To the Editor:
More than 1.5 million patients are diagnosed with a pleural effusion annually in the United States (1). Point-of-care ultrasound is commonly used to categorize pleural effusions radiologically as simple, complex septated, complex nonseptated, or homogeneously echogenic; however, the utility of ultrasound to differentiate transudative versus exudative pleural effusions is less clear. Certain sonographic features, including septations, loculations, and fibrinous stranding, have been associated with exudative pleural effusions (2, 3), but conflicting reports exist on whether exudates have increased fluid echogenicity (2, 4). We hypothesize that combining quantitative measurement of pleural fluid echogenicity and qualitative characteristics can improve identification of exudates and guide decisions about drainage. This exploratory study evaluated the utility of measuring pleural fluid echogenicity quantitatively using computerized image pixel density to differentiate transudative versus exudative pleural effusions.
Methods
A retrospective observational study was conducted of subjects with a pleural effusion who underwent thoracentesis between January 2012 and December 2015 at a university-affiliated county teaching hospital and Veterans Affairs hospital. The institutional review board (HSC20120034H) approved this study.
All subjects were hospitalized in a medical ward or intensive care unit. Inclusion criteria were age of more than 18 years, pleural effusion drained by thoracentesis, recorded preprocedure pleural fluid ultrasound images, and availability of pleural fluid laboratory studies. Subjects with missing pleural fluid laboratory studies, low-quality ultrasound images, or unknown final diagnosis were excluded.
A preprocedure ultrasound examination was performed on all subjects in a sitting or semi-recumbent position with the transducer oriented longitudinally on the chest wall. A 5–1 MHz phased-array transducer was used (Fujifilm-Sonosite ultrasound machine) with an abdominal preset in “Gen” mode with tissue harmonic imaging turned on (3.7 MHz). The quality of all recorded ultrasound images was reviewed by the principal investigator before inclusion in the analysis.
Pleural fluid echogenicity was quantified by measuring pixel density using image processing software (ImageJ; National Institutes of Health). The pixel density was measured by tracing a central area of the pleural effusion and reported on a scale of 0–100 (Appendix, Figure 1). Two operators blinded to pleural effusion laboratory data independently measured the pixel density, and the mean value was used.
Figure 1.
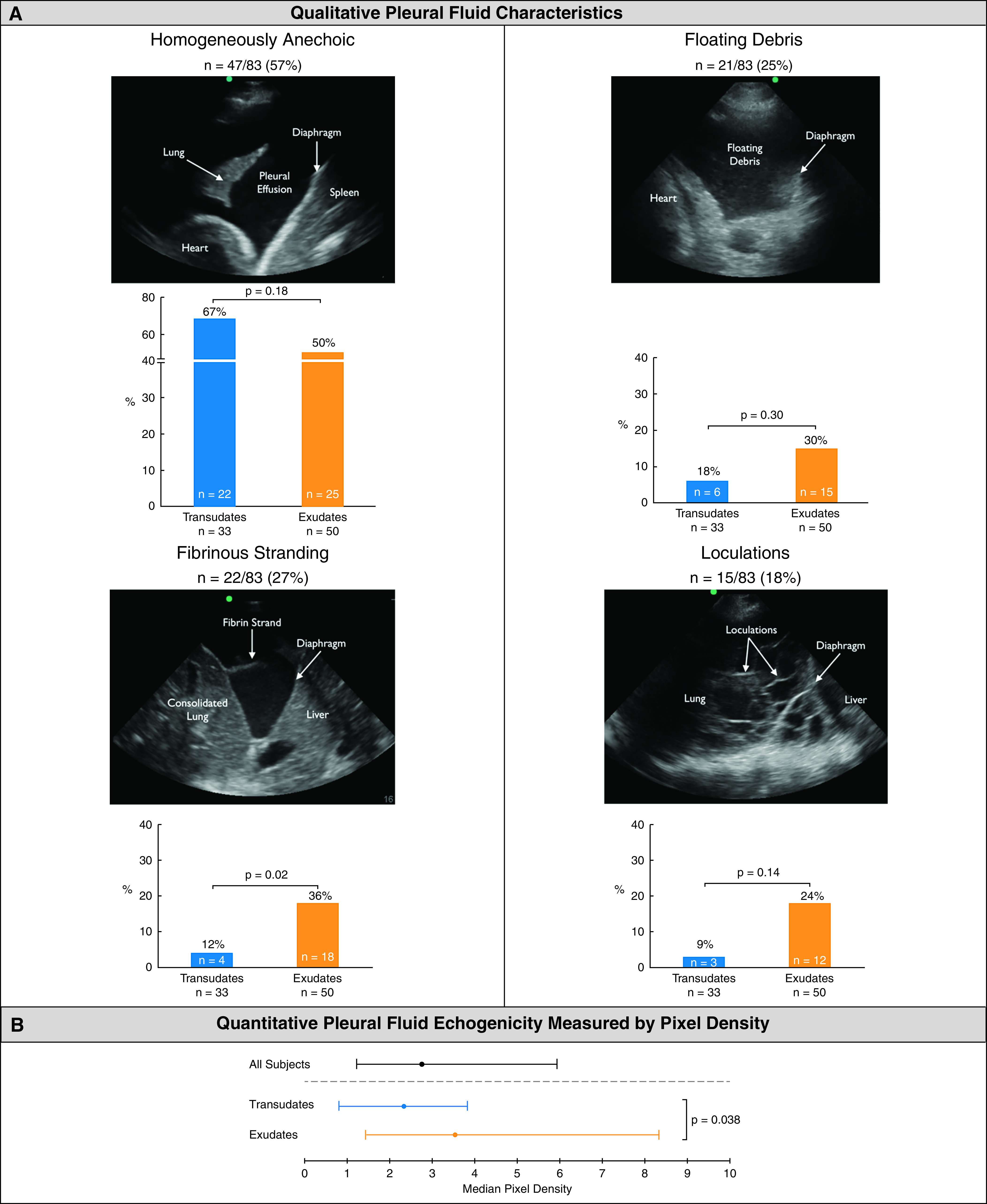
Qualitative and quantitative pleural fluid characteristics by ultrasound. (A) Pleural effusions were qualitatively characterized as being homogeneously anechoic or as having floating debris, fibrinous stranding, or loculations. Most homogeneously anechoic pleural effusions were transudates, whereas those with floating debris, fibrinous stranding, or loculations were mostly exudates. (B) Pleural fluid echogenicity was quantified by measuring pixel density. Exudates had a significantly higher median pixel density compared with transudates.
Demographics and pleural fluid laboratory data were abstracted from the medical record. Pleural fluid was analyzed for pH, glucose, lactate dehydrogenase (LDH), total protein, bacterial cultures, acid-fast stain, and cell count. Pleural effusions were categorized as exudative if one or more of Light’s criteria (5) were met: 1) pleural fluid LDH/serum LDH ratio > 0.6; 2) pleural fluid protein/serum protein ratio > 0.5; and/or 3) pleural fluid LDH of more than two-thirds of the upper limit of the normal level of serum LDH. A pleural fluid ultrasound score was calculated based on the following criteria: floating debris (+1), fibrinous stranding (+1), loculations (+1), pixel density of 3–10 (+1), and pixel density of at least 10 (+2).
Medians of continuous variables were compared with the Mann-Whitney test, and Fisher exact test was used to compare proportions of dichotomous variables. Pearson correlation coefficient was calculated to assess associations between pixel density and LDH or protein. Statistical calculations were done using R version 3.6.3 (R Foundation for Statistical Computing).
Results
Data were analyzed from 83 subjects. Median age was 61 years, and 76% were male. Most pleural effusions were exudative (60%) per Light’s criteria. Etiologies included cirrhosis, cancer (nonlung primary), pneumonia, lung cancer, and heart failure. Exudates had higher median white blood cell count, pleural fluid protein, LDH, and respective pleural/serum ratios compared with transudates (Table 1).
Table 1.
Characteristics of the study population and pleural fluid
| Total Cohort (n = 83) | Transudate (n = 33) | Exudate (n = 50) | |
|---|---|---|---|
| Demographics | |||
| Age, yr | 61.0 (55.0–68.0) | 57.0 (55.0–67.5) | 62.0 (47.8–70.3) |
| Sex, male | 63 (76) | 23 (70) | 40 (80) |
| Etiology of pleural effusion | |||
| Cirrhosis* | 28 (34) | 19 (58) | 9 (18) |
| Heart failure | 8 (10) | 6 (18) | 2 (4) |
| Cancers combined* | 24 (29) | 4 (12) | 20 (40) |
| Lung cancer | 9 (11) | 1 (3) | 8 (16) |
| Nonlung cancer | 15 (18) | 3 (9) | 12 (24) |
| Pneumonia* | 11 (13) | 1 (3) | 10 (20) |
| ESRD | 5 (6) | 2 (6) | 3 (6) |
| Other | 7 (8) | 1 (3) | 6 (12) |
| Pleural fluid lab values | |||
| pH (n = 44) | 7.4 (7.3–7.5) | 7.4 (7.3–7.7) | 7.4 (7.2–7.5) |
| Glucose, g/dl (n = 58) | 117 (102–154) | 138 (110–161) | 115 (96–137) |
| RBC count (n = 77)* | 2,520 (1,000–15,188) | 1,025 (<1,000–3,300) | 3,406 (1,094–63,250) |
| WBC count (n = 77)* | 500 (200–1,708) | 197 (113–433) | 1,150 (451–2,799) |
| LDH, IU/L* | 113 (67–232) | 56 (48–86) | 191 (111–368) |
| Protein, g/dl (n = 82)* | 3.0 (<2–3.9) | <2 (<2–3.0) | 3.8 (3.0–4.4) |
| LDH ratio, pleural:serum (n = 78)* | 0.60 (0.34–1.19) | 0.32 (0.20–0.42) | 1.03 (0.61–1.74) |
| Protein ratio, pleural:serum (n = 82)* | 0.50 (0.40–0.61) | 0.38 (0.33–0.43) | 0.58 (0.51–0.67) |
| Pleural fluid ultrasound | |||
| Homogeneously anechoic | 47 (57) | 22 (67) | 25 (50) |
| Floating debris | 21 (25) | 6 (18) | 15 (30) |
| Fibrinous stranding* | 22 (27) | 4 (12) | 18 (36) |
| Loculations | 15 (18) | 3 (9) | 12 (24) |
| Pleural fluid pixel density*† | |||
| Median | 2.74 (1.23–5.94) | 2.32 (0.82–3.81) | 3.53 (1.43–8.34) |
| Mean | 5.10 (3.62–6.57) | 2.74 (1.96–3.53) | 6.65 (4.32–8.97) |
Definition of abbreviations: ESRD = end-stage renal disease; LDH = lactate dehydrogenase; RBC = red blood cell; WBC = white blood cell.
Median and interquartile range (25th–75th percentile) reported for continuous variables, with Mann-Whitney test used for P value unless otherwise noted. Count and percentage reported for categorical variables, with Fisher exact test used for P value.
Statistically significant difference between transudates and exudates, with a P value < 0.05.
Mean and 95% confidence interval reported for pleural fluid pixel density, with t test used for P value.
Qualitatively, exudates had more fibrinous stranding than transudates on ultrasound. Quantitatively, the median pleural fluid pixel density was higher in exudates versus transudates (3.53 vs. 2.32; P = 0.038) (Figure 1). A pixel density cutoff of more than 10 was only seen with exudates. Sensitivity of pixel density of 9.5 for an exudate was 22% (Positive Likelihood Ratio, 7.3), whereas specificity was 97% (Negative Likelihood Ratio, 0.8). A significant correlation was seen between pleural fluid pixel density versus LDH (r = 0.42; P = 0.042) and pixel density versus protein (r = 0.30; P = 0.036) of exudative effusions.
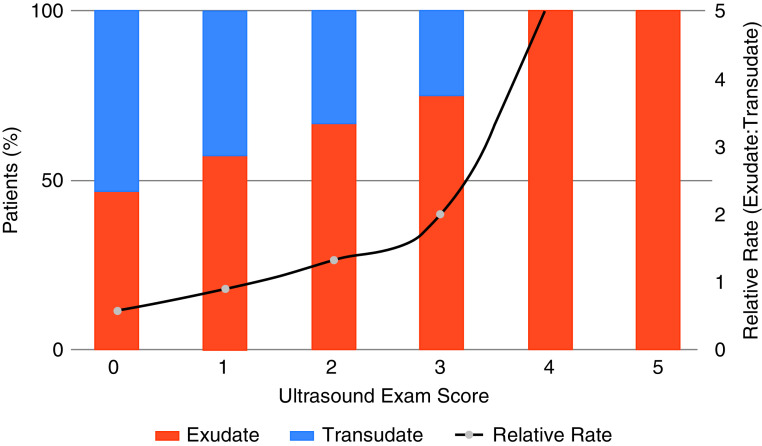
Pleural fluid ultrasound score of at least 3 indicated a very high likelihood of being exudative and relatively low likelihood (<10%) of being transudative (Figure 2).
Figure 2.
Relative rate of exudates to transudates by pleural fluid ultrasound score. A pleural fluid ultrasound score was calculated based on presence of floating debris (+1), fibrinous stranding (+1), loculations (+1), pixel density of 3–10 (+1), and pixel density of at least 10 (+2). A pleural fluid ultrasound score of 3 or higher was associated with a high likelihood of an exudative effusion.
Discussion
We have demonstrated that pleural fluid echogenicity on ultrasound can be quantified using computerized pixel density measurement. Pleural fluid pixel density correlated with LDH and total protein of exudative effusions. A novel pleural fluid ultrasound score combining qualitative and quantitative characteristics may help differentiate transudates versus exudates noninvasively.
Controversy exists on the utility of qualitative pleural fluid characteristics to differentiate transudative versus exudative pleural effusions. Early studies reported 100% of transudates were simple and anechoic (2, 6); however, subsequent studies revealed only 45% of transudates were anechoic and 55% were complex nonseptated (3). Although exudates often have increased pleural fluid echogenicity compared with transudates, 33% of exudates were simple and anechoic in one study (2). A study by radiologists concluded that qualitative assessments of pleural fluid echogenicity had low specificity for identifying exudates (4).
Few studies have quantitatively assessed pleural fluid echogenicity by measuring image pixel density. One study measured pixels to calculate a hypoechogenicity index, which correlated well with LDH, cell count, pH, and mean effusion pixels; however, correlation with classification as a transudate and exudate was not evaluated. Our study demonstrated median pleural fluid echogenicity measured by pixel density is higher in exudates, and a pleural ultrasound score of at least 3 had a high likelihood of being exudative. All pleural effusions with an average pixel density of more than 10 were exudative.
A small study observed an association between hypoechogenicity index and duration of chest tube and hospitalization for parapneumonic effusions (7). Additional studies are needed to assess the utility of pixel density measurement to guide clinical management decisions (8).
Our study has limitations. As a retrospective study, we were unable to control all variables related to image acquisition that could affect pleural fluid echogenicity measurement. Key findings of this exploratory study were determined post hoc and should be confirmed in a larger prospective trial.
In conclusion, pleural fluid echogenicity measured by image pixel density was significantly higher in exudative versus transudative pleural effusions. Correlation of pleural fluid pixel density versus LDH and protein was seen in exudative effusions. A pleural fluid ultrasound score of at least 3 combining qualitative and quantitative assessments can help differentiate exudates from transudates. Prospective studies validating our findings are needed, and, if validated, sonographic technical parameters should be included as part of the documentation of the comprehensive ultrasound assessment of pleural effusions.
Footnotes
Supported by the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative grant HX002263-01A1 (N.J.S.). This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System in San Antonio, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author Contributions: N.J.S., Z.S.D., S.A., A.E., K.C.P., M.J.M., M.I.V., S.B.S., J.I.P., and M.I.R. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript. N.J.S., Z.S.D., M.I.V., and K.C.P. performed lung ultrasound examinations to acquire pleural fluid images, and N.J.S., K.C.P., S.A., and A.E. reviewed ultrasound images of each subject. N.J.S. has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This letter has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Feller-Kopman D, Light R. Pleural disease. N Engl J Med . 2018;378:740–751. doi: 10.1056/NEJMra1403503. [DOI] [PubMed] [Google Scholar]
- 2. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol . 1992;159:29–33. doi: 10.2214/ajr.159.1.1609716. [DOI] [PubMed] [Google Scholar]
- 3. Chen HJ, Tu CY, Ling SJ, Chen W, Chiu KL, Hsia TC, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol . 2008;34:362–369. doi: 10.1016/j.ultrasmedbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 4. Asciak R, Hassan M, Mercer RM, Hallifax RJ, Wrightson JM, Psallidas I, et al. Prospective analysis of the predictive value of sonographic pleural fluid echogenicity for the diagnosis of exudative effusion. Respiration . 2019;97:451–456. doi: 10.1159/000496153. [DOI] [PubMed] [Google Scholar]
- 5. Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med . 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 6. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci . 2004;1019:585–592. doi: 10.1196/annals.1297.110. [DOI] [PubMed] [Google Scholar]
- 7. Varsamas C, Kalkanis A, Gourgoulianis KI, Malli F. The use of a novel quantitative marker of echogenicity of pleural fluid in parapneumonic pleural effusions. Can Respir J . 2020;2020:1283590. doi: 10.1155/2020/1283590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu CJ, Yang PC, Chang DB, Luh KT. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. AJR Am J Roentgenol . 1992;159:695–701. doi: 10.2214/ajr.159.4.1529829. [DOI] [PubMed] [Google Scholar]