Abstract
BACKGROUND:
Afatinib is an oral, irreversible ErbB family blocker indicated for first-line treatment of metastatic non-small cell lung cancer (NSCLC) in patients with exon 19 deletion mutations or exon 21 substitutions in the epidermal growth factor receptor (EGFR). Afatinib is also approved for the treatment of metastatic squamous NSCLC following progression on platinum-based chemotherapy. Common afatinib-associated toxicities include gastrointestinal and dermatologic events, which can be dose limiting.
OBJECTIVES:
In this review, we describe clinical trial experience with afatinib, as well as best practices and practical approaches to the management of afatinib-associated adverse events in EGFR mutation-positive NSCLC.
METHODS:
Safety and tolerability data from phase 3 trials of afatinib were reviewed, together with real-life experience from our own clinical practice.
FINDINGS:
Patient education, combined with early assessment and effective management of afatinib-related adverse events as well as dose-reduction strategies, allows patients to continue treatment and maximize the clinical benefits of afatinib.
Keywords: afatinib, non-small cell lung cancer, adverse events, dose reduction, patient education
Introduction
Oncology treatments are designed to provide safety while achieving maximum efficacy. In the era of targeted therapies, patients often continue treatment chronically for extended durations. Chronic treatment may cause different toxicities than those reported in clinical trials and, because tolerability varies among patients, dose optimization schemes can help to balance long-term clinical benefit with safety (Dy & Adjei, 2013). Oncology nurses and other advanced oncology practitioners are instrumental in achieving optimal clinical outcomes through patient education, early assessment, and management of potential adverse events (AEs); strategies include supportive care and dose interruption/modifications.
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer mortality worldwide (World Health Organization, 2017). The mutated form of the epidermal growth factor receptor (EGFR/HER1/ErbB1) is the best characterized oncogenic driver in NSCLC, and activating mutations have been reported in 10–50% of cases; most commonly exon 19 deletions (del19; 60%), and exon 21 (L858R) substitutions (35%) where leucine is replaced by arginine at position 858 (Chan & Hughes, 2015). Targeted therapies to inhibit mutant EGFR include tyrosine kinase inhibitors such as gefitinib, erlotinib, osimertinib, and afatinib. Afatinib (GILOTRIF®; Boehringer Ingelheim; Ingelheim, Germany) is an oral, irreversible inhibitor of EGFR and all other members of the ErbB family of tyrosine kinases (HER2 [ErbB2], HER3 [ErbB3] and HER4 [ErbB4]). Afatinib is indicated for the first-line treatment of metastatic NSCLC in patients whose disease harbors common EGFR mutations (del19 or L858R), as identified by a US Food and Drug Administration-approved test (Boehringer Ingelheim, 2016a). Afatinib is also approved in Europe for EGFR tyrosine kinase inhibitor (TKI)-naive adult patients with locally advanced/metastatic NSCLC with activating EGFR mutation(s), including less common mutations in exon 18 (G719X) and exon 21 (L861Q) (Boehringer Ingelheim, 2016b). Phase 3 afatinib trials showed improved efficacy versus traditional gold-standard chemotherapy, and a manageable safety profile in patients with advanced EGFR mutation-positive NSCLC (Sequist et al., 2013; Y. L. Wu et al., 2014). A similar safety profile was observed with afatinib in patients with SCC of the lung (Soria et al., 2015). The safety profile of afatinib is similar to that of first-generation EGFR-targeted therapies and primarily includes gastrointestinal and dermatologic AEs (Park et al., 2016; Sequist et al., 2013; Soria et al., 2015; Y. L. Wu et al., 2014). These AEs can be bothersome to the patient; education, early detection, and effective management is required to optimize the benefits of afatinib therapy. Herein we describe clinical trial experience with afatinib, as well as best practices and additional practical approaches to the management of afatinib-associated AEs among patients with EGFR mutation-positive NSCLC.
Afatinib Safety Profile: Randomized Clinical Trial Experiences
The first-line use of afatinib in patients with EGFR mutation-positive NSCLC is supported by two randomized phase 3 trials, LUX-Lung 3 (LL3) and LUX-Lung 6 (LL6), and a randomized phase 2b trial, LUX-Lung 7 (LL7) (Sequist et al,. 2013; Wu et al., 2014; Park et al., 2016). Briefly, in both LL3 and LL6, afatinib significantly improved median progression-free survival (PFS) and overall survival (OS; in patients with del-19 positive tumors only) versus platinum-doublet chemotherapy in this setting (Sequist et al., 2013; Wu et al., 2014; J. C. H. Yang et al., 2015). In LL7, PFS was significantly improved with afatinib versus gefitinib in patients with treatment-naïve advanced EGFR mutation-positive NSCLC (Park et al., 2016). The use of afatinib in patients with squamous cell carcinoma (SCC) of the lung following failure of chemotherapy is supported by the phase 3 LUX-Lung 8 (LL8) trial, which demonstrated improved PFS and OS with afatinib versus erlotinib (TARCEVA®; Genentech, South San Francisco, CA) in this setting (Soria et al., 2015).
Common treatment-related AEs
Treatment-related AEs reported for ≥15% of patients in any of the LL3, 6, 7, and 8 trials are shown in Table 1. Across all trials, the most common TRAEs (all grades) were diarrhea (70–95%), rash/acne (67–89%), and stomatitis/mucositis (29–72%). Fewer grade ≥3 AEs were reported in LL6 than LL3, possibly because of the higher average patient enrollment per site in LL6, allowing the medical teams greater drug experience to improve their use of AE mitigation strategies in subsequent patients. Additionally, patient populations differed between LL3 (Caucasians, Eastern Asians) and LL6 (Southeast Asians, South Koreans, and Chinese). Higher EGFR mutation rates have been reported for Asians versus other ethnicities (Dearden, Stevens, Wu, & Blowers, 2013); consequently, physicians from those regions may be more used to managing EGFR TKI-related toxicities.
Table 1.
Most common treatment-related AEs, frequency of dose reductions, and frequency of discontinuations due to AEs in LL3, 6, 7, and 8
| LUX-Lung 3 (N=229) | LUX-Lung 6 (N=239) | LUX-Lung 7 (N=160) | LUX-Lung 8 (N=392) | |||||
|---|---|---|---|---|---|---|---|---|
| All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | |
| Total TRAEs | - | 112 (48.9) | 236 (98.7) | 86 (36.0) | 156 (97.5) | 50 (31.3) | 366 (93.4) | 104 (26.5) |
| Diarrhea | 218 (95.2) | 33 (14.4) | 211 (88.3) | 13 (5.4) | 144 (90.0) | 20 (12.5) | 274 (69.9) | 41 (10.5) |
| Rash/acne* | 204 (89.1) | 37 (16.2) | 193 (80.8) | 35 (14.6) | 142 (88.8) | 15 (9.4) | 263 (67.1) | 23 (5.9) |
| Stomatitis/mucositis* | 165 (72.1) | 20 (8.7) | 124 (51.9) | 13 (5.4) | 103 (64.4) | 7 (4.4) | 113 (28.8) | 16 (4.1) |
| Paronchyia* | 130 (56.8) | 26 (11.4) | 78 (32.6) | 0 | 89 (55.6) | 3 (1.9) | 41 (10.5) | 2 (0.5) |
| Dry skin | 67 (29.3) | 1 (0.4) | - | - | 52 (32.5) | 0 | 34 (8.7) | 2 (0.5) |
| Decreased appetite | 47 (20.5) | 7 (3.1) | 24 (10.0) | 3 (1.3) | 25 (15.6) | 1 (0.6) | 50 (12.8) | 3 (0.8) |
| Pruritis | 43 (18.8) | 1 (0.4) | 26 (10.9) | 1 (0.4) | 37 (23.1 | 0 | 32 (8.2) | 1 (0.3) |
| Nausea | 41 (17.9) | 2 (0.9) | 18 (7.5) | 0 | 26 (16.3) | 2 (1.3) | 52 (13.3) | 4 (1.0) |
| Fatigue* | 40 (17.5) | 3 (1.3) | 24 (10.0) | 1 (0.4) | 33 (20.6) | 9 (5.6) | 62 (15.8) | 6 (1.5) |
| Vomiting | 39 (17.0) | 7 (3.1) | 23 (9.6) | 2 (0.8) | 17 (10.6) | 0 | 31 (7.9) | 3 (0.8) |
| Frequency of dose reductions | 120 (52.4) | 67 (28.0) | 63 (39.4) | 104 (26.5) | ||||
| Frequency of discontinuations due to AEs | 23 (10.0) | 21 (8.8) | 18 (11.3) | 68 (17.3) | ||||
Includes events that occurred in ≥15% of patients treated with afatinib in any trial.
AE, adverse event
group term
Tolerability guided dose modifications
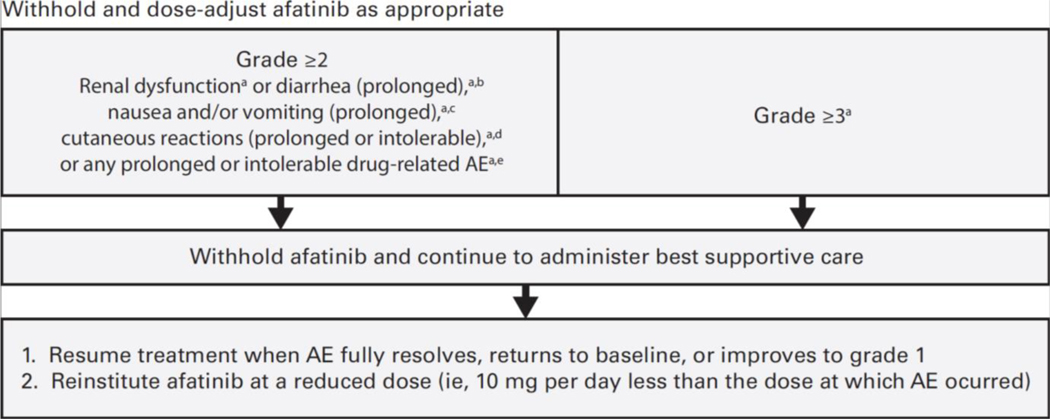
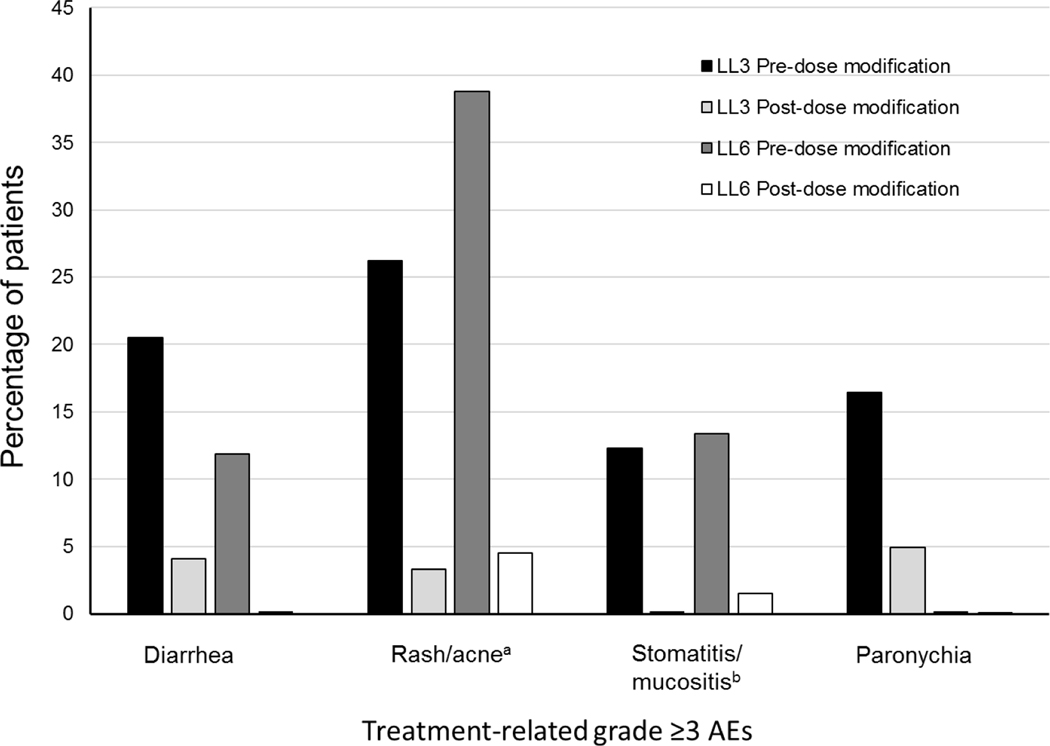
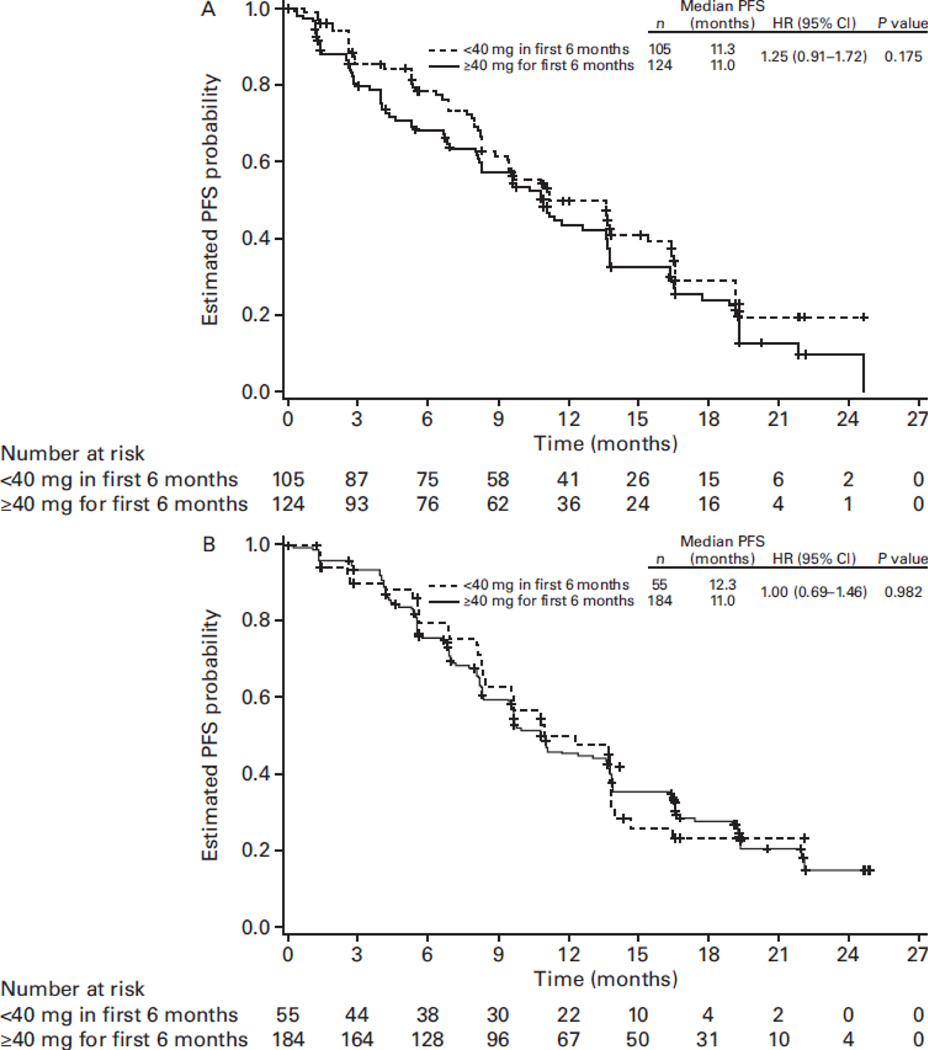
In all four studies, patients started afatinib at 40 mg daily and underwent a predefined dose-modification scheme in cases of grade ≥3 or select grade ≥2 treatment-related AEs (TRAEs) (Figure 1) (Park et al., 2016; Sequist et al., 2013; Soria et al., 2015; Wu et al., 2014). Afatinib treatment was interrupted and supportive care administered until the AE fully resolved, returned to baseline, or improved to grade 1. Subsequently, afatinib was resumed at 10 mg less than the dose at which the AE occurred. Afatinib was permanently discontinued for AEs that did not improve after dose interruption, or severe/intolerable AEs occurring at 20 mg/day; confirmed interstitial lung disease (ILD); severe drug-induced hepatic impairment; persistent ulcerative keratitis; symptomatic left ventricular dysfunction; life-threatening bullous, blistering, or exfoliative skin lesions. The incidence of dose reductions across the four trials is presented in Table 1. Post-hoc analyses of LL3 and 6 showed that tolerability-guided dose reductions reduced AE incidence and severity (Figure 2); the incidence of grade 3 AE recurrence across both trials was also low, at 0.4–1.7% (Wu et al.). Moreover, tolerability-guided dose adjustment effectively reduced TRAEs without reducing therapeutic efficacy: median PFS was similar between patients who dose reduced during the first 6 months of treatment and those who did not (LL3: 11.3 vs 11.0 months; Figure 3) (Yang et al., 2016).
Figure 1. Dose-modification scheme for afatinib-related grade ≥2 AEs.
AE, adverse event. aNational Cancer Institute Common Terminology Criteria for Adverse Events, v3.0. bGrade ≥2 diarrhea persisting ≥48 hours while taking antidiarrheal medication. cGrade ≥2 nausea and/or vomiting persisting for ≥7 days despite antiemetic treatment/hydration. dGrade 2 cutaneous reactions persisting >7 days. eGrade 2 drug-related AE persisting ≥7 days.
Figure 2. Incidence of grade 3 AEs pre- and post-dose modification in LUX-Lung 3 (LL3) and LUX-Lung 6 (LL6).
AE, adverse event; aGrouped term (dermatitis acneiform, skin fissures, folliculitis, skin exfoliation, dermatitis, erythema, skin reaction, rash pustular, skin ulcer, rash maculopapular, rash pruritic, dermatosis, drug eruption, skin toxicity, acne pustular, exfoliative rash, rash erythematous, rash follicular, rash generalized, rash macular, skin disorder, skin erosion, skin lesion, eczema). bGrouped term (mucosal inflammation, mouth ulceration, dry mouth, tongue ulceration, aphthous stomatitis, glossitis, glossodynia, mucous membrane disorder, oral mucosal erythema, throat irritation).
Figure 3. PFS in patients who had dose reductions within the first 6 months and those who remained on afatinib ≥40 mg once daily in LUX-Lung 3 (A) and LUX-Lung 6 (B).
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival. Figure reproduced from J. C-H. Yang. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103–2110, by permission of Oxford University Press.
Discontinuations due to drug-related AEs
The frequency of drug-discontinuation due to AEs for all four trials is presented in Table 1. In both LL3 and LL6, drug-related discontinuations due to the most common AEs were low. In LL3, of the most common afatinib-associated AEs, only diarrhea (1.3%) and paronychia (0.9%) led to treatment discontinuation (Sequist et al., 2013). In LL6, no patient permanently discontinued afatinib because of diarrhea; 2.1% discontinued due to rash/acne (Wu et al., 2014). The low discontinuation rate may have been attributable to effective utilization of the tolerability-guided dose modification protocol resulting in infrequent AE recurrence and allowing prolonged afatinib treatment. Taken together, the low discontinuation rate, low AE recurrence rate after dose reduction, and equivalent efficacy in those who dose-reduced indicate that careful monitoring, early recognition and proactive management of AEs, alongside dose optimization, if necessary can provide patients the best opportunity to continue afatinib and maintain both their quality of life and anticancer benefits.
Managing Toxicities: Real-World Clinical Practice Experience with Afatinib
AE rates reported during clinical studies may not necessarily equate to those in real-world clinical settings. Clinicians with greater familiarity with a particular agent will be able to better manage its specific safety and tolerability profile. Therefore, clinicians and patients using afatinib need to be properly informed in order to manage common AEs through supportive care measures and dose optimization. In terms of dose optimization, suspending afatinib dosing until AE resolution and reinstating at a lower dose is key to the successful early management of moderate to severe (grades 2 and 3) toxicities (Boehringer Ingelheim, 2016a; Boehringer Ingelheim, 2016b; Yang et al., 2016). In some cases, temporary interruption of dosing for 7–14 days may be sufficient; if symptoms improve, the patient may be able to resume full-dose afatinib in conjunction with supportive care.
Management of AEs via Supportive Care Measures
Provision of patient education, and frequent communication between patients and their clinical teams (suggested twice weekly during the first cycle), are essential to early detection and timely management of AEs. Strategies used successfully in clinical trials and in the clinic for the management of common afatinib-related AEs are described below. Routine monitoring, prevention, and early treatment are important in managing common afatinib-related AEs, as is prophylactic treatment in some cases.
Dermatologic AEs
Dermatologic AEs are frequent in patients receiving EGFR TKIs, because EGFR plays a critical role in skin physiology. EGFR inhibition leads to a cascade of cellular events resulting in cutaneous AEs such as rash, dry skin, pruritus, and inflammation of nail/periungual tissues (e.g., paronychia) (Califano et al., 2015). While these events are generally mild to moderate in severity (and manageable resulting in low discontinuation rates), they may impact quality of life and increase the risk of non-compliance and/or drug discontinuation (Charles et al., 2016; Rosen et al., 2013).
Specific management strategies for dermatologic afatinib-associated AEs have been proposed, depending on the AE and its severity (Lacouture et al., 2013). Topical steroids (e.g., alclometasone 0.05%, fluticasone propionate 0.05%, or hydrocortisone acetate 2.5%) and topical antibiotics (e.g., clindamycin 1–2%, erythromycin 1–2%, or metronidazole 1%), combinations of which have been found to resolve the rash completely within two weeks, are recommended for grade 1 papulopustular/acneiform rash (Fabbrocini et al., 2015). For grades ≥2 papulopustular/acneiform rash, topical steroids alongside a six-week course of oral antibiotics (e.g., doxycycline 100 mg, minocycline 100 mg, or oxytetracycline 500 mg, each twice daily) are recommended (Lacouture et al., 2013). Prophylaxis with oral antibiotics is also effective, reducing the incidence and severity of afatinib-related rash by >60% (Arrieta et al., 2015). Prophylactic measures, such as topical steroids and/or antibiotics, are also effective with other EGFR-targeting therapies, decreasing the incidence of rash by 40–50% relative to reactive treatment administered upon rash occurrence (Lacouture et al., 2010; Melosky et al., 2014). In another study, in patients treated with erlotinib for metastatic NSCLC who also received prophylactic minocycline, reactive treatment at rash initiation, or treatment only if grade 3 rash developed (control group), the overall incidence of rash was similar amongst groups (range, 82–84%), but incidence of grade 3 rash differed significantly between prophylactic and control arms (12% vs 28%; P = 0.0455) and between reactive and control arms (8% vs 28%; P = 0.0092) (Melosky et al., 2015). Furthermore, time on therapy was longer with prophylactic minocycline, and median OS was greater in prophylactic and reactive arms, although this was not considered a significant difference (Melosky et al., 2015).
For xerosis and skin fissures in the hands and feet, twice-dailyuse of prophylactic moisturizers containing ammonium lactate 12%, salicylic acid 3–6%, or urea 10–20% are recommended (Lacouture et al., 2013). Specifically, suggested treatments for xerosis, including over-the-counter moisturizing cream, and topical steroids (e.g., triamcinolone acetonide 0.025%, desonide 0.05%, alclometasone 0.05%, or fluticasone propionate 0.05%) may be required for grade ≥3 eczematous areas (Lacouture et al., 2013). Alfa-hydroxy acid-containing moisturizers may be particularly helpful for fingertip fissures. Specific management strategies for pruritus based on severity grade are included in Table 2.
Table 2.
RECOMMENDATIONS FOR MANAGEMENT OF AFATINIB-ASSOCIATED AEs
| PRURITUS (Lacouture et al., 2013) | DERMATOLOGIC ADVERSE EVENTS (Boehringer Ingelheim, 2014; Lacouture et al., 2013) | STOMATITIS/ORAL MUCOSITIS (Lalla et al., 2013; McGuire et al., 2013) | PARONYCHIA (Lacouture et al., 2013) |
|---|---|---|---|
| Recommendations for grade 1: • Topical steroid twice daily or topical antipruriticsa 4× /day Recommendations for grade 2: • Topical steroid twice daily or topical antipruriticsa 4×/day and oral antihistaminesb Recommendations for grade ≥3: • Oral antihistaminesb or GABA agonistsc or aprepitant or tricyclicsd |
Recommendations for any grade: • Early intervention with emollients (alcohol free), topical or oral (e.g., tetracycline class) antibiotics, topical or oral steroids, tacrolimus ointment, or antihistamines • Protective clothes that cover the head, face, hands, arms, and legs • Sunscreen (SPF 15) when outside; every 4 hours in sun exposure areas • Skin creams and lotions that moisturize the skin and prevent dryness, and use hypoallergenic products that do not have perfumes or preservatives • Mild bath soap that will not irritate the skin; take a bath or shower in warm (not hot) water • Wash sheets, clothing, and undergarments in mild soaps • To relieve itching, place a cool washcloth or some ice over the area that itches, rather than scratching For any grade, avoid: • Sun exposure, especially direct sunlight between 10 am and 4 pm • Certain fabrics (e.g., wool, synthetics) that can make skin itch; recommend wearing loose-fitting cotton clothing or other soft fabrics and switching to cotton bed sheets • Overheating the house, as warm dry air can make skin dry, and suggest using a humidifier |
Recommendations for any grade: • Topical steroids (e.g., dexamethasone mouth rinse), viscous lidocaine, or magic mouthwash (i.e., antihistamine or local anesthetic, antifungal, corticosteroid, and antacid) • Practice good mouth care, gently brushing teeth and gums with a soft toothbrush, and rinsing with warm salt water after every meal and at bedtime • Eat foods cold or at room temperature; hot and warm food can irritate a tender mouth • Eat soft, soothing, and moist food; suggest avoiding rough or coarse foods • Drink plenty of water and use a straw to drink liquids • Lip balm or petroleum jelly for dry lips • Numb the mouth with ice chips or flavored ice pops, as needed For any grade, avoid: • Salty, spicy, acidic, or irritating foods and juices |
Recommendations for grade 1: • Topical antibiotics/antiseptics,e vinegar soaks,f and topical ultra-potent steroidsRecommendations for grade 2: • Topical antibiotics,e vinegar soaks,f silver nitrate application weekly, and topical ultra-potent steroids with dermatology consultation Recommendations for grade ≥3: • Topical antibiotics,e vinegar soaks,f silver nitrate application weekly/nail avulsion, and systemic antibioticsg |
AE, adverse event; GABA, gamma-aminobutyric acid; SPF, sun protection factor.
Examples of topical antipruritics: pramoxine 1% cream or doxepin 5% cream.
Examples of antihistamines: levocetirizine 5 mg 4×/day, desloratadine 5 mg 4×/day, diphenhydramine 25–50 mg 3×/day, hydroxyzine 25 mg 3×/day, or fexofenadine 60 mg 3×/day.
Examples of GABA agonists (adjust for renal impairment): gabapentin 300 mg or pregabalin 50–75 mg, every 8 hours.
Examples of tricyclics: doxepin 25–50 mg every 8 hours or aprepitant three doses (125 mg on day 1, 80 mg on days 2 and 3).
Examples of topical antibiotics/antiseptics: clindamycin 1%, erythromycin 1%, tetracycline 1%, or chloramphenicol 1%, iodine ointment.
Vinegar soaks consist of soaking fingers or toes in a solution of white vinegar in water 1:1 for 15 minutes every day.
Systemic antibiotics include tetracyclines and antimicrobials (the potent P-glycoprotein inhibitor of erythromycin should be avoided).
Non-pharmacologic approaches may also help reduce dermatologic AEs during afatinib therapy; these include avoidance of prolonged sun exposure, routine use of UVA/UVB-protective zinc-based sunscreen (sun protection factor >30), frequent use of moisturizing creams, and use of fragrance-free soaps and detergents (Table 2).
Paronychia
Strategies for managing paronychia are included in Table 2. Partial or complete nail avulsion can be effective for persistent paronychia unresponsive to other methods, such as topical antibiotics/antiseptics (e.g., clindamycin 1%, erythromycin 1%, tetracycline 1%, or chloramphenicol 1%), vinegar soaks (1:1 solution of white vinegar and water), topical ultra-potent steroids (eg. clobetasol propionate, applied to the nail bed twice daily), or silver nitrate (applied weekly) (Melosky & Hirsh., 2014; Relhan et al., 2014). Supportive care measures include warm water in vinegar (1:1) soaks to the affected lesion for 15 minutes/day, and avoiding nail biting, aggressive manicures/pedicures, irritating substances and prolonged exposure to water (Califano et al., 2015). High-risk patients (including high-risk diabetic and immunosuppressed patients, or those repeatedly exposed to moist environments) should be monitored carefully to ensure nails are dry and clean.
Diarrhea
Diarrhea is the most common AE associated with EGFR TKI therapy, and is thought to be caused by multiple factors, including excessive chloride secretion resulting in secretory diarrhea (Hirsh et al., 2014; Yang et al., 2013). Diarrhea typically occurs during the initial weeks of afatinib treatment, and may become evident as early as 2–3 days after initiation of afatinib, so patients must be closely monitored, instructed early on diet, and given anti-motility agents, to prevent dose reduction or permanent therapy discontinuation (Yang et al., 2013). The preferred first-line pharmacologic treatment is loperamide at an initial dose of 4 mg at onset of diarrhea, then 2 mg after every episode until bowel movements cease for 12 hours (maximum of 20 mg/day) (Yang et al., 2013); if loperamide use is maximized, diphenoxylate/atropine (5 mg/0.5 mg every 6 hours) may be added (Hirsh et al., 2014; Walko & Grande 2014). Patients with persistent diarrhea may also require temporary treatment interruption and gastroenterology consultation. If grade 2 diarrhea persists for >48 hours, intravenous fluids/electrolytes (for ≥24 hours), a stool panel for infection, and imaging should be considered. Dietary changes are also useful, including avoiding milk products, uncooked vegetables, caffeine, alcohol, fiber, and spicy foods, and eating smaller, more frequent meals. Increasing intake of water and other clear liquids (to 8–10 glasses per day) is key to preventing dehydration (J.C. Yang et al., 2013). Additional therapies used for treatment-resistant diarrhea include tincture of opium and octreotide.
Stomatitis/Mucositis
For early-stage/mild stomatitis/mucositis secondary to afatinib, or damage to the mucosal layer of the gastrointestinal tract (Al-Dasooqi et al., 2013), topical management is usually adequate (Table 2). This includes good oral hygiene (regular brushing, flossing and rinsing); avoiding hot, acidic, spicy, or salty foods; and drinking plenty of water (McGuire et al., 2013). Patients can also use topical corticosteroids (e.g. triamcinolone acetonide 0.05–0.5%, flucinolone acetonide 0.025–0.05% or clobetasol propionate 0.025%) as gels or pastes, or a dexamethasone (0.5 mg/5 mL) elixir, which has been shown to reduce the incidence of stomatitis related to the mammalian target of rapamycin (mTOR) inhibitor everolimus (Belenguer-Guallar et al., 2014; Rugo et al., 2017). A baking soda rinse can also help maintain oral hygiene (Choi & Kim 2012).
Topical anesthetics, including lidocaine (1% cream or 2% gel/spray), polidocanol paste, and benxocaine lozenges, can help manage pain associated with oral ulcerations, while dexamethasone mouthwash (0.5 mg/5 mL) can be used for more severe ulcerative stomatitis. Patients should rinse three times/day, rinsing around the mouth for 1 minute, then spitting out the rinse. This is most effective when done after meals, with no food/drink/other rinse for ≥30 minutes after the procedure. Patients should be warned of the potential development of oral candidiasis, a common side effect with topical steroid mouth rinses (Patil et al., 2015). Oral candidiasis can be managed using a topical (e.g. clotrimazole lozenges 10 mg [four/day]) or systemic antifungal agent (fluconazole 100 mg/day for 14 days) (Lalla, Patton, & Dongari-Bagtzoglou, 2013). Painful and difficult swallowing may result if mucositis extends toward the back of and beyond the oral cavity. Examination of the nasal mucosa of patients reporting nosebleeds while taking afatinib may reveal nasal vestibulitis; topical mupirocin may be useful in this situation (Ruiz et al., 2015).
Conclusions
The AE profile of afatinib is consistent with that of other EGFR inhibitors, with the most common TRAEs including diarrhea, rash/acne, stomatitis/mucositis, and paronychia. These events are generally mild to moderate in severity and manageable but, if untreated, may impact quality of life and lead to afatinib discontinuation. Patient education (pre-treatment), frequent communication, vigilant assessment of AEs, and proactive utilization of management strategies (including supportive care measures and proper dose modification), allow patients experiencing clinical benefit to manage AEs and continue afatinib therapy. Patients should notify their nurse/provider if the prescribed interventions are not effective within a specified time. Temporary interruption of the afatinib dose can be particularly effective in the early management of moderate to severe toxicities, and may be sufficient in many cases, allowing afatinib to be resumed at full dose with supportive care, after a few days “off”.
Afatinib AE management has been further evaluated in a phase 3b, non-randomized, open-label, two-cohort study of patients with EGFR mutation-positive advanced lung adenocarcinoma (ClinicalTrials.gov NCT01814553). Patients in the ‘reactive’ cohort followed Afatinib Diarrhea Assessment and Management (ADAM) guidelines and received loperamide at the first sign of diarrhea, while patients in the ‘prophylactic’ cohort received loperamide from the first day of afatinib treatment. Results from this trial may provide additional guidance to help manage afatinib-related diarrhea.
In conclusion, results from the afatinib phase 3 clinical trial program combined with real-world clinical practice experience highlight that patient education, frequent communication, routine monitoring, early recognition, proactive management, and adherence to the recommended dose-interruption/reduction scheme are important strategies to maximize clinical benefits during afatinib therapy. Similar strategies have been recommended by a UK–based multidisciplinary panel on the prevention and management of cutaneous and gastrointestinal AEs associated with EGFR TKI therapy (Califano et al., 2015). Early recognition and nursing interventions optimize symptom management and reinforce compliance with supportive care measures and dose-reduction schemes, thereby helping patients stay on therapy longer. Oncology nurses and other advanced oncology practitioners should educate their patients on the prevention and management of common afatinib-related AEs before treatment starts, and explain that dose interruption/reduction and/or supportive care measures can allow TRAEs to be managed while maintaining the therapeutic benefits of afatinib.
IMPLICATIONS FOR PRACTICE.
Understand that tolerability should be assessed early in patients with EGFR mutation-positive NSCLC or those with SCC of the lung, who are treated with afatinib
Educate patients about common toxicities and the existence of management-strategies prior to treatment initiation, and anticipate the need for early intervention to manage afatinib-related AEs
Implement appropriate dose-reduction schemes and supportive care measures, as needed, to ensure patients can remain on treatment and maintain therapeutic benefits of afatinib
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing and editorial support provided by Lauren Fink, PhD, of MedErgy, and editorial and formatting assistance provided by GeoMed, an Ashfield company, was contracted and compensated by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Research Support: The authors acknowledge the support of all trial investigators, clinical trial support staff, and all patients and their families participating in these trials. Dr. Lacouture is funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
Previous Presentation: The content has been previously presented, in part, at the 40th Annual Congress of the Oncology Nursing Society; April 23–26, 2015; Orlando, FL.
Financial Disclosures:
R. L. Edwards: None
C. Andan: Current affiliation: Boehringer Ingelheim Pharmaceuticals, Inc, Ridgefield, CT; experience with afatinib-treated patients described herein and manuscript development occurred prior to joining Boehringer Ingelheim.
R.V. Lalla: Received compensation from Boehringer Ingelheim for participation in a symposium at the 39th Annual Congress of the Oncology Nursing Society (2014).
M.E. Lacouture: Consulting for AstraZeneca, Boehringer Ingelheim.
D. O’Brien: Employee of Boehringer Ingelheim Pharmaceuticals, Inc.
L.V. Sequist: Consulting fees from AstraZeneca, Ariad, Bristol-Myers Squibb, and Genentech; uncompensated consulting for Boehringer Ingelheim, Clovis, Merrimack, Novartis.
Job descriptions:
Rebecca L. Edwards, DNP, APRN, ACNP, ACHPN, AOCNP is an Assistant Professor at the University of Alabama at Birmingham School of Nursing.
Christine Andan, BSN, RN, is an Oncology Clinical Nurse Educator for Boehringer Ingelheim Pharmaceuticals, Inc. in Ridgefield, CT
Rajesh V. Lalla, DDS, PhD, DABOM, is an Associate Professor of Oral Medicine and Associate Dean for Research at the University of Connecticut School of Dental Medicine in Farmington, CT.
Mario E. Lacouture, MD is a board-certified dermatologist at the Memorial Sloan Kettering Cancer Center, NY
Dennis O’Brien: MD, is an Executive Director and Pharmacovigilance Therapeutic Area Head for Oncology at Boehringer-Ingelheim
Lecia V. Sequist, MD, MPH, is an Associate Professor of Medicine at Harvard Medical School and a thoracic medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, MA
References
- Al-Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ, … Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). (2013). Emerging evidence on the pathobiology of mucositis. Support Care Cancer, 21(11), 3233–3241. [DOI] [PubMed] [Google Scholar]
- Arrieta O, Vega-Gonzalez MT, López-Macias D, Martinez-Hernandez JN, Bacon-Fonseca L, Macedo-Perez EO, … de la Garza-Salazar J (2015). Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer, 88(3), 282–288. [DOI] [PubMed] [Google Scholar]
- Belenguer-Guallar et al. Treatment of recurrent aphthous stomatitis. A literature review. J Clin Exp Dent. 2014 Apr; 6(2): e168–e174. Published online 2014. Apr 1. doi: 10.4317/jced.51401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer Ingelheim. (2014). Investigator Brochure: Proactive approaches to understanding and addressing adverse events. (Reprinted from: Not in File). [Google Scholar]
- Boehringer Ingelheim. (2016a). GILOTRIF® (afatinib) tablets, for oral use [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharm. (Reprinted from: Not in File). [Google Scholar]
- Boehringer Ingelheim. (2016b). GIOTRIF 20 mg film-coated tablets Summary of Product Characteristics. Ingelheim am Rhein, Germany: Boehringer Ingelheim International GmbH. (Reprinted from: In File). [Google Scholar]
- Califano R, Tariq N, Compton S, Fitzgerald DA, Harwood CA, Lal R, … Nicolson M (2015). Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs, 75(12), 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BA, & Hughes BG (2015) Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res, 4(1), 36–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles et al. Impact of dermatologic adverse events induced by targeted therapies on quality of life. Crit Rev Oncol Hematol. 2016. May;101:158–68. doi: 10.1016/j.critrevonc.2016.03.003. Epub 2016 Mar 5 [DOI] [PubMed] [Google Scholar]
- Choi & Kim. Sodium Bicarbonate Solution versus Chlorhexidine Mouthwash in Oral Care of Acute Leukemia Patients Undergoing Induction Chemotherapy: A Randomized Controlled Trial. Asian Nurs Res (Korean Soc Nurs Sci). 2012. Jun;6(2):60–6. doi: 10.1016/j.anr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Dearden S, Stevens J, Wu YL, & Blowers D. (2013). Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol, 24(9), 2371–2376. DOI: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy GK, & Adjei AA (2013). Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin, 63(4), 249–279. [DOI] [PubMed] [Google Scholar]
- Fabbrocini et al. Acneiform Rash Induced by EGFR Inhibitors: Review of the Literature and New Insights. Skin Appendage Disord. 2015. Mar; 1(1): 31–37. doi: 10.1159/000371821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh et al. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol, Vol. 21, pp. 329–336; doi: 10.3747/co.21.2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, … Yassine M. (2010). Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol, 28(8), 1351–1357. [DOI] [PubMed] [Google Scholar]
- Lacouture ME, Schadendorf D, Chu CY, Uttenreuther-Fischer M, Stammberger U, O’Brien D, & Hauschild A. (2013). Dermatologic adverse events associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther, 13(6), 721–728. [DOI] [PubMed] [Google Scholar]
- Lalla RV, Patton LL, & Dongari-Bagtzoglou A. (2013). Oral candidiasis: pathogenesis, clinical presentation, diagnosis and treatment strategies. J Calif Dent Assoc, 41(4), 263–268. [PubMed] [Google Scholar]
- McGuire DB, Fulton JS, Park J, Brown CG, Correa ME, Eilers J, … Lalla RV (2013). Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer, 21(11), 3165–3177. [DOI] [PubMed] [Google Scholar]
- Melosky B, Anderson H, Burkes RL, Chu Q, Hao D, Ho V, … Laskin JJ (2015). Pan Canadian rash trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol, 34(8), 810–815. [DOI] [PubMed] [Google Scholar]
- Melosky B, Anderson H, Burkes RL, Chu QS, Hao D, Ho V, … Laskin JJ (2014). Pan-Canadian rash trial with EGFR inhibitors. J Clin Oncol, 32(5s). [DOI] [PubMed] [Google Scholar]
- Melosky & Hirsh. Management of Common Toxicities in Metastatic NSCLC Related to Anti-Lung Cancer Therapies with EGFR–TKIs. Front Oncol. 2014; 4: 238. doi: 10.3389/fonc.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, … Paz-Ares L (2016). Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol, 17(5), 577–589. [DOI] [PubMed] [Google Scholar]
- Patil et al. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front Microbiol. 2015; 6: 1391. Published online 2015. Dec 17. doi: 10.3389/fmicb.2015.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relhan et al. Management of Chronic Paronychia. Indian J Dermatol. 2014. Jan-Feb; 59(1): 15–20. doi: 10.4103/0019-5154.123482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013. Aug;14(4):327–33. doi: 10.1007/s40257-013-0021-0. [DOI] [PubMed] [Google Scholar]
- Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, … Litton JK (2017). Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol, 18(5), 654–662. DOI: 10.1016/S1470-2045(17)30109-2 [DOI] [PubMed] [Google Scholar]
- Ruiz et al. Nasal vestibulitis due to targeted therapies in cancer patients. Support Care Cancer. 2015. Aug;23(8):2391–8. doi: 10.1007/s00520-014-2580-x. Epub 2015 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, … Schuler M (2013). Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol, 31(27), 3327–3334. [DOI] [PubMed] [Google Scholar]
- Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, … Goss GD. (2015). Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol, 16(8), 897–907. [DOI] [PubMed] [Google Scholar]
- Walko & Grande. Management of Common Adverse Events in Patients Treated With Sorafenib: Nurse and Pharmacist Perspective. Semin Oncol. 2014. Feb;41 Suppl 2:S17–28. doi: 10.1053/j.seminoncol.2014.01.002 [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cancer Fact Sheet. Feb, 2017. Available at http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed January 15, 2018
- Wu Y, Geater SL, Mok T, O’Byrne KJ, Schuler MH, Sequist LV, … Zazulina V (2014). Epidermal Growth Factor Receptor (EGFR)-Mediated Adverse Events (AEs) in Patients (pts) with EGFR-Mutation Positive (EGFR M+) Non-Small Cell Lung Cancer (NSCLC) Treated With Afatinib (A). Int J Radiation Oncol, 90(5), S41. [Google Scholar]
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, … Geater SL. (2014). Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol, 15(2), 213–222. [DOI] [PubMed] [Google Scholar]
- Yang JC, Reguart N, Barinoff J, Kohler J, Uttenreuther-Fischer M, Stammberger U, … Cohen EE (2013). Diarrhea associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther, 13(6), 729–736. [DOI] [PubMed] [Google Scholar]
- Yang JC, Sequist LV, Zhou C, Schuler M, Geater SL, Mok T, … Wu YL (2016). Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol, 26(11), 2103–2110. [DOI] [PubMed] [Google Scholar]
- Yang JCH, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, … Sequist L (2015). Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol, 16(2), 141–151. [DOI] [PubMed] [Google Scholar]