pulmonary consolidations being the most common finding.
Table 2.
COVID-19 hospitalization admission vital signs, laboratory and imaging.
| Entire cohort (n = 56) | Home discharge (n = 42) | Severe outcome (n = 14) | P-value | ||
|---|---|---|---|---|---|
| Time from onset of symptoms to admission, mean (SD), days | 8.7 (5.3) | 8.1 (5.4) | 10.2 (4.6) | 0.1 | |
| Weight (kg) | 86.13 (16.4) | 86.04 (18) | 86.31 (13.5) | 0.94 | |
| Admission vital signs and laboratory, mean (SD) | Temperature (°C) | 37.51 (0.9) | 37.35 (0.8) | 37.97 (1) | 0.04 |
| Heart rate (BPM) | 88.59 (15.5) | 88.83 (16) | 87.86 (14.2) | 0.81 | |
| SaO2 (%) | 92 (6.9) | 92.76 (7.2) | 89.79 (5.2) | 0.03 | |
| Systolic BP (mmHg) | 136.27 (23.4) | 135.17 (24) | 139.57 (21.8) | 0.37 | |
| Diastolic BP (mmHg) | 76.75 (11.3) | 76.33 (11.8) | 78 (10.1) | 0.51 | |
| Hb (g%) | 13.09 (1.97) | 13.19 (2.02) | 12.79 (1.85) | 0.52 | |
| WBC (103/μL) | 7.54 (3.9) | 7.79 (4) | 6.81 (3.9) | 0.24 | |
| Neutrophils to lymphocytes ratio (NLR) | 9.6 (18.6) | 7.23 (6.4) | 16.6 (35.3) | 0.36 | |
| Platelets (103/μL) | 191.7 (62.1) | 196.2 (59.8) | 178.4 (58.9) | 0.34 | |
| CRP (mmol/L) | 104.6 (86) | 101 (92.4) | 101.9 (58.8) | 0.27 | |
| INR | 1.04 (0.1) | 1.03 (0.1) | 1.07 (0.1) | 0.16 | |
| Venous pH | 7.39 (0.06) | 7.38 (0.06) | 7.39 (0.05) | 0.57 | |
| Chest x-ray imaging, No. (%) | Pulmonary consolidations | 42 (80.8) | 29 (76.3) | 13 (92.9) | 0.25 |
| Pleural effusion | 2 (3.8) | 0 (0) | 2 (14.3) | 0.07 | |
| Enlarged cardia | 9 (17.3) | 5 (13.2) | 4 (28.6) | 0.23 | |
| Length of stay, median (IQR), days | 6 (4–9.8) | 5.5 (4–8.3) | 9.5 (7.8–17.8) | 0.001 | |
Bold indicates significant.
The administered COVID-19-related medical treatment (dexamethasone, Remdesivir, Proton pump inhibitor [PPI], Clexane, Actemra) was similar for the hospitalized groups (data not shown). The length of stay (LOS) was significantly longer in the severe outcomes group compared to the home discharge (median [interquartile range, IQR], 9.5 [7.75 to 17.75] vs. 5.5 [4 to 8.25] days, P = .001). Six patients in the severe outcomes group (42.9%) required further care in an internal medicine ward or in a dedicated medical facility after being discharged from the COVID-19 ward, 6 (42.9%) patients required ICU hospitalization, and 2 (14.3%) patients died from COVID-19.
Thirty-one home discharge patients (73.8%) and 34 severe outcomes (81%) patients participated in both the 14- and 28-day follow-ups. Loss-to-follow-up rates were higher in the severe outcome group, with only 4 patients (28.6%) responding to the 14-day follow-up call and 5 (35.7%) responding to the 28-day follow-up call, possibly due to continued hospitalization and treatment elsewhere. There were no group differences in COVID-19-related symptoms at 14 or 28 days following discharge. According to the TLVMC databases, while no mortality was reported in our cohort during the follow-up period, 2 patients from the home discharge group required hospitalization during the follow-up period at 6 and 11 days post-discharge (data not shown).
3.3. Enrolment FeNO measurements and implications for severe outcomes
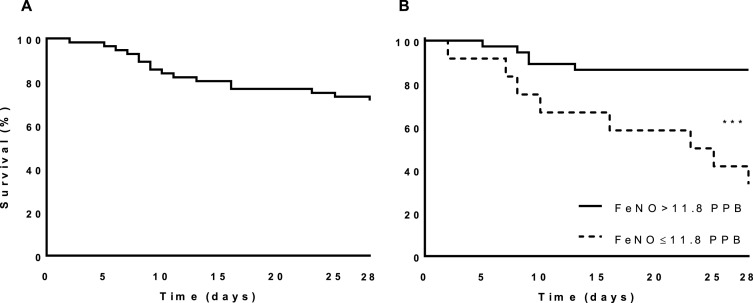
As shown in Fig. 1 , the admission FeNO measurements were significantly lower in the severe outcomes group (15.9 ± 14.7 vs. 25.8 ± 13.8 PPB for the home discharge group, P = .008). Inter-variable Spearman's ρ correlation analysis was performed to assess the association of admission FeNO with severe outcomes, length of hospital stay, age, gender and asthma. Of these, enrolment FeNO measurement was found to correlate significantly only with severe outcomes (ρ = −0.39, P = .006) and age (ρ = −0.36, P = .01), with both correlations being negative and with intermediate potency. The Kaplan-Meier survival analysis for severe outcomes is shown in Fig. 2 . The mean ± SE time from recruitment to the occurrence of a severe outcome for the entire cohort was 23.9 ± 1.2 days. Since there is no established range for normal FeNO levels, we selected the lower quartile of the entire cohort (11.8 PPB) as the cutoff of a low admission FeNO measurement. Using this definition, the mean ± SD time to a severe outcome was shorter for the low admission FeNO measurement group compared to the higher admission FeNO measurement group (19.3 ± 3 and 24.4 ± 1.1 days, respectively, log-rank P = .0002; a difference of 5.2 days, 95% CI 1.1 to 4.3). The univariate binary logistic regression model revealed that an admission FeNO measurement of 11.8 PPB or less was a significant risk factor for the prediction of a severe outcome, with an odds ratio (OR) of 12.8 (95% CI: 2.78 to 58.88, P = .001) and an area under curve of 0.752 in the ROC analysis (Fig. 3 ). Overall, an admission FeNO of ≤11.8 PPB had a sensitivity of 61.5% and a specificity of 88.9% to predict a severe outcome. The small severe outcome sample size precluded multivariate model assessment.
Fig. 1.
Study admission FeNO measurements and hospitalization outcomes. Box plot of FeNO measurements for the home discharge and the severe outcome groups. Lower and upper whiskers represent 5th and 95th percentiles, respectively. **P < .01.
Fig. 2.
Kaplan-Meier survival analysis. (A) Survival analysis for the entire cohort. (B) Survival analysis for the low (dashed line) and higher (solid line) FeNO measurement groups. The cutoff for admission FeNO measurements was determined as being 11.8 PPB. ***P < .001.
Fig. 3.
Receiver operating characteristics (ROC) analysis. ROC analysis of an admission FeNO measurement ≤11.8 PPB predictive model for severe outcomes.
4. Discussion
In this study, we assessed the efficacy and feasibility of an easily obtained admission FeNO measurement as a biomarker for disease severity trajectory among hospital-admitted COVID-19 patients. In our cohort of 56 patients, that FeNO measurement was significantly lower among patients with severe hospitalization outcomes as determined by death, ICU admission, or hospitalizatiion in a medical facility for continued care, compared with that of patients who were discharged to their homes. Furthermore, an admission FeNO measurement equal to or lower than 11.8 PPB was found to be associated with an earlier occurrence of severe hospitalization outcomes, with an FeNO level ≤11.8 PPB serving as a significant risk factor for these events in a univariate logistic regression model.
The FeNO level is a broadly used method for diagnosis and surveillance of various diseases, mainly respiratory [[9], [10], [11], [12], [13]]. In an attempt to use FeNO measurements as a diagnostic tool for the identification of COVID-19 disease, 2 studies compared FeNO levels of healthy controls to those of COVID-19 patients [25,26]. Contrary to our results, both of those studies showed mildly increased FeNO levels for the COVID-19 patients compared to the controls, however, most of the studied patients had a mild COVID-19 disease course, with many being outpatients. This implies that a high FeNO may serve a marker of a competent inflammatory airway response [20,23], and defining COVID-19 patients with low FeNO levels as being unable to amount such a response, which could suggest an added risk factor, as also suggested by our results.
Other clinical scores for the prediction and identification of severe acute COVID-19, such as the neutrophil-lymphocyte ratio (NLR) [27], early CRP [28], and neutrophilia and coagulation dysfunction [3], have been previously described with ORs and hazard ratios ranging from 1.14 to 1.61. Examination of these clinical scores of our cohort failed to reveal a significant difference between the 2 study groups. In comparison, the admission FeNO measurement was found to be a superior tool for the identification of severe acute COVID-19 cases, with an OR of 12.8, all the while being an easier, more rapid, noninvasive, and more traceable tool.
Some limitations to this study bear mention. In spite of the study team's efforts, recruitment of COVID-19 patients within the wards proved to be difficult, limiting the sample size. The small sample size also limited our ability to relate our results to age differences between the groups, although FeNO measurements reportedly do not seem to differ across different ages in adult populations [29]. Additionally, it took place in a single medical center, and a single Vivatmo-me device was used for all measurements. Lastly, our study population did not include smokers or reported COPD patients, which may limit the applicability of our results to wider populations. At the same time, however, the absence of smokers in this study serves to further strengthen our results by essentially removing that confounder which is known to reduce FeNO levels [30,31]. Further studies are warranted to determine disease-specific population nomograms as well as assess the precise efficacy and sensitivity of FeNO measurements of COVID-19 severity across wider populations, possibly alongside correlation to pulmonary function tests.
Our results suggest that bedside FeNO measurements during hospitalization could serve as a support tool for decision-making among medical personnel when considering home discharge from the ED, COVID-19 ward hospitalization, or ICU admission of confirmed COVID-19 cases. Furthermore, given the portability, ease of use, and rapid results of FeNO measurement devices, the use of FeNO in the context of COVID-19 could be further expanded to community clinics and remote locations where auxiliary tests may not be readily available.
5. Conclusion
In conclusion, low FeNO measurements may indicate a susceptibility of patients to respiratory complications of COVID-19, a clinical entity that may overlap with other inflammatory airway conditions, such as active asthma, pulmonary co-infections, and smoking-related respiratory disorders. This tool is affordable, easy to use at bedside, and provides immediate measurements that may have clinical applications even in remote and low-resource facilities. While requiring further validation and conformation in larger cohorts, our results support the use of FeNO measurements as a another resource for the clinician's decision-making process in the management of COVID-19 patients.
Funding
None declared.
Clinical trial registration number
MOH_2020-11-15_009509 Institutional ethics committee approval0355-20-TLV.
Authors’ contributions
YL, IB, YY, RR, IH, MF, and GFL conceived and designed the study. YL, NY, MB, IA, and ML supervised the study and the data collection. YL undertook patient recruitment, data management, and statistical analysis, including quality control. YL drafted the manuscript, and all authors contributed substantially to its revision. YL takes responsibility for the integrity of the paper.
Declaration of competing interest
None declared.
Acknowledgements
The authors thank the BOSCH corporation and its staff for the donation of 2 Vivatmo-me devices and a generous amount of single-use mouthpieces. We also acknowledge the valuable contribution of Ms. Polina Farber.
References
- 1.Gomes C. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) Braz. J. Implantol. Heath Sci. 2020;2(3) [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wu C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangcharoensathien V., et al. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York State. BMJ. 2021;372:n83. doi: 10.1136/bmj.n83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin C., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jiehe He Huxi Zazhi. 2020;43(3):203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Wu C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo Marin B., et al. Predictors of COVID-19 severity: a literature review. Rev. Med. Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thudium R.F., et al. Fraction of exhaled nitric oxide levels are elevated in people living with HIV compared to uninfected controls suggesting increased eosinophilic airway inflammation. Clin. Infect. Dis. 2020;71(12):3214–3221. doi: 10.1093/cid/ciz1223. [DOI] [PubMed] [Google Scholar]
- 10.Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manna A., et al. Clinical application of exhaled nitric oxide measurement in pediatric lung diseases. Ital. J. Pediatr. 2012;38:74. doi: 10.1186/1824-7288-38-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashir A., et al. Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FE(NO)) and other volatiles in exhaled breath. J. Breath Res. 2011;5(3) doi: 10.1088/1752-7155/5/3/037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z., et al. Assessment of ventilator-associated pneumonia by combining 8-isoprostane and nitric oxide levels in exhaled breath condensate with the clinical pulmonary infection score. J. Int. Med. Res. 2020;48(5) doi: 10.1177/0300060520922472. 300060520922472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J., et al. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61(3):681–689. doi: 10.1161/HYPERTENSIONAHA.111.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai F., et al. Angiotensin II AT1 receptor alters ACE2 activity, eNOS expression and CD44-hyaluronan interaction in rats with hypertension and myocardial fibrosis. Life Sci. 2016;153:141–152. doi: 10.1016/j.lfs.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Malinovschi A., et al. Application of nitric oxide measurements in clinical conditions beyond asthma. Eur. Clin. Respir. J. 2015;2:28517. doi: 10.3402/ecrj.v2.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G., et al. ACE2 and the homolog collectrin in the modulation of nitric oxide and oxidative stress in blood pressure homeostasis and vascular injury. Antioxidants Redox Signal. 2017;26(12):645–659. doi: 10.1089/ars.2016.6950. [DOI] [PubMed] [Google Scholar]
- 18.Skrupky L.P., et al. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit. Care Med. 2012;40(1):281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson L.E., et al. Endogenous nitric oxide is present in the exhaled air of rabbits, Guinea pigs and humans. Biochem. Biophys. Res. Commun. 1991;181(2):852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 20.Thudium R.F., et al. Fraction of exhaled nitric oxide levels are elevated in people living with human immunodeficiency virus compared to uninfected controls, suggesting increased eosinophilic airway inflammation. Clin. Infect. Dis. 2020;71(12):3214–3221. doi: 10.1093/cid/ciz1223. [DOI] [PubMed] [Google Scholar]
- 21.https://www.vivatmo.com/en/products/ Bosch. Vivtamo me. [cited 2021; Available from:
- 22.Bosch. Vivtamo me technical specifications 2021. https://www.vivatmo.com/media/pdf/vivatmo_me_techisches_datenblatt/f09g100196_technical_data_sheet_vivatmome_en_v8-0.pdf Available from:
- 23.Dweik R.A., et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath I., et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur. Respir. J. 2017;49(4) doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- 25.Balcı A., et al. A predictive and prognostic marker in COVID-19 patients: exhaled nitric oxide (FENO) Acta Med. Mediterr. 2021:841–846. [Google Scholar]
- 26.Yang L., et al. 2021. Ultrafast Preliminary Screening of COVID-19 by Machine Learning Analysis of Exhaled NO. [Google Scholar]
- 27.Imran M.M., et al. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int. J. Clin. Pract. 2021;75(4):e13698. doi: 10.1111/ijcp.13698. [DOI] [PubMed] [Google Scholar]
- 28.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malerba M., et al. Values in elderly people for exhaled nitric oxide study. Rejuvenation Res. 2016;19(3):233–238. doi: 10.1089/rej.2015.1706. [DOI] [PubMed] [Google Scholar]
- 30.Ahovuo-Saloranta A., Csonka P., Lehtimaki L. Basic characteristics and clinical value of FeNO in smoking asthmatics-a systematic review. J. Breath Res. 2019;13(3) doi: 10.1088/1752-7163/ab0ece. [DOI] [PubMed] [Google Scholar]
- 31.Dolovich M.B., et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American college of chest physicians/American college of asthma, allergy, and immunology. Chest. 2005;127(1):335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]