Abstract
Bacteriophages have been widely used as surrogates for human enteric viruses in many studies on virus transport and fate. In this investigation, the fates of three bacteriophages, MS2, R17, and φX174, were studied in a series of dynamic batch experiments. Both MS2 and R17 readily underwent inactivation in batch experiments where solutions of each phage were percolated through tubes packed with varying ratios of glass and Teflon beads. MS2 and R17 inactivation was the result of exposure to destructive forces at the dynamic air-water-solid interface. φX174, however, did not undergo inactivation in similar studies, suggesting that this phage does not accumulate at air-water interfaces or is not affected by interfacial forces in the same manner. Other batch experiments showed that MS2 and R17 were increasingly inactivated during mixing in polypropylene tubes as the ionic strength of the solution was raised (φX174 was not affected). By the addition of Tween 80 to suspensions of MS2 and R17, phage inactivation was prevented. Our data suggest that viral inactivation in simple dynamic batch experiments is dependent upon (i) the presence of a dynamic air-water-solid interface (where the solid is a hydrophobic surface), (ii) the ionic strength of the solution, (iii) the concentration of surface active compounds in the solution, and (iv) the type of virus used.
Virus contamination of groundwater resources has been well documented (9, 13, 32). In order to better predict the vulnerability of groundwater to viral contamination, a greater understanding of virus adsorption to soil and its influence on transport and fate is needed. The traditional method for determining virus adsorption to soil is the batch equilibrium method, which describes the partitioning of virus particles between solid and liquid phases at equilibrium. In order to reduce cost and time of analysis, both male-specific and somatic bacteriophages (including both RNA and DNA phages) have been routinely used as surrogates for human pathogenic viruses in many soil adsorption and transport studies (5, 6, 8, 12, 18, 26). In a recent investigation of the adsorption of two bacteriophages to three different soils, we reported a novel virus loss mechanism (27) similar to that reported for virus inactivation at air-water interfaces (AWIs) (2, 28–31). We concluded that the observed MS2 loss in polypropylene (PP) vessels was the result of virus inactivation due to forces associated with the presence of an AWI in the tube during mixing. However, the locus of inactivation was determined not to be the AWI itself, but rather the triple-phase-boundary (TPB), the interface at which the gas, liquid, and solid (tube wall) phases intersected (27).
Previous work has shown that exposing viral suspensions to AWIs via shaking, bubbling, or aerosolization leads to virus inactivation (2, 28–31). Inactivation in the presence of an AWI is dependent upon the virus first reaching the AWI (31). This process is greatly enhanced in a dynamic system, or one in which the AWI is continuously being regenerated. The strength of attraction between a virus particle and the AWI is influenced by the ionic strength of the suspending medium as well as the particle’s relative hydrophobicity. Raising the ionic strength of the solution has been shown to increase electrostatic attractions between colloid-sized particles (e.g., latex and polystyrene beads, clays, bacteria, and viruses) and the AWI (31, 33, 34, 36). Also, particles with greater surface hydrophobicities have a higher affinity for adsorption to AWIs than do hydrophilic particles (10, 22, 33, 34, 36). When surface active compounds (e.g., peptone, amino acids, surfactants, etc.) are added to a dynamic system, they accumulate at the AWI, thereby preventing viruses from reaching it and being inactivated (2, 28, 31).
This study provides evidence to further substantiate our finding that viral inactivation observed in dynamic batch systems (27) is directly related to virus interaction with the TPB and not only the AWI (as others have concluded). A series of dynamic batch experiments was systematically used to study the fate of three different bacteriophages (MS2, R17, and φX174) in both glass and PP batch systems under conditions of (i) varying levels of exposure to a hydrophobic TPB, (ii) varying ionic strengths, and (iii) varying surfactant concentrations.
MATERIALS AND METHODS
Bacteriophage stocks and enumeration.
Three different bacteriophages were used in this study as models for human enteric viruses. MS2 (host, Escherichia coli ATCC 15597), R17 (host, E. coli ATCC 25868), and φX174 (host, E. coli ATCC 13706) were obtained from the American Type Culture Collection. MS2 and R17 are male-specific, single-stranded RNA phages (7), while φX174 is a somatic, single-stranded DNA phage (1).
Each bacteriophage was grown on a lawn of its host by using the agar overlay method (3), then harvested, resuspended in phosphate-buffered saline (PBS), centrifuged, and filtered as previously described (27), with the exception that the phage were not filtered through 0.20- or 0.05-μm-pore-size membrane filters. Phage stock concentrations typically ranged from 1010 to 1012 PFU ml−1.
Phage were enumerated according to the PFU method (3) with the bacterial hosts mentioned above. One milliliter of the sample and 1 ml of the host bacterium (in the logarithmic-growth phase) were combined in a tube of molten (45°C) tryptic soy agar (TSA) (Difco Laboratories, Detroit, Mich.) and poured onto TSA plates to be incubated overnight at 37°C. Each sample was replica plated, with countable numbers of plaques ranging from 25 to 250 per plate.
Batch experiments with varying amounts of TPB.
A series of batch experiments was designed to quantitatively validate the previously described relationship (27) between phage inactivation and the amount of hydrophobic TPB within a batch system. Glass tubes (Pyrex screw-cap; 16 by 125 mm) (Fisher Scientific, Los Angeles, Calif.) were filled with Teflon (radius, ∼5.6 mm) (Norton Performance Plastics, Akron, Ohio) and/or glass beads (radius, ∼5.3 mm) (Corning Inc., Corning, N.Y.) in order to vary the amount of hydrophobic and hydrophilic surface areas within the system. Glass tubes (as opposed to PP) were chosen for this study so that phage inactivation at the hydrophobic TPB would be limited to the Teflon beads and would not involve the tube surface, an area which could not be quantified as easily as the surfaces of the beads. The number of Teflon beads placed in each tube ranged from 0 (all glass beads) to 60 (all Teflon beads). Beads were added so that both types were distributed as evenly as possible throughout the tube. Five-milliliter aliquots of each phage in PBS (104 to 105 PFU ml−1) were dispensed into a portion of the remaining void space within the tubes, thereby creating a porous matrix in which solid, liquid, and air phases were represented. The tubes were mixed for 3 h at 7 ± 1°C by end-over-end rotation (∼20 rpm) on a tube rotator (Fisher Scientific) so that the solution percolated between the beads. A series of control tubes (filled to capacity) containing 15 Teflon beads and no AWI were also rotated. After 3 h, virus solutions were removed and titered as described above. Between experiments, glass tubes were cleaned as previously described (27).
Batch experiments with varying ionic strengths.
All batch experiments in which the ionic strength was varied were performed both in 15-ml PP centrifuge tubes (Fisher Scientific) and in the glass tubes described above. Each bacteriophage was individually tested (in both tube types) in a series of batch experiments in which the total ionic strength of the solution was varied from 0.022 mol liter−1 (phage stock diluted in deionized water) to 2.052 mol liter−1 by changing the amount of NaCl added. The pH of the solution was adjusted to 7.45 with 1 or 5 mmol of Na2HPO4 liter−1. The bacteriophage were diluted (104 to 105 PFU ml−1) in each solution, dispensed into the batch tubes (10-ml aliquots), and mixed for 3 h at 7 ± 1°C by end-over-end rotation as described above. Control tubes for each ionic strength were not rotated. Since the total capacity of the glass (∼17 ml) and PP (∼15 ml) tubes was greater than the volume of solution added (10 ml), an AWI was always present in the tubes during mixing. After 3 h, mixing was stopped and the total PFU remaining in solution was determined.
Batch experiments with varying surfactant concentrations.
Stock solutions of Tween 80 (T-80) (Aldrich Chemical Co., Milwaukee, Wis.), an anionic detergent, were serially diluted over a concentration range of 1.0 to 10−8% (vol/vol) in PP tubes by using PBS as the diluent. Aliquots of phage were added to each tube (final concentration, 104 to 105 PFU ml−1) so that the final volume of liquid added was 10 ml, leaving an AWI present. The contents of the tubes were mixed for 3 h at 7 ± 1°C as described above. A series of nonrotated tubes acted as controls.
To determine the AWI activity of T-80, surface tension measurements were made on static 20-ml aliquots of each dilution (7 ± 1°C) according to the Wilhelmy slide method (4) with a TS9000 surface tensiometer (NIMA, Coventry, United Kingdom).
RESULTS
Batch experiments with varying TPBs.
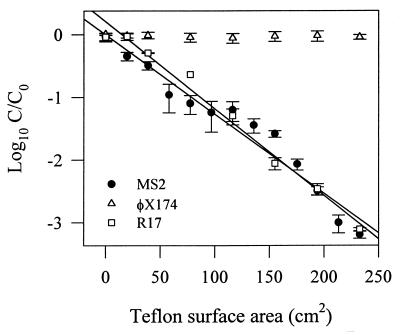
Figure 1 shows the relationship between the Teflon surface area and bacteriophage concentration (C/C0) after mixing in glass tubes packed with varying ratios of glass and Teflon beads. The concentration of φX174 after mixing did not change as the number of Teflon beads increased. In comparison, the MS2 and R17 concentrations clearly decreased (r2 = 0.953 and 0.985, respectively) as the Teflon-to-glass bead ratio increased, or as the amount of hydrophobic TPB increased. The non-AWI controls demonstrated no substantial loss of MS2, R17, or φX174 during the 3-h mixing period (data not shown).
FIG. 1.
Fates of MS2, R17, and φX174 during batch experiments in which glass tubes were packed with varying numbers of Teflon and glass beads. A surface area of 0 cm2 implies that tubes were filled only with glass beads. An AWI was present in all batch experiments. Data are means from three experiments; error bars represent the standard deviations from the means. r2 values from the regression analysis are given in the text.
Batch experiments with varying ionic strengths.
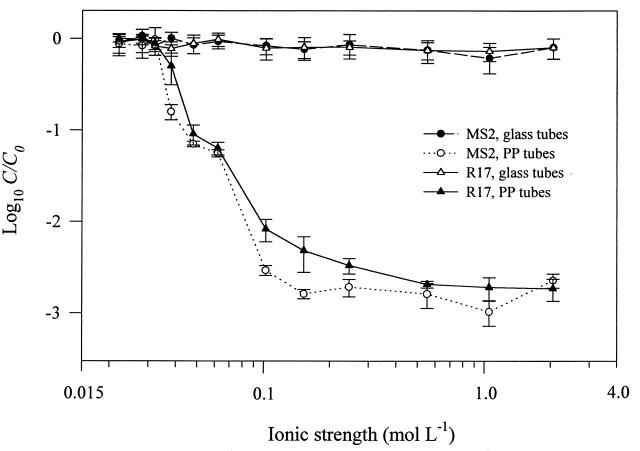
Figure 2 presents the results of MS2 and R17 batch experiments performed in glass and PP tubes at varying ionic strengths. MS2 and R17 inactivation in the PP tubes clearly increased as the ionic strength of the solution increased. There was a particularly sharp decrease in C/C0 between 0.032 and 0.102 mol liter−1. In the same experiment performed in glass tubes, no substantial loss of MS2 or R17 was observed; C/C0 values were consistent with those for the static controls (data not shown), which also demonstrated no loss of phage. φX174 demonstrated no inactivation at any of the ionic strengths tested (data not shown).
FIG. 2.
MS2 and R17 batch experiments performed at varying ionic strengths in glass and PP tubes with an AWI present. Data are means from three experiments; error bars represent the standard deviations from the means.
Batch experiments with varying surfactant concentrations.
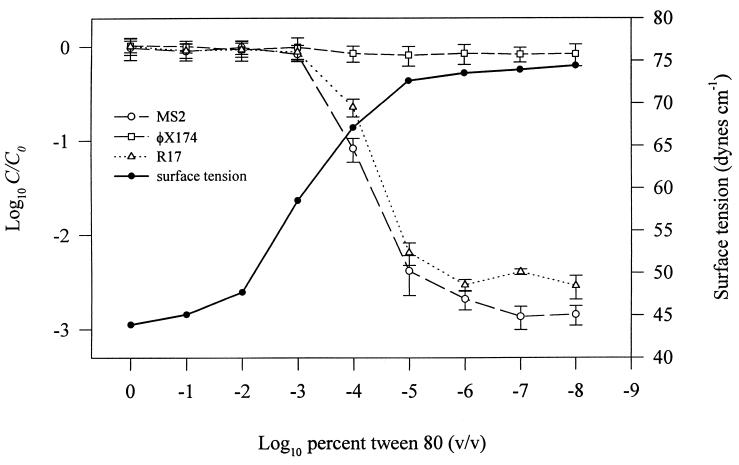
The relative bacteriophage concentration (C/C0) and solution surface tension (in dynes per centimeter) are plotted against decreasing T-80 concentration in Fig. 3. Mean values of φX174 C/C0 were consistently near 0.9 over the entire range of T-80 concentrations tested. In contrast, MS2 and R17 concentrations decreased as the T-80 concentration decreased. C/C0 values for MS2 and R17 began to decline after the amount of T-80 reached a threshold level of protection at approximately 10−3% (Fig. 3). The roughly 3-order-of-magnitude loss of MS2 and R17 at the lowest T-80 concentrations was consistent with the maximum amount of phage loss demonstrated in Fig. 1 and 2 (after 3 h of mixing). In the static controls no loss of any of the three bacteriophages was seen (data not shown) over the range of T-80 concentrations tested.
FIG. 3.
Fates of MS2, R17, and φX174 during batch experiments with varying concentrations of T-80. An AWI was present in all batch experiments. Also shown are measurements of the static solution surface tension for each concentration of T-80 used. Data are means from three experiments; error bars represent the standard deviations from the means.
Also demonstrated in Fig. 3 is a decrease in solution surface tension (in dynes per centimeter) as the concentration of T-80 increases. At a T-80 concentration of 1.0%, the measured solution surface tension was 43.9 ± 0.4 dynes cm−1. After serial dilution to 10−5%, the measured surface tension increased to nearly the same level as that of pure PBS (74.9 ± 0.3 dynes cm−1).
DISCUSSION
Our results demonstrate that bacteriophage inactivation in a simple batch system may be influenced by the presence of both a dynamic AWI and a hydrophobic solid surface within the batch system. These two factors combine to form a dynamic TPB capable of rendering virus particles noninfectious, an observation we recently reported (27). Consequently, a more definitive assessment of the factors influencing viral inactivation at interfaces is needed in order to design more effective soil batch adsorption experiments.
In the varying TPB batch experiments, MS2, R17, and φX174 were exposed to varying numbers of glass and Teflon beads. This allowed us to observe bacteriophage behavior in the presence of two distinctly different TPBs: the air-water-glass TPB and the air-water-Teflon TPB. Although the amount of TPB could not be directly quantified because of the dynamics of the system, it is clear that increasing the number of Teflon beads results in a greater total air-water-Teflon TPB within the tube. Therefore, Fig. 1 indirectly relates inactivation of MS2 and R17 to the amount of air-water-Teflon TPB. The absence of virus inactivation in the non-AWI controls (data not shown) clearly demonstrates the importance of the AWI. Without its presence in the system, a TPB does not form, resulting in no inactivation of MS2 or R17. Speculation that phage inactivation was occurring at the AWI, as others have shown (2, 28–31), is not supported by these experiments. If the AWI were strictly the locus of inactivation, then MS2 and R17 inactivation would have occurred independent of the type of beads within the tube.
The ionic strength of the solution is also a factor contributing to phage inactivation in the presence of a dynamic TPB where the solid is hydrophobic. This was demonstrated in the experiments performed at varying ionic strengths in PP tubes (Fig. 2). MS2 and R17 both underwent greater inactivation as ionic strength increased. The effect of increasing ionic strength cannot be attributed to salt toxicity, as inactivation of MS2 and R17 did not occur in the static PP controls (data not shown) or in the dynamic glass experiments (Fig. 2). The data are consistent with previous evidence showing increased adsorption to the AWI as ionic strength is elevated. This type of behavior has been observed for various colloidal particles, such as viruses, bacteria, clay, and polystyrene and latex spheres (31, 33, 34, 36), as well as for individual proteins (24). As the ionic strength of the solution increases, the particle is increasingly attracted to the AWI because of a decrease in the size of the electrostatic double layer (31, 34, 36). In Fig. 2, the increase in MS2 and R17 inactivation at higher ionic strengths is the result of greater phage sorption at the AWI, resulting in exposure to inactivating forces at the air-water-PP TPB. The lack of phage inactivation in the PP tubes at low ionic strengths is the result of electrostatic repulsion between the AWI and the phage. No inactivation of MS2 or R17 occurred in the glass tubes because the forces at the air-water-glass TPB are different from those at the air-water-PP TPB (27), regardless of the ionic strength of the solution.
Data from the surfactant experiments (Fig. 3) conclusively show that viruses must reach the AWI before being inactivated. This is demonstrated by relating the amount of T-80 present at the AWI (indicated by the surface tension of the solution) to the level of phage inactivation. Organic solutes (e.g., T-80) within an aqueous system produce a decrease in solution surface tension by displacing water molecules from the AWI and replacing them with solute molecules. Since organic solutes have lower surface energy than water molecules, solution surface tension will decrease in proportion to the amount of organic solute accumulated at the interface (17). This suggests that at T-80 solution surface tensions below ∼60 dynes cm−1, there is sufficient accumulation of T-80 at the AWI to deny bacteriophage access to the interface, thereby preventing inactivation (Fig. 3). As the solution surface tension approaches that of pure PBS (∼75 dynes cm−1), phage interaction with the AWI increases, resulting in greater exposure to the TPB. We have also observed similar protection against inactivation using tryptic soy broth and peptone (data not shown). Other investigators have demonstrated a protective influence from peptone, amino acids, and various surfactants against phage inactivation upon exposure to AWIs (2, 29, 31). While the mechanism of protection is the same, the locus of inactivation (AWI versus hydrophobic TPB) is clearly different.
φX174 did not undergo inactivation in any of the experiments performed here or previously (27), suggesting that either this phage is resistant to forces at the hydrophobic TPB or it does not partition at the AWI to the same extent as MS2 and R17. A previous study ranked 15 animal viruses and bacteriophages on the basis of relative hydrophobicity (23). It was determined that φX174 was the most hydrophilic, while MS2 was the most hydrophobic, of the viruses tested. This suggests that φX174 would not be attracted to the AWI or would be only weakly attracted. The interaction of a virus particle with an AWI is strongly influenced by the virus’s amphipathicity, the result of localized hydrophobic and hydrophilic regions on the surfaces of the capsid proteins (15). Amphipathic molecules accumulate at AWIs with the hydrophobic end orienting into the nonpolar air phase while the hydrophilic end remains in the aqueous phase (25). This suggests that φX174, because of its dominant hydrophilicity, will not readily accumulate at AWIs, while MS2 will. Interaction of φX174 with the AWI cannot be completely ruled out, since it has been demonstrated that some hydrophilic particles may experience attraction to AWIs (34). In the event that φX174 did accumulate at the AWI during our investigation, the data strongly suggest that it is much more resistant to forces at the hydrophobic TPB than MS2 or R17.
In this report we have presented evidence demonstrating the influence of the AWI and the hydrophobic TPB on bacteriophage fate during batch experiments. These findings have immediate application to work involving the determination of virus adsorption to soil (27) or in any type of quantitative batch experiment in which a virus suspension is maintained in a dynamic state. The results are particularly important given that PP is considered the standard container type for virus storage and experimentation because relatively little virus adsorption to this material has been observed (16).
We also suggest that these findings have application beyond simple bench scale studies. Knowledge of a virus’s relative attractiveness to an AWI and ability to withstand interfacial forces might be helpful in predicting virus inactivation during transport through unsaturated soil, where the AWI is a significant component of the system. Previous work indicates that bacteria accumulate at AWIs within unsaturated porous media (35), and virus particles will undoubtedly behave similarly. Although the soil environment clearly differs from the systems described herein, a virus particle moving through unsaturated soil will experience similar interfacial forces. A number of studies have shown that virus removal during unsaturated flow is greater than during saturated flow (14, 19–21). A recent study also demonstrates that MS2 undergoes inactivation during flow through unsaturated soil columns whereas φX174 does not (11). This study also found that φX174 underwent extensive reversible adsorption. Based on our findings, this suggests that MS2 is inactivated in unsaturated soils upon exposure to destructive air-water-interfacial forces, while φX174, which will not accumulate at the AWI, remains in solution, where the potential for soil adsorption is much greater.
ACKNOWLEDGMENT
This research was supported by a grant from the Kearney Foundation of Soil Science.
REFERENCES
- 1.Ackerman H-W, DuBow M S. Viruses of prokaryotes. Vol. 2. Boca Raton, Fla: CRC Press; 1987. [Google Scholar]
- 2.Adams M H. Surface inactivation of bacterial viruses and of proteins. J Gen Physiol. 1948;31:417–432. doi: 10.1085/jgp.31.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959. [Google Scholar]
- 4.Adamson A W. Physical chemistry of surfaces. 4th ed. New York, N.Y: Wiley Interscience; 1982. [Google Scholar]
- 5.Bales R C, Li S, Maguire K M, Yahya M T, Gerba C P. MS-2 and poliovirus transport in porous media: hydrophobic effects and chemical perturbations. Water Resour Res. 1993;29:957–963. [Google Scholar]
- 6.Burge W D, Enkiri N K. Virus adsorption by five soils. J Environ Qual. 1978;7:73–76. [Google Scholar]
- 7.Brown F, editor. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses. Arch Virol. 1991;1991(Suppl. 2):1–450. [Google Scholar]
- 8.Goyal S M, Gerba C P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979;38:241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal S M, Keswick B H, Gerba C P. Viruses in groundwater beneath sewage irrigated cropland. Water Res. 1984;18:299–302. [Google Scholar]
- 10.Hermansson M, Kjelleberg S, Korhonen T K, Stenstrom T-A. Hydrophobic and electrostatic characterization of surface structures of bacteria and its relationship to adhesion to an air-water interface. Arch Microbiol. 1982;131:308–312. [Google Scholar]
- 11.Jin, Y. 1997. Personal communication.
- 12.Jin Y, Yates M V, Thompson S S, Jury W A. Sorption of viruses during flow through saturated sand columns. Environ Sci Technol. 1997;31:548–555. [Google Scholar]
- 13.Keswick B H, Gerba C P. Viruses in groundwater. Environ Sci Technol. 1980;14:1290–1297. [Google Scholar]
- 14.Lance J C, Gerba C P. Virus movement in soil during saturated and unsaturated flow. Appl Environ Microbiol. 1984;47:335–337. doi: 10.1128/aem.47.2.335-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mix T W. The physical chemistry of membrane-virus interactions. Dev Ind Microbiol. 1974;15:136–142. [Google Scholar]
- 16.Moore R S, Taylor D H, Sturman L S, Reddy M M, Fuhs G W. Poliovirus adsorption by 34 minerals and soils. Appl Environ Microbiol. 1981;42:963–975. doi: 10.1128/aem.42.6.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers D. Surfaces, interfaces and colloids. New York, N.Y: VCH Publishers; 1990. [Google Scholar]
- 18.Pieper A P, Ryan J N, Harvey R W, Amy G L, Illangasekare T H, Metge D W. Transport and recovery of bacteriophage PRD1 in a sand and gravel aquifer: effect of sewage-derived organic matter. Environ Sci Technol. 1997;31:1163–1170. [Google Scholar]
- 19.Powelson D, Simpson J R, Gerba C P. Virus transport and survival in saturated and unsaturated flow through soil columns. J Environ Qual. 1990;19:396–401. [Google Scholar]
- 20.Powelson D K, Gerba C P. Virus removal from sewage effluents during saturated and unsaturated flow through soil columns. Water Res. 1994;28:2175–2181. [Google Scholar]
- 21.Powelson D K, Gerba C P. Fate and transport of microorganisms in the vadose zone. In: Wilson L G, Everett L G, Cullen S J, editors. Handbook of vadose zone characterization and monitoring. Boca Raton, Fla: CRC Press, Inc.; 1995. pp. 123–125. [Google Scholar]
- 22.Powelson D K, Mills A L. Bacterial enrichment at the gas-water interface of a laboratory apparatus. Appl Environ Microbiol. 1996;62:2593–2597. doi: 10.1128/aem.62.7.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields, P. A., and S. R. Farrah. Unpublished data.
- 24.Song K B, Damodaran S. Influence of electrostatic forces on the adsorption of succinylated β-lactoglobulin at the air-water interface. Langmuir. 1991;7:2737–2742. [Google Scholar]
- 25.Stumm W, Morgan J J. Aquatic chemistry. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 26.Taylor D H, Bellamy A R, Wilson A T. Interaction of bacteriophage R17 and reovirus type III with the clay mineral allophane. Water Res. 1980;14:339–346. [Google Scholar]
- 27.Thompson S S, Flury M, Yates M V, Jury W A. Role of the air-water-solid interface in bacteriophage sorption experiments. Appl Environ Microbiol. 1998;64:304–309. doi: 10.1128/aem.64.1.304-309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trouwborst T, de Jong J C, Winkler K C. Mechanism of inactivation in aerosols of bacteriophage T1. J Gen Virol. 1972;15:235–242. doi: 10.1099/0022-1317-15-3-235. [DOI] [PubMed] [Google Scholar]
- 29.Trouwborst T, Winkler K C. Protection against aerosol-inactivation of bacteriophage T1 by peptides and amino acids. J Gen Virol. 1972;17:1–11. doi: 10.1099/0022-1317-17-1-1. [DOI] [PubMed] [Google Scholar]
- 30.Trouwborst T, de Jong J C. Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Appl Microbiol. 1973;26:252–257. doi: 10.1128/am.26.3.252-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trouwborst T, Kuyper S, de Jong J C, Plantinga A D. Inactivation of some bacterial and animal viruses by exposure to liquid-air interfaces. J Gen Virol. 1974;24:155–165. doi: 10.1099/0022-1317-24-1-155. [DOI] [PubMed] [Google Scholar]
- 32.Vaughn J M, Landry E F, Baranosky L J, Beckwith C A, Dahl M C, Delihas N C. Survey of human virus occurrence in wastewater-recharged groundwater on Long Island. Appl Environ Microbiol. 1978;36:47–51. doi: 10.1128/aem.36.1.47-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan J, Wilson J L. Colloid transport and the gas-water interface in porous media. In: Sabatini D A, Knox R C, editors. Transport and remediation of subsurface contaminants. Washington, D.C: American Chemical Society; 1992. pp. 55–70. [Google Scholar]
- 34.Wan J, Wilson J L. Visualization of the role of the gas-water interface on the fate and transport of colloids in porous media. Water Resour Res. 1994;30:11–23. [Google Scholar]
- 35.Wan J, Wilson J L, Kieft T L. Influence of the gas-water interface on transport of microorganisms through unsaturated porous media. Appl Environ Microbiol. 1994;60:509–516. doi: 10.1128/aem.60.2.509-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams D F, Berg J C. The aggregation of colloidal particles at the air-water interface. J Colloid Interface Sci. 1992;152:218–229. [Google Scholar]