Abstract
PURPOSE:
To determine the incidence of cosmetic-related lacrimal sac black deposits (LSBDs) in primary-acquired nasolacrimal duct obstruction (PANDO) biopsies and the role of LSBD in the pathogenesis of PANDO, in addition to their association with dry eye disease (DED).
METHODS:
A clinicopathological study included all patients who underwent surgical management of PANDO. We excluded patients in whom lacrimal sac biopsy was not taken during the surgery. Lacrimal sac tissues were evaluated for the presence of LSBD and related inflammation, with correlation to the demographics, clinical presentation, and pre-operative clinical assessment of dry eye. P <0.05 was considered statistically significant.
RESULTS:
Of the 177 PANDO specimens, black deposit aggregates were noted in the sac stroma of 61 lacrimal sac specimens (34.5%; 95% confidence interval: 27.5–47.5). LSBDs were significantly more common in females (P < 0.001). The age, residence, past ailments, and laterality were not associated with LSBD. Dry eye was more common with LSBD (P = 0.004). Other presenting symptoms were not significantly associated with LSBD. The stromal black deposits in biopsies were mostly extracellular or in macrophages. The LSBD in only 10 specimens demonstrated birefringence. Energy dispersive spectroscopy determined that carbon and sulfur were the main elements in the black aggregates.
CONCLUSION:
Cosmetic-related LSBD is unlikely to play a role in the pathogenesis of PANDO. However, they were significantly associated with DED.
Keywords: Cosmetics, dacryocystitis, deposits, dry eye, lacrimal sac, pigment
INTRODUCTION
Primary-acquired nasolacrimal duct obstruction (PANDO) is a common condition seen in an oculoplastic service. The etiology behind this chronic inflammation usually remains unknown. Intraoperative examination of the sac wall and histopathological examination might assist in determining the etiology of the underlying inflammation.
In our practice, we frequently encounter intraoperative black deposits in the lacrimal sac, and it has been hypothesized that these might play a role in the development of PANDO. Intraoperative detection of abnormal dark discoloration in chronically inflamed tissue warrants careful assessment and raises the suspicion of a melanocytic tumor. However, among all tumors of the lacrimal sac, melanoma is rarely diagnosed.[1] Melanomas account for approximately 4%‒5% of lacrimal sac tumors.[2] The other differential diagnoses of pigmentation in the lacrimal sac include melanosis, cosmetic agents, and drug deposits.[1]
Although the term pigmentation implies a clinically observable finding in relation to melanocyte activity, several studies used it to report cosmetic-related dark discoloration, mainly in the conjunctiva,[3,4,5,6,7] and only few articles reported these cosmetic-related pigmentations in the lacrimal sac.[8,9] The incidence of lacrimal sac black deposits (LSBDs) caused by cosmetic agents and whether this observation is just a chance finding or plays a role in nasolacrimal duct obstruction remain unknown. In addition, eye cosmetics have been proposed to be a principal cause of instability of the lipid layer of the tear film, which leads to symptomatic dry eye.[10,11] Deposition of this material in the lacrimal sac might indicate overuse of the eye cosmetics, especially that the use of the eyeliner (kohl) is popular among women in Saudi Arabia.[12] To our knowledge, there are no published studies to assess the association of the deposition of cosmetic materials in the lacrimal sac with dry eye disease (DED).
The aim of the current study is to determine the incidence of black deposits in the lacrimal sac biopsies, describe the pathologic findings, determine their composition, and correlate the presence of these black deposits with the clinical findings of PANDO and preoperative assessment of DED.
METHODS
A clinicopathological study was conducted at King Khaled Eye Specialist Hospital (KKESH) of all patients who underwent surgical management of PANDO, either by dacryocystorhinostomy (DCR) or dacryocystectomy (DCT) for the last 17 years. We excluded patients in whom lacrimal sac biopsy was not taken during the surgery as well as the patients in whom inadequate histopathological tissue was noted. This study was approved by the KKESH Research Ethical Committee.
Data collected included patient age, gender, residence, nationality, past medical history, and ocular profile related to chronic dacryocystitis at initial presentation. Preoperative assessment of DED was routinely done to determine if patient would undergo DCR or DCT. It was based on the diagnostic criteria of DED by the Japanese Dry Eye Society, which included three categories of subjective symptoms, abnormalities of tears, and epithelial damage.[13] All patients underwent evaluation of basal aqueous tear production using Schirmer Test 1 and ocular surface fluorescein staining. Patients were considered having DED if they had Schirmer stri P value lower than 5 mm in 5 min with topical anesthesia and significant fluorescent staining.
All histological slides of surgical specimens were re-evaluated for this study by one author who was masked to the clinical information. In histological specimens, where there was a question regarding black deposits or type of inflammatory response, an additional ophthalmic pathologist examined the slides. Quality checks on random slides were also performed on the tissue slides to ensure that the data quality was consistent and robust. The histological examination of surgical specimens includes checking of the presence of LSBD, determining their location in the tissue (intra- or extra-cellular), and documenting presence or absence of inflammation around them, a foreign body reaction to them, and whether the particles demonstrated birefringence under the polarized light. In this manuscript, the term “pigmented sac” was used when black deposits were found in the connective tissue of the subepithelial stroma of the lacrimal sac. Scanning electron microscopy (JSM – IT300; JEOL Ltd., Tokyo, Japan) and energy dispersive spectroscopy (EDS), X-Max; Oxford Instrument System, Oxfordshire, United Kingdom) were used to determine the nature of black deposits in the lacrimal sac in two specimens.[14]
Data were collected using a pretested data collection form. Data were collected on an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA). Data analysis was performed using Statistical Package for the Social Studies (SPSS 23; IBM Corp., New York, NY, USA). For quantitative variables with normal distribution, the mean and standard deviation were calculated. If the data distribution was not normal, the median, 25% quartile, and minimum and maximum values were calculated. For qualitative variables, the frequencies and percentage proportions were calculated. To compare the success in subgroups, P < 0.05 was considered statistically significant.
RESULTS
The study sample was comprised of 253 patients with PANDO who were evaluated and managed by the oculoplastics service at KKESH. Histopathological tissues that met the inclusion criteria were available for 177 patients. LSBDs were noted in 61 (34.5%; 95% confidence interval: 27.5–47.5) patients. The demographics and clinical profile are presented in Table 1. The age was comparable between patients with pigmented versus nonpigmented lacrimal sacs. There were more females with LSBD (83.6%) than males (P < 0.001). The age, residence, nationality, past medical history, and laterality were not associated with LSBD in the connective tissue of the biopsy specimens. There was also no significant difference in the clinical profile at presentation in patients with LSBD versus without LSBD, except for dry eye at presentation. Dry eye was statistically significantly more common with LSBD (P < 0.004).
Table 1.
Demographic and clinical profile at presentation of patients with pigmented versus nonpigmented lacrimal sac
| Quantitative | Pigmented sac (n=61), n (%) | Non pigmented sac (n=116), n (%) | Validation |
|---|---|---|---|
| Age | |||
| Mean | 57.5 | 50.0 | 0.03 |
| SD | 20.3 | 24.1 | |
| Gender | |||
| Male | 10 (16.4) | 65 (56.0) | <0.001 |
| Female | 51 (83.6) | 51 (44.0) | |
| Nationality | |||
| Saudi | 58 (95.1) | 111 (95.7) | 0.6 |
| Other | 3 (4.9) | 4 (3.4) | |
| Past medical history | |||
| Yes | 25 (41.0) | 36 (31.0) | 0.2 |
| No | 36 (59.0) | 80 (69.0) | |
| Eye affected | |||
| Right | 35 (57.4) | 56 (48.3) | 0.2 |
| Left | 23 (37.7) | 48 (41.4) | |
| Both | 3 (4.9) | 10 (8.6) | |
| Epiphora | |||
| Present | 44 (72.1) | 81 (69.8) | 0.7 |
| Absent | 16 (26.2) | 34 (29.3) | |
| Discharge | |||
| Present | 41 (67.2) | 80 (69.0) | 0.6 |
| Absent | 21 (34.4) | 35 (30.2) | |
| Conjunctivitis | |||
| Present | 5 (8.2) | 9 (7.8) | 0.9 |
| Absent | 56 (91.8) | 107 (92.2) | |
| Dacryocystitis | |||
| Present | 33 (54.1) | 66 (56.9) | 0.7 |
| Absent | 28 (45.9) | 50 (43.1) | |
| Regurgitation | |||
| Present | 30 (49.2) | 58 (50.0) | 0.9 |
| Absent | 31 (50.8) | 58 (50.0) | |
| Preseptal/orbital cellulitis | |||
| Present | 1 (1.6) | 3 (2.6) | 0.75 |
| Absent | 60 (98.4) | 113 (97.4) | |
| Blepharitis | |||
| Present | 7 (11.5) | 5 (4.3) | 0.09 |
| Absent | 54 (88.5) | 111 (95.7) | |
| Dry eye | |||
| Present | 28 (45.9) | 28 (24.1) | 0.004 |
| Absent | 33 (54.1) | 88 (75.9) |
SD: Standard deviation
Histopathological studies revealed black-pigmented aggregates that were noted in the sac stroma [Figure 1]. They were largely extracellular with black deposits in some areas engulfed by macrophages [Figure 1]. A typical foreign body reaction granuloma (with foreign body giant cells) reaction was not seen in the specimens examined. Chronic inflammatory infiltrates were noted in the sac stroma in both pigmented and nonpigmented lacrimal sacs. Stromal fibrosis in pigmented and nonpigmented sacs showed no differences on qualitative assessment. LSBD birefringence was checked in all specimens. LSBD birefringence was noted in ten specimens. In the most of specimens that did not demonstrate birefringence, the LSBD particles were either have small size or surrounded by tissue scarring that appeared to interfere with the birefringence.
Figure 1.
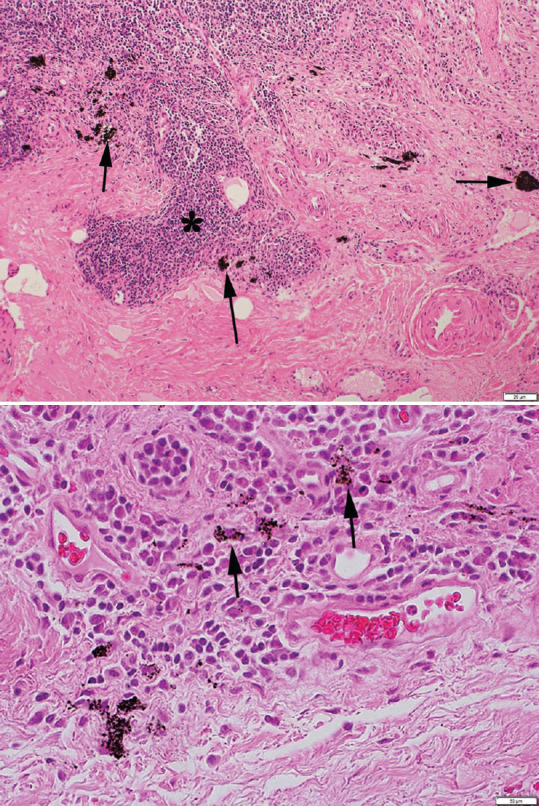
Top. Microphotograph showing a biopsy from lacrimal sac excision. Note the dense collagenous scar in the stroma that is infiltrated with lymphocytes and plasma cells (*). Clumps of black deposits of varying size are seen in the extracellular space of the stroma (arrows). (Hematoxylin and eosin; magnification bar at lower right corner). Bottom. Scarring and chronic inflammation and high magnification. Note that some black deposits are engulfed by macrophages (arrows). (Hematoxylin and eosin; magnification bar at lower right corner)
The nature of the LSBD was determined using EDS. Scanning electron microscopy of the tissue black deposits is shown in Figure 2a. EDS analysis showed that carbon (C) 65% and sulfur (S) 18% were the major elements in the black deposits [Figure 2b].
Figure 2.
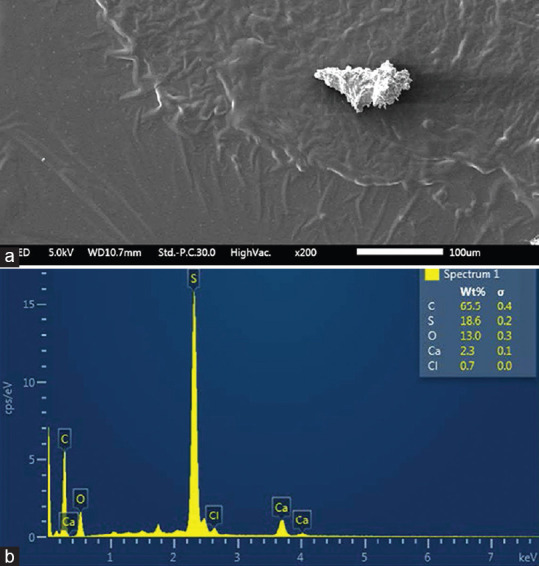
(a): Scanning electron micrograph showing a black clump in the tissue sample (×200). (b): Energy dispersive spectroscopy showing that carbon and sulfur are the major elements in the black deposits
DISCUSSION
In the current study, LSBDs were noted in 61 (34.5%) histological specimens. Although several reports have demonstrated cosmetic-related pigmentation in the conjunctiva,[3,4,5,6,7] there are very few reports of cosmetic-related lacrimal sac pigmentation.[9,11] To the best of our knowledge, the current study reports the largest sample size on this topic.
The black deposits were determined to be related to cosmetic agents after careful assessment by histological examination and electron microscopy. The LSBDs in all cases were found in the subepithelial stroma. In contrast, the pigmentation related to melanosis of the lacrimal sac was confined to sac epithelium with characteristic intraepithelial melanocytes that tend to accumulate in the sac wall.[1] The histopathological features of the LSBD in this study were similar to previously reported cases of lacrimal sac pigmentation due to mascara, as well as to the conjunctival pigmentations due to mascara, in which there is accumulation of black pigment granules in and around stromal cells or as free pigment granules in the tissue.[4,8]
A wide variety of causes have been attributed to the development of PANDO, including inflammatory, traumatic, mechanical, or neoplastic factors.[15] However, idiopathic chronic inflammation remains the main cause.[15] Although black deposits were observed in over one-third of the lacrimal sac biopsies, based on the histological findings and statistical analysis, we believe that they were not directly related to the pathogenesis of PANDO. The possible mechanism by which these black deposits could cause nasolacrimal duct obstruction is a foreign body granulomatous reaction, resulting in stricture of the duct. However, the particles were inert and found to be present in the extracellular space or engulfed by macrophages without a typical foreign body granulomatous reaction in the tissue nor fibrosis. Thus, they were unlikely to be the cause for an increased risk of inflammatory changes in the sac. In addition, they were small, and we believe even in large aggregates would be unlikely to cause mechanical ductal obstruction. Ali and Paulsen have extensively studied the etiopathogenesis of PANDO and concluded the complexity of its occurrence due to multiple factors such as micro-environmental, hormonal, vascular, and tears biochemical features.[16]
We found a significant association of preoperative DED and presence of LSBD. This observation is similar to some reports in the literature; three patients were described with mascara-induced pigmentation of the bulbar conjunctiva found to have a short tear break-up time and deficient tear aqueous layer,[6] as well as a patient with a history of long-term use of mascara also had a DED.[3] Eye cosmetics have been proposed to be a principal cause of instability of the lipid layer of the tear film.[10,11] Studies using infrared spectroscopy have reported that eye cosmetics might significantly increase medium viscosity, destabilizing the tear film, resulting in higher evaporation rates and dryness.[17] Other complications reported with cosmetics were mascara-laden dacryolith, and conjunctival mascaroma; which is a pigmented mass in the conjunctiva that initially causes a toxic follicular reaction, and later, results in a chronic follicular-papillary reaction.[7]
Energy dispersive analysis (EDS) of our specimens indicated that C 65% and S 18% were the major elements without the presence of the lead. Hidayat et al.[9] described eight cases from the KKESH with lacrimal sac pigmentation that were analyzed as well using EDS of all biopsy specimens. In their study, high lead content was found in the patients’ tissues, in addition to other less frequent elements including C, S, and silver. The differences in analysis between studies could be due to the different periods in time that the two studies were conducted. At the time of Hidayat et al[9] study, which was conducted in 1997, the commonly used kind of eyeliner (kohl surma) was known to have high lead content.[12,14] However, the composition of indigenous eyeliners such as “Kohl” can be variable. It is also possible that that the composition of cosmetics might have improved due to a more stringent regulatory environment by the FDA that has labeled Kohl, Kajal, Surma, and similar materials as illegal color additives.[18] Al-Hazza and Krahn using EDS determined that in 21 Kohl specimens originating from various parts of Saudi Arabia, India, and the Middle East, the composition of the pigment was variable.[14] Although lead was a predominant metal detected in 14/21 specimens, C levels more than 60% were detected in six Kohl samples. Furthermore, some specimens showed the presence of sulfur. Our analysis was limited to two specimens. It is likely that the two specimens subjected to EDS were not large enough to make a definitive conclusion regarding the elements that might be present in periocular cosmetic-pigmented agents in our study.
Three case reports revealed birefringence of the mascara granules under polarization microscopy.[8,19,20] The LSBD in this study showed birefringence when the particle size was large but lacked birefringence in specimens in which the black particles were small and/or surrounded by dense scar tissue. It is possible that these factors might have affected the foreign bodies’ property to exhibit birefringence. Furthermore, the lack of birefringence could be due to the absence of lead in some of the specimens in which newer cosmetic eyeliners were used. In fact, mascara consists of fine needle-like birefringent particles of plastic which give body to the mascara to increase the length and size of the lashes which is lacking in eyeliners.[21] In our cases, the histologic appearance of the particles did not resemble mascara. In our population, we believe that the use of eyeliner rather than mascara is more common, which might be one of many reasons to explain the paucity of birefringence in our samples. However, it is difficult to confirm this hypothesis due to the retrospective nature of our study.
It is interesting that our patients with PANDO also had DED, in contrary to what would be expected since in the presence of PANDO an increased tear lake and possible alleviation of objective signs of dryness might happen. A reduction in Schirmer strip wetting in the presence of PANDO suggests that these patients have severe DED preoperatively, and this dryness is indirectly related to their PANDO due to tear proteomics as previously reported.[16] The fact that our study is retrospective and that we did not perform further biochemical testing to link the molecular pathways of DED and PANDO to the disruptions caused by these deposits are our two main limitations of our study and hypothesis.
CONCLUSION
Cosmetic-related LSBD is unlikely to play a direct role in the pathogenesis of PANDO. However, they were significantly associated with DED, and these combined interrelated factors are worth consideration in the pathogenesis of PANDO. Additional prospective testing of patients using frequent periocular cosmetics, biochemical testing of tear composition in these cases, and examination for the effects of all this on the ocular surface might be useful to support our observations and to conclude that both associated factors play an actual role in the etiopathogenesis of PANDO.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jakobiec FA, Stagner AM, Sutula FC, Freitag SK, Yoon MK. Pigmentation of the lacrimal sac epithelium. Ophthalmic Plast Reconstr Surg. 2016;32:415–23. doi: 10.1097/IOP.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 2.Krishna Y, Coupland SE. Lacrimal sac tumors – A review. Asia Pac J Ophthalmol (Phila) 2017;6:173–8. doi: 10.22608/APO.201713. [DOI] [PubMed] [Google Scholar]
- 3.Ciolino JB, Mills DM, Meyer DR. Ocular manifestations of long-term mascara use. Ophthalmic Plast Reconstr Surg. 2009;25:339–41. doi: 10.1097/IOP.0b013e3181ab443e. [DOI] [PubMed] [Google Scholar]
- 4.Reese AB. Pigmentation of the palpebral conjunctiva resulting from mascara. Trans Am Ophthalmol Soc. 1946;44:113–6. [PubMed] [Google Scholar]
- 5.Shields PW, Jakobiec FA, Stagner AM, Yoon MK. Spitz nevus arising in the eyelid of a teenager. Surv Ophthalmol. 2016;61:228–35. doi: 10.1016/j.survophthal.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Davis LJ, Paragina S, Kincaid MC. Mascara pigmentation of the bulbar conjunctiva associated with rigid gas permeable lens wear. Optom Vis Sci. 1992;69:66–71. doi: 10.1097/00006324-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Haddad R, Zehetbauer G. Problems arising from the use of cosmetics on the lid margin (author's transl) Klin Monbl Augenheilkd. 1980;177:829–31. doi: 10.1055/s-2008-1057739. [DOI] [PubMed] [Google Scholar]
- 8.Clifford L, Jeffrey M, Maclean H. Lacrimal sac pigmentation due to mascara. Eye (Lond) 2011;25:397–8. doi: 10.1038/eye.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidayat AA, Weatherhead RG, al-Rajhi A, Johnson FB. Conjunctival and lacrimal sac pigmentation by kohl (eyeliner) Br J Ophthalmol. 1997;81:418. doi: 10.1136/bjo.81.5.415d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozato PA, Pisella PJ, Baudouin C. The lipid layer of the lacrimal tear film: Physiology and pathology. J Fr Ophtalmol. 2001;24:643–58. [PubMed] [Google Scholar]
- 11.Ng A, Evans K, North RV, Jones L, Purslow C. Impact of eye cosmetics on the eye, adnexa, and ocular surface. Eye Contact Lens. 2016;42:211–20. doi: 10.1097/ICL.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ashban RM, Aslam M, Shah AH. Kohl (surma): A toxic traditional eye cosmetic study in Saudi Arabia. Public Health. 2004;118:292–8. doi: 10.1016/j.puhe.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Shimazaki J Japanese Dry Eye Society. Definition and diagnosis of dry eye 2006 [in Japanese] Atarashii Ganka. 2007;24:181–4. [Google Scholar]
- 14.Al-Hazzaa SA, Krahn PM. Kohl: A hazardous eyeliner. Int Ophthalmol. 1995;19:83–8. doi: 10.1007/BF00133177. [DOI] [PubMed] [Google Scholar]
- 15.Koturović Z, Knežević M, Rašić DM. Clinical significance of routine lacrimal sac biopsy during dacryocystorhinostomy: A comprehensive review of literature. Bosn J Basic Med Sci. 2017;17:1–8. doi: 10.17305/bjbms.2016.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MJ, Paulsen F. Etiopathogenesis of primary acquired nasolacrimal duct obstruction: What we know and what we need to know. Ophthalmic Plast Reconstr Surg. 2019;35:426–33. doi: 10.1097/IOP.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 17.Hunter M, Bhola R, Yappert MC, Borchman D, Gerlach D. Pilot study of the influence of eyeliner cosmetics on the molecular structure of human meibum. Ophthalmic Res. 2015;53:131–5. doi: 10.1159/000371852. [DOI] [PubMed] [Google Scholar]
- 18. [Last accessed 2018 Feb 13];Administration UFaD. Kohl, Kajal, Al.Kahal, Surma, Tiro, Tozali, or Kwalli: By Any Name, Beware of Lead Poisoning. Available from: https://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm137250.htm . [Google Scholar]
- 19.Pao KY, Murchison AP, Eagle RC., Jr Unilateral non-pigmented palpebral conjunctival lesions due to cosmetics use. Ophthalmic Plast Reconstr Surg. 2012;28:e107–8. doi: 10.1097/IOP.0b013e31823c0282. [DOI] [PubMed] [Google Scholar]
- 20.Shields JA, Marr BP, Shields CL, Eagle RC., Jr Conjunctival mascaroma masquerading as melanoma. Cornea. 2005;24:496–7. doi: 10.1097/01.ico.0000148289.53323.fb. [DOI] [PubMed] [Google Scholar]
- 21.Lee’s WR. Ophthalmic histopathology. 3rd edition,. Fiona Roberts and Chee Koon Thum (Editors): Springer-Verlag London as part of Springer Science & Business Media. 2014 DOI: 10.1007/978-1-4471-2476-4_1. [Google Scholar]


