Abstract
BACKGROUND
Sotorasib showed anticancer activity in patients with KRAS p.G12C–mutated advanced solid tumors in a phase 1 study, and particularly promising anticancer activity was observed in a subgroup of patients with non–small-cell lung cancer (NSCLC).
METHODS
In a single-group, phase 2 trial, we investigated the activity of sotorasib, administered orally at a dose of 960 mg once daily, in patients with KRAS p.G12C–mutated advanced NSCLC previously treated with standard therapies. The primary end point was objective response (complete or partial response) according to independent central review. Key secondary end points included duration of response, disease control (defined as complete response, partial response, or stable disease), progression-free survival, overall survival, and safety. Exploratory biomarkers were evaluated for their association with response to sotorasib therapy.
RESULTS
Among the 126 enrolled patients, the majority (81.0%) had previously received both platinum-based chemotherapy and inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1). According to central review, 124 patients had measurable disease at baseline and were evaluated for response. An objective response was observed in 46 patients (37.1%; 95% confidence interval [CI], 28.6 to 46.2), including in 4 (3.2%) who had a complete response and in 42 (33.9%) who had a partial response. The median duration of response was 11.1 months (95% CI, 6.9 to could not be evaluated). Disease control occurred in 100 patients (80.6%; 95% CI, 72.6 to 87.2). The median progression-free survival was 6.8 months (95% CI, 5.1 to 8.2), and the median overall survival was 12.5 months (95% CI, 10.0 to could not be evaluated). Treatment-related adverse events occurred in 88 of 126 patients (69.8%), including grade 3 events in 25 patients (19.8%) and a grade 4 event in 1 (0.8%). Responses were observed in subgroups defined according to PD-L1 expression, tumor mutational burden, and co-occurring mutations in STK11, KEAP1, or TP53.
CONCLUSIONS
In this phase 2 trial, sotorasib therapy led to a durable clinical benefit without new safety signals in patients with previously treated KRAS p.G12C–mutated NSCLC. (Funded by Amgen and the National Institutes of Health; CodeBreaK100 ClinicalTrials.gov number, NCT03600883.)
CONSIDERABLE PROGRESS HAS BEEN made in the treatment of non–small-cell lung cancer (NSCLC) in recent years, with a substantial reduction in mortality.1,2 This progress is attributable largely to improvements in systemic therapy for advanced disease, including the approvals of targeted therapies for patients with specific oncogenic driver mutations and of checkpoint inhibitors, either as monotherapy or in combination with chemotherapy, for patients without an actionable driver mutation.3–6 However, the prognosis in patients with advanced NSCLC receiving second or subsequent lines of therapy is unsatisfactory, with 6 to 20% of such patients having a response and with a median progression-free survival of 2 to 4 months associated with chemotherapy agents or checkpoint inhibitors.7–10 For patients whose disease progresses after the use of platinum-based chemotherapy and checkpoint inhibitors, chemotherapy with docetaxel, with or without antiangiogenic therapy, or single-agent pemetrexed remains the standard care.11,12
Activating mutations in Kirsten rat sarcoma viral oncogene homologue (KRAS) are found in 25 to 30% of non–squamous-cell NSCLCs, representing the most prevalent genomic driver event in NSCLC.13–15 KRAS-mutated NSCLCs constitute a molecularly diverse and clinically heterogeneous group, and standard treatment options provide only modest clinical benefit.16–18 Among all KRAS mutations, the KRAS p.G12C single-nucleotide variation, with glycine substituted by cysteine at codon 12, is the most frequent variant in NSCLC, with a prevalence of approximately 13% in lung adenocarcinomas.13
The KRAS protein is a guanosine triphosphatase (GTPase) that serves as a molecular switch by cycling between active guanosine triphosphate (GTP)–bound and inactive guanosine diphosphate (GDP)–bound states in response to extracellular stimuli. The KRAS p.G12C mutation favors the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS, leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth.19 The picomolar affinity of KRAS for GTP and the high intracellular concentration of this trinucleotide, coupled with the lack of binding pockets on GTP-bound KRAS and the consequent failure of direct KRAS-targeting approaches, led to a long-standing notion that mutant KRAS is “undruggable.”20 This view persisted for almost four decades until several breakthrough structural and mechanistic studies established the conceptual foundation for the clinical development of covalent and selective KRASG12C inhibitors.19,21–23
Sotorasib is a small molecule that specifically and irreversibly inhibits KRASG12C. Sotorasib covalently binds to a pocket of the switch II region that is present only in the inactive GDP-bound conformation, trapping KRASG12C in the inactive state and inhibiting KRAS oncogenic signaling.24 The phase 1 portion of the CodeBreaK100 trial, which involved patients with pretreated advanced solid tumors harboring the KRAS p.G12C mutation, showed encouraging safety and efficacy of sotorasib monotherapy, and particularly promising anticancer activity was observed in the subgroup of patients with NSCLC.25 Here, we report results from the phase 2 portion of the CodeBreaK100 trial (aimed at defining a particular indication for use), which involved patients with KRAS p.G12C–mutated advanced NSCLC. The phase 1 cohorts and the phase 2 portion were analyzed separately; therefore, data from the patients in the phase 1 cohorts are not included in the current article.
METHODS
PATIENTS
We conducted a multicenter, single-group, open-label, phase 2 trial to evaluate the efficacy and safety of sotorasib as monotherapy in patients with locally advanced or metastatic KRAS p.G12C–mutated NSCLC. Key inclusion criteria for this trial were an age of 18 years or older; pathologically documented, locally advanced or metastatic NSCLC with the KRAS p.G12C mutation confirmed on central laboratory testing with the use of the therascreen KRAS RGQ PCR Kit; disease progression after the receipt of anti–programmed death 1 (PD-1) or anti–programmed death ligand 1 (PD-L1) immunotherapy or platinum-based combination chemotherapy or after the receipt of both immunotherapy and platinum-based combination chemotherapy; an Eastern Cooperative Oncology Group performance-status score of 0 to 1 (on a scale from 0 to 5, with higher numbers indicating greater disability); and measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
Key exclusion criteria were active untreated brain metastases, the receipt of more than three previous lines of therapy, the receipt of systemic anticancer therapy within 28 days before the initiation of sotorasib therapy, the receipt of therapeutic or palliative radiation therapy within 2 weeks before the initiation of sotorasib therapy, and previous treatment with a direct KRASG12C inhibitor. The full inclusion and exclusion criteria are listed in the protocol, available with the full text of this article at NEJM.org.
TrIAL DESIGN AND END POINTS
Sotorasib was administered at a dose of 960 mg orally once daily. Treatment with sotorasib continued until the occurrence of progressive disease, the development of unacceptable side effects, or withdrawal of consent.
The primary end point was objective response (complete or partial response) as assessed by blinded, independent, central radiologic review. Tumor response was assessed by independent central review according to RECIST, version 1.1, with the use of contrast-enhanced computed tomography or magnetic resonance imaging.
Key secondary end points were duration of response, disease control (defined as complete response, partial response, or stable disease, according to RECIST, version 1.1; minimum time interval for the determination of stable disease, 5 weeks), time to response, progression-free survival, overall survival, and safety. Adverse events were graded with the use of the Common Terminology Criteria for Adverse Events, version 5.0. In accordance with the protocol, response-related end points were evaluated in patients who had received at least one dose of sotorasib and had at least one measurable lesion at baseline as assessed by independent central review according to RECIST, version 1.1. In the exploratory analyses, candidate biomarkers were evaluated by means of molecular analysis of blood and tumor-tissue specimens for their association with tumor response to sotorasib therapy. Further details are included in the Supplementary Methods section in the Supplementary Appendix, available at NEJM.org.
TRIAL OVERSIGHT
The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation and the principles of the Declaration of Helsinki. The protocol and amendments were approved by the institutional review board at each participating site and regulatory authorities of participating countries. All the patients provided written informed consent. The trial was designed by employees of Amgen (the main sponsor) in collaboration with the investigators. The data were collected by investigators, assessed by independent central review, and analyzed by statisticians employed by Amgen. A medical writer employed by Amgen wrote the first draft of the manuscript and provided editorial assistance. All the authors contributed to the interpretation of the data and to the preparation of the manuscript. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
We planned that the phase 2 portion of this trial would include 105 patients with NSCLC. We calculated that this sample size would provide the trial with approximately 90% probability that the lower limit of the 95% confidence interval for objective response would exceed the bench-mark response of 23% among patients with pre-treated NSCLC. This benchmark was shown in the phase 3 REVEL trial, which tested the combination of ramucirumab plus docetaxel as second-line treatment in patients with advanced NSCLC after disease progression during platinum-based therapy.26
A data review team continuously assessed safety and made recommendations to the main sponsor regarding the continuation of the trial. The data review team also oversaw futility analyses that were conducted in a continuous manner with the use of Bayesian predictive probability, starting after 25 patients completed 7 weeks of the trial and occurring after every 10 additional patients could be evaluated for response after completing at least 7 weeks of the trial. The criterion for moving forward with the trial was at least an 80% probability that the true response rate would exceed the benchmark. Futility was met if, with the enrollment of the planned sample, the probability of reaching the criterion was less than 5%.
Response was summarized with the use of frequency counts and percentages, with exact 95% confidence intervals calculated by the Clopper–Pearson method. Descriptive summaries of the percentages of patients with a response and 95% Clopper–Pearson exact confidence intervals according to biomarker subgroup are provided. Time-to-event end points were summarized with the use of Kaplan–Meier estimates and 95% confidence intervals.
Results
Patients
A total of 126 patients with previously treated KRAS p.G12C–mutated NSCLC were enrolled from August 13, 2019, to February 5, 2020, and received at least one dose of sotorasib. According to independent central review, 2 patients did not have measurable lesions at baseline and were ineligible for response assessment. Among the remaining 124 patients, 1 did not have centrally confirmed KRAS p.G12C mutation; this patient had stable disease and was included in the response assessments as prespecified in the protocol.
The data-cutoff date was March 15, 2021. The median follow-up was 15.3 months (range, 1.1 to 18.4+; the plus sign indicates that the value includes data that were censored at data cutoff). The median duration of treatment was 5.5 months (range, 0.2 to 17.8). A total of 88 patients (69.8%) received sotorasib for 3 months or more, 60 (47.6%) for 6 months or more, and 41 (32.5%) for 9 months or more. Dose reduction occurred in 26 patients (20.6%). As of the data-cutoff date, 103 patients (81.7%) had discontinued treatment with sotorasib; disease progression (in 83 patients [65.9%]) and adverse events regardless of attribution (in 11 [8.7%]) were the most common reasons for discontinuation (Fig. S1 in the Supplementary Appendix).
The characteristics of the patients at baseline are summarized in Table 1, with further details provided in Table S1. Among the 126 enrolled patients, the median age was 63.5 years (range, 37 to 80), and 117 (92.9%) were current or former smokers. Patients had received a median of two previous lines of systemic anticancer therapy. Previous therapies included platinum-based chemotherapy (in 113 patients [89.7%]), checkpoint inhibitors (in 116 [92.1%]), and antiangiogenic therapies (in 25 [19.8%]). A total of 102 patients (81.0%) had received both platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Patients (N = 126) |
|---|---|
| Median age (range) — yr | 63.5 (37–80) |
| Female sex — no. (%) | 63 (50.0) |
| Race — no. (%)† | |
| White | 103 (81.7) |
| Asian | 19 (15.1) |
| Black | 2 (1.6) |
| Other | 2 (1.6) |
| Smoking history — no. (%) | |
| Never smoked | 6 (4.8) |
| Current smoker | 15 (11.9) |
| Former smoker | 102 (81.0) |
| Missing data | 3 (2.4) |
| ECOG performance-status score — no. (%)‡ | |
| 0 | 38 (30.2) |
| 1 | 88 (69.8) |
| Brain metastasis — no. (%) | |
| Yes | 26 (20.6) |
| No | 100 (79.4) |
| Histologic subtype — no. (%) | |
| Squamous-cell carcinoma | 1 (0.8) |
| Adenocarcinoma | 120 (95.2) |
| Large-cell carcinoma | 3 (2.4) |
| Bronchoalveolar carcinoma | 2 (1.6) |
| Metastatic disease — no. (%) | |
| Yes | 122 (96.8) |
| No | 4 (3.2) |
| No. of previous lines of anticancer systemic therapy — no. (%) | |
| 1 | 54 (42.9) |
| 2 | 44 (34.9) |
| 3 | 28 (22.2) |
| Type of previous systemic anticancer therapy — no. (%) | |
| Chemotherapy§ | 115 (91.3) |
| Platinum-based chemotherapy | 113 (89.7) |
| Checkpoint inhibitor | 116 (92.1) |
| Anti–PD-1 or anti–PD-L1 agent | 115 (91.3) |
| Platinum-based chemotherapy and PD-1 or PD-L1 inhibitor | 102 (81.0) |
| Antiangiogenic monoclonal antibodies | 25 (19.8) |
| Targeted small molecules¶ | 9 (7.1) |
| Other║ | 1 (0.8) |
P ercentages may not total 100 because of rounding. PD-1 denotes programmed death 1, and PD-L1 programmed death ligand 1.
R ace was reported by the patient.
P erformance-status scores on the Eastern Cooperative Oncology Group (ECOG) scale range from 0 to 5, with higher numbers in§dicating greater disability.
T wo patients who did not receive platinum-based chemotherapy received pemetrexed, docetaxel, gemcitabine, and vinorelbine.
Targeted small molecules included capmatinib, nintedanib, trametinib, vorolanib, RMC-4630, sitravatinib, and cobimetinib.
The other previous anticancer systemic therapy was an investigational agent.
Efficacy
Among the 124 patients who were evaluated for a response, 4 (3.2%) had a complete response and 42 (33.9%) had a partial response; thus, an objective response occurred in 46 patients (37.1%; 95% confidence interval [CI], 28.6 to 46.2). Disease control occurred in 100 patients (80.6%; 95% CI, 72.6 to 87.2) (Table 2). Tumor shrinkage of any magnitude was observed in 102 patients (82.3%); among all the patients who had a response, the median best percentage decrease from baseline in tumor burden (defined as the sum of the longest diameters of all target lesions) was 60% (Fig. 1A). Disease progression was the best overall response in 20 patients. A total of 4 patients either could not be evaluated for a response (2) or had missing scans (2). Percentages of patients with an objective response were consistent across prespecified subgroups defined according to the number of previous lines of therapy and according to previous receipt of anti–PD-1 or anti–PD-L1 therapy (Table S2).
Table 2.
Tumor Response to Sotorasib Therapy According to Independent Central Review.*
| Variable | Patients (N = 124) |
|---|---|
| Objective response — % (95% CI)† | 37.1 (28.6–46.2) |
| Disease control — % (95% CI)‡ | 80.6 (72.6–87.2) |
| Best response — no. (%) | |
| Complete response | 4 (3.2) |
| Partial response | 42 (33.9) |
| Stable disease | 54 (43.5) |
| Progressive disease | 20 (16.1) |
| Could not be evaluated | 2 (1.6) |
| Missing scan | 2 (1.6) |
| Median duration of objective response (95% CI) — mo§ | 11.1 (6.9–NE) |
| Kaplan–Meier estimate of objective response (95% CI) — % | |
| At 3 mo | 90.5 (76.7–96.3) |
| At 6 mo | 70.8 (54.3–82.2) |
| At 9 mo | 57.3 (40.4–71.0) |
NE denotes could not be evaluated.
Objective response was defined as a complete or partial response.
Disease control was defined as a complete response, partial response, or stable disease.
The median duration of objective response was calculated on the basis of the 46 patients who had a complete or partial response.
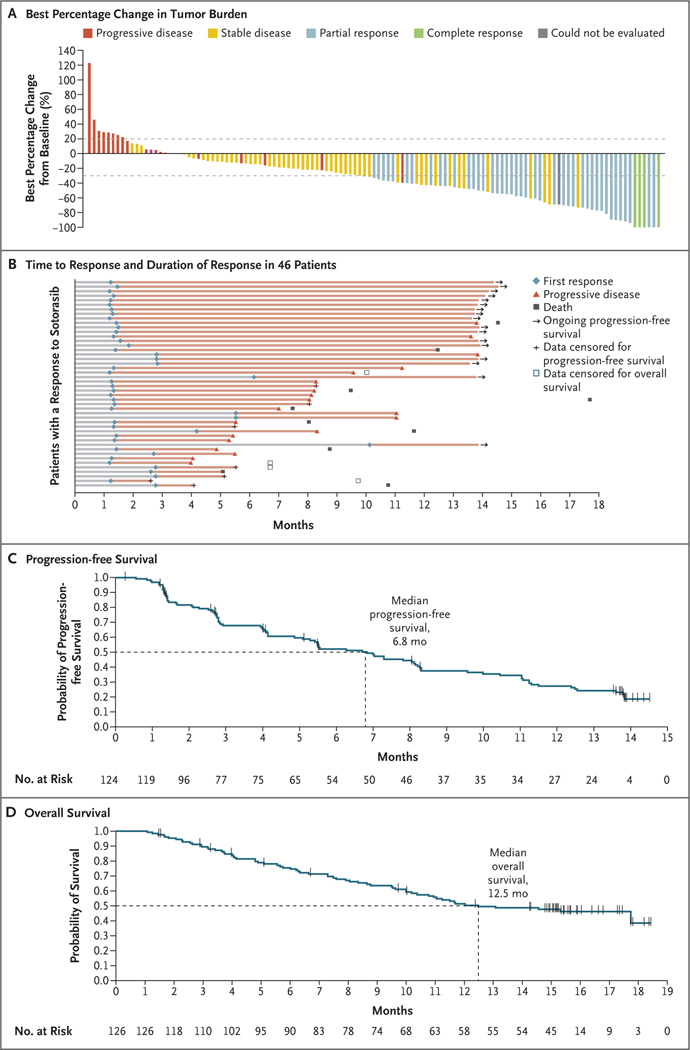
Figure 1. Efficacy of Sotorasib Therapy.
Panel A shows the best percentage decrease from baseline in the tumor burden (defined as the sum of the longest diameters of all target lesions) in 121 of 124 patients with non–small-cell lung cancer who had available post-baseline measurements of target lesions. The 3 patients whose data were excluded from the graph include 2 who had missing scans and 1 who had no measurement in target lesions and had progressive disease in nontarget lesions (progressive disease as the best overall response). Panel B shows the time to response, duration of response, and patient status as of the data-cutoff date for all 46 patients who had an objective response to sotorasib therapy. Panel C shows the Kaplan–Meier curve of progression-free survival among all 124 patients who could be evaluated for a response according to central review. Panel D shows the Kaplan–Meier curve of overall survival among all 126 patients enrolled in the trial. Tick marks in Panels C and D indicate censored data.
Among the 46 patients with an objective response, the median time to response was 1.4 months (range, 1.2 to 10.1), and the median duration of response was 11.1 months (95% CI, 6.9 to could not be evaluated). A response was observed at the first tumor assessment, at approximately week 6, in 33 patients (71.7%) with a response. As of the data-cutoff date, 16 patients with a response (34.7%) were continuing to receive treatment without disease progression (Fig. 1B). Among patients with a response, the Kaplan–Meier estimate of duration of response was 90.5% (95% CI, 76.7 to 96.3) at 3 months, 70.8% (95% CI, 54.3 to 82.2) at 6 months, and 57.3% (95% CI, 40.4 to 71.0) at 9 months.
The median progression-free survival among the 124 patients who could be evaluated was 6.8 months (95% CI, 5.1 to 8.2) (Fig. 1C). The Kaplan–Meier estimate of progression-free survival was 52.2% (95% CI, 42.6 to 60.9) at 6 months and 37.5% (95% CI, 28.4 to 46.5) at 9 months. The median overall survival among all 126 enrolled patients was 12.5 months (95% CI, 10.0 to could not be evaluated) (Fig. 1D). In an analysis of response according to assessment by the local investigator, which included all 126 patients, 2 patients (1.6%) had a complete response, 37 (29.4%) had a partial response, 69 (54.8%) had stable disease, and 15 (11.9%) had disease progression (Table S3).
Exploratory Biomarkers
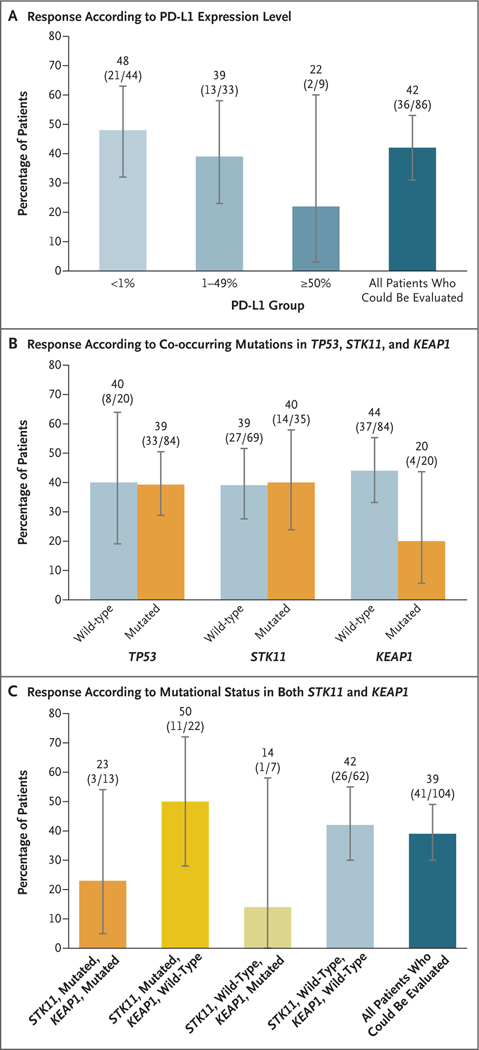
In the descriptive exploratory analyses, we evaluated the potential association between response to sotorasib therapy and baseline tumor PD-L1 expression level, tumor mutational burden, and mutations in STK11, KEAP1, and TP53, which are among the most prevalent genes with co-occurring mutations in KRAS-mutated NSCLC (Figs. 2, S2, and S3 and Tables S6 and S7).27 Among the 86 patients who were assessed for PD-L1 expression, objective response and tumor shrinkage were observed across the range of baseline PD-L1 expression levels, with 48% (95% CI, 32 to 63) of the patients in the PD-L1–negative group (tumor proportion score, <1%) having a response, as well as 42% (95% CI, 31 to 53) of the overall population of patients who could be evaluated (Fig. 2A). Among the 84 patients who were assessed for tumor mutational burden, a response was seen in 42% (95% CI, 30 to 55) of the patients in the subgroup with a low tumor mutational burden (<10 mutations per megabase) and in 40% (95% CI, 16 to 68) of those in the subgroup with a high tumor mutational burden (≥10 mutations per megabase) (Table S7).
Figure 2. Exploratory Biomarker Analyses.
Panel A shows the percentages of patients with an objective response associated with sotorasib therapy in subgroups categorized according to programmed death ligand 1 (PD-L1) expression level. A total of 86 patients with available tissue data were evaluated. Panel B shows the percentages of patients with an objective response in subgroups categorized according to the mutational status of TP53, STK11, and KEAP1, and Panel C the percentages of patients with an objective response in subgroups categorized according to the mutational status of STK11 and KEAP1. In these analyses, 104 patients with available tissue data, plasma data, or both were evaluated. In all panels, I bars represent 95% confidence intervals.
Among the 104 patients who were assessed for co-occurring genomic alterations, efficacy was seen in the subgroups with mutated STK11, KEAP1, or TP53 (Fig. 2B). A response was seen in 50% (95% CI, 28 to 72) of the patients in the subgroup with mutated STK11 and wild-type KEAP1 and in 39% (95% CI, 30 to 49) of the overall population of patients who could be evaluated. Among patients with mutated KEAP1, a response was seen in 23% (95% CI, 5 to 54) of those in the subgroup with both mutated STK11 and KEAP1 and in 14% (95% CI, 0 to 58) of those in the subgroup with wild-type STK11 and mutated KEAP1 (Fig. 2C).
Safety
Safety data for all 126 patients are summarized in Tables 3, S4, and S5. Adverse events of any grade, regardless of attribution, were observed in 125 patients (99.2%); the most common adverse events were diarrhea (in 64 patients [50.8%]), nausea (in 39 [31.0%]), fatigue (in 32 [25.4%]), arthralgia (in 27 [21.4%]), increase in the aspartate aminotransferase level (in 27 [21.4%]), and increase in the alanine aminotransferase level (in 26 [20.6%]). The worst grade of adverse event was grade 3 in 53 patients (42.1%), grade 4 in 4 patients (3.2%), and grade 5 in 20 patients (15.9%).
Table 3.
Adverse Events.*
| Event | All Patients (N = 126) | ||||
|---|---|---|---|---|---|
| Any Grade | Grade 1 or 2 | Grade 3 | Grade 4 | Fatal | |
| number of patients (percent) | |||||
| Adverse event | 125 (99.2) | 48 (38.1) | 53 (42.1) | 4 (3.2) | 20 (15.9) |
| Treatment-related adverse event | 88 (69.8) | 62 (49.2) | 25 (19.8) | 1 (0.8) | 0 |
| Treatment-related adverse event leading to dose modification | 28 (22.2) | 8 (6.3) | 20 (15.9) | 0 | 0 |
| Treatment-related adverse event leading to discontinuation of therapy | 9 (7.1) | 4 (3.2) | 4 (3.2) | 1 (0.8) | 0 |
| Treatment-related adverse event of any grade occurring in >5% of the patients or that was grade ≥3 |
|||||
| Diarrhea | 40 (31.7) | 35 (27.8) | 5 (4.0) | 0 | 0 |
| Nausea | 24 (19.0) | 24 (19.0) | 0 | 0 | 0 |
| Alanine aminotransferase increase | 19 (15.1) | 11 (8.7) | 8 (6.3) | 0 | 0 |
| Aspartate aminotransferase increase | 19 (15.1) | 12 (9.5) | 7 (5.6) | 0 | 0 |
| Fatigue | 14 (11.1) | 14 (11.1) | 0 | 0 | 0 |
| Vomiting | 10 (7.9) | 10 (7.9) | 0 | 0 | 0 |
| Blood alkaline phosphatase increase | 9 (7.1) | 8 (6.3) | 1 (0.8) | 0 | 0 |
| Maculopapular rash | 7 (5.6) | 7 (5.6) | 0 | 0 | 0 |
| Hypokalemia | 5 (4.0) | 4 (3.2) | 1 (0.8) | 0 | 0 |
| Drug-induced liver injury | 3 (2.4) | 1 (0.8) | 2 (1.6) | 0 | 0 |
| γ-Glutamyltransferase increase | 3 (2.4) | 0 | 3 (2.4) | 0 | 0 |
| Lymphocyte count decrease | 3 (2.4) | 2 (1.6) | 1 (0.8) | 0 | 0 |
| Dyspnea | 2 (1.6) | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Pneumonitis | 2 (1.6) | 0 | 1 (0.8) | 1 (0.8) | 0 |
| Abnormal hepatic function | 2 (1.6) | 1 (0.8) | 1 (0.8) | 0 | 0 |
| Lymphopenia | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Neutropenia | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Hepatotoxic event | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Drug hypersensitivity | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Cellulitis | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Lipase increased | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Increase in liver-function level† | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Neutrophil count decrease | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
| Abnormal aminotransferase level‡ | 1 (0.8) | 0 | 1 (0.8) | 0 | 0 |
For patients who had an adverse event of multiple grades, the worst grade is reported. Adverse events were graded with the use of the Common Terminology Criteria for Adverse Events, version 5.0, which incorporates certain elements of Medical Dictionary for Regulatory Activities (MedDRA) terminology.
The MedDRA preferred term “increased liver-function test” was used for this event.
The MedDRA preferred term “transaminases abnormal” was used for this event.
A total of 88 patients (69.8%) reported adverse events of any grade that were considered by the investigators to be related to treatment (treatment-related adverse events). The worst grade of treatment-related adverse event was grade 3 in 25 patients (19.8%) and grade 4 in 1 patient (0.8%; pneumonitis and dyspnea); no treatment-related adverse events of grade 5 were reported. The most frequent treatment-related adverse events were diarrhea (in 40 patients [31.7%]), nausea (in 24 [19.0%]), increase in the alanine aminotransferase level (in 19 [15.1%]), increase in the aspartate aminotransferase level (in 19 [15.1%]), and fatigue (in 14 [11.1%]). Treatment-related adverse events led to dose modification (dose interruption, reduction, or both) in 28 patients (22.2%) and to the discontinuation of therapy in 9 (7.1%). The most common treatment-related adverse events that led to dose modification were diarrhea (in 10 patients [7.9%]), increase in the aspartate aminotransferase level (in 10 [7.9%]), increase in the alanine aminotransferase level (in 9 [7.1%]), increase in the blood alkaline phosphatase level (in 3 [2.4%]), and nausea (in 3 [2.4%]).
Discussion
The highly selective and irreversible KRASG12C inhibitor sotorasib showed clinical efficacy with reversible toxic effects, mainly of grade 1 or 2, in the phase 1 portion of the CodeBreaK100 trial.25 In the NSCLC cohort of the current phase 2 portion of this trial, an objective response was observed in 37.1% of the patients, with a median duration of response of 11.1 months. The median progression-free survival was 6.8 months, and the median overall survival was 12.5 months. In addition, tumor shrinkage and disease control were observed in the majority of patients. These data provide further evidence in support of the clinical use of sotorasib in patients with KRAS p.G12C–mutated NSCLC.
With the incorporation of immunotherapies into the first-line treatment of advanced NSCLC, the current standard care for patients with newly diagnosed KRAS-mutated NSCLC commonly involves an immune-checkpoint inhibitor, either in combination with chemotherapy or as monotherapy.4–6 However, for patients whose disease progresses after immunotherapy and platinum doublet chemotherapy, few effective second-line options are available. Single-agent chemotherapy with pemetrexed or docetaxel, a standard care in this context, yields unsatisfactory outcomes, with less than 10% of patients having a response and with a median progression-free survival of less than 4 months.12,17,18,26 Survival was longer with the addition of ramucirumab (antibody to vascular endothelial growth factor receptor 2) or nintedanib (a broadly acting receptor tyrosine kinase inhibitor) to docetaxel therapy than with the use of docetaxel alone in the REVEL trial and the LUME–Lung 1 trial, respectively.26,28 Combination therapy with ramucirumab plus docetaxel led to a median progression-free survival of 4.5 months and to a response in 23% of the patients, a percentage that was used as the benchmark response in this trial of sotorasib.26 In our trial, the majority of the patients (81.0%) had advanced NSCLC that had been previously treated with both checkpoint inhibitors and platinum-based chemotherapy; nonetheless, sotorasib treatment induced rapid and durable responses that were also observed across all PD-L1 expression level subgroups. Although it is not possible to compare results across different trials, the efficacy that was associated with sotorasib therapy appears to exceed that with ramucirumab plus docetaxel, which was previously reported in the REVEL trial (i.e., the lower boundary of the 95% confidence interval for objective response exceeded that for the benchmark response), and improved outcomes in this population of patients.
The percentage of patients with an objective response that was associated with sotorasib therapy in our trial appears to be lower than that associated with tyrosine kinase inhibitors that have been approved for the treatment of NSCLCs with targetable driver mutations. This finding could potentially be attributable to the inherent molecular heterogeneity of KRAS-mutated tumors, which may predispose tumors to adapt quickly to the selective pressure of KRASG12C inhibtion.29,30 In addition, the genome damage that has been associated with tobacco carcinogens and that is commonly seen with KRAS p.G12C mutations may provide alternative pathways to drive tumor growth.31 Future investigations are expected to shed light on mechanisms of adaptation or resistance, as well as to inform the development of combination strategies to enhance the anticancer activity of KRASG12C inhibitors. Given that patients with active untreated brain metastases were excluded from this trial, the efficacy of sotorasib in the treatment of patients with central nervous system metastases remains to be further investigated.
Co-occurring genomic alterations in KRAS-mutated tumors have an effect on the tumor biology and response to systemic therapies.16,32,33 In our exploratory analyses, the activity of sotorasib was observed across a spectrum of prevalent co-occurring mutations, including STK11 and KEAP1, both of which are associated with inferior treatment outcomes and a poor prognosis in patients with NSCLC.18,27,33–37 A numerically higher response was seen among patients with STK11-mutated tumors that were wild-type for KEAP1 than in other subgroups or among all patients who could be evaluated. This finding is noteworthy because inactivating genomic alterations in STK11 confer primary resistance to PD-1 and PD-L1 blockade and docetaxel in patients with KRAS-mutated NSCLC.18,33 A response to sotorasib therapy was also observed in patients with KEAP1-mutated tumors, although at a lower percentage than among patients with wild-type KEAP1. These exploratory analyses were not statistically powered, and the 95% confidence intervals overlap across subgroups; therefore, the results should be interpreted with caution. Future prospective studies are warranted to identify subgroups of patients who may benefit differently from sotorasib therapy.
In a result that was consistent with the safety findings of the phase 1 study, treatment with sotorasib produced primarily grade 1 and 2 side effects in this trial, mainly low-grade hepatic and gastrointestinal toxic effects, and there were no new safety signals. The percentages of patients who had a dose modification or who discontinued treatment were low, with only 7.1% of patients discontinuing treatment.
In the phase 2 portion of this trial, sotorasib therapy led to a rapid and durable clinical benefit in patients with KRAS p.G12C–mutated NSCLC. A phase 3 trial to compare sotorasib therapy with docetaxel therapy in patients with previously treated, locally advanced, unresectable or metastatic NSCLC with a KRAS p.G12C mutation is under way (CodeBreaK200 ClinicalTrials.gov number, NCT04303780). In addition, efforts are ongoing to investigate sotorasib in combination therapies (CodeBreaK101; NCT04185883) and to identify patients who may benefit from sotorasib regimens in the context of first-line treatment.
Supplementary Material
Acknowledgments
Supported by Amgen and by a Cancer Center Core Grant (P30 CA008748, to Dr. Li) at Memorial Sloan Kettering Cancer Center from the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their families for participating in this trial and Yang Li (of Amgen) for medical writing assistance with an earlier version of the manuscript.
References
- 1.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 2020; 383:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021; 71:7–33. [DOI] [PubMed] [Google Scholar]
- 3.Black RC, Khurshid H. NSCLC: an update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013) 2015; 98: 25–8. [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 2018;378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 5.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393:1819–30. [DOI] [PubMed] [Google Scholar]
- 6.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med 2017; 376: 2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540–50. [DOI] [PubMed] [Google Scholar]
- 9.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallis AG, Agelaki S, Agelidou A, et al. A randomized phase III study of the docetaxel/carboplatin combination versus docetaxel single-agent as second line treatment for patients with advanced/ metastatic non-small cell lung cancer. BMC Cancer 2010; 10:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095–103. [DOI] [PubMed] [Google Scholar]
- 12.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–97. [DOI] [PubMed] [Google Scholar]
- 13.Biernacka A, Tsongalis PD, Peterson JD, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet 2016; 209:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008; 14:5731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open 2013; 3(4): e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015; 5: 860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jänne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA 2017; 317: 1844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Daemen A, Nickles D, et al. NRF2 activation promotes aggressive lung cancer and associates with poor clinical outcomes. Clin Cancer Res 2021; 27: 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503: 548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov 2014; 13: 828–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrem JM, Shokat KM. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discov 2016;15: 771–85. [DOI] [PubMed] [Google Scholar]
- 22.Lito P, Solomon M, Li L-S, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 2016; 351: 604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patricelli MP, Janes MR, Li L-S, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov 2016;6 : 316–29. [DOI] [PubMed] [Google Scholar]
- 24.Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019; 575: 217–23. [DOI] [PubMed] [Google Scholar]
- 25.Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020; 383:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384:665–73. [DOI] [PubMed] [Google Scholar]
- 27.Arbour KC, Jordan E, Kim HR, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res 2018; 24:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, doubleblind, randomised controlled trial. Lancet Oncol 2014; 15: 143–55. [DOI] [PubMed] [Google Scholar]
- 29.Scheffler M, Ihle MA, Hein R, et al. K-ras mutation subtypes in NSCLC and associated co-occuring mutations in other oncogenic pathways. J Thorac Oncol 2019; 14: 606–16. [DOI] [PubMed] [Google Scholar]
- 30.Xue JY, Zhao Y, Aronowitz J, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature 2020; 577: 421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan S, Shen R, Ang DC, et al. Mo-lecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012; 18: 6169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoulidis F, Heymach JV. Co-occur-ring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 2019; 19: 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 2018; 8: 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negrao MV, Lam VK, Reuben A, et al. PD-L1 expression, tumor mutational burden, and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non-small cell lung cancer. J Thorac Oncol 2019; 14:1021–31. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Su C, Ren S, Zhou C, Jiang T. Pan-cancer analysis of KEAP1 mutations as biomarkers for immunotherapy outcomes. Ann Transl Med 2020; 8: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong Y, Hellyer JA, Stehr H, et al. Role of KEAP1/NFE2L2 mutations in the chemotherapeutic response of patients with non-small cell lung cancer. Clin Cancer Res 2020; 26: 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Fleur L, Falk-Sörqvist E, Smeds P, et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer 2019; 130:50–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.