Abstract
PURPOSE:
To characterize demographic, clinical, and histopathologic features of ocular adnexal lymphoma (OAL) at a single institution.
METHODS:
Retrospective review of all patients with pathologic diagnosis of OAL between 2015 and 2020.
RESULTS:
There were 133 patients with OAL, with a median age of 65 years (range 23–97) and a slight female predominance (male: female = 1:1.46), (n = 79, 59%). The majority of tumors were non-Hodgkin B-cell lymphomas (n = 131, 99%), most frequently Extranodal Marginal Zone B-Cell Lymphoma (EMZL, n = 93, 70%), followed by follicular lymphoma (n = 21, 16%), chronic lymphocytic leukemia/small lymphocytic lymphoma (n = 7, 5%), diffuse large B-cell lymphoma (n = 5, 4%), and mantle cell lymphoma (n = 5, 4%). The most frequently involved sites included the orbit (n = 85, 64%) and conjunctiva (n = 43, 32%). Information was available on oncologic staging in 78 (59%), treatment in 82 (62%), and follow-up in 75 (56%) patients. By the Ann-Arbor classification system, patients were classified as IE (54/78, 69%), IIE (9/78, 12%), IIIE (6/78, 8%), and IVE (9/78, 12%). The most common treatments included external beam radiotherapy (standard and ultra-low-dose) (48/82, 59%), biologics (22/82, 27%), and surgical excision with cryotherapy (14/82, 17%) (some patients had >1 therapy). Median follow-up time was 24 months (range 0–221 months). Recurrence was observed in 13% (10/75) with a median time to recurrence of 60 months (95% confidence interval 47–73 months). Excision with cryotherapy as a sole treatment modality was associated with earlier recurrence (P = 0.003).
CONCLUSION:
In this largest single-center study of OAL, we found that most OAL were Ann-Arbor Stage IE EMZL, occurring in older patients with a female predominance. Early recurrence was noted in tumors treated with excision and cryotherapy alone.
Keywords: Ocular adnexal lymphoma, Ocular adnexal lymphoma pathology, ocular adnexal lymphoma recurrence, ocular adnexal lymphoma staging, ocular adnexal lymphoma treatment
INTRODUCTION
Lymphomas are malignant tumors derived from clonal expansion of B or T lymphocytes, and rarely natural killer (NK) lymphocytes. B-cell lymphomas are broadly divided into Hodgkin and non-Hodgkin subtypes and can arise in a lymph node or in an extranodal site.[1] Lymphomas are further classified according to the presumed cell of origin as defined by the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues.[1] Although pathogenesis of ocular adnexal lymphoma (OAL) is multifactorial, a major proposed mechanism is a clonal expansion of lymphocytes induced by persistent antigenic stimulation from chronic inflammation, analogous to a process that has been described in other mucosal-associated lymphoid tissue sites.[2,3,4]
Ophthalmic lymphomas can involve the intraocular tissues or the OA. Intraocular lymphomas are rare and include vitreoretinal diffuse large B-cell lymphomas (DLBCL) associated with central nervous system lymphoma, and choroidal lymphoma associated with systemic lymphoma.[5,6,7] OAL can be primary, arising in the eyelids, conjunctiva, lacrimal gland, lacrimal sac, extraocular muscles, and orbital soft tissue, or can involve these structures secondarily. Compared to all lymphomas collectively, OAL is rare, with an incidence of 0.28 cases per 100,000 patients and comprising only 1%–2% of all lymphomas and 8% of all extranodal lymphomas. However, it is important to note that OAL is the most common orbital malignancy in adults, accounting for 10% of all orbital mass lesions, and this percentage can increase up to 24% in patients aged 60 years or older.[8,9,10,11]
OAL predominantly affects patients in the fifth to seventh decade of life, with a median age of 65 years and female predominance, with female-to-male ratio of 1.5–2:1.[12,13,14,15] The most frequent involved site is the superior orbit (40%), followed by conjunctiva (35%–40%), lacrimal gland (10%–15%), and the eyelid (10%).[12,13,14,16] Demirci et al. reviewed 160 cases of orbital lymphoproliferative tumors and noted involvement of the superior orbit in 64%, inferior 9%, temporal 8%, nasal 8%, and 11% central.[11] The most common presenting signs were orbital mass (67%), proptosis (54%), restriction of extraocular motility (39%), conjunctival swelling with a “salmon-patch” appearance (22%), and symptoms of irritation and tearing.[12,13,14,16,17,18,19]
Of all OALs, 98% are B-cell lymphomas, with extranodal marginal zone B-cell lymphoma (EMZL) being the most common subtype (49%-64%), followed by follicular lymphoma (FL) (10%–23%), and DLBCL (8%–10%).[13,20,21] The less frequent subtypes include mantle cell lymphoma (MCL) (5%–9%) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (4%–7%). T-cell lymphomas are extremely rare accounting for <2% of all OALs.[13,20,21]
The objectives of this study were to analyze the distribution of OAL at our institution by demographics, location, subtype, treatment, and outcome and to compare our data with the prior literature.
METHODS
Study design
A retrospective review of patients with the pathologic diagnosis of OAL managed by the Pathology, Oncology, and Oculoplastic and Orbital Surgery Services at a single tertiary care center between January 01, 2015 and January 01, 2020, was performed. The study was approved by the Institutional Review Board and followed the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability act of 1996 in the United States.
Tissue selection/biopsy Specimens
The pathologic diagnoses were rendered based on the integration of morphologic, immunohistochemical, immunophenotypic, and molecular genetic parameters in accordance with the current World Health Organization classification of lymphomas.[1] Immunohistochemical panels for B-cell and T-cell lymphoma included the following panel of antibodies: CD3, CD5, CD4, CD8, CD7, CD10, CD20, CD21, CD23, CD30, CD56, CCND1 (cyclin D-1, BCL-1), BCL-2, BCL-6, MUM1, CD138, kappa, lambda, c-MYC, and Ki-67. Flow cytometry was performed with standard panels for B-cell and T-cell lymphoma. Molecular genetic studies included the polymerase chain reaction for B-cell and T-cell clonality, fluorescence in situ hybridization for lymphoma subtyping, targeted next generation sequencing with a 40 gene lymphoma panel (complete list of genes available at https://www.pennmedicine.org/-/media/academic%20departments/personalized%20diagnostics/pdfs/canfy1819117cpdphysicianbrochure2018updatev2.ashx?la= en) or targeted MYD88 mutation analysis.
Clinical data
The demographic and clinical data collected included age, sex, presenting signs and symptoms, clinical local disease extent, biopsy site, tumor laterality, results of systemic oncologic workup, management, and outcome.
The patients were staged in accordance with the Ann Arbor staging system for extranodal lymphoma and the tumour node metastasis (TNM) classification for OAL specified by the American Joint Committee on Cancer (AJCC) Cancer.[22,23]
The tumors were classified into 3 categories: Primary, disseminated, and secondary. Primary OAL was defined as lymphoma limited to the OA and the adjacent regional site (unilateral preauricular or sub-mandibular lymph nodes or nearby structures such as the parotid gland) and no prior history of lymphoma elsewhere. Disseminated OAL was defined OAL with concurrent involvement of lymph nodes outside of regional area or in noncontiguous tissues or organs external to the OA (submandibular gland, lung, liver, spleen, kidney, breast, and bone marrow) at the time of diagnosis. Secondary OAL was defined as known systemic lymphoma of any kind, with secondary involvement of the OA. Progression of disease was defined as transformation from a low-grade to a high-grade lymphoma or progression to a higher Ann Arbor or AJCC TNM stage after a period of remission. Recurrence of the disease was defined as lymphoma that re-manifested after successful treatment and a period of remission (no evidence of lymphoma on follow-up testing and imaging).
Oncologic workup (imaging, laboratory studies, bone marrow biopsy) and management including treatment modalities (excision and cryotherapy, systemic chemo-and/or biologic therapy, radiation, and allogenic bone marrow stem cell transplant [BMT] or combination therapies) were documented. Outcome information included recurrence status, time to recurrence, disease progression, disease-related mortality, and date of last follow-up.[24,25]
Statistical analysis
Demographic and clinical data of patients with recurrent and non-recurrent OA lymphoma were summarized and proportions were analyzed via Chi-square test or Fisher exact test. Continuous variables including age, time to recurrence, and time to last follow-up were expressed as a mean, median, and range, and means were compared using the Student's t-test. The survival distribution for patients with recurrent OAL was plotted using Kaplan–Meier curves and factors significantly affecting survival time were compared using log-rank test at a 5% level of significance. Univariable Cox-proportional hazards regression with hazard ratios and 95% confidence intervals (95% CI) was employed to evaluate factors predictive of time to survival at a 5% level of significance. All statistical analyses were performed using IBM SPSS Version 24 (Armonk, NY, USA: IBM Corp. IBM Corp. Released 2016.).
RESULTS
Tissue selection/biopsy Specimens
Review of pathology records identified a total of 170 biopsies suspicious for lymphoproliferative disease between 2015 and 2020. Of these, 133 (78%) were diagnosed as OAL [Figure 1]. Histopathologic findings were supported by immunohistochemical panels in 126 (95%), flow cytometry in 57 (43%), and molecular genetic studies in 30 (22%) cases.
Figure 1.
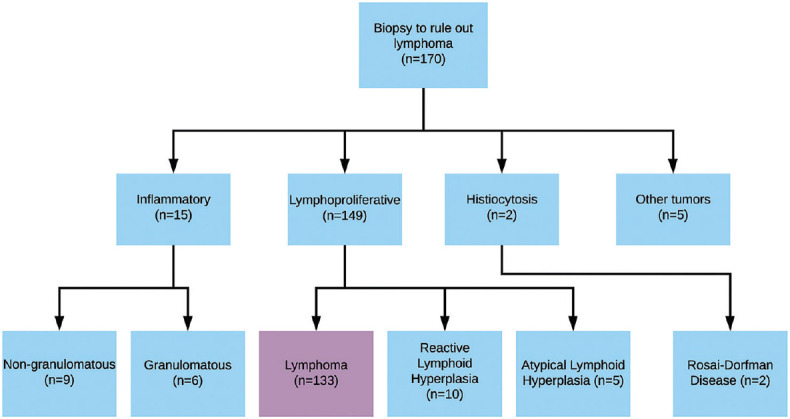
Flow diagram of biopsies with clinical concern for orbital and ocular adnexal lymphoproliferative disease at single institution between 2015 and 2020, with final histological diagnoses
Overall ocular adnexal lymphoma clinical features
OAL occurred at the median age of 65 years (range 23–97 years) with a female predominance (79/133, 59%) [Table 1]. The mean time from clinical symptoms to consultation was 12 months (interquartile range 6–45 months).
Table 1.
Demographics of patients with ocular adnexal lymphoma by subtype (n=133)
| Type of lymphoma | n (%) | Median age (years) | Age range (years) | Male:female ratio |
|---|---|---|---|---|
| Marginal zone lymphoma | 93 (70) | 64 | 23-92 | 42:51 (0.8) |
| Follicular lymphoma | 21 (16) | 71 | 34-97 | 5:16 (0.3) |
| Small lymphocytic lymphoma | 7 (5) | 69 | 66-85 | 2:5 (0.4) |
| Diffuse large B-cell lymphoma | 5 (4) | 76 | 64-89 | 1:4 (0.3) |
| Mantle cell lymphoma | 5 (4) | 61 | 51-76 | 4:1 (4) |
| T-cell lymphoma | 2 (1) | 34 | 26-41 | 0:2 (0) |
| Total | 133 (100) | 65 | 23-97 | 54:79 (0.7) |
Laterality was known in 131 of 133 (98%) patients. 52 of 131 (40%) lesions involved the left OA, 60 of 131 (46%) involved right OA, and 19 of 131 (14%) demonstrated bilateral involvement [Table 2]. Presenting signs included proptosis, decreased visual acuity, “salmon-patch” mass, and tearing.
Table 2.
Ocular adnexal lymphoma presentation by histological location (n=133)
| n (%) | |
|---|---|
| Orbital fibroadipose tissue | 49 (37) |
| Conjunctiva | 43 (32) |
| Lacrimal gland | 18 (13) |
| Eyelid | 2 (2) |
| Lacrimal sac | 2 (2) |
| Extraocular muscle | 1 (1) |
| Mixed location | 18 (13) |
| Orbit, conjunctiva | 10 (7) |
| Orbit, caruncle | 2 (1) |
| Orbit, conjunctiva, lacrimal gland | 2 (1) |
| Orbit, tenon fascia | 1 (1) |
| Orbit, skin, extraocular muscle | 1 (1) |
| Orbit, lacrimal gland | 1 (1) |
| Orbit, extraocular muscle | 1 (1) |
| Laterality (n=131) | |
| Left | 52 (40) |
| Right | 60 (46) |
| Bilateral | 19 (14) |
The orbit and the conjunctiva were the most frequently involved OA sites, accounting for 50% and 32% of 133 cases, respectively [Table 2].
Of 78 patients with staging information, 58 (74%) had primary OAL, 16 (21%) had disseminated lymphoma, and 4 (5%) had secondary lymphoma [Table 3]. The Ann-Arbor and AJCC classification data are summarized in Table 4.
Table 3.
Distribution of ocular adnexal lymphoma presentation by systemic disease status (n=78)
| Primary, n (%) | Disseminated, n (%) | Secondary, n (%) | |
|---|---|---|---|
| Marginal zone lymphoma | 44 (85) | 7 (13) | 1 (2) |
| Follicular lymphoma | 10 (63) | 5 (31) | 1 (6) |
| Small lymphocytic lymphoma | 3 (75) | 0 | 1 (25) |
| Diffuse large B-cell lymphoma | 1 (50) | 1 (50) | 0 |
| Mantle cell lymphoma | 0 | 2 (67) | 1 (33) |
| T-cell lymphoma | 0 | 1 (100) | 0 |
| Total | 58 (74) | 16 (21) | 4 (5) |
Table 4.
Staging of ocular adnexal lymphoma by subtype (n=78)
| All, n (%) | EMZL, n (%) | FL, n (%) | SLL, n (%) | DLBCL, n (%) | MCL, n (%) | TCL, n (%) | |
|---|---|---|---|---|---|---|---|
| Ann arbor stage | |||||||
| IE | 54 (69) | 44 (83) | 7 (44) | 3 (75) | 0 | 0 | 0 |
| IIE | 9 (12) | 5 (9) | 3 (19) | 0 | 1 (50) | 0 | 0 |
| IIIE | 6 (8) | 1 (2) | 4 (25) | 0 | 1 (50) | 0 | 0 |
| IVE | 9 (12) | 3 (6) | 2 (13) | 1 (25) | 0 | 2 (100) | 1 (100) |
| AJCC T-stage | |||||||
| T1 | 27 (35) | 23 (44) | 4 (25) | 0 | 0 | 0 | 0 |
| T2 | 49 (63) | 28 (54) | 12 (75) | 4 (100) | 2 (100) | 3 (100) | 0 |
| T3 | 2 (3) | 1 (1) | 0 | 0 | 0 | 0 | 1 (100) |
| T4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AJCC N-stage | |||||||
| N0 | 55 (71) | 42 (81) | 7 (43) | 4 (100) | 0 | 1 (33) | 1 (100) |
| N1 | 8 (10) | 4 (8) | 3 (19) | 0 | 1 (50) | 0 | 0 |
| N2 | 4 (5) | 4 (8) | 0 | 0 | 0 | 0 | 0 |
| N3 | 11 (14) | 2 (3) | 6 (38) | 0 | 1 (50) | 2 (67) | 0 |
| AJCC M-stage | |||||||
| M0 | 71 (91) | 51 (98) | 15 (94) | 3 (75) | 1 (50) | 1 (34) | 0 |
| M1 | 7 (9) | 1 (2) | 1 (6) | 1 (25) | 1 (50) | 2 (64) | 1 (100) |
| M2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
EMZL=Extranodal marginal zone B-cell lymphoma; FL=Follicular lymphoma; SLL=Small lymphocytic lymphoma; DLBCL=Diffuse large B-cell lymphoma; TCL=T-cell lymphoma; AJCC=American joint committee on cancer staging system; MCL=Mantle cell lymphoma
Ocular adnexal lymphoma subtypes
Of 133 lymphomas, 131 (99%) were B-cell lymphomas and the remaining 2 (1%) were T-cell lymphoma [Table 1].
Extranodal Marginal Zone B-cell lymphoma throughout
EMZL was by far the most common lymphoma of the OA, constituting 93 of 133 (70%) of all cases [Table 1]. EMZL was more common in females, with a male: female ratio 1:1.2, and a median age of 64 years (range 23–92). Most EMZL lymphomas (44/52, 85%) were primary, Ann Arbor stage IE (44/53, 83%), involved the conjunctiva alone (AJCC T1, 23/52, 44%) or orbit (AJCC T2, 28/52, 54%), and had low frequency of distal nodal involvement (AJCC N3, 2/52, 3%). None of the patients with EMZL had extranodal involvement outside of OA [Table 4].
Follicular lymphoma
FL was the second most common OAL, constituting 21/133 (16%) of all cases. The majority (19/21, 90%) of FL were low-grade, with only two (2/21, 10%) high-grade (Grade 3) cases. FL was more common in females, with a M:F ratio 1:3.2 and a median age of 71 years (range 34–97) [Table 1]. Similar to EMZL, most FL were primary (10/16, 63%) and confined to the OA and regional sites [10/16, 63% stage IE and IIE, Table 4]. However, isolated conjunctival involvement was less frequent in FL (AJCC T1, 4/16, 25%) when compared to EMZL. Additionally, FL had a higher tendency for disseminated disease (5/16, 31%), higher Ann Arbor stage IIIE (4/16, 25%), and a higher potential for distal nodal metastasis (N3 6/16, 38%) and extranodal metastasis (1/16, 6%) [Table 4]. Of two high-grade FL, one was secondary FL, Ann Arbor stage IIIE and the second high-grade FL was stage IE.
Chronic lymphocytic leukemia/small lymphocytic lymphoma
CLL/SLL was the third most common OAL, comprising 7/133 (5%) of all cases [Table 1]. CLL/SLL was more common in females, with a male: female ratio 1:2.5, and a median age of 69 years (range 66–85) [Table 1]. Of 4 patients with staging information, 3 lymphomas were primary Ann Arbor stage IE (75%). All tumors affected the orbit (T2). One patient (25%) with secondary CLL/SLL had extranodal metastasis [Table 4].
Diffuse large B-Cell lymphoma
DLBCL was the fourth most common OAL, comprising 5/133 (4%) of all cases. DLBCL was more common in females, with a male: female ratio 1:4, and a higher median age of 76 years (range 64–89) when compared to EMZL, although the difference was not statistically significant (76 vs. 64, P = 0.06). By Hans algorithm, 3/5 (60%) DLBCL were of non-germinal center B-cell (non-GCB) type and 2/5 (40%) DLBCL were of GCB type.[1] None of these high-grade lymphomas had rearrangements in MYC/BCL-2/BCL-6 genes characteristic of double-hit or triple-hit lymphoma.[1] Of 2 patients with staging information, one had primary disease and one had disseminated disease at presentation with distal nodal and extranodal metastasis [Tables 3 and 4].
Mantle cell lymphoma
MCL was the fourth most common OAL, together with DLBCL, constituting 5/133 (4%) of all lymphomas. MCL was the only lymphoma with male predilection (male: female ratio 4:1) and had a median age of 61 years (range 51–76). Of 3 patients with available staging information, all had advanced stage IVE lymphoma, presenting with either disseminated disease (2/3, 67%) or as a secondary orbital involvement of a known systemic disease (1/3, 33%) [Tables 3 and 4].
T-cell lymphoma
T-cell lymphoma was the least frequent OAL subtype, constituting 2/133 (1%) of all lymphomas. Both affected patients were females with a median age of 34 years (range 26–41) [Table 1]. One patient with known staging information had disseminated Ann Arbor Stage IV disease at the time of diagnosis [Tables 3 and 4].
Treatment, follow-up and recurrence
Treatment information was available on 82 of 133 (62%) patients [Table 5 and Supplementary Digital Content Figures 1 (189.3KB, tif) -3 (191.2KB, tif) ]. Treatment was administered as a monotherapy in 72 patients (88%) or as a combination therapy in 10 patients (12%). The most common treatment modality was external beam radiotherapy (EBRT) (48/82, 59%), administered as a monotherapy (42/48, 88%) or in combination (6/48, 12%). The dose of EBRT varied from 24 to 30 Gy fractioned in 15 sessions or an ultra-low-dose (4 Gy) radiotherapy (ULDR), divided in two sessions (also known as “Boom-Boom” technique).[26] The second most common treatment modality was biologic agents (22/82, 27%), comprising the CD20-targeted antibody therapy (rituximab) in 21 patients and Bruton tyrosine kinase inhibitor (ibrutinib) in one patient with CLL/SLL. Fourteen of 82 patients (17%) were managed by excision with cryotherapy followed by observation. Seven of 82 (9%) patients were managed by chemotherapy [Table 5].
Table 5.
Treatment of ocular adnexal lymphomas by subtype and staging
| Treatment (n=82) Ann arbor stage | All subtypes total, n (%) | EMZL | FL low grade | FL high-grade | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||
| IE | IIE | IIIE | IVE | N/A | Total | IE | IIE | IIIE | IVE | Total | IE | IIE | IIIE | IVE | Total | ||
| EBRT | 38 (46) | 24 | 2 | 5 | 31 | 2 | 1 | 3 | 1 | 1 | |||||||
| Surgical excision with cryotherapy* | 14 (17) | 8 | 4 | 12 | 0 | ||||||||||||
| Biologics | 14 (17) | 6 | 3 | 1 | 10 | 1 | 1 | 1 | 3 | ||||||||
| ULDR | 4 (5) | 0 | 1 | 1 | 1 | 2 | 3 | ||||||||||
| Chemotherapy | 2 (2) | 1 | 1 | 0 | |||||||||||||
| EBRT+biologics | 5 (6) | 0 | 2 | 2 | 1 | 1 | 2 | ||||||||||
| Chemotherapy+biologic | 3 (4) | 1 | 1 | 1 | 1 | ||||||||||||
| EBRT+chemotherapy | 1 (1) | 1 | 1 | 0 | |||||||||||||
| Chemotherapy+BMT | 1 (1) | 0 | 0 | 0 | |||||||||||||
| 59 | 12 | ||||||||||||||||
*Surgical excision with cryotherapy as a sole treatment modality, followed by observation. EBRT=External beam radiation therapy; ULDR=Ultra-low-dose radiotherapy; BMT=Bone marrow transplant; EMZL=Extranodal marginal zone lymphoma; FL=Follicular lymphoma; N/A=Staging information not available; MCL=Mantle cell lymphoma; Extranodal marginal zone B-cell lymphoma" throughout
Treatment was generally dependent on lymphoma staging and subtype [Table 5 and Supplementary Digital Content Figures 1 (189.3KB, tif) -3 (191.2KB, tif) ] as well as systemic involvement. Some patients received multiple therapies. The stage IE OAL was generally treated with EBRT or ULDR (32/50 (64%). The patients with stage IIIE and IVE lymphoma underwent more aggressive therapy, including systemic biologics (6/12, 50%), chemotherapy (3/12, 25%), combination chemotherapy and allogenic BMT (1/12, 8%). One patient with stage IVE CLL/SLL was treated with palliative therapy because of advanced age and disseminated disease. Most patients with EMZL were treated with radiotherapy (34/59, 58%) or excision with cryotherapy followed by observation (12/59, 20%). The excision and cryotherapy as the sole therapy was a therapeutic choice in elderly, frail, and debilitated patients, who were not good candidates for other ancillary treatment modalities. Five of 15 (33%) of patients with bilateral EMZL received biologic therapy [Table 5]. The more aggressive lymphomas were more frequently treated with systemic therapy, such as chemotherapy in combination with either biologics or BMT in 2 of 3 MCLs and either chemotherapy or biologics in 2 of 3 DLBCLs [Table 5].
Recurrent ocular adnexal lymphoma
Follow-up information was available on 75 of 133 (56%) patients. Mean patient follow-up was 24 months (95% CI 15–33 months), with a range of 0–221 months. Disease progression was documented in one patient, who had bilateral conjunctival and right orbital EMZL with disseminated nodal involvement, which transformed to DLBCL 29 months after diagnosis. Local recurrence occurred in 10 patients (13%) with a median time to recurrence of 60 months [95% CI 47–73 months, Supplementary Digital Content Figure 4 (202.3KB, tif) ]. The recurrent tumors were predominantly EMZL (9/10, 90%) and less frequently, FL, Grade ½ of 3 (1/10, 10%). Recurrent lesions were localized to the orbit (5/10, 50%) and conjunctiva (5/10, 50%), with most arising at the site of primary OAL (8/10, 80%). Multiple tumor recurrences were noted in 2 patients (2/10, 20%). Recurrent tumors were initially treated with a wide range of modalities including chemotherapy and biologics (2/9, 22%), chemotherapy and radiation (1/9, 11%), biologic therapy alone (2/9%) and excisional biopsy and cryotherapy alone (1/9, 11%) Recurrent tumors were managed with biologic therapy (3/7, 43%) and radiotherapy (4/7, 57%). At last follow-up, disease remission was achieved in 5/9 (56%) and stable disease in 4/9 (44%) patients. Excision and cryotherapy as a sole treatment modality was associated with earlier recurrence (Log rank P = 0.003).
DISCUSSION
Demographics and subtype
This retrospective study identified 133 patients with OAL at a single tertiary care center. To our knowledge, this is the largest reported single-center study analyzing clinical, pathological and treatment data in patients with OAL.
Most patients in this cohort were older adults with a median age of 65 years, with a female predominance overall (59%, female: male ratio 1.5:1). This sex distribution was present in most subtypes of lymphoma with the exception of MCL, consistent with previous studies.[12,13,14,15] DLBCL had the oldest age at presentation with a median of 76 years, also in line with published literature.[27] Autoimmune disease, which has a documented association with OAL, was noted in only one patient with EMZL in this series, which likely reflects a regional variation and an incomplete clinical data in our cohort.[2,3]
Patients with OAL commonly present with a mass lesion on clinical examination or imaging, associated with proptosis, ptosis, diplopia, decreased visual acuity, “salmon-patch” mass, and tearing.[11] The frequent mild nature of presenting symptoms may account for prolonged time to diagnosis, with an average time from symptoms to consultation of 12 months in our cohort. This is compatible with a previous study by Demirci et al. in which 160 patients were assessed and found the mean duration of symptoms at 6 months (range 0–12 months).[11] The insidious clinical course of OAL is hypothesized to be secondary to the high tumor cellularity and low extracellular connective stroma, which allows the tumor to mold rather than distort to the globe and adjacent orbital structures.[28]
The OAL subtype distribution in this cohort was in line with previously described series. Nearly all lymphomas were of the B-cell type (99%), with the majority being low-grade lymphomas (EMZL [70%] and FL [16%]). Although the frequency of CLL/SLL in our study was slightly higher than DLBCL (5% vs. 4%, respectively) as compared with previously published data, the frequencies for these two lymphomas are known to range between 5% and 10%, which is in keeping with our study results.[13,17,20,21,29]
The overall distribution of OAL in this study was consistent with previously published data.[11,29,30] Over half of OAL involved the orbital fibroadipose tissue (64%), with 37% confined to the orbital fibroadipose tissue alone, 14% involving the lacrimal gland alone, and 14% involving the orbital soft tissue and other sites. The second most common location was conjunctiva, accounting for 32% of lymphoma in our series. Bilateral involvement was seen in 14% of patients and was not associated with lymphoma subtype (P = 0.366).
Ann Arbor is the most commonly used staging system for non-Hodgkin lymphoma. By Ann Arbor staging, most patients with OAL fall into IE category. The Ophthalmic Oncology Task Force of the American Joint Committee on Cancer 8th edition has proposed a new TNM staging system for OAL, which is more site-specific and allows for better delineation of the local extent of tumor.[22] However, this differentiation is not helpful for predicting systemic prognosis and has not been widely adopted.[31] In our study, most OAL were Ann Arbor stage IE (54/78, 69%) and T1 (27/78, 35%) or T2 (49/78, 63%), N0 (55/78, 71%), and M0 (71/78, 91%) by AJCC classification, reflecting the predominance of EMZL lymphoma [Table 4], similar to the previously published data.[31]
Treatment
The management of OAL is heterogeneous, as reflected by multiple treatment regimens in this study. The level of evidence for optimal management of OAL is generally low, leading to ambiguity on the optimal treatment of these patients. Most therapeutic decisions are based on tumor size, extent, subtype, and systemic involvement. Prior studies that included data on multiple histologic subtypes of OAL or non-EMZL lymphomas documented lower local control rates, disease-free survival rates, and overall survival rates when compared with the studies focused solely on EMZL.[32] The adverse outcomes of higher-grade lymphomas justify the need for more aggressive systemic therapy, usually as a combination therapy. In our study, high-grade lymphomas with available information (DLBCL and MCL) were predominantly treated with chemotherapy, EBRT + biologics, EBRT + chemotherapy, or chemotherapy + BMT.
For indolent and low-grade OALs, we prefer local therapies with surgical excision and/or radiotherapy, whereas if systemic involvement is present, we tend to employ systemic biologics or chemotherapy. Aggressive subtypes (MCL, DLBCL) are treated with systemic therapy, even when localized to the OA. Patients with advanced disease (stage IIIE and IVE) and systemic involvement underwent more aggressive treatments for OAL, including targeted biologic therapy (33%), systemic chemotherapy (8%), and combination therapies, such as allogenic BMT (8%). However, management was overall quite heterogeneous, in part reflective of a lack of randomized clinical studies and international consensus guidelines.
The heterogeneity in OAL management also stems from the need for individualized therapy, which is based on the patient-specific variables, tumor-related factors (size, location, symptoms, histopathology, and disease extent), and treatment risk-benefit considerations, which should all be balanced to optimize results. The most important prognosticators for survival after OAL are histological subtype and the extent of systemic involvement; when evaluating treatment regimens, these two factors must be taken into consideration.[20,33] Currently, a complete diagnostic workup includes tumor biopsy, computed tomography (CT) or magnetic resonance imaging (MRI), skull base to midthigh positron emission tomography (PET)/CT, bone-marrow biopsy, and hematology/chemistry laboratory studies.[32,34,35]
Excision with cryotherapy
Adequate tumor biopsy is mandatory for accurate diagnosis. Of note, enough tissue should be excised for both histopathology/immunohistochemistry (formalin-fixed) and flow cytometry (fresh, nonfixed tissue). Although “excision” has been attempted in circumscribed and easily accessible tumors, lymphoma is an infiltrative malignancy, with potential for local recurrence (up to 25% at 4 years) following excision without adjuvant therapy.[36] The propensity for local OAL recurrence following excision and cryotherapy as the sole treatment modality was validated in this series; adjunctive EBRT in localized, indolent cases should be considered in most cases unless the patient is of very advanced age. Furthermore, attempts at excision of diffuse lesions can lead to surgical morbidity and have been shown to have null effect on survival.[37]
The “wait and watch” approach for indolent OAL subtypes has been evaluated in several studies.[15,31] In a retrospective study by Tanimoto et al., patients with asymptomatic EMZL who were initially managed with biopsy or surgical excision alone showed results similar to those reported with immediate radiotherapy in terms of disease progression, high-grade transformation, and lymphoma-related mortality.[37] It is therefore reasonable to consider a conservative approach with frequent follow-up in elderly, asymptomatic patients or those with severe comorbidities, since the risk of progression to higher grade lymphoma or death related to lymphoma is low.
Radiotherapy
Adjuvant therapy can help control local recurrence in low-grade lymphomas. EBRT is the treatment of choice for the vast majority of localized low-grade OAL, with a potential for local disease control as high as 100% and a low recurrence rate that does not exceed 15%.[38,39,40,41] The reliance on EBRT is seen in our series, with half of the patients managed with this therapeutic modality. According to the current National Cancer Center Network consensus guidelines, the suggested radiation dose is 20–30 Gy.[42] Early radiation toxicity is manageable and typically consists of cutaneous or conjunctival inflammatory reactions, while long-term complications are observed in 50% patients and include cataract formation (30%–50%) and xerophthalmia (20%-40%).[42] The two main radiation doses in our study were standard-dose radiation, which varies from 24 to 30 Gy fractioned in 15 sessions, and an ULDR, which consists of (4 Gy) in 2 sessions. Some literature suggests that complete response to ULDR may be initially achievable in the near term, but long-term implications for recurrence and survival have yet to be determined.[43] Although a debate exists about the optimal dose, the small sample size of the cohort with recurrent lymphoma precluded statistical comparison of these two radiation schemes.
Biologic therapy
Rituximab (Mabthera/Rituxan, Roche, Basel, Switzerland) is a chimeric IgG1 monoclonal antibody directed against CD20 surface antigen on B lymphocytes, thought to mediate its anti-lymphoma effect through antibody and complement-dependent cytotoxicity and inducing apoptosis.[44,45] Twenty seven percent of patients in our study received rituximab, either as monotherapy (17%) or in combination (10%) with other treatments. Patients with bilateral lymphoma in this cohort were more likely to receive rituximab when compared to those with unilateral disease (38% vs. 10%, respectively P = 0.0709). Single-institution studies have shown response rates in the range of 30%–87% with rituximab monotherapy for the treatment of OAL, although long-term local control may be less than with regional radiotherapy.[45,46,47,48,49,50,51,52,53,54]
Ibrutinib (Imbruvica, AbbVie, North Chicago, USA), is an irreversible, small-molecule inhibitor of Bruton tyrosine kinase, which stimulates pathways essential for B-cell survival and proliferation. Initially approved as a first-line therapy for CLL/SLL and as a therapy for relapsed/refractory MCL, ibrutinib has been shown to have efficacy in other B-cell lymphomas, including lymphoplasmacytic lymphoma, EMZL, and FL.[55] In our cohort, one patient with CLL/SLL was managed with ibrutinib as a first-line therapy with excellent response.
Chemoimmunotherapy
The level of evidence supporting the use of chemoimmunotherapy as a treatment for OAL is low and limited to small prospective studies of patients that did not respond to local therapy.[56] In our study, the lack of consensus guidelines was evidenced by the high variability in the treatment regimens of these patients.
The frequently used chemotherapy regimens include CHOP (cyclophosphamide, adriamycin, procarbazine, and prednisone), COP/CVP (cyclophosphamide, vincristine and prednisone), C-MOPP (cyclophosphamide, vincristine, pro-carbazine and prednisone) and chlorambucil.[26,36,57,58] The most preferred agent is chlorambucil, as it can be used in combination with other agents, causing low toxicity.[17] A recent IELSG19 trial, the largest randomized trial for EMZL lymphoma, compared chlorambucil vs. rituximab-chlorambucil combination.[56] This trial demonstrated a higher complete remission rate in the combined arm (78% vs. 65%) and better 5-year event-free survival (68% versus 50%), suggesting that combination therapy is more effective.[56]
CONCLUSION
In this 5-year retrospective single-institution study, we characterized orbital and ocular adnexal lymphomas in terms of the clinical presentation, histopathologic findings, management, and outcomes. The vast majority of OALs were low-grade B-cell lymphomas with a low risk of dissemination or high-grade transformation. Most tumors were successfully treated with local therapies, such as EBRT, while higher grade or higher stage lesions required more aggressive systemic treatments, including biologics, chemotherapy or immunotherapy, highlighting the individualized approach in OAL management. Finally, long-term follow-up of indolent OALs is necessary to monitor for local recurrence, systemic spread, or transformation to a high-grade histopathology. Excision and cryotherapy as sole treatment modalities were associated with shorter interval to recurrence in this analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Management of ocular adnexal lymphoma by lymphoma subtype
Management of ocular adnexal lymphoma by treatment type
Management of ocular adnexal lymphoma by Ann Arbor stage
Recurrence- free survival of patients with orbital and ocular adnexal lymphomas
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. 4th ed. Vol. 1. Lyon: International Agency for Research on Cancer (IARC); 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; pp. 363–7. [Google Scholar]
- 2.Wöhrer S, Troch M, Streubel B, Zwerina J, Skrabs C, Formanek M, et al. MALT lymphoma in patients with autoimmune diseases: A comparative analysis of characteristics and clinical course. Leukemia. 2007;21:1812–8. doi: 10.1038/sj.leu.2404782. [DOI] [PubMed] [Google Scholar]
- 3.Ponzoni M, Govi S, Licata G, Mappa S, Giordano Resti A, Politi LS, et al. A reappraisal of the diagnostic and therapeutic management of uncommon histologies of primary ocular adnexal lymphoma. Oncologist. 2013;18:876–84. doi: 10.1634/theoncologist.2012-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coupland SE. Molecular pathology of lymphoma. Eye (Lond) 2013;27:180–9. doi: 10.1038/eye.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CC. Primary intraocular lymphoma: Clinical features, diagnosis, and treatment. Clin Lymphoma. 2003;4:30–1. doi: 10.1016/s1526-9655(11)70005-7. [DOI] [PubMed] [Google Scholar]
- 6.Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Exp Ophthalmol. 2008;36:564–78. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 7.Davis JL. Diagnosis of intraocular lymphoma. Ocul Immunol Inflamm. 2004;12:7–16. doi: 10.1076/ocii.12.1.7.28072. [DOI] [PubMed] [Google Scholar]
- 8.Bairey O, Kremer I, Rakowsky E, Hadar H, Shaklai M. Orbital and adnexal involvement in systemic non-Hodgkin's lymphoma. Cancer. 1994;73:2395–9. doi: 10.1002/1097-0142(19940501)73:9<2395::aid-cncr2820730924>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Moslehi R, Coles FB, Schymura MJ. Descriptive epidemiology of ophthalmic and ocular adnexal non-Hodgkin's lymphoma. Expert Rev Ophthalmol. 2011;6:175–80. doi: 10.1586/eop.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111:997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Demirci H, Shields CL, Karatza EC, Shields JA. Orbital lymphoproliferative tumors: Analysis of clinical features and systemic involvement in 160 cases. Ophthalmology. 2008;115:1626–31. doi: 10.1016/j.ophtha.2008.02.004. 1631.e1-3. [DOI] [PubMed] [Google Scholar]
- 12.White WL, Ferry JA, Harris NL, Grove AS., Jr Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa-associated lymphoid tissue type. Ophthalmology. 1995;102:1994–2006. doi: 10.1016/s0161-6420(95)30764-6. [DOI] [PubMed] [Google Scholar]
- 13.Coupland SE, Krause L, Delecluse HJ, Anagnostopoulos I, Foss HD, Hummel M, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998;105:1430–41. doi: 10.1016/S0161-6420(98)98024-1. [DOI] [PubMed] [Google Scholar]
- 14.Knowles DM, Jakobiec FA, McNally L, Burke JS. Lymphoid hyperplasia and malignant lymphoma occurring in the ocular adnexa (orbit, conjunctiva, and eyelids): A prospective multiparametric analysis of 108 cases during 1977 to 1987. Hum Pathol. 1990;21:959–73. doi: 10.1016/0046-8177(90)90181-4. [DOI] [PubMed] [Google Scholar]
- 15.Andrew NH, Coupland SE, Pirbhai A, Selva D. Lymphoid hyperplasia of the orbit and ocular adnexa: A clinical pathologic review. Surv Ophthalmol. 2016;61:778–90. doi: 10.1016/j.survophthal.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Demirci H, Shields CL, Shields JA, Honavar SG, Mercado GJ, Tovilla JC. Orbital tumors in the older adult population. Ophthalmology. 2002;109:243–8. doi: 10.1016/s0161-6420(01)00932-0. [DOI] [PubMed] [Google Scholar]
- 17.Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114:501–10. doi: 10.1182/blood-2008-12-195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannami T, Yoshino T, Oshima K, Takase S, Kondo E, Ohara N, et al. Clinical, histopathological, and immunogenetic analysis of ocular adnexal lymphoproliferative disorders: Characterization of malt lymphoma and reactive lymphoid hyperplasia. Mod Pathol. 2001;14:641–9. doi: 10.1038/modpathol.3880366. [DOI] [PubMed] [Google Scholar]
- 19.Ferreri AJ, Dolcetti R, Du MQ, Doglioni C, Resti AG, Politi LS, et al. Ocular adnexal MALT lymphoma: An intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann Oncol. 2008;19:835–46. doi: 10.1093/annonc/mdm513. [DOI] [PubMed] [Google Scholar]
- 20.Auw-Haedrich C, Coupland SE, Kapp A, Schmitt-Gräff A, Buchen R, Witschel H. Long term outcome of ocular adnexal lymphoma subtyped according to the REAL classification. Revised European and American Lymphoma. Br J Ophthalmol. 2001;85:63–9. doi: 10.1136/bjo.85.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldini L, Blini M, Guffanti A, Fossati V, Colombi M, La Targia ML, et al. Treatment and prognosis in a series of primary extranodal lymphomas of the ocular adnexa. Ann Oncol. 1998;9:779–81. doi: 10.1023/a:1008327301372. [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK. 8th ed. Springer - New York, NY, USA: Springer International Publishing; 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 23.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 24.Skarin AT, Dorfman DM. Non-Hodgkin's lymphomas: Current classification and management. CA Cancer J Clin. 1997;47:351–72. doi: 10.3322/canjclin.47.6.351. [DOI] [PubMed] [Google Scholar]
- 25.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 26.Kennerdell JS, Flores NE, Hartsock RJ. Low-dose radiotherapy for lymphoid lesions of the orbit and ocular adnexa. Ophthalmic Plast Reconstr Surg. 1999;15:129–33. doi: 10.1097/00002341-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: An analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol. 2014;89:310–4. doi: 10.1002/ajh.23638. [DOI] [PubMed] [Google Scholar]
- 28.Eagle R. Eye Pathology an Atlas and Text. 3rd ed. Philadelphia, PA, USA: Wolters Kluwer; 2017. [Google Scholar]
- 29.Ferry JA, Fung CY, Zukerberg L, Lucarelli MJ, Hasserjian RP, Preffer FI, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31:170–84. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- 30.Alkatan HM, Alaraj A, El-Khani A, Al-Sheikh O. Ocular adnexal lymphoproliferative disorders in an ophthalmic referral center in Saudi Arabia. Saudi J Ophthalmol. 2013;27:227–30. doi: 10.1016/j.sjopt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graue GF, Finger PT, Maher E, Della Rocca D, Della Rocca R, Lelli GJ, Jr, et al. Ocular adnexal lymphoma staging and treatment: American Joint Committee on Cancer versus Ann Arbor. Eur J Ophthalmol. 2013;23:344–55. doi: 10.5301/ejo.5000224. [DOI] [PubMed] [Google Scholar]
- 32.Yen MT, Bilyk JR, Wladis EJ, Bradley EA, Mawn LA. Treatments for ocular adnexal lymphoma: A report by the American academy of ophthalmology. Ophthalmology. 2018;125:127–36. doi: 10.1016/j.ophtha.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 33.Nola M, Pavletic SZ, Weisenburger DD, Smith LM, Bast MA, Vose JM, et al. Prognostic factors influencing survival in patients with B-cell small lymphocytic lymphoma. Am J Hematol. 2004;77:31–5. doi: 10.1002/ajh.20137. [DOI] [PubMed] [Google Scholar]
- 34.Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol. 2019;64:45–66. doi: 10.1016/j.survophthal.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Thuro BA, Ning J, Peng SA, Pace ST, Dudeja G, Ozgur O, et al. Rates of positive findings on positron emission tomography and bone marrow biopsy in patients with ocular adnexal lymphoma. Ophthalmic Plast Reconstr Surg. 2017;33:355–60. doi: 10.1097/IOP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 36.Uno T, Isobe K, Shikama N, Nishikawa A, Oguchi M, Ueno N, et al. Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: A multiinstitutional, retrospective review of 50 patients. Cancer. 2003;98:865–71. doi: 10.1002/cncr.11539. [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto K, Kaneko A, Suzuki S, Sekiguchi N, Maruyama D, Kim SW, et al. Long-term follow-up results of no initial therapy for ocular adnexal MALT lymphoma. Ann Oncol. 2006;17:135–40. doi: 10.1093/annonc/mdj025. [DOI] [PubMed] [Google Scholar]
- 38.Bolek TW, Moyses HM, Marcus RB, Jr, Gorden L, 3rd, Maiese RL, Almasri NM, et al. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys. 1999;44:31–6. doi: 10.1016/s0360-3016(98)00535-5. [DOI] [PubMed] [Google Scholar]
- 39.Le QT, Eulau SM, George TI, Hildebrand R, Warnke RA, Donaldson SS, et al. Primary radiotherapy for localized orbital MALT lymphoma. Int J Radiat Oncol Biol Phys. 2002;52:657–63. doi: 10.1016/s0360-3016(01)02729-8. [DOI] [PubMed] [Google Scholar]
- 40.Liao SL, Kao SC, Hou PK, Chen MS. Results of radiotherapy for orbital and adnexal lymphoma. Orbit. 2002;21:117–23. doi: 10.1076/orbi.21.2.117.7192. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M, Kojima M, Shioya M, Tamaki Y, Saitoh J, Sakurai H, et al. Treatment results of radiotherapy for malignant lymphoma of the orbit and histopathologic review according to the WHO classification. Int J Radiat Oncol Biol Phys. 2003;57:172–6. doi: 10.1016/s0360-3016(03)00506-6. [DOI] [PubMed] [Google Scholar]
- 42.Yahalom J, Illidge T, Specht L, Hoppe RT, Li YX, Tsang R, et al. Modern radiation therapy for extranodal lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:11–31. doi: 10.1016/j.ijrobp.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Pinnix CC, Dabaja BS, Milgrom SA, Smith GL, Abou Z, Nastoupil L, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck. 2017;39:1095–100. doi: 10.1002/hed.24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 45.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: Ways to improve rituximab efficacy. Blood. 2004;104:2635–42. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 46.Tuncer S, Tanyıldız B, Basaran M, Buyukbabani N, Dogan O. Systemic rituximab immunotherapy in the management of primary ocular adnexal lymphoma: Single institution experience. Curr Eye Res. 2015;40:780–5. doi: 10.3109/02713683.2014.959605. [DOI] [PubMed] [Google Scholar]
- 47.Morgensztern D, Rosado MF, Serafini AN, Lossos IS. Somatostatin receptor scintigraphy in MALT lymphoma of the lacrimal gland treated with rituximab. Leuk Lymphoma. 2004;45:1275–8. doi: 10.1080/10428190310001641233. [DOI] [PubMed] [Google Scholar]
- 48.Nückel H, Meller D, Steuhl KP, Dührsen U. Anti-CD20 monoclonal antibody therapy in relapsed MALT lymphoma of the conjunctiva. Eur J Haematol. 2004;73:258–62. doi: 10.1111/j.1600-0609.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferreri AJ, Ponzoni M, Martinelli G, Muti G, Guidoboni M, Dolcetti R, et al. Rituximab in patients with mucosal-associated lymphoid tissue-type lymphoma of the ocular adnexa. Haematologica. 2005;90:1578–9. [PubMed] [Google Scholar]
- 50.Benetatos L, Alymara V, Asproudis I, Bourantas KL. Rituximab as first line treatment for MALT lymphoma of extraocular muscles. Ann Hematol. 2006;85:625–6. doi: 10.1007/s00277-006-0134-0. [DOI] [PubMed] [Google Scholar]
- 51.Heinz C, Merz H, Nieschalk M, Mueller-Miny H, Koch P, Heiligenhaus A. Rituximab for the treatment of extranodal marginal zone B-cell lymphoma of the lacrimal gland. Br J Ophthalmol. 2007;91:1563–4. doi: 10.1136/bjo.2007.115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conconi A, Martinelli G, Thiéblemont C, Ferreri AJ, Devizzi L, Peccatori F, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102:2741–5. doi: 10.1182/blood-2002-11-3496. [DOI] [PubMed] [Google Scholar]
- 53.Raderer M, Jäger G, Brugger S, Püspök A, Fiebiger W, Drach J, et al. Rituximab for treatment of advanced extranodal marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue lymphoma. Oncology. 2003;65:306–10. doi: 10.1159/000074641. [DOI] [PubMed] [Google Scholar]
- 54.Lossos IS, Morgensztern D, Blaya M, Alencar A, Pereira D, Rosenblatt J. Rituximab for treatment of chemoimmunotherapy naive marginal zone lymphoma. Leuk Lymphoma. 2007;48:1630–2. doi: 10.1080/10428190701457949. [DOI] [PubMed] [Google Scholar]
- 55.Aalipour A, Advani RH. Bruton tyrosine kinase inhibitors: A promising novel targeted treatment for B cell lymphomas. Br J Haematol. 2013;163:436–43. doi: 10.1111/bjh.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zucca E, Conconi A, Laszlo D, López-Guillermo A, Bouabdallah R, Coiffier B, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol. 2013;31:565–72. doi: 10.1200/JCO.2011.40.6272. [DOI] [PubMed] [Google Scholar]
- 57.Daibata M, Nemoto Y, Togitani K, Fukushima A, Ueno H, Ouchi K, et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br J Haematol. 2006;132:651–2. doi: 10.1111/j.1365-2141.2005.05943.x. [DOI] [PubMed] [Google Scholar]
- 58.Gayed I, Eskandari MF, McLaughlin P, Pro B, Diba R, Esmaeli B. Value of positron emission tomography in staging ocular adnexal lymphomas and evaluating their response to therapy. Ophthalmic Surg Lasers Imaging. 2007;38:319–25. doi: 10.3928/15428877-20070701-08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Management of ocular adnexal lymphoma by lymphoma subtype
Management of ocular adnexal lymphoma by treatment type
Management of ocular adnexal lymphoma by Ann Arbor stage
Recurrence- free survival of patients with orbital and ocular adnexal lymphomas


