Abstract
Background
Postmastectomy breast reconstruction is an essential element of multidisciplinary breast cancer care but may be underutilized.
Methods
This retrospective study analyzed mastectomy patients (2018–2021) at an urban hospital. Multivariable logistic regression was performed, and a mixed-effects logistic regression model was constructed to determine patient-level factors (age, race, body mass index, comorbidities, smoking status, insurance, type of surgery) and provider-level factors (breast surgeon gender, participation in multidisciplinary breast clinic) that influence reconstruction.
Results
Overall, 167 patients underwent mastectomy. The reconstruction rate was 35%. In multivariable analysis, increasing age (odds ratio [OR] 0.95; 95% confidence interval [CI] 0.91–0.99) and Medicaid insurance (OR 0.18; 95% CI 0.06–0.53) relative to private insurance were negative predictors, whereas bilateral mastectomy was a positive predictor (OR 7.07; 95% CI 2.95–17.9) of reconstruction. After adjustment for patent age, race, insurance, and type of surgery, female breast surgeons had 3.7 times greater odds of operating on patients who had reconstruction than males (95% CI 1.20–11.42).
Conclusion
Both patient- and provider-level factors have an impact on postmastectomy reconstruction. Female breast surgeons had nearly four times the odds of caring for patients who underwent reconstruction, suggesting that a more standardized process for plastic surgery referral is needed.
Postmastectomy breast reconstruction, an important element of multidisciplinary breast cancer care, improves psychosocial outcomes for mastectomy patients.1 In recent decades, the United States has experienced an increase in postmastectomy reconstruction rates, largely motivated by federal legislation mandating insurance coverage for patients undergoing mastectomy who opt for reconstruction.2–4 More recently, in 2010, New York State passed the Breast Cancer Provider Discussion Law, mandating discussion of insurance coverage for reconstruction and expedient plastic surgery referral.5
Despite these measures, postmastectomy reconstruction rates remain variable, and postmastectomy reconstruction may be underutilized among certain patient populations.6 Numerous studies have sought to identify patient-, hospital-, and system-level factors that predict which patients will undergo reconstruction. Patient factors found to influence reconstruction include race, age, insurance, hospital type, type of surgery, geographic location, body mass index, and number of comorbidities.7–19
In this study, we sought to evaluate trends in postmastectomy breast reconstruction at an urban hospital with a newly established multidisciplinary breast program (MDC) and to identify patient- and provider-level factors that may predict which patients undergo reconstruction.
Materials and Methods
Patient Cohort
Patients undergoing uni- or bilateral mastectomy between January 2018 and December 2021 at New York-Presbyterian Brooklyn Methodist Hospital (NYPBMH) were identified from the institutional tumor registry. As an academic hospital in Brooklyn, New York, NYPBMH serves a large proportion of minority and Medicaid patients.
In January of 2020, NYPBMH launched its MDC program with the goal of providing more comprehensive breast cancer care. The program, located at our main hospital, consisted of a multidisciplinary breast cancer clinic allowing patients to be seen by surgical oncology, medical oncology, radiation oncology, and a genetic counselor within the same day. Additionally, the program involved implementation of a standardized discussion of each case, which included whether plastic surgery referral was to be made.
This retrospective analysis was approved by the institutional review board. For this type of study, no formal consent was required. This dataset and its analysis were performed according to the ethical standards of the institutional research committee and the Helsinki declaration.
Electronic medical records were reviewed to gather the following variables: age, self-reported race, body mass index (BMI), smoking status (ever or current smoker), comorbidities for determination of the Charlson Comorbidity Index (CCI),20 insurance status (Medicaid, Medicare, or private), type of surgery (uni- or bilateral mastectomy), male or female breast surgeon, type of reconstruction (implant, autologous, none), and whether the patient’s breast surgeon participated in the MDC clinic.
Statistical Analysis
Descriptive statistics were used to characterize the study sample with respect to demographic and clinical factors of interest. Continuous variables are represented as median (interquartile range) and categorical variables as n (%). Fisher’s exact test and the Wilcoxon rank-sum test were used to examine the association between demographic/clinical factors of interest and reconstruction (yes or no). Uni- and multivariable logistic regression analyses were performed to evaluate patient-level factors predictive of reconstruction. Multicollinearity was assessed using the variance inflation factor (VIF) and adjusted VIF and determined not to be an issue.
To determine whether surgeon-level factors were associated with reconstruction, a mixed-effects logistic regression model was constructed, in which the breast surgeon ID was entered as the random effect. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from the multivariable models.
All p values were two-sided, with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in R Version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the Study Population
From January 2018 to December 2021, 167 patients underwent uni- or bilateral mastectomy at NYPBMH and were analyzed. The median age was 62 years, and half of patients (50%) self-identified as black. The median BMI was 29 kg/m2, and the median CCI was 2. Most of the patients were insured by Medicare (41%). Of the 167 patients, 22% were former or current smokers. More than half of the patients (57%) had a male surgeon, and 34% had a breast surgeon that participated in MDC. The overall reconstruction rate was 35%, and most of the reconstructions were implant-based (Table 1).
Table 1.
Characteristics of the study population (n = 167)
| Characteristic |
n = 167 n (%) |
|---|---|
| Age at diagnosis (years) | |
| Median (IQR) | 62 (51–70) |
| Mean ± SD | 61 ± 13 |
| Range | 30–91 |
| Race | |
| White | 37 (22) |
| Asian | 21 (13) |
| Black | 84 (50) |
| Hispanic | 14 (8.4) |
| Other | 11 (6.6) |
| Insurance | |
| Private | 55 (33) |
| Medicaid | 44 (26) |
| Medicare | 68 (41) |
| BMI (kg/m2) | |
| Median (IQR) | 29 (25–33) |
| Mean ± SD | 30 ± 7 |
| Range | 15–52 |
| Charlson Comorbidity Index | |
| Median (IQR) | 2.00 (1.00–3.00) |
| Mean ± SD | 2.09 ± 1.64 |
| Range | 0.00–7.00 |
| Ever/former smoker | 37 (22) |
| MDC surgeon | 57 (34) |
| Surgeon gender | |
| Male | 96 (57) |
| Female | 71 (43) |
| Type of surgery | |
| Unilateral mastectomy | 119 (71) |
| Bilateral mastectomy | 48 (29) |
| Type of reconstruction | |
| Autologous | 5 (3) |
| Implant | 53 (32) |
| None | 190 (65) |
| Any reconstruction | 58 (35) |
IQR, interquartile range; SD, standard deviation; BMI, body mass index; MDC, multidisciplinary breast clinic
Characteristics of Patients Undergoing Postmastectomy Breast Reconstruction
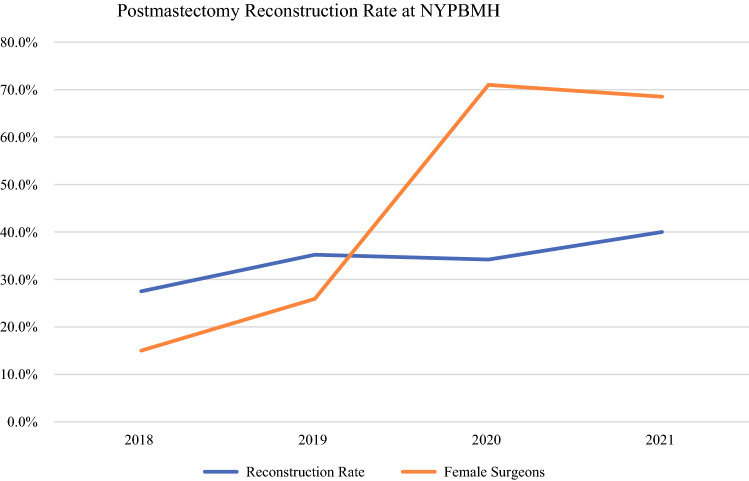
A gradual increase in reconstruction rate was observed during the study period, from 27.5% at the beginning in 2018 to 40% in 2021. This was accompanied by an increase in the proportion of patients who had surgery performed by a female breast surgeon (Fig. 1).
Fig. 1.
Postmastectomy breast reconstruction and female surgeon volume at New York Presbyterian–Brooklyn Methodist Hospital over time (2018–2021).
The patients who underwent reconstruction differed from those who did not with respect to nearly every variable. The patients undergoing reconstruction were younger (median age, 56 vs 66 years; p < 0.001) and more likely to be privately insured (p < 0.001), with a slightly higher BMI (median BMI, 30 vs 28 kg/m2; p = 0.027) and a lower CCI (median C CI 1 vs 2; p < 0.001). Slightly more than half of the patients (57%) who underwent reconstruction had a female breast surgeon compared with only 35% of those who did not undergo reconstruction (p = 0.008). Of the patients undergoing reconstruction, 57% had a bilateral mastectomy compared with only 14% of the patients who did not have reconstruction (p < 0.001). More than 90% of the reconstructions were implant-based (Table 2).
Table 2.
Characteristics of patients stratified by whether postmastectomy breast reconstruction was performed
| Characteristic | Reconstruction (n = 58) n (%) |
No reconstruction (n = 109) n (%) |
p Valuea |
|---|---|---|---|
| Age at diagnosis (years) | |||
| Median (IQR) | 56 (44–63) | 66 (53–73) | <0.001 |
| Race | |||
| White | 12 (21) | 25 (33) | 0.056 |
| Asian | 2 (3.4) | 19 (17) | |
| Black | 34 (59) | 50 (46) | |
| Hispanic | 7 (12) | 7 (6.4) | |
| Other | 3 (5.2) | 8 (7.3) | |
| Insurance | |||
| Private | 34 (59) | 21 (19) | <0.001 |
| Medicaid | 9 (16) | 35 (32) | |
| Medicare | 15 (26) | 53 (49) | |
| BMI | |||
| Median (IQR) | 30 (26–35) | 28 (24–32) | 0.027 |
| Charlson Comorbidity Index | |||
| Median (IQR) | 1.00 (0.00–2.00) | 2.00 (1.00–4.00) | <0.001 |
| Ever/former smoker | 18 (31) | 19 (17) | 0.052 |
| MDC surgeon | 23 (40) | 34 (31) | 0.31 |
| Surgeon gender | |||
| Male | 25 (43) | 71 (65) | 0.008 |
| Female | 33 (57) | 38 (35) | |
| Type of surgery | |||
| Unilateral mastectomy | 25 (43) | 94 (86) | <0.001 |
| Bilateral mastectomy | 33 (57) | 15 (14) | |
| Reconstruction type | |||
| Autologous | 5 (8.6) | 0 (0) | <0.001 |
| Implant | 53 (91) | 0 (0) | |
| None | 0 (0) | 109 (100) |
IQR, interquartile range; BMI, body mass index; MDC, multidisciplinary breast clinic
aFisher’s exact test, Wilcoxon rank-sum test
Patient Factors Predictive of Postmastectomy Breast Reconstruction
In the univariate analysis, the negative predictors of reconstruction were increasing patient age (OR 0.94; 95% CI 0.91–0.96), increasing CCI (OR 0.60; 95% CI 0.46–0.75), no smoking history (OR 0.47; 95% CI 0.22–0.99), and Medicaid (OR 0.16; 95% CI 0.006–0.38) or Medicare insurance (vs private insurance) (OR 0.17; 95% CI 0.0–0.38). Type of surgery also was significantly associated with reconstruction. The patients undergoing bilateral mastectomy had 8.27 times greater odds of undergoing reconstruction than the patients undergoing unilateral mastectomy (95% CI 3.97–18.0). In the multivariable analysis, the patient-level factors that retained significance were age (OR 0.95; 95% CI 0.91–0.99), Medicaid insurance (OR 0.18; 95% CI 0.06–0.53), and bilateral mastectomy (OR 7.07; 95% CI 2.95–17.9) (Table 3).
Table 3.
Uni- and multivariate logistic regression analyses of patient factors associated with reconstruction
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age at diagnosis (years) | 0.94 | 0.91–0.96 | <0.001 | 0.95 | 0.91–0.99 | 0.031 |
| Race | ||||||
| White | 1.0 (Ref) | – | – | 1.0 (Ref) | – | – |
| Asian | 0.22 | 0.03–0.93 | 0.065 | 0.29 | 0.03–1.67 | 0.19 |
| Black | 1.42 | 0.64–3.63 | 0.40 | 1.57 | 0.54–4.86 | 0.42 |
| Hispanic | 2.08 | 0.59–7.47 | 0.25 | 2.10 | 0.41–10.7 | 0.37 |
| Other | 0.78 | 0.15–3.27 | 0.75 | 1.23 | 0.18–7.35 | 0.83 |
| BMI (kg/m2) | 1.05 | 1.00–1.10 | 0.054 | 1.04 | 0.98–1.10 | 0.22 |
| Charlson Comorbidity Index | 0.60 | 0.46–0.75 | <0.001 | |||
| Ever/former smoker | ||||||
| Yes | 1.0 (Ref) | – | – | |||
| No | 0.47 | 0.22–0.99 | 0.046 | |||
| Insurance | ||||||
| Private | 1.0 (Ref) | – | – | 1.0 (Ref) | – | – |
| Medicaid | 0.16 | 0.006–0.38 | <0.001 | 0.18 | 0.06–0.53 | 0.003 |
| Medicare | 0.17 | 0.08–0.38 | <0.001 | 0.33 | 0.11–1.01 | 0.053 |
| Type of mastectomy | ||||||
| Unilateral | 1.0 (Ref) | – | – | 1.0 (Ref) | – | – |
| Bilateral | 8.27 | 3.97–18.0 | <0.001 | 7.07 | 2.95–17.9 | <0.001 |
OR, odds ratio; CI, confidence interval; BMI, body mass index
Provider Factors Predictive of Postmastectomy Breast Reconstruction
In the mixed-effects logistic regression model, age, race, insurance, surgeon gender, and type of surgery were added as fixed effects, and surgeon ID was added as the random effect. Female breast surgeons had 3.7 times greater odds of treating patients who had reconstruction than male breast surgeons, after adjustment for patient's age, race, insurance, and type of surgery (95% CI 1.20–11.42; Table 4). A surgeon-level mixed model also was conducted, and surgeon participation in MDC status was not significant after adjustment for surgeon gender (OR 0.62; 95% CI 0.18–2.19) and thus was not included in the final model.
Table 4.
Mixed-effects model of patient and provider factors associated with postmastectomy reconstruction
| OR | 95% CI | p value | |
|---|---|---|---|
| Age at diagnosis (years) | 0.94 | 0.90–0.99 | 0.017 |
| Race | |||
| White | 1.0 (Ref) | – | – |
| Asian | 0.12 | 0.02–0.93 | 0.043 |
| Black | 1.65 | 0.51–5.40 | 0.405 |
| Hispanic | 1.62 | 0.27–9.64 | 0.597 |
| Other | 1.72 | 0.27–10.94 | 0.564 |
| Insurance | |||
| Private | 1.0 (Ref) | – | – |
| Medicaid | 0.13 | 0.04–0.44 | 0.001 |
| Medicare | 0.40 | 0.12–1.33 | 0.136 |
| Surgeon gender | |||
| Male | 1.0 (Ref) | – | – |
| Female | 3.70 | 1.20–11.42 | 0.023 |
| Type of mastectomy | |||
| Unilateral | 1.0 (Ref) | – | – |
| Bilateral | 5.41 | 2.11–13.88 | <0.001 |
OR, odds ratio; CI, confidence interval
Discussion
In this single-institution study to investigate patterns of postmastectomy breast reconstruction, we demonstrated an increase in our reconstruction rate over time, from 27.5 to 40%. Patient-level factors including age, insurance status, and type of surgery were important predictors of reconstruction. Notably, a significant disparity was noted in the likelihood of reconstruction based on breast surgeon gender, with female breast surgeons having nearly four times greater odds of operating on patients who had reconstruction than male breast surgeons.
The impact of patient age and race on the likelihood of reconstruction has been well studied.3,8–12,18,21 Retrospective series have consistently shown that older patients and minorities are less likely to undergo reconstruction than younger white patients.2,11,13,14,16,17,21,22 For example, a study by Alderman et al.13 of more than 3000 patients from the Surveillance, Epidemiology, and End Results (SEER) database found significant variability in the rates of reconstruction when patients were stratified by race. The reconstruction rate was 40.9% for whites versus 33.5% for African Americans.
In another analysis of mastectomy patients in Pennsylvania, Yang et al.15 found that even after federal and state policy changes designed to improve access to reconstruction, and after adjustment for potential confounders, reconstruction was less likely to be performed for black patients (OR 0.66; 95% CI 0.55–0.80), Asian patients (OR 0.30; 95% CI 0.18–0.49), and patients from mixed or other races (OR 0.29; 95% CI 0.16–0.51). As in previous studies, we found that increasing age was a negative predictor of patients who would undergo reconstruction.
Although we did not find race to be a significant predictor in our multi- or univariate models, we did observe a borderline significant difference in the racial distribution between the patients undergoing reconstruction and those not undergoing reconstruction. Our data demonstrated a greater proportion of black patients having reconstruction relative to white patients. However, it should be noted that our population comprised a large proportion of racial/ethnic minorities, including 50% black patients, in contrast to prior studies that analyzed predominantly white populations.
We also found that Medicaid insurance was a negative predictor of patients who would undergo reconstruction, as in other studies.11,12,19,22 The Women’s Health and Cancer Rights Act of 1998 was designed to provide protections for patients who choose breast reconstruction after mastectomy. The law states that coverage must be provided for all stages of reconstruction, including reconstruction of the other breast to produce a symmetric appearance, as well as coverage for prostheses and treatment of complications such as lymphedema. However, the law applies only to group health plans and individual health insurance policies. Medicaid coverage is less certain, leaving this as an area that remains in need of further study.4
Differences were observed in CCI and smoking status between the patients who underwent reconstruction and those who did not. The higher median CCI among the patients not undergoing reconstruction may be reflective of surgeon or patient hesitancy to attempt additional surgery in the presence of multiple comorbidities. A borderline significant difference was observed regarding smoking status, with a smoking history reported by a greater proportion of patients undergoing reconstruction (31% vs 17%; p = 0.052). Although findings have shown smoking be an independent predictor of postoperative complications in breast reconstruction, we did not delineate current versus former smoker.23 We suspect that this finding may have been because a large subset of our patients listed as smokers were former smokers and not current smokers, leading to less hesitancy by surgeons to proceed with reconstruction.
Overall, 34% of our patients were seen by a breast surgeon who participated in the new MDC. The variety in surgeon participation in the recently established clinic is largely due to the various locations at which providers see patients and the centralized location of the clinic at the main hospital campus. Surprisingly, in our study we found that the breast surgeon participation in the MDC clinic did not differ significantly between the patients who underwent reconstruction and those who did not.
In contrast, previous studies have shown that a dedicated oncoplastic multidisciplinary meeting may lead to increased reconstruction rates. For example, one retrospective analysis of 229 mastectomy patients from 2014 to 2016 found an increase in the reconstruction rate, from 28 beforehand to 42% afterward.24 It should be noted that a dedicated plastic surgeon did not actively participate in the weekly MDC clinic, which may explain this unexpected finding.
Most striking was the finding that female surgeons were more than three times more likely to have patients undergoing reconstruction than male breast surgeons. Although a large body of data exists identifying patient-level factors associated with reconstruction, few have reported on the impact of surgeon gender. One survey-based study examined factors associated with surgeons’ propensity to refer breast cancer mastectomy patients to plastic surgeons. Among 456 general surgeons, only 24% referred more than 75% of their patients to plastic surgery. However, this high referral propensity was independently associated with women surgeons (OR 2.3; p = 0.03).25
Another survey of surgeons in Wisconsin found that 40% of breast surgeons did not refer all mastectomy patients for reconstruction due to concern about cancer recurrence and advanced age.26 Similar to our findings, in 2015, Iskandar et al.7 reported factors influencing both the incidence and type of breast reconstruction at an urban multidisciplinary cancer center. In their analysis of 258 mastectomy patients, they found that patients who had a female breast surgeon were more than five times more likely to undergo reconstruction (OR 5.17; 95% CI 3.01–8.89; p = 0.001). Additionally, they examined the impact of having a breast surgeon who participated in the institution’s multidisciplinary cancer center and found that this was not significantly associated with the likelihood of reconstruction.
We believe the significant disparity in reconstruction rates observed for patients of female versus male surgeons warrants further study to design targeted interventions both to empower patients and to educate providers and reduce provider-level barriers. Furthermore, these findings warrant implementation of a standardized process for plastic surgery referral. Finally, incorporating on-site plastic surgeons into the MDC clinic may improve referral and access of patients to both implant-based and autologous reconstruction options.
Study limitations
Our study had limitations inherent to the size and retrospective nature of our analysis and its generalizability outside our institution. We were not able to ascertain status of referral to a plastic surgeon. We could only determine whether patients underwent reconstruction. Although surgeon gender was found to be an important predictor despite no influence of surgeon participation in the MDC clinic, we recognize that our data did not account for variability in fellowship training, which may have influenced the results. For example, general surgeons performing breast surgery may be less likely to offer reconstruction than surgeons with dedicated breast surgical oncology fellowship training. However, we did perform a mixed-effects model in which the surgeon was considered the random effect, thereby accounting for individual practice patterns and training.
To evaluate the theoretical concern that patients of male versus female breast surgeons may be inherently different, we compared patient characteristics and found that the magnitude of the difference in age and comorbidities was unlikely to have an impact on the eligibility of patients to undergo reconstruction. However, with respect to insurance, we found that male providers saw significantly fewer Medicaid patients despite findings showing Medicaid to be a negative predictor of reconstruction in the multivariate analysis (17% males vs 39% females). In practice, patient reasons for choosing one breast surgeon over another are complex and may be linked to the referral process, clinic appointment availability, and individual patient preferences that cannot be controlled, which was a limitation of this study.
Given the extension of our study period through December 2021, we recognize that we may not have captured patients undergoing delayed reconstruction that may have occurred later. This may have led to an underestimation of the true reconstruction rate. The study period also overlapped with the height of the COVID-19 pandemic in the Northeast, which had an impact on the availability of reconstruction for patients undergoing oncologic surgery.
Although we found a significant disparity in the reconstruction patterns of male and female breast surgeons, we did not gather data on surgeon age or academic position within the institution (e.g., fellowship-trained or full-time status). We also did not capture complication rates or long-term outcomes of reconstruction. Finally, we could not determine the reasons why patients who may have been offered plastic surgery referral chose to decline it.
Conclusions
Our data demonstrated an increase in the rate of postmastectomy breast reconstruction at our institution during recent years and identified important patient- and provider-level factors influencing whether reconstruction was performed. Importantly, a significant difference was noted regarding breast surgeon gender, with female breast surgeons showing nearly four times greater odds of having patients who underwent reconstruction. No significant differences were noted between groups regarding surgeon participation in the MDC clinic.
These findings are consistent with those of previous studies and add to the growing body of literature that serves to identify and eliminate disparities in postmastectomy breast reconstruction. This study also may help inform the development of strategies to provide more uniform plastic surgery referral for patients undergoing mastectomy as an essential element of multidisciplinary breast cancer care.
Acknowledgement
This work was supported by the multidisciplinary breast program at New York Presbyterian-Brooklyn Methodist Hospital and the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. Dr. Lisa Newman receives funding from the Susan G. Komen and Fashion Footwear Association of New York Charitable Foundation. Dr. Vivian Bea receives funding from the American Cancer Society and Pfizer.
Disclosures
There are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilkins EG, Cederna PS, Lowery JC, Davis JA, Kim HM, Roth RS, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106(5):1014–1025. doi: 10.1097/00006534-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Pappas L, Neumayer L, Agarwal J. An analysis of immediate postmastectomy breast reconstruction frequency using the Surveillance, Epidemiology, and End Results database. Breast J. 2011;17(4):352–358. doi: 10.1111/j.1524-4741.2011.01105.x. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher JR, Taylor LJ, Tucholka JL, Poore S, Eggen A, Steiman J, et al. Socioeconomic factors associated with post-mastectomy immediate reconstruction in a contemporary cohort of breast cancer survivors. Ann Surg Oncol. 2017;24(10):3017–3023. doi: 10.1245/s10434-017-5933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services. 2021. https://www.cms.gov/CCIIO/Programs-and-Initiatives/Other-Insurance-Protections/whcra_factsheet. Accessed 15 Dec 2021.
- 5.The New York State Senate. 2010. https://www.nysenate.gov/legistlation/bills/2009/S6993. Accessed 24 Nov 2021.
- 6.Ballard TNS, Zhong L, Momoh AO, Chung KC, Waljee JF. Improved rates of immediate breast reconstruction at safety net hospitals. Plast Reconstr Surg. 2017;140(1):1–10. doi: 10.1097/PRS.0000000000003412. [DOI] [PubMed] [Google Scholar]
- 7.Iskandar ME, Dayan E, Lucido D, Samson W, Sultan M, Dayan JH, et al. Factors influencing incidence and type of postmastectomy breast reconstruction in an urban multidisciplinary cancer center. Plast Reconstr Surg. 2015;135(2):270e–276e. doi: 10.1097/PRS.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Wang M, Chapgar A. Factors associated with reconstruction in patients undergoing mastectomy. Am Surg. 2020;86:134–139. doi: 10.1177/000313482008600233. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M, Scott SK, Menck HR, Mustoe TA, Winchester DP. Factors influencing the use of breast reconstruction postmastectomy: a National Cancer Database study. J Am Coll Surg. 2001;192(1):1–8. doi: 10.1016/s1072-7515(00)00747-x. [DOI] [PubMed] [Google Scholar]
- 10.Wolfswinkel EM, Lopez SN, Weathers WM, Qashqai S, Wang T, Hilsenbeck SG, et al. Predictors of post-mastectomy reconstruction in an underserved population. J Plast Reconstr Aesthet Surg. 2013;66(6):763–769. doi: 10.1016/j.bjps.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Kruper L, Holt A, Xu X, Duan L, Henderson K, Bernstein L, et al. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Ann Surg Oncol. 2011;18(8):2158–2165. doi: 10.1245/s10434-011-1580-z. [DOI] [PubMed] [Google Scholar]
- 12.Sisco M, Du H, Warner JP, Howard MA, Winchester DP, Yao K. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. J Am Coll Surg. 2012;215(5):658–666. doi: 10.1016/j.jamcollsurg.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Alderman AK, Hawley ST, Janz NK, Mujahid MS, Morrow M, Hamilton AS, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayyar A, Strassle PD, Reddy KG, Jameison DI, Moses CG, Roughton MC, et al. Variations in the utilization of immediate post-mastectomy breast reconstruction. Am J Surg. 2019;218(4):712–715. doi: 10.1016/j.amjsurg.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Yang RL, Newman AS, Reinke CE, Lin IC, Karakousis GC, Lin IC, Karakousis GC, Czerniecki BJ, et al. Racial disparities in immediate breast reconstruction after mastectomy: impact of state and federal health policy changes. Ann Surg Oncol. 2013;30(2):399–406. doi: 10.1245/s10434-012-2607-9. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Richards CA, Kalinsky K, Wilde ET, Lu YS, Ascherman JA, et al. Influence of health insurance, hospital factors, and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat. 2012;136(2):535–545. doi: 10.1007/s10549-012-2273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortina CS, Bergom CR, Kijack J, Thorgerson AA, Spencer Huang C, Kong AL. Postmastectomy breast reconstruction in women aged 70 and older: an analysis of the National Cancer Database (NCDB) Surgery. 2021;170(1):30–38. doi: 10.1016/j.surg.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karunanayake M, Bortoluzzi P, Chollet A, Lin JC. Factors influencing the rate of post-mastectomy breast reconstruction in a Canadian Teaching Hospital. Plast Surg Oakv. 2017;25(4):242–248. doi: 10.1177/2292550317728034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoudi E, Giladi AM, Wu L, Chung KC. Effect of federal and state policy changes on racial/ethnic variation in immediate postmastectomy breast reconstruction. Plast Reconstr Surg. 2015;135(5):1285–1294. doi: 10.1097/PRS.0000000000001149. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Kruper L, Xu X, Henderson K, Bernstein L. Disparities in reconstruction rates after mastectomy for ductal carcinoma in situ (DCIS): patterns of care and factors associated with the use of breast reconstruction for DCIS compared with invasive cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-2010-y. [DOI] [PubMed] [Google Scholar]
- 22.Restrepo DJ, Boczar D, Huayllani MT, et al. Influence of race, income, insurance, and education on the rate of breast reconstruction. Anticancer Res. 2019 doi: 10.21873/anticanres.13428. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin SJ, McCarthy CM, Pusic AL, Bui D, Howard M, Disa JJ, et al. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg. 2005;55(1):16–19. doi: 10.1097/01.sap.0000168282.81348.b3. [DOI] [PubMed] [Google Scholar]
- 24.El Gammal MM, Lim M, Uppal R, Sainsbury R. Improved immediate breast reconstruction as a result of oncoplastic multidisciplinary meeting. Breast Cancer (Dove Med Press). 2017;9:293–296. doi: 10.2147/BCTT.S133800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alderman AK, Hawley ST, Waljee J, Morrow M, Katz SJ. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109(9):1715–1720. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]
- 26.Stacey DH, Spring MA, Breslin TM, Rao VK, Gutowski KA. Exploring the effect of the referring general surgeon's attitudes on breast reconstruction utilization. WMJ. 2008;107:292–297. [PubMed] [Google Scholar]