Abstract
Context
Red ginseng polysaccharide (RGP) is an active component of the widely used medicinal plant Panax ginseng C. A. Meyer (Araliaceae), which has displayed promising activities against cancer cells. However, the detailed molecular mechanism of RGP in ferroptosis is still unknown.
Objective
This study evaluates the effects of RGP in cancer cells.
Materials and methods
A549 and MDA-MB-231 cells were used. Cell proliferation was measured by CCK-8 assay after being treated with RGP at concentrations of 0, 50, 100, 200, 400, 800 and 1600 μg/mL at 0, 12, 24 and 48 h. Lipid reactive oxygen species (ROS) levels were assessed by C11-BODIPY assay. The control group was treated with PBS.
Results
RGP inhibited human A549 (IC50: 376.2 μg/mL) or MDA-MB-231(IC50: 311.3 μg/mL) proliferation and induced lactate dehydrogenase (LDH) release, promoted ferroptosis and suppressed the expression of GPX4. Moreover, the effects of RGP were enhanced by the ferroptosis inducer erastin, while abolished by ferroptosis inhibitor ferrostatin-1.
Discussion and conclusions
Our study is the first to demonstrate (1) the anticancer activity of RGP in human lung cancer and breast cancer. (2) RGP presented the anti-ferroptosis effects in lung and breast cancer cells via targeting GPX4.
Keywords: Lung cancer, breast cancer, traditional Chinese medicine
Introduction
The use of medicinal herbs and their derivatives has emerged as an easily available complementary treatment in the field of cancer therapy, and the efficacy of herbal medicines has been extensively explored in human cell lines and animal models, as well as clinical trials (Ho and Tan 2011). Red ginseng is a common name for Panax ginseng C. A. Meyer (Araliaceae) that has been widely used in traditional herbal medicine in Asia, and has increasingly gained popularity in Western countries, despite that the underlying mechanism has not been clearly defined (Radad et al. 2006; Ho and Tan 2011; Wang CZ et al. 2016). Red ginseng polysaccharide (RGP) is one of the active components from red ginseng. A previous report has demonstrated that RGP is a promising immune-stimulating modifiers and has potential value for tumour therapy (Zhou et al. 2014). Moreover, a high level of RGAP is closely associated with the increased activity of the immune system; RGP is believed to have the function to activate immune activity (Youn et al. 2020). However, the detailed molecular mechanism of PGP is still not fully understood.
Lung cancer is the most common cancer with poor prognosis worldwide, and breast cancer is the most prevalent cancer diagnosed among women (Siegel et al. 2020). Although extensive efforts have been focussed on research and therapeutic regimens for these cancers, favourable clinical efficacy has yet to be achieved (Duda et al. 1999; Corbit et al. 2006; Park et al. 2011; Zhou et al. 2014; Ahuja et al. 2018; Yu et al. 2018). Therefore, exploring and developing novel therapeutic strategies is eagerly desired for patients with lung or breast cancer.
Ferroptosis is a non-apoptotic type of regulated and controlled cell death that is biochemically and genetically disparate from other major cell deaths, such as apoptosis, autophagy and necrosis (Dixon 2017; Mou et al. 2019; Xu et al. 2019). It is iron-dependent and is accompanied by the accumulation of lipid reactive oxygen species (ROS) due to ROS imbalance. Ferroptosis is characterized by the downregulation of the antioxidant peroxidase glutathione peroxidase 4 (GPX4) which regulates clearance of ROS, followed by the accumulation of lipid peroxidation products (Friedmann Angeli et al. 2014; Dixon and Stockwell 2019; Seibt et al. 2019). Ferroptosis inducers include artesunate/erastin and sulfasalazine (SAS), which act directly or indirectly on glutathione peroxidase (GPXs) through diverse pathways, resulting in decreased cell antioxidant capacity and ROS accumulation and eventually causing oxidative cell death (Dolma et al. 2003; Dixon et al. 2012). It has been reported that ferritinophagy-mediated ferroptosis is an essential mechanism contributing to sepsis-induced cardiac injury. Targeting ferroptosis is recognized as a therapeutic strategy for its treatment (Li N et al. 2020). Moreover, targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity (Li X et al. 2020). Moreover, the induction of ferroptosis has been implicated in cancer cell resistance to chemotherapies or targeted therapies (Hangauer et al. 2017; Friedmann Angeli et al. 2019).
Currently, ferroptosis-inducing drugs are gaining attention and exhibiting promising potential in cancer therapy. Here, we evaluated the therapeutic implication of RGP in cancer cells, characterized its ferroptosis induction effect, and propose a rational therapeutic approach for disease treatment.
Materials and methods
Chemicals
Red ginseng polysaccharide was synthesized and purchased from JRDUN Biological Technology Co., Ltd. (Shanghai, China). Ferrostatin-1 (F129882) and erastin (E126853) were obtained from Aladdin (Shanghai, China).
Cell culture
A549 (human non-small cell lung cancer cell line) and MDA-MB-231 (human triple-negative breast cancer cell line) cells were bought from the Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM medium supplemented with 10% foetal bovine serum and 1% penicillin–streptomycin, and incubated in a humidified atmosphere at 37 °C with 5% CO2.
GPX4 overexpression
The full-length human GPX4 cDNA sequence was cloned into pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA) by Genewiz (Suzhou, China). The plasmids were verified by restriction enzyme digestion and Sanger sequencing. GPX4 (oeGPX4) or empty vector constructs were transfected into A549 and MDA-MB-231 cells using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions (Ma et al. 2014).
Cell proliferation assay
Cell proliferation was assessed by CCK-8 assay (Jiancheng Bioengineering, Nanjing, China). Cells were seeded at 3000 cells per well in opaque 96-well plates at 37 °C overnight. Then, cells were cocultured with RGP in different concentrations for 0, 12, 24 and 48 h, respectively, including 0, 50, 100, 200, 400, 800 and 1600 µg/mL. After that, 10 μL of CCK-8 was dispensed to each well at various time points, followed by 3 h incubation in the dark. The absorbance was detected at 450 nm with a microplate reader (model 550, BioRad, Richmond, CA). The cell viability was calculated by the normalization of mean optical densities (ODs) to the negative control using the following equation: OD450nm at 0, 12, 24 and 48 h/average OD450nm at 0 h in 0 µg/mL group.
Detection of lactate dehydrogenase (LDH)
Lactate dehydrogenase release was assessed using LDH release assay kit (Jiancheng Bioengineering, Nanjing, China). Briefly, 1 × 104 A549 and MDA-MB-231 cells were plated in 96-well plates, and RGP (200 μg/mL) was added to the cells and incubated for 48 h at 37 °C. Then, the medium solution was transferred to new 96-well plates and LDH reaction mix was added according to the instruction of the manufacturer. Absorbance was detected at 450 nm with a plate reader (model 550, BioRad, Richmond, CA).
Lipid peroxidation assay using flow cytometry
Lipid ROS levels were assessed by using C11-BODIPY assay following the manufacturer’s protocol (D3861, Thermo Fisher Scientific, Waltham, MA). Cells were treated with RGP (200 μg/mL) for 48 h at 37 °C as indicated above, then 10 mM C11-BODIPY-containing medium was replenished for 1 h. Cells were then collected by trypsinization and washed with PBS containing 1% BSA. Lipid ROS levels were determined with a flow cytometer (FACSCanto™ II, BD Biosciences, San Jose, CA).
Immunoblotting
Total protein lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer containing proteinase inhibitor (Beyotime, Shanghai, China). Proteins were separated on sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gels and transferred onto nitrocellulose membranes (Millipore, Billerica, MA). After blocking with 5% non-fat milk, membranes were incubated with primary antibodies at 4 °C for overnight. Subsequent to washing, membranes were incubated with anti-rabbit IgG HRP-conjugated secondary antibody (A0208, Beyotime, Shanghai, China) at room temperature for 1 h. Enhanced chemiluminescence (ECL) system (MilliporeSigma, Burlington, MA) was used for signal detection. Antibodies used were GPX4 (Ab125066, Abcam, Cambridge, UK), GAPDH (60004-1-1G, Proteintech, Wuhan, China).
Statistical analysis
Statistical analyses were carried out using GraphPad Prism software (version 6.0, San Diego, CA). Student’s t-test and analysis of variance (ANOVA) were used to conduct the data analysis. A p value of less than 0.05 was considered statistically significant.
Results
Red ginseng polysaccharide prevented cancer cell proliferation
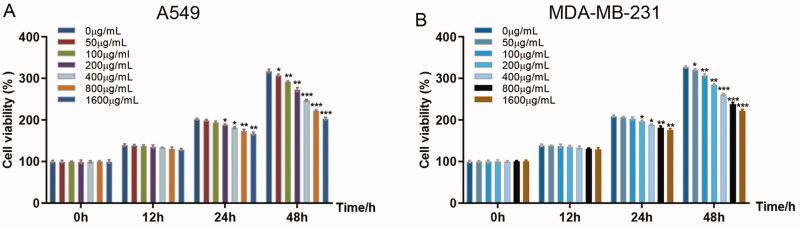
To evaluate the anticancer activity of RGP, human non-small cell lung cancer cell line A549 and human triple-negative breast cancer cell line MDA-MB-231 were treated with serial concentrations of RGP. CCK-8 cell viability assay showed that starting from 200 μg/mL treatment, RGP significantly inhibited cancer cell proliferation at 24 h in both A549 and MDA-MB-231 cells (Figure 1(A,B)). Furthermore, longer incubation revealed that as low as 50 μg/mL of RGP treatment resulted in potent anticancer activity at 48 h. RGP therefore displays potent anticancer activity against cancer cells, suggesting its potential as a therapeutic drug. Importantly, our results suggested the IC50 RGP in human A549 or MDA-MB-231 is 376.2 or 311.3 μg/Ml, respectively. Taken together, the following analysis was conducted by using RGP at the concentration of 200 μg/mL.
Figure 1.
RGP inhibited cancer cell proliferation in A549 and MDA-MB-231 cells. Lung cancer cell line A549 (A) and breast cancer cell line MDA-MB-231 (B) were incubated with increasing concentrations of RGP for 24 and 48 h. Cell viability was measured using CCK-8 assay and normalized to 0 h using the following equation: OD450 nm at 0, 12, 24 and 48 h/average OD450 nm at 0 h in 0 µg/mL group. *p < 0.05, **p < 0.01 and ***p < 0.001.
RGP treatment leads to LDH release and ferroptosis induction
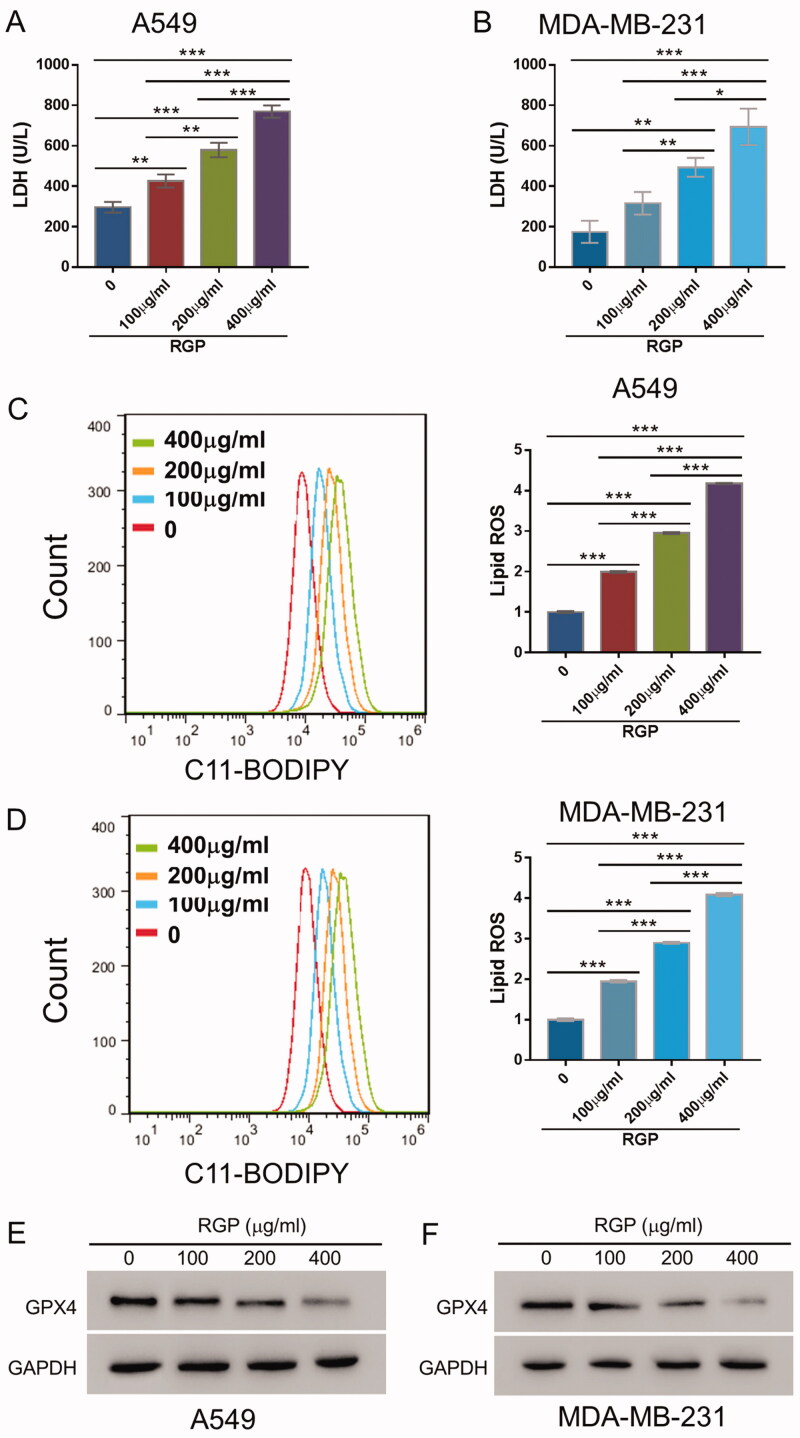
To further understand the physiological and therapeutic basis of RGP, we measured the levels of LDH release upon RGP treatment given that cytosolic LDH is promptly released into the cell medium when the plasma membrane and cell membrane were compromised. As the concentration of RGP increased, the cellular cytotoxicity was gradually increasing in both A549 and MDA-MD-231 cells (Figure 2(A,B)).
Figure 2.
RGP treatment dose-dependently resulted in LDH release, ROS accumulation and GPX4 downregulation in A549 and MDA-MB-231 cells. A549 and MDA-MB-231 cells were treated with 0, 100, 200 and 400 μg/mL of RGP and incubated for 48 h. (A, B) Levels of LDH release were measured. (C, D) Lipid ROS accumulation was determined by BODIPYTM 581/591 C11 using a flow cytometer. (E, F) GPX4 expression was examined by Western blotting. *p < 0.05, **p < 0.01 and ***p < 0.001.
Since ROS-induced lipid peroxidation is well-defined mechanism in ferroptosis induction, we employed BODIPY 581/591 undecanoic acid to detect ROS upon RGP treatment. As shown by flow cytometry, notable shifts in C11-BODIPY signal following RGP treatments were observed in both A549 and MDA-MD-231 cells, indicating ROS induction (Figure 2(C,D)).
Furthermore, the expression of GPX4, a phospholipid hydroperoxidase that protects cells from membrane lipid peroxidation and an essential regulator of ferroptosis, was significantly reduced in response to RGP treatment in a dose-dependent manner (Figure 2(E,F)). Together, these data demonstrated that RGP treatment induced significant cellular toxicities in cancer cells and ferroptosis, resulting in LDH release, accumulation of lipid ROS, and GPX4 downregulation.
RGP treatment elicits its effects through ferroptosis induction
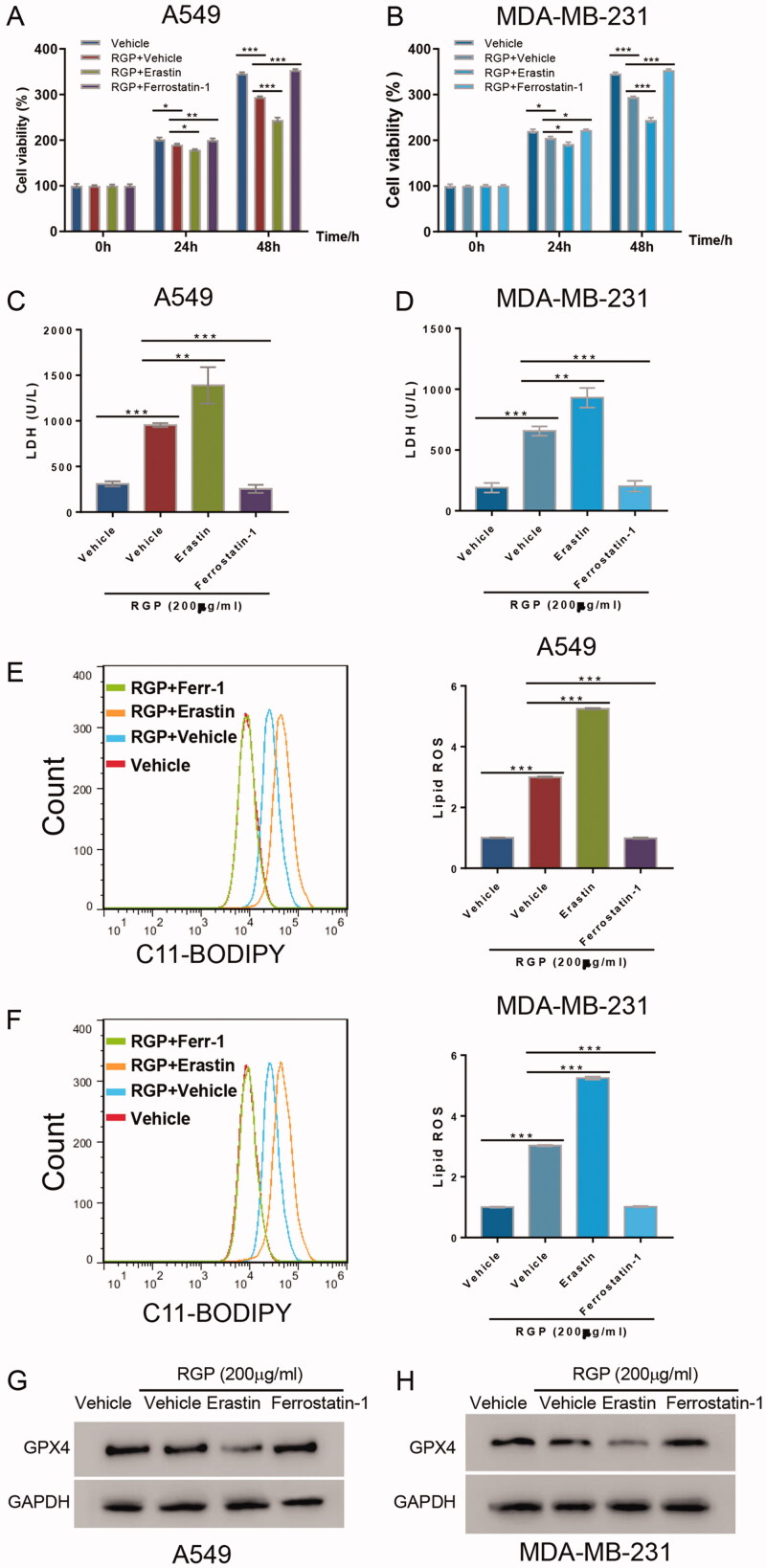
To verify RGP treatment-induced ferroptosis, we introduced ferrostatin-1, a ferroptosis inhibitor, and erastin, a ferroptosis inducer, in both A549 and MDA-MB-231 cells. Cells were treated with either 200 μg/mL of RGP itself, or combined with either ferrostatin-1 or erastin for 48 h. Consistently, 200 μg/mL of RGP treatment exhibited ferroptotic cell deaths in A549 and MD-MB-231 cells. Correspondingly, 35 μM of erastin treatment synergized with RGP and significantly suppressed cell proliferation. In contrast, when combined with 2 μM of ferrostatin-1, RGP-induced ferroptosis was abolished (Figure 3(A,B)).
Figure 3.
Ferroptosis induction mediated the underlying effects of RGP treatment. A549 and MDA-MB-231 cells were pre-treated with 200 μg/mL of RGP, and then ferrostatin-1 (2 μM) or erastin (35 μM) was added to cells. (A, B) Cell viability assay, (C, D) LDH release assay and (E, F) C11-BODIPY assay were performed. (G, H) GPX4 expression level was determined. *p < 0.05, **p < 0.01 and ***p < 0.001.
Likewise, the release of LDH and the accumulation of lipid ROS resulting from RGP treatment were significantly inhibited by ferrostain-1 treatment but elevated by erastin treatment, as shown by LDH release assay (Figure 3(C,D)) and C11-BODIPY assay (Figure 3(E,F)), respectively.
As for GPX4, treatment with erastin strongly suppressed GPX4 expression while treatment with ferrostatin-1 abolished RGP-induced GPX4 downregulation (Figure 3(G,H)). Collectively, these data demonstrated the anticancer activity of RGP via ferroptosis induction.
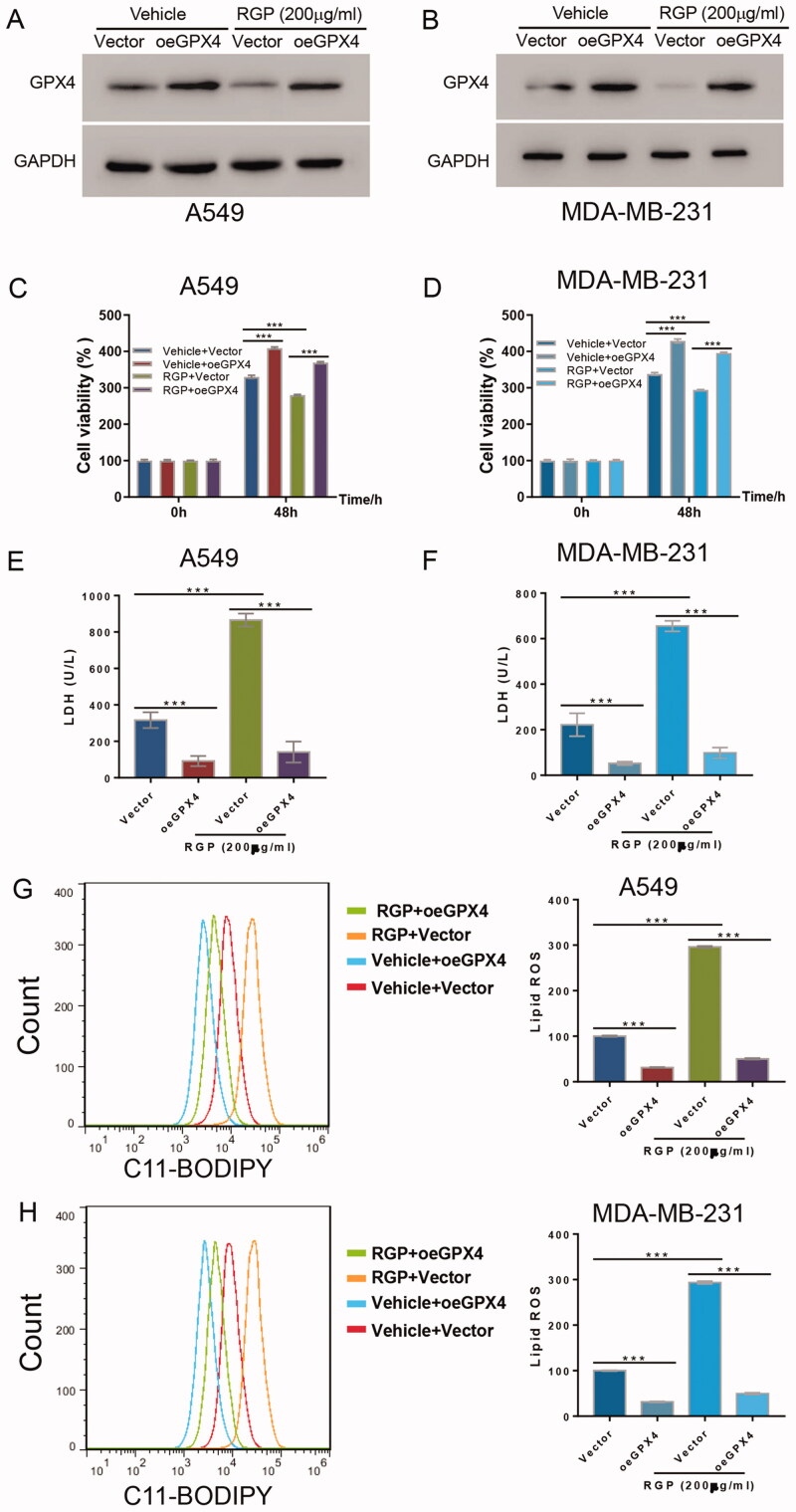
Enforced GPX4 expression abrogated the anticancer activity of RGP
Given that GPX4 expression was significantly reduced upon RGP treatment, we assessed the effects that would be elicited when GPX4 expression was restored. Western blot confirmed overexpression of GPX4 in both A549 and MDA-MB-231 cells (Figure 4(A,B)). Consistently, 200 μg/mL of RGP downregulated the expression of GPX4 in cells transduced with vector control, and this effect was disrupted in GPX4 overexpressing cells (oeGPX4) (Figure 4(A,B)). More importantly, the overexpression of GPX4 itself stimulated cell proliferation in both cell lines. While RGP treatment inhibited cell viability as expected, oeGPX4 abrogated RGP activity and facilitated cell viability when compared to the control (Figure 4(C,D)). Similarly, GPX4 overexpression led to the decline of LDH release in both cell lines without RGP treatment. Moreover, oeGPX4 significantly abolished RGP-induced LDH release in cells with RGP treatment (Figure 4(E,F)). Strikingly, oeGPX4 reversed the accumulation of ROS in cells with or without RGP treatment in spite of the enhanced accumulation of lipid ROS upon RGP treatment, as demonstrated by C11-BODIPY assay (Figure 4(G,H)).
Figure 4.
GPX4 overexpression abrogated the effects of RGP. GPX4 or empty vector was overexpressed in A549 and MDA-MB-231 cells, and cells were incubated for 48 h, with or without 200 μg/mL of RGP treatment. (A, B) Western blotting, (C, D) cell viability assay and (E, F) LDH release assay were performed. (G, H) Lipid ROS accumulation was determined according to C11-BODIPY signal shift. ***p < 0.001.
Taken together, GPX4 abrogated the anticancer activity of RGP and prevented the lipid hydroperoxides primed ferroptosis.
Discussion
Ferroptosis is a type of programmed and controlled cell death first proposed by Dixon through a chemical screening (Dixon et al. 2012). Emerging evidence has indicated the application of suppressing ferroptosis induction in cancer treatment (Lu et al. 2017; Mou et al. 2019; Ye et al. 2020). Additionally, drug resistance and evasion of cell death in tumour cells could be overcome by introducing and maintaining ferroptosis (Hangauer et al. 2017), suggesting the potential of ferroptosis-inducing agents in cancer therapy.
Ginseng has been traditionally administrated as a medicine in Asia for centuries (Helms 2004). Red ginseng polysaccharide is an active component from red ginseng that has been explored extensively in cancer treatment. Previous reported that a neutral polysaccharide fraction (WGPN) inhibits S180 tumour growth in a bell-shaped dose-response curve and can be used as a potential adjuvant for chemotherapeutic drugs (Ni et al. 2010). Moreover, ginseng polysaccharides also provide anti-fatigue activity (Wang J et al. 2010). In this report, we evaluated the activity of RGP in lung and breast cancer cells, and investigated the underlying mechanisms. Our findings suggested that RGP treatment significantly inhibited the proliferation and promoted ferroptosis induction in lung or breast cancer cells resulting in LDH release and ROS accumulation and thus highlighting its potential in cancer treatment.
Considering the essential role of GPX4 in ferroptosis, we therefore assessed the effects of RGP treatment on GPX4 expression. Interestingly, we found that RGP treatment downregulated GPX4 expression and demonstrated that GPX4 overexpression conferred drug resistance. We therefore propose that targeting GPX4 is a promising therapeutic approach in the treatment of lung and breast cancers. Importantly, it was the first time to illustrated the role of RGP in the ferroptosis in lung or breast cancer cells. Moreover, RGP suppressed the progression of lung or breast cancer cells that might target GPX4. Our findings not only enhanced the understating into the effects of RGP in the progression of lung and breast cancer cells but also provided evidence to indicate its potential role in developing the therapy for patient with lung or breast cancer.
Conclusions
We evaluated the anticancer activity of RGP in lung and breast cancer cells, and characterized the ferroptosis induction underlying its basis of action. Our findings implied that RGP could serve as a cancer-prevention agent in lung cancer and breast cancer. Furthermore, we revealed a rational approach to remove cancer cell protection from ferroptosis by targeting GPX4. Meanwhile, our long-term goals are to carry out animal experiments to assess the in vivo anticancer activity of RGP, and explore and improve the strategy using combination treatment options.
Funding Statement
This study was supported by Scientific Research Projects for Basic Scientific Research in Heilongjiang Provincial Universities (2020-KYYWF-0802), Natural Science Foundation of Heilongjiang Province (LH2019H062) and Applied Technology Research and Development Programme in Mudanjiang (HT2020NS085). This study was also supported by The Doctoral Scientific Research Foundation of Mudanjiang Medical University; Scientific Research Projects for Basic Scientific Research in Heilongjiang Provincial Universities.
Disclosure statement
The authors declare that they have no competing interests.
References
- Ahuja A, Kim JH, Kim JH, Yi YS, Cho JY.. 2018. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 42(3):909–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit R, Ebbs S, King ML, Murphy LL.. 2006. The influence of lead and arsenite on the inhibition of human breast cancer MCF-7 cell proliferation by American ginseng root (Panax quinquefolius L.). Life Sci. 78(12):1336–1340. [DOI] [PubMed] [Google Scholar]
- Dixon SJ. 2017. Ferroptosis: bug or feature? Immunol Rev. 277(1):150–157. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149(5):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Stockwell BR.. 2019. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 3(1):35–54. [Google Scholar]
- Dolma S, Lessnick SL, Hahn WC, Stockwell BR.. 2003. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 3(3):285–296. [DOI] [PubMed] [Google Scholar]
- Duda RB, Zhong Y, Navas V, Li MZ, Toy BR, Alavarez JG.. 1999. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol. 72(4):230–239. [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Krysko DV, Conrad M.. 2019. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 19(7):405–414. [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. 2014. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 16(12):1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. 2017. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 551(7679):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms S. 2004. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 9(3):259–274. [PubMed] [Google Scholar]
- Ho CC, Tan HM.. 2011. Rise of herbal and traditional medicine in erectile dysfunction management. Curr Urol Rep. 12(6):470–478. [DOI] [PubMed] [Google Scholar]
- Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, Wu H, Deng W, Shen D, Tang Q.. 2020. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 160:303–318. [DOI] [PubMed] [Google Scholar]
- Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, Guan KL, Xiong Y, Liu J, Yuan HX.. 2020. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 40(6):1378–1394. [DOI] [PubMed] [Google Scholar]
- Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B.. 2017. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 8:992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KL, Nyamtsengel V, Bao WL, Lian MY, Wang WP, Wang YF, Wang X, Wang ZG.. 2014. Overexpression of protein kinase B/AKT induces phosphorylation of p70S6K and 4E-BP1 in goat fetal fibroblasts. Genet Mol Res. 13(4):9931–9938. [DOI] [PubMed] [Google Scholar]
- Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B.. 2019. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 12(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Zhang X, Wang B, Chen Y, Han H, Fan Y, Zhou Y, Tai G.. 2010. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J Med Food. 13(2):270–277. [DOI] [PubMed] [Google Scholar]
- Park D, Bae DK, Jeon JH, Lee J, Oh N, Yang G, Yang YH, Kim TK, Song J, Lee SH, et al. 2011. Immunopotentiation and antitumor effects of a ginsenoside Rg3-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 31(3):397–405. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Liu L, Rausch WD.. 2006. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 100(3):175–186. [DOI] [PubMed] [Google Scholar]
- Seibt TM, Proneth B, Conrad M.. 2019. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 133:144–152. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A.. 2020. Cancer statistics, 2020. CA Cancer J Clin. 70(1):7–30. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Anderson S, Du W, He TC, Yuan CS.. 2016. Red ginseng and cancer treatment. Chin J Nat Med. 14(1):7–16. [DOI] [PubMed] [Google Scholar]
- Wang J, Li S, Fan Y, Chen Y, Liu D, Cheng H, Gao X, Zhou Y.. 2010. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J Ethnopharmacol. 130(2):421–423. [DOI] [PubMed] [Google Scholar]
- Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J.. 2019. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 23(8):4900–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Liu W, Zhuo Q, Hu Q, Liu M, Sun Q, Zhang Z, Fan G, Xu W, Ji S, et al. 2020. Ferroptosis: final destination for cancer? Cell Prolif. 53(3):e12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn SH, Lee SM, Han CK, In G, Park CK, Hyun SH.. 2020. Immune activity of polysaccharide fractions isolated from Korean red ginseng. Molecules. 25(16):3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Roh HS, Baek KH, Lee S, Kim S, So HM, Moon E, Pang C, Jang TS, Kim KH.. 2018. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J Ginseng Res. 42(4):562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Shi H, Jiang G, Zhou Y, Xu J.. 2014. Antitumor activities of ginseng polysaccharide in C57BL/6 mice with Lewis lung carcinoma. Tumour Biol. 35(12):12561–12566. [DOI] [PubMed] [Google Scholar]