Abstract
Purpose
Cytomegalovirus (CMV) reactivation in immunocompetent critically ill patients is common and relates to a worsening outcome. In this large observational study, we evaluated the incidence and the risk factors associated with CMV reactivation and its effects on mortality in a large cohort of patients affected by coronavirus disease 2019 (COVID-19) admitted to the intensive care unit (ICU).
Methods
Consecutive patients with confirmed SARS-CoV-2 infection and acute respiratory distress syndrome admitted to three ICUs from February 2020 to July 2021 were included. The patients were screened at ICU admission and once or twice per week for quantitative CMV-DNAemia in the blood. The risk factors associated with CMV blood reactivation and its association with mortality were estimated by adjusted Cox proportional hazards regression models.
Results
CMV blood reactivation was observed in 88 patients (20.4%) of the 431 patients studied. Simplified Acute Physiology Score (SAPS) II score (HR 1031, 95% CI 1010–1053, p = 0.006), platelet count (HR 0.0996, 95% CI 0.993–0.999, p = 0.004), invasive mechanical ventilation (HR 2611, 95% CI 1223–5571, p = 0.013) and secondary bacterial infection (HR 5041; 95% CI 2852–8911, p < 0.0001) during ICU stay were related to CMV reactivation. Hospital mortality was higher in patients with (67.0%) than in patients without (24.5%) CMV reactivation but the adjusted analysis did not confirm this association (HR 1141, 95% CI 0.757–1721, p = 0.528).
Conclusion
The severity of illness and the occurrence of secondary bacterial infections were associated with an increased risk of CMV blood reactivation, which, however, does not seem to influence the outcome of COVID-19 ICU patients independently.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06716-y.
Keywords: COVID-19, Cytomegalovirus reactivation, Mechanical ventilation, Sepsis
Take-home message
| In critically ill patients affected by coronavirus disease 2019 (COVID-19), the Cytomegalovirus (CMV) blood reactivation is frequent, and its risk depends on the severity of illness and the development of secondary bacterial infections. CMV reactivation is associated with prolonged hospital stay and higher mortality, but its role in worsening patient outcomes and the appropriate strategy for its management remain to be clarified |
Introduction
In critically ill patients, the reactivation of Cytomegalovirus (CMV) and other Herpesviridae has been reported with a rate ranging between 20 and 70%, and it is associated with increased risk of secondary infections and mortality [1–4]. Although the risk factors remain to be defined, profound dysfunction of the immune response is the key mechanism leading to viral reactivation in previous immunocompetent patients with critical illness [5]. In this context, the immunosuppression induced by SARS-CoV-2 direct pathogenic effects, the unregulated host response and the use of drugs to modulate such response (e.g., steroids and immunomodulators) make critically ill patients affected by coronavirus disease 2019 (COVID-19) at high risk for viral reactivation [6–9]. A recent large observational study indicated that the Herpes simplex 1 virus reactivated in around a quarter of COVID-19 patients requiring mechanical ventilation and impacted secondary infections and mortality [10]. Unfortunately, in this population, very little data are available on CMV reactivation which is one of the most pathogenetic viruses and seems to be closely related to worse outcomes in other intensive care unit (ICU) populations [11, 12]. As from animal model and in vivo studies, CMV reactivation may have significant clinical effects by direct organ damage, down-regulation of the immune response and boosting a hyper-inflammatory response that may further aggravate the ongoing inflammatory processes in sepsis, in acute respiratory distress syndrome and, perhaps, in COVID-19 disease [13–17]. However, the lack of survival benefits from prophylactic or pre-emptive anti-CMV strategies generated extensive discussion on the role of CMV reactivation that is often considered only a marker of clinical complexity not requiring specific treatment in immunocompetent critically ill patients [18–20].
This large observational study aimed to evaluate the incidence and the risk factors associated with CMV reactivation and its effects on mortality risk in a large cohort of COVID-19 patients admitted to ICU for severe respiratory failure.
Methods
In this observational study using prospectively collected data, we included all the patients admitted to the three COVID-19 ICUs of the University Hospital of Modena with laboratory-confirmed SARS-CoV-2 infection and moderate to severe acute distress respiratory syndrome (ARDS) from February 22nd, 2020, to July 21st 2021 [21]. Patients with age < 18 years, ICU length of stay (LOS) < 24 h, limitation of care or do not resuscitate order were excluded from the study. The Institutional Ethics Committee of Area Vasta Emilia Nord (EC AVEN) approved the study (approval number 396/2020/OSS/AOUMO—CoV-2 MO-Study). Due to the observational nature, written informed consent was not required.
Treatment protocol
All the patients received standard ICU and supportive care as recommended by the World Health Organization (WHO) guidelines [22] and specific therapies according to national [23] and local protocol for COVID-19 treatment, including dexamethasone, low-molecular weight heparin for prophylaxis of deep vein thrombosis according to individual bodyweight and renal function. In addition, the local protocol allowed the use of steroids (methylprednisolone 2 mg/kg/day) to prevent the onset of pulmonary fibrosis in patients who maintained a PaO2/FiO2 ratio < 150 mmHg for at least 7 days of mechanical ventilation [24]. Since March 2020, the local management protocol has included Tocilizumab (TOCI) option in patients with moderate or severe ARDS and the need for mechanical ventilation (non-invasive or invasive). From the end of March 2020, all patients who received TOCI or a high dose of steroids received standard prophylaxis with Acyclovir. The standard supportive management in ICU did not significantly change during the study period.
Data collection
Patients' demographics, Sequential Organ Failure Assessment (SOFA) score, Simplified Acute Physiology Score II (SAPS II) and standard laboratory including coagulation and inflammatory variables were collected at ICU admission. In addition, the need for invasive mechanical ventilation, therapy with steroids, tocilizumab (also before ICU admission) and ganciclovir, the CMV blood reactivation and the occurrence of new bacterial infections were collected during ICU stay.
As for the ICU protocol, patients were screened at ICU admission and twice (in invasive mechanically ventilated patients) or once per week for bacterial colonization in the rectum, respiratory (if tracheal intubation) and urinary tract, respiratory (if tracheal intubation) and serum Galactomannan, serum Beta-d-glucan and quantitative CMV-DNAemia in the blood (see protocol in the supplementary material). The CMV reactivation was set for a DNAemia > 62 UI/ml in the whole blood, the detection threshold of the method used (Abbott, RealTime CMV). In patients with CMV blood reactivation, in case of suspected CMV-related pneumonia, ganciclovir was initiated after detection of CMV-DNA in the broncho-alveolar lavage. The clinical suspicion of CMV pneumonia was based on the following elements: new worsening of pulmonary gas exchange, modification of chest X-ray or computed tomography compatible with new interstitial pneumonia, CMV blood reactivation and no other causes of pneumonia/worsening of pulmonary gas exchange. Ganciclovir dosage was set based on renal function and continued for at least 10 days. Secondary infections were defined according to international guidelines [25, 26] and divided into hospital-acquired pneumonia (HAP), including also ventilator-associated pneumonia and bloodstream infection (BSI). Probable invasive pulmonary Aspergillosis was defined according to definitions from the recent consensus document [27]. According to the WHO International Standard for Human CMV, all microbiological samples were analyzed in the local Microbiology and Virology laboratory [28].
Data analysis
The risk factors associated with CMV blood reactivation within 60 days after ICU admission were estimated by Cox proportional hazards regression model adjusted for covariates with p value < 0.1 at unadjusted analysis. Similarly, we used a Cox proportional hazards regression model including variables with p value < 0.1 at unadjusted analysis to evaluate the independent association of CMV blood reactivation with mortality censored at day 60. An additional sensitivity analysis with the same procedure described above was performed only in the population with CMV-related pneumonia treated with ganciclovir to evaluate the independent association of CMV pneumonia with mortality censored at day 60. To further evaluate the association of CMV blood reactivation with mortality censored at day 60, we performed a secondary analysis by matching patients with and without CMV blood reactivation (1:1) using a propensity score estimated by multivariable logistic-regression model that included as covariates the risk factors for developing CMV reactivation; the nearest-neighbor method was applied to propensity-score matching analysis.
Non-parametric and χ2 tests were used as appropriate for the comparisons of demographic and baseline values, outcomes in patients with and without CMV blood reactivation, and survivors and no-survivors. All results were expressed as median (range) for continuous variables and as frequency (percentage) for categorical variables. All tests were two-tailed with a p value < 0.05 considered significant. SPSS version 22.0 package (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis.
Results
In the study period, 493 patients were admitted to the three ICUs, and 431 patients met the study's inclusion criteria. Blood CMV reactivation was observed in 88 patients (20.4%) with a median onset of 17 days (IQR 5–26) after ICU admission. Interestingly, blood CMV reactivation was observed upon ICU admission in 19 out of 88 patients (21.6%). Thirty patients out of 88 (34.1%) received ganciclovir because of clinical signs of CMV-related pneumonia, and CMV-DNA detected in the broncho-alveolar lavage.
Patients with CMV reactivation showed older age (p < 0.001), higher SAPS II scores (p < 0.001), lower platelet count (p < 0.001) and higher procalcitonin (p = 0.019) than patients without CMV reactivation at ICU admission (Table 1). A larger proportion of patients with CMV reactivation received steroids (i.e., dexamethasone, methylprednisolone or both) (p = 0.005), required invasive mechanical ventilation (p < 0.001), and developed secondary bacterial infections and probable invasive pulmonary Aspergillosis during ICU stay (Table 1). The hospital mortality was larger (p < 0.001) in patients with CMV reactivation than without reactivation. Among patients with CMV reactivation, patients with CMV-related pneumonia and treated with ganciclovir showed higher (p = 0.063) mortality (24/30; 80%) than patients without signs of CMV-related clinical pneumonia (35/58; 30%) (Table 2).
Table 1.
Demographics, severity scores and laboratory results at ICU admission in all the patients and in patients with or without cytomegalovirus (CMV) blood reactivation
| All patients (n = 431) |
No CMV reactivation (n = 343) |
CMV reactivation (n = 88) |
p value | |
|---|---|---|---|---|
| Age (years; median, IQR) | 65 (56–72) | 63 (54–72) | 69 (64–75) | < 0.001 |
| Sex, male (n, %) | 323 (74.9) | 248 (72,3) | 75 (85.2) | 0.013 |
| Hypertension (n, %) | 222 (51.7) | 174 (50.9) | 48 (55.7) | 0.408 |
| Diabetes (n, %) | 96 (22.4) | 71 (20.8) | 25 (28.4) | 0.128 |
| Immunosuppressiona (n, %) | 68 (15.9) | 47 (13.7) | 21 (23.9) | 0.021 |
| BMI (kg/m2; median, IQR) | 29 (26–33) | 29 (26–33) | 29 (26–33) | 0.738 |
| SAPSII (median, IQR) | 35 (30–40) | 34 (28–39) | 37 (34–44) | < 0.001 |
| D-dimer (ng/ml; median, IQR) | 1470 (820–2840) | 1400 (820–2660) | 1805 (855–4760) | 0.166 |
| LDH (U/L; median, IQR) | 808 (632–1086) | 786 (633–1081) | 874 (623–1148) | 0.260 |
| Lymphocyte count (109/L; median, IQR) | 0.65 (0.46–0.91) | 0.66 (0.47–0.90) | 0.61 (0.41–0.96) | 0.307 |
| Platelet count (109/L; median, IQR) | 219 (169–286) | 227 (182–291) | 186 (140–247) | < 0.001 |
| C-reactive protein (mg/dl; median, IQR) | 7.2 (2.1–17.6) | 7.5 (2.6–17.5) | 6.2 (1.2–18.4) | 0.408 |
| Procalcitonin (ng/ml; median, IQR) | 0.20 (0.10–0.60) | 0.2 (0.1–0.5) | 0.3 (0.11–1.15) | 0.019 |
| PaO2/FiO2 (mmHg; median, IQR) | 104 (83–138) | 104 (84–140) | 106 (83–135) | 0.870 |
BMI body mass index; SOFA simplified organ failure assessment; SAPSII Simplified Acute Physiology Score II; LDH lactate dehydrogenase
aIncluding active hematological malignancies, neoplastic diseases, AIDS, transplants
Table 2.
Treatments provided and infections during ICU stay, intensive care and hospital mortality in all the patients and in patients with or without cytomegalovirus blood reactivation
| All patients (n = 431) |
No CMV reactivation (n = 343) |
CMV reactivation (n = 88) |
p value | |
|---|---|---|---|---|
| Invasive mechanical ventilation (n, %) | 276 (64) | 197 (57.4) | 79 (89.8) | < 0.001 |
| Steroids (n, %) | 393 (91.4) | 306 (89.5) | 87 (98.9) | 0.005 |
| Tocilizumab (n, %) | 356 (82.6) | 283 (82.5) | 73 (83) | 0.921 |
| Acyclovir prophylaxis (n, %) | 318 (73.8) | 246 (71.7) | 72 (81.8) | 0.055 |
| Ganciclovir treatment (n, %) | 30 (6.9) | 30 (34.1) | < 0.001 | |
| Onset Time of CMV reactivation (days; median, IQR) | 17 (5–26) | 17 (5–26) | ||
| New bacterial infections (n, %) | 157 (37.3) | 90 (26.9) | 67 (77) | < 0.001 |
| HAP (n, %) | 133 (32.7) | 75 (23.4) | 58 (67.4) | < 0.001 |
| HAP Bacterial species (n, %) | 119 (89.5) | 64 (89.3) | 55 (94.8) | 0.344 |
| S. aureus | 38 (28.6) | 24 (32) | 14 (24.1) | |
| Pseudomonas aeruginosa | 34 (25.6) | 16 (21.3) | 18 (31) | |
| Klebsiella spp | 24 (18) | 14 (18.7) | 10 (17.2) | |
| Other entobacterales | 15 (11.3) | 6 (8) | 9 (15.5) | |
| Other | 8 (6) | 4 (5.3) | 4 (6.9) | |
| Not identified | 14 (10.5) | 11 (14.7) | 3 (5.2) | |
| Multidrug resistanta | 56 (47.1) | 29 (45.3) | 27 (49.1) | 0.681 |
| BSI (n, %) | 65 (16) | 32 (10) | 33 (38.4) | < 0.001 |
| Onset time of new bacterial infection (days; median, IQR) | 8 (3–14) | 5 (1–9) | 12 (6–17) | < 0.001 |
| Probable Invasive Pulmonary Aspergillosis (n, %) | 74 (17.1) | 45 (13.1) | 29 (33) | < 0.001 |
| ICU LOS (days; median, IQR) | 7 (4–16) | 6 (3–11) | 31 (12–49) | < 0.001 |
| Hospital LOS (days; median, IQR) | 23 (15–37) | 21 (13–31) | 40 (25–57) | < 0.001 |
| ICU Mortality (n, %) | 111 (25.8) | 65 (19) | 46 (52.3) | < 0.001 |
| Hospital mortality (n, %) | 143 (33.2) | 84 (24.5) | 59 (67) | < 0.001 |
CMV cytomegalovirus; HAP hospital-acquired pneumonia; BSI bloodstream infection; ICU intensive care unit; LOS length of stay; Other enterobacterales: E. coli, Serratia M, Proteus M
aMultidrug resistant according to Magiorakos AP et al. Clin Microbiol Infect. 2012 Mar;18(3):268–81
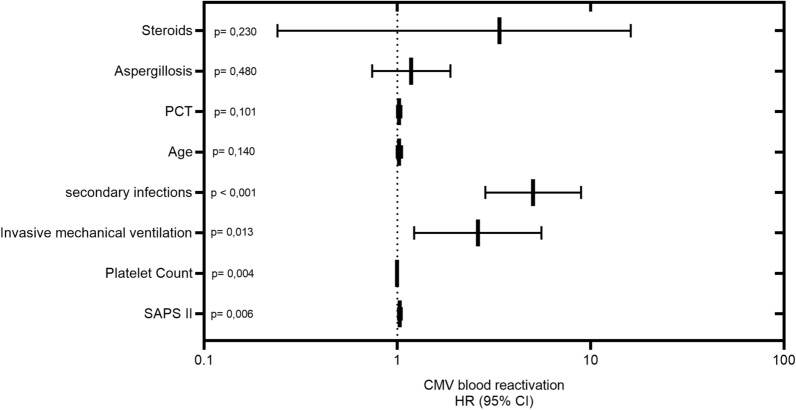
Adjusted analysis by Cox regression model showed that factors related to risk of CMV reactivation within 60 days after ICU admission were SAPS II and platelet count at ICU admission and need of invasive mechanical ventilation and occurrence of secondary bacterial during ICU stay (Fig. 1). Interestingly, CMV reactivation during ICU stay occurred later (median 9, IQR 1–15 days) than the occurrence of bacterial infection in 43 patients (64.1%), at the same time (± 48 h) in 7 patients (10.4%) and earlier (median 11, IQR 9–15 days) in 17 patients (25.4%) (Fig. 1S1).
Fig. 1.
Association between risk factors and cytomegalovirus (CMV) blood reactivation. Hazard ratio and 95% confidential interval as obtained by adjusted Cox regression analysis; p values are also reported
Unadjusted analysis indicated that CMV reactivation, together with many other variables, was related to an increased mortality risk at day 60. However, the adjusted analysis did not confirm the relationship between CMV reactivation and mortality at day 60 (Table 3). Similarly, the sensitivity analysis performed in patients only in patients with CMV-related pneumonia and treated with ganciclovir did not show any independent relationship between CMV pneumonia and mortality at day 60 (HR 1248; 95% CI 0.732–2129; p = 0.415). The secondary analysis on the 168 patients with and without CMV reactivation matched (1:1) for the individual propensity to develop CMV blood reactivation also indicated no association between CMV blood reactivation and mortality at day 60 (HR 1105; CI 0.738–1640; p = 0,638) (Table 1 and 2 S1). The fifty (56.8%) patients with CMV blood maximal viral load > 500 IU showed similar characteristics, treatments (but Ganciclovir therapy) and mortality than 38 patients with maximal viral load < 500 IU (Table 3S1).
Table 3.
Unadjusted and adjusted analysis for mortality censored at day 60
| Survived (n = 288) |
Not survived (n = 143) |
Unadjusted HR (95% CI) p value |
Adjusted HR (95% CI) p value |
|
|---|---|---|---|---|
| Age (years; median, IQR) | 63 (53–71) | 69 (62–75) | 1.043 (1.025–1.061); p < 0.001 | 1.01 (0.989–1.031); p = 0.359 |
| SAPSII (median, IQR) | 33 (28–38) | 37 (34–44) | 1.042 (1.029–1.055); p < 0.001 | 1.022 (1.004–1.041); p = 0.017 |
| LDH (U/L; median, IQR) | 773 (620–1050) | 909 (671–1245) | 1.001 (1–1.001); p < 0.001 | 1 (1–1.001); p = 0.212 |
| platelet count (109/L; median, IQR) | 233 (188–297) | 188 (139–248) | 0.994 (0.992–0.996); p < 0.001 | 0.996 (0.994–0.998); p = 0.001 |
| PaO2/FiO2 (mmHg; median, IQR) | 109 (86–143) | 99 (81–129) | 0.994 (0.990–0.999); p = 0.01 | 0.998 (0.994–1.002) p = 0.354 |
| Invasive mechanical ventilation (n, %) | 140 (48.6) | 136 (95.1) | 14.904 (6.56–33.84); p < 0.001 | 9.02 (3.58–22.87); p < 0.001 |
| New bacterial infection (n, %) | 58 (20.1) | 99 (69.2) | 4.638 (3.174–6.777); p < 0.001 | 2.571 (1.665–3.972); p < 0.001 |
| Probable Invasive Pulmonary Aspergillosis (n, %) | 38 (13.2) | 36 (25.2) | 2.041 (1.389–3.001); p < 0.001 | 1.023 (0.675–1.54); p = 0.916 |
| CMV reactivation (n, %) | 29 (10.1) | 59 (41.3) | 2.53 (1.771–3.613); p < 0.001 | 1.141 (0.757–1.721); p = 0.528 |
Only factors with p < 0.1 at unadjusted analysis are reported
SAPSII Simplified Acute Physiology Score II: LDH lactate dehydrogenase; CMV cytomegalovirus
Discussion
This large cohort observational study showed that CMV blood reactivation occurs in about 20% of COVID-19 patients admitted to ICU for respiratory failure, and 30% of these patients received anti-CMV treatment for suspected CMV pneumonia. High severity scores at ICU admission, the requirement of invasive mechanical ventilation and the development of secondary bacterial infections during ICU stay increase the risk of CMV blood reactivation. Furthermore, COVID-19 patients with CMV blood reactivation showed increased mortality compared to patients without reactivation, but the CMV blood reactivation and the occurrence of CMV-related pneumonia did not seem to increase the risk of mortality at day 60 independently. To our knowledge, this is the most extensive study published so far on the occurrence, risk factors and impact of CMV reactivation in ICU patients with respiratory failure caused by SARS-CoV-2. The data provided should be considered high quality because it originated from a prospective clinical protocol used from the beginning of the COVID-19 surge.
As in our cohort, the previous small studies reported a CMV reactivation in about a quarter of COVID-19 critical patients, with about 50–60% of these patients showing at least one Herpesviridae reactivation [6, 8, 29]. In COVID-19 patients with a high rate of pre-existing immune defect, Epstein–Barr virus (EBV) was the Herpesviridae with the most frequent reactivation [29]. Unfortunately, our protocol did not include surveillance of EBV reactivation and then, only a few patients were screened for it. Similarly, we are not able to provide robust data on HSV-1 reactivation because very early during the first wave 2020, we introduced acyclovir prophylaxis in all the ICU patients after two cases of fatal liver failure related to HSV-1 and the high incidence of HSV-1 reactivation observed (published elsewhere) [9, 30]. Consequently, we withhold the protocol for systematic surveillance of HSV-1 that was evaluated only in patients with high clinical suspicion of infection.
In immunocompetent no-COVID-19 critically ill patients, numerous risk factors have been associated with the risk of CMV reactivation with a relationship for sepsis and mechanical ventilation. Although in our cohort many demographic characteristics, admission parameters and treatments were related to CMV reactivation in the crude analysis, as in no-COVID-19 patients, the adjusted analysis indicated that only invasive mechanical ventilation and the occurrence of new bacterial infection, that in critically ill patients frequently causes sepsis, increased the risk of CMV reactivation during ICU stay. The association observed between low platelets count and CMV reactivation may be explained by the pathobiology of SARS-CoV-2 infection that comprises persistent viral replication/viremia, uncontrolled inflammation, immune system impairment, and progressive involvement the endothelium with severe disturbances of coagulation processes leading to multiple thrombotic events [31]. Therefore, as for bacterial sepsis, the reduction of platelets indicates the degree of the hemostasis and the immune-inflammatory response impairment [32].
Interestingly, the incidence of CMV reactivation in COVID-19 appears to be lower than that reported in no-COVID-19 immunocompetent critically ill patients with sepsis, in whom it ranges between 30 and 60% depending on the time of screening, specimen and methods used [11, 33]. Several factors may theoretically explain this difference, but the severity scores of COVID-19 patients and the impact of SARS-CoV-2 infections on the immune response are usually less severe in the first 15 days than those in patients with sepsis from bacterial infections admitted to ICU [34]. These aspects, combined with younger age, fewer pre-existing comorbidities and the shorter duration of ICU stay, may justify the reduced occurrence of CMV reactivation in COVID-19 patients compared to the septic ICU population [13].
Therapy with steroids has been suggested as a potential risk for CMV reactivation in ICU patients with a low grade of certainty [35]. In our cohort, steroid therapy was administered more frequently in patients with CMV reactivation, but the adjusted analysis did not confirm this association. On the contrary, a relationship between CMV reactivation and the occurrence of new bacterial infections, the most hospital/ventilator acquired pneumonia, came out in our patients. The association between CMV and bacterial infections and their causal effect have been long debated in non-COVID-19 ICU patients [13, 33]. On one side, it is well known that sepsis profoundly deranges the immune mechanisms controlling viral reactivation with increased levels of IL-10, lymphopenia and reduced activity of T cells, natural killer and Th1 T cells [36–38]. On the other side, CMV reactivation may further induce immune suppression by complex mechanisms involving TNF-alfa, interleukin1-beta and cellular mediated response [5] with consequent augmented risk for secondary infections. Similar to previous reports in COVID-19 and non-COVID-19 patients [8, 13], the CMV reactivation occurred in median 2 weeks after ICU admission and, noteworthy, only in a quarter of the patients occurred before secondary bacterial infection. Therefore, we believe reasonable to suppose that in COVID-19 patients, the development of secondary bacterial infections increases the risk of CMV reactivation rather than the opposite. CMV reactivation in immunocompetent ICU patients has also been indicated as a risk factor for invasive pulmonary Aspergillosis [39]. In fact, our patients with CMV reactivation also showed an increased (+ 20%) incidence of invasive pulmonary Aspergillosis compared to patients without CMV reactivation. Nevertheless, the adjusted analysis did not show a significant association, and, in addition, probable invasive pulmonary Aspergillosis was diagnosed at least 1 week before CMV reactivation in most of our patients.
The negative impact of CMV reactivation on the outcome of immunocompetent critically ill patients has been reported from several observational studies from at least 30 years. A recent meta-analysis reported 2.5-fold increase in ICU mortality (10 studies, n = 970 patients), prolonged duration of mechanical ventilation (7 studies, n = 683 patients; mean difference 6.6 days 95% CI 3.1–10.1) and increased length of ICU stay (9 studies, n = 973 patients; mean difference 8.2 days 95% CI 6.1–10.2) associated with CMV reactivation[33]. Nevertheless, the true effect of CMV in the critically ill patient is still objective of large debate without a definitive answer. As described in the introduction, several animal and in-vivo studies described the putative mechanisms for direct and indirect pathogenicity of CMV in the ICU population, but numerous recent interventional trials failed to demonstrate any survival improvement by anti-CMV-specific therapeutic strategies. A specific potential role of CMV reactivation in worsening COVID-19 disease has been theorized due to its capacity to reinforce and perpetuate hyper-inflammatory response and induce immune suppression with persisting SARS-CoV-2 viremia and secondary infections [17]. Our COVID-19 cohort detected increased mortality at ICU and hospital discharge, but the adjusted analysis did not confirm this association. Similar results were also observed in the sub-group of patients treated with ganciclovir because of suspected CMV-related pneumonia. Therefore, as hypostatized in the critically ill non-COVID-19 population, the CMV reactivation might be considered just a marker of disease severity rather than a factor capable of modifying outcomes in COVID-19 ICU patients. The lack of specific serial measurements related to CMV pathogenicity (e.g., IL-1Beta, IL-6, TNF-alfa) and the difficulties in diagnosing CMV pneumonia in patients with COVID-19 severe interstitial pneumonia limit any further consideration on the potential effects of CMV reactivation in these patients.
Beyond the limitations reported above, our study has other limitations. First, the study included patients admitted to 3 ICUs by the same hospital with potential limitations in generalization to other settings. Second, as in many other observational studies [33], we included all the ICU admitted patients and not only the CMV-seropositive population because seropositivity was not systematically evaluated. Third, the clinical protocol did not include systematic analysis of the respiratory sample for CMV reactivation, which was deserved only to patients with high suspicion of CMV pneumonia. This can underestimate the actual number of CMV reactivation. However, due to the uncertain significance of CMV detection in the respiratory tract, the CMV viremia is commonly used in high-quality interventional trials [18]. Last, our data did not indicate an association between CMV reactivation and the use of immune-suppressive therapies. However, the large number of patients treated with steroids (91%) and tocilizumab (84%) may have limited the sensitivity of our analysis.
Conclusions
In COVID-19 critically ill patients, the CMV blood reactivation is frequent and depends on the severity of illness and the occurrence of secondary bacterial infections but not on steroids and cytokine blocking agents. The patients with CMV reactivation showed prolonged hospital stay and higher mortality than patients without reactivation. Nevertheless, the lack of independent association between CMV reactivation and mortality leaves open the question of its role and the appropriate strategy for its monitoring and management.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The members of Modena COVID-19 Working Group (MoCo19) are: Massimo Girardis, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti, Lucia Serio, Annamaria Ghirardini, Marco Sita, Stefano De Julis, Lara Donno, Lorenzo Dall'Ara, Fabrizio Di Salvo, Carlotta Farinelli, Laura Rinaldi, Ilaria Cavazzuti, Andrea Ghidoni, Antonio Buono, Elena Ferrari, Daniela Iseppi, Anna Maria Ardito, Irene Coloretti, Sophie Venturelli, Elena Munari, Martina Tosi, Erika Roat, Ilenia Gatto, Marco Sarti, Andrea Cossarizza, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, Milena Nasi, Annamaria Paolini, Marcello Pinti, Cristina Mussini, Giovanni Guaraldi, Marianna Meschiari, Alessandro Cozzi-Lepri, Jovana Milic, Marianna Menozzi, Erica Franceschini, Gianluca Cuomo, Gabriella Orlando, Vanni Borghi, Antonella Santoro, Margherita Di Gaetano, Cinzia Puzzolante, Federica Carli, Andrea Bedini, Luca Corradi, Enrico Clini, Roberto Tonelli, Riccardo Fantini, Ivana Castaniere, Luca Tabbì, Giulia Bruzzi, Chiara Nani, Fabiana Trentacosti, Pierluigi Donatelli, Maria Rosaria Pellegrino, Linda Manicardi, Antonio Moretti, Morgana Vermi, Caterina Cerbone, Monica Pecorari, William Gennari, Antonella Grottola, Giulia Fregni Serpini.
Author contributions
GI, BE, FE, MM, GM: study conception, design, data interpretation, draft, final approval of submitted version. CI, FC, AC, CVSM, PM, GW, TR: data acquistion. Analysis and interpretation. BS, GG, MC, CE, CA: critical revision of the draft.
Declarations
Conflicts of interest
All the authors declare non-financial interests directly or indirectly related to the work submitted for publication.
Footnotes
The members of the Modena Covid-19 Working Group (MoCo19) are listed in the Acknowledgments section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Massimo Girardis, Email: girardis.massimo@unimo.it.
the Modena COVID-19 Working Group:
Massimo Girardis, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti, Lucia Serio, Annamaria Ghirardini, Marco Sita, Stefano De Julis, Lara Donno, Lorenzo Dall’Ara, Fabrizio Di Salvo, Carlotta Farinelli, Laura Rinaldi, Ilaria Cavazzuti, Andrea Ghidoni, Antonio Buono, Elena Ferrari, Daniela Iseppi, Anna Maria Ardito, Irene Coloretti, Sophie Venturelli, Elena Munari, Martina Tosi, Erika Roat, Ilenia Gatto, Marco Sarti, Andrea Cossarizza, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, Milena Nasi, Annamaria Paolini, Marcello Pinti, Cristina Mussini, Giovanni Guaraldi, Marianna Meschiari, Alessandro Cozzi-Lepri, Jovana Milic, Marianna Menozzi, Erica Franceschini, Gianluca Cuomo, Gabriella Orlando, Vanni Borghi, Antonella Santoro, Margherita Di Gaetano, Cinzia Puzzolante, Federica Carli, Andrea Bedini, Luca Corradi, Enrico Clini, Roberto Tonelli, Riccardo Fantini, Ivana Castaniere, Luca Tabbì, Giulia Bruzzi, Chiara Nani, Fabiana Trentacosti, Pierluigi Donatelli, Maria Rosaria Pellegrino, Linda Manicardi, Antonio Moretti, Morgana Vermi, Caterina Cerbone, Monica Pecorari, William Gennari, Antonella Grottola, and Giulia Fregni Serpini
References
- 1.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imlay H, Dasgupta S, Boeckh M, et al. Risk factors for cytomegalovirus reactivation and association with outcomes in critically ill adults with sepsis: a pooled analysis of prospective studies. J Infect Dis. 2021;223:2108–2112. doi: 10.1093/infdis/jiaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hraiech S, Bonnardel E, Guervilly C, et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care. 2019;9:142. doi: 10.1186/s13613-019-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libert N, Bigaillon C, Chargari C, et al. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed J. 2015;38:70–76. doi: 10.4103/2319-4170.132905. [DOI] [PubMed] [Google Scholar]
- 5.Imlay H, Limaye AP. Current understanding of cytomegalovirus reactivation in critical illness. J Infect Dis. 2020;221:S94–S102. doi: 10.1093/infdis/jiz638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Balc'h P, Pinceaux K, Pronier C, et al. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care. 2020;24:530. doi: 10.1186/s13054-020-03252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moniz P, Brito S, Póvoa P. SARS-CoV-2 and cytomegalovirus co-infections-a case series of critically ill patients. J Clin Med. 2021;10:2792. doi: 10.3390/jcm10132792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonnet A, Engelmann I, Moreau A-S, et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51:296–299. doi: 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschini E, Cozzi-Lepri A, Santoro A, et al. Herpes simplex virus re-activation in patients with SARS-CoV-2 pneumonia: a prospective, observational study. Microorganisms. 2021;9:1896. doi: 10.3390/microorganisms9091896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer A, Buetti N, Houhou-Fidouh N, et al. HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. Crit Care. 2021;25:417. doi: 10.1186/s13054-021-03843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Omari A, Aljamaan F, Alhazzani W, et al. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann Intensive Care. 2016;6:110. doi: 10.1186/s13613-016-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildermans J, De Vlieger G. Cytomegalovirus: a troll in the ICU? Overview of the literature and perspectives for the future. Front Med (Lausanne) 2020;7:188. doi: 10.3389/fmed.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papazian L, Hraiech S, Lehingue S, et al. Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016;42:28–37. doi: 10.1007/s00134-015-4066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiche L, Forel J-M, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37:1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 15.Barry SM, Johnson MA, Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transpl. 2000;26:591–597. doi: 10.1038/sj.bmt.1702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papazian L, Thomas P, Bregeon F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998;88:935–944. doi: 10.1097/00000542-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Söderberg-Nauclér C. Does reactivation of cytomegalovirus contribute to severe COVID-19 disease? Immun Ageing. 2021;18:12. doi: 10.1186/s12979-021-00218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papazian L, Jaber S, Hraiech S, et al. Preemptive ganciclovir for mechanically ventilated patients with cytomegalovirus reactivation. Ann Intensive Care. 2021;11:33. doi: 10.1186/s13613-020-00793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley NJ, Owen A, Shiels SC, et al. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial. JAMA Intern Med. 2017;177:774–783. doi: 10.1001/jamainternmed.2017.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limaye AP, Stapleton RD, Peng L, et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA. 2017;318:731–740. doi: 10.1001/jama.2017.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . COVID-19 clinical management: living guidance. Geneva: World Health Organization; 2021. [Google Scholar]
- 23.Mussini C, Falcone M, Nozza S, et al. Therapeutic strategies for severe COVID-19: a position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT) Clin Microbiol Infect. 2021;27:389–395. doi: 10.1016/j.cmi.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 25.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 26.Manian FA. IDSA guidelines for the diagnosis and management of intravascular catheter-related bloodstream infection. Clin Infect Dis. 2009;49:1770–1771. doi: 10.1086/648113. [DOI] [PubMed] [Google Scholar]
- 27.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary Aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fryer JF, Heath AB, Anderson R, et al. Collaborative study to evaluate the proposed 1st [first] WHO international standard for human cytomegalovirus (HCMV) for nucleic acid amplification (NAT)-based assays. Geneva: World Health Organization; 2010. [Google Scholar]
- 29.Saade A, Moratelli G, Azoulay E, Darmon M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect Dis Now. 2021;51:676–679. doi: 10.1016/j.idnow.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busani S, Bedini A, Biagioni E, et al. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies. Clin Infect Dis. 2021;73:e252–e255. doi: 10.1093/cid/ciaa1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini SF, Behnam-Roudsari S, Alavinia G, et al. Diagnostic and prognostic value of Sepsis-Induced coagulopathy and International Society on Thrombosis and Hemostasis scoring systems in COVID-19-associated disseminated intravascular coagulopathy. J Res Med Sci. 2021;26:102. doi: 10.4103/jrms.JRMS_1295_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachance P, Chen J, Featherstone R, Sligl WI. Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:029. doi: 10.1093/ofid/ofx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loftus TJ, Ungaro R, Dirain M, et al. Overlapping but disparate inflammatory and immunosuppressive responses to SARS-CoV-2 and bacterial sepsis: an immunological time course analysis. Front Immunol. 2021;12:792448. doi: 10.3389/fimmu.2021.792448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaber S, Chanques G, Borry J, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127:233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 36.Rubio I, Osuchowski MF, Shankar-Hari M, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:e422–e436. doi: 10.1016/S1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 37.Venet F, Davin F, Guignant C, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 38.Clari MA, Aguilar G, Benet I, et al. Evaluation of cytomegalovirus (CMV)-specific T-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. J Med Virol. 2013;85:1802–1810. doi: 10.1002/jmv.23621. [DOI] [PubMed] [Google Scholar]
- 39.Kuo C-W, Wang S-Y, Tsai H-P, et al. Invasive pulmonary aspergillosis is associated with cytomegalovirus viremia in critically ill patients: a retrospective cohort study. J Microbiol Immunol Infect. 2021;S1684–1182(21):00057–58. doi: 10.1016/j.jmii.2021.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.