Abstract
Rationale
Few studies have assessed personal exposure to pollutants and lung function among adults with chronic obstructive pulmonary disease (COPD). Blood eosinophil level may be a biomarker of airway inflammation and pollution susceptibility.
Objectives
To evaluate if daily pollutant exposures are associated with lung function and if associations are modified by eosinophil level in COPD.
Methods
We recruited 30 former smokers with moderate to severe COPD living in the Boston area and followed them for up to 4 nonconsecutive months in different seasons. Participants measured morning lung function and carried a portable air quality monitor daily. Previous-day exposure to pollutants (particulate matter ⩽2.5 μm in aerodynamic diameter, nitrogen dioxide [NO2], and ozone) were measured by portable and community monitors. We constructed multilevel linear mixed-effects models with random intercepts for person and observation month, adjusted for temperature, humidity, age, sex, race, height, weight, income, and season, to assess associations of previous-day pollutant exposure with lung function and effect modification by eosinophil count (<150/μl vs. ⩾150/μl).
Results
A total of 3,314 observations with exposure and lung function data were collected. Each interquartile range (5.1 parts per billion [ppb])–higher previous-day personal exposure to NO2 was associated with an 11.3 ml (95% confidence interval [CI], −18.7 to −4.0) lower forced expiratory volume in 1 second (FEV1) and an 18.0 ml (95% CI, −32.0 to −4.2) lower forced vital capacity. Personal and community-level exposure to particulate matter ⩽2.5 μm in aerodynamic diameter and community-level NO2 were negatively associated with FEV1 among the 55.2% of participants with the higher eosinophil level (Pinteraction < 0.05).
Conclusions
Our study highlights the need to address air pollution exposure among patients with COPD. Future research is needed to verify if eosinophil level is a biomarker for susceptibility to air pollution in COPD.
Keywords: chronic obstructive pulmonary disease, lung function, air pollution, nitrogen dioxide, eosinophilic
Exposure to higher levels of outdoor air pollutants, including particulate matter ⩽2.5 μm in aerodynamic diameter (PM2.5), ozone (O3), and nitrogen dioxide (NO2), is associated with lower lung function, hospitalization for respiratory illness, and premature mortality in the general population (1–4). There is compelling evidence from administrative data that outdoor levels of PM2.5, O3, and NO2 trigger hospitalization for chronic obstructive pulmonary disease (COPD) (5, 6). A small number of studies have evaluated effects of ambient pollution on lung function among those with COPD, whose lung function is already compromised, and findings have been less consistent (7–9). In COPD, blood eosinophil level is a biomarker for more active airway inflammation (10–12) and greater clinical response to antiinflammatory treatments such as inhaled corticosteroids and anti-eosinophilic drugs (10, 13, 14). It could be a biomarker for more airway inflammation in response to inhaled pollutants. However, to date, no studies of patients with COPD have assessed differential susceptibility to air pollution by eosinophil level.
People with moderate to severe COPD are relatively frail and spend most of their time at home, where they are exposed to pollution from both outdoor and indoor sources (15). Although outdoor pollution enters the home and affects indoor pollution levels, there are also indoor sources of particulate and gaseous pollutants from routine cooking and heating that may have harmful respiratory effects (16). These indoor sources of exposure are generally ignored in studies that use only central monitoring data, and they may be key determinants of personal pollution exposure, especially among older adults with COPD and other chronic diseases who spend their time at home.
We designed SPACE (Study of Pollution and COPD Exacerbation) to evaluate if personal pollutant exposures are associated with daily lung function among adults with COPD. We recruited 30 former smokers with COPD living in an urban environment and followed them each for 4 nonconsecutive months in different seasons over a year, during which participants measured their personal exposure to PM2.5, NO2, and O3 by portable personal air quality monitor (PAM) and their daily lung function by office-grade spirometer. We also assessed Boston community-level outdoor pollution levels and their associations with personal exposures and lung function. We hypothesized that both personal and community-level daily exposure to these pollutants would be associated with lower daily lung function, especially among patients with COPD with higher eosinophil levels.
Methods
Study Population
The study population consists of 30 former smokers with COPD who were recruited as part of SPACE at Beth Israel Deaconess Medical Center in Boston, Massachusetts. To be eligible, study participants were required to have a home address within 50 km of the Harvard air pollution supersite in Boston, Massachusetts, and a clinical diagnosis of COPD with at least moderate Global Initiative for Chronic Obstructive Lung Disease stage II airflow obstruction, defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of <70% and FEV1 <80% predicted, using National Health and Nutrition Examination Survey (NHANES) III prediction equations (17). Participants with a history of lung cancer, interstitial lung disease, or bronchiectasis were ineligible to participate.
Data Collection
Participants entered the study between February 24, 2017, and January 17, 2019. At study entry, demographic information, height, weight, past medical history, medication history, and baseline measures of lung function were obtained and participants were instructed on how to use a portable spirometer and PAM. They were then observed for up to four nonconsecutive 30-day periods in different seasons over 12 months. Participants measured their lung function daily in the morning before taking any medications, using a portable EasyOne Plus Diagnostic Spirometer. This device meets American Thoracic Society guidelines and has built-in quality assurance and incentive software. Participants also completed a validated electronic daily symptom questionnaire to report any changes in baseline respiratory symptoms, including nasal discharge and/or congestion, wheeze, sore throat, cough, fever, dyspnea, sputum purulence, and sputum amount, as previously described (18). At the end of follow-up, the 30 participants contributed up to 3,314 observation days.
Exposure Assessment
We measured personal pollutant exposures using PAMs, and we measured community-level pollutant exposures using state-owned stationary monitors in Boston. For PM2.5 and NO2, we averaged daily concentrations from state-owned monitors in Boston. For O3 we used the state monitor in the Roxbury neighborhood of Boston, which measures O3. Figure E1 in the online supplement shows the locations of the stationary Boston monitors, which are clustered within a 2.5 km radius. Median (interquartile range [IQR]) distance of participants’ home address from the Boston Harvard Supersite monitor was 14.9 km (5.3). The PAMs, developed by Atmospheric Sensors Ltd. (Cambridge area, UK) (model 520) in collaboration with investigators in the UK, were equipped with Sensirion SHT-25 sensors for temperature and relative humidity, Alphasense electrochemical for NO2 and O3, and an Alphasense optical particle counter for PM2.5 (19, 20). During repeated calibration periods in different seasons, the 24-hour average exposure measures of the PAMs were evaluated and calibrated by colocating one or multiple PAMs at stationary monitors in Boston. PAMs were calibrated for PM2.5 by placing each monitor for an average duration of 48 days adjacent to the Harvard Supersite monitor, Beta Attenuation Monitor (model 1020; Met One Instruments, Inc.), using a U.S. Environmental Protection Agency (EPA) equivalent method. The state-owned stationary monitor in Roxbury, Massachusetts, was used to calibrate the PAMs for NO2, O3, temperature, and relative humidity. Because it was not feasible to co-locate all the monitors at the state-owned Roxbury site, a reference PAM was calibrated at the site across all seasons for a total of 55 days, and the remaining eight PAMs were placed side-by-side the reference PAM in indoor and outdoor settings for a mean duration of 49 days. Regarding PM2.5, PAM measurements were consistently higher than the Harvard Beta Attenuation Monitor by a mean (standard deviation) difference of 6.5 (1.4) μg/m3. We generally found good agreement between PAMs for all exposures (mean adjusted R2 = 0.7) and with stationary monitors (mean adjusted R2 = 0.8) in linear regression models. Mean adjusted R2 was 0.6 for PM2.5, 0.7 for NO2, 0.7 for O3, 0.8 for temperature, and 0.9 for relative humidity. More details on the PAM calibrations are in Table E1. All personal 24-hour exposure measures for PM2.5, NO2, O3, temperature, and relative humidity collected during the course of the study were adjusted according to the calibration formulas for each PAM before data analysis. Information about the PAM technology, reproducibility of pollutant measurements, and agreement with stationary monitors in diverse settings has been published by investigators in the UK who developed the PAMs for respiratory health research (19, 21).
Statistical Analyses
We calculated 24-hour exposure to pollutants from the PAMs (personal exposure) and outdoor state-owned stationary monitors in the Boston area (community-level exposure). To evaluate predictors of personal exposure, we used unadjusted multilevel linear mixed-effects models to test if daily outdoor (community-level) exposures were associated with daily personal exposure to PM2.5, NO2, O3, temperature, and humidity, using the mixed model structure described below. We evaluated the shape of each of these relationships by constructing and plotting generalized additive mixed models with penalized splines. We used linear models to test if gas cooking was associated with average personal NO2. Because gas and particle pollution from fuel combustion by home and neighborhood heating systems may enter the home, we tested if gas heating was associated with personal NO2, and fuel oil heating with personal PM2.5, compared with electric heating during the home heating season (November to April) in Boston (22). We constructed multilevel linear mixed-effects models to assess for associations between previous-day 24-hour exposure to individual pollutants (PM2.5, NO2, and O3) and lung function (FEV1 and FVC). The model is as follows:
where Yimt is any continuous indicator of lung function for participant i at observation month m on day t; polimt indicates any pollutant exposure average, and cimt represents a vector of potential confounders (temperature, relative humidity, age, sex, race, height, weight, total pack-year smoking history, season, income, and education). The term wi denotes a participant-specific random effect and was used to account for intra-individual correlations among repeated measurements recorded on the same person. The term vim denotes a participant-observation month-specific random effect that allows daily observations from a single observation month within the same person to be more highly correlated than observations from different observation months for the same person. The residuals eimt are normally distributed errors, assumed to follow an autoregressive (AR) (1) process, modeling the serial correlation among the daily time series within the same person’s observation month. When assessing the association between exposure to air pollution and lung function, we examined exposure to air pollution based on previous-day 24-hour average of pollutant concentrations preceding the morning spirometry testing. We selected this exposure average a priori, based on our prior findings of pollution exposure and lung function in adults (3). We performed secondary analyses examining 2- to 7-day moving averages of pollution exposure and FEV1. Season was categorized as winter, spring, summer, and fall, based on the calendar start dates of each observation month. We also used sine and cosine functions of the date to estimate the amplitude and phase of the seasonal cycle and evaluated if findings differed when adjusting for season as sine and cosine terms. To determine if any associations with lung function were explained by COPD disease severity, treatment, or symptoms, we ran models additionally adjusting for inhaler use and restricting to days without worsening respiratory symptoms or COPD exacerbation, as defined by the daily questionnaire tool (18, 23). We adjusted for inhaler use at study entry using three variables: any inhaled corticosteroid, any long-acting bronchodilator, and any short-acting bronchodilator (β-agonist or anti-muscarinic). As we have previously reported, participants rarely changed their inhaler medication use or received steroids or antibiotics during follow-up (18). For community-level O3, we performed secondary analyses restricting to the warm EPA monitoring season for O3 (April to September) (3, 24) and examining associations with 8-hour maximal concentrations to correspond with the National Ambient Air Quality Standard monitoring standard for O3.
Based on evidence that patients with eosinophilic COPD have more active and dynamic airway inflammation and more responsiveness to anti-inflammatory treatments (12, 25–27), we tested for effect modification of the associations between previous-day exposure to air pollutants and FEV1 by absolute eosinophil count at baseline visit (<150 cells/μl vs. ⩾150 cells/μl) (10–12). This cutoff was selected a priori based on findings from clinical trials that an eosinophil count of ⩾150 cells/μl (or the equivalent of 2%) identifies a subset of patients with COPD who are more likely to benefit from inhaled steroid and anti-eosinophilic treatments (13, 14, 27). Because the sources of personal pollutant exposures may vary by season (e.g., based on indoor heating or window opening), we also evaluated effect modification of personal pollutant exposures with lung function by Boston indoor heating season (November to April) versus warm season (May to October) (18). In fully adjusted models, we included the exposure, effect modifier, and exposure–effect modifier interaction. We used the Wald test for cross-product terms to determine statistical significance, with a P value less than 0.05 indicating statistical evidence of effect modification. In models with evidence of effect modification, we report stratum-specific associations with air pollution exposure using fully adjusted models. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc.), Stata v.12 (for figures) (StataCorp.), and RStudio Version 1.3.1093 (RStudio).
Results
Study Participants
Baseline participant characteristics are summarized in Table 1 (N = 30). Participants had an average age of 71.1 ± 8.4 years and were 80% non-Hispanic White and 20% non-Hispanic Black or African American, with a slight majority of females. Household income and education status were generally low. A large majority of participants had a household income of less than $50,000, and fewer than one-third of participants had attained a bachelor’s degree of education. Participants were former heavy smokers with more than 50 pack-years of smoking on average. Most participants used gas (43%) or oil (33%) for home heating, and 43% used gas for cooking. Baseline lung function was poor, with a mean FEV1% predicted of 54% and mean FVC% predicted of 80%. A slight majority of participants had high blood eosinophil count defined as ⩾150/μl at study entry.
Table 1.
Baseline characteristics of participants (N = 30)
| Mean ± SD or n (%) | |
|---|---|
| Demographics | |
| Age, yr | 71.1 ± 8.4 |
| Height, cm | 165.1 ± 10.2 |
| Weight, kg | 85.3 ± 17.7 |
| Sex | |
| Male | 14 (46.7) |
| Female | 16 (53.3) |
| Race | |
| White, non-Hispanic | 24 (80.0) |
| Black or African American, non-Hispanic | 6 (20.0) |
| Income | |
| <$25,000 | 10 (33.3) |
| $25,000–$49,999 | 10 (33.3) |
| ⩾$50,000 | 9 (30.0) |
| Other or missing | 1 (3.3) |
| Education | |
| Up to grade 12 or GED | 8 (26.7) |
| Some college—associate’s degree | 13 (43.3) |
| Bachelor’s degree and above | 9 (30.0) |
| Total pack-year smoking history | 54.4 ± 30.7 |
| Home fuel use | |
| Heating | |
| Gas | 13 (43.3) |
| Electric | 5 (16.7) |
| Oil | 10 (33.3) |
| Other or unknown | 2 (6.7) |
| Cooking | |
| Gas | 12 (40.0) |
| Electric | 18 (60.0) |
| Baseline clinical measures | |
| FEV1, L | 1.3 ± 0.5 |
| FEV1 % predicted | 54.3 ± 14.5 |
| FVC, L | 2.5 ± 0.9 |
| FVC % predicted | 79.9 ± 15.8 |
| FEV1/FVC | 51.1 ± 11.5 |
| Eosinophil count | |
| ⩾150 cells/μl | 16 (55.2) |
| <150 cells/μl | 13 (44.8) |
| Baseline medication use | |
| Inhaled corticosteroid | |
| Yes | 18 (60.0) |
| No | 12 (40.0) |
| Long-acting bronchodilator | |
| Yes | 23 (76.7) |
| No | 7 (23.3) |
| Short-acting bronchodilator | |
| Yes | 23 (76.7) |
| No | 7 (23.3) |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GED = General Educational Diploma; SD = standard deviation.
Exposure Distributions and Associations
Table 2 displays the distribution of personal and community-level exposure to pollutants. Median previous-day 24-hour personal exposure to PM2.5 (8.8 μg/m3) was higher than median community-level PM2.5 by stationary monitor (6.0 μg/m3), although both values are within the EPA 24-hour PM2.5 standard of 35 μg/m3 and the annual standard of 12 μg/m3. Median previous-day personal and community-level NO2 concentrations were equivalent (∼7 parts per billion [ppb]). Median personal exposure to O3 was very low (9.6 ppb) and less than half the outdoor community-level O3 concentration (26.3 ppb). Table 3 shows the associations between daily outdoor community-level and daily personal exposure to pollutants, temperature, and humidity using mixed effects models. Although outdoor concentrations of the pollutants and temperature and humidity were positively associated with personal exposure levels measured by the PAM, the magnitude of these associations was modest. For example, a 10 μg/m3 higher community-level concentration of PM2.5 was associated with a 2.0 μg/m3 (95% confidence interval [CI], 0.9–3.2) higher personal PM2.5 exposure on the same day. A 10 ppb higher community-level concentration of NO2 was associated with a 1 ppb (95% CI, 0.6 to 1.3) higher personal NO2 exposure. Penalized splines of these associations demonstrated near-linear associations for PM2.5, O3, temperature, and humidity across the range of exposure, whereas personal NO2 showed a positive association at lower (<10 ppb) and higher (>15 ppb) outdoor NO2 concentrations and a negative association in the intermediate range (10–15 ppb) (Figure E2). Gas cooking fuel in the home was associated with a 3.6 ppb (95% CI, 1.3 to 5.8) higher average personal exposure to NO2 (P = 0.003). During the home heating season (November to April), personal NO2 exposure was higher (3.0 ppb; 95% CI, −0.8 to 6.8) among participants with gas, and personal PM2.5 exposure was higher (5.6 μg/m3; 95% CI, −4.7 to 15.9) among participants with fuel oil compared with electric heating, although these CIs crossed the null.
Table 2.
Previous-day community-level and personal exposure to air pollutants, temperature, and humidity among 30 participants
| Exposure | n (obs) | Median | 25th Percentile | 75th Percentile | IQR |
|---|---|---|---|---|---|
| PM2.5, μg/m3 | |||||
| Community-level | 3,314 | 6.0 | 4.4 | 7.9 | 3.6 |
| Personal | 2,803 | 8.8 | 7.5 | 11.3 | 3.8 |
| NO2, ppb | |||||
| Community-level | 3,314 | 7.1 | 5.1 | 9.7 | 4.6 |
| Personal | 2,803 | 6.8 | 3.8 | 8.9 | 5.1 |
| O3, ppb | |||||
| Community-level | 3,297 | 26.3 | 20.5 | 32.4 | 11.8 |
| Personal | 2,803 | 9.6 | 7.3 | 12.5 | 5.2 |
| Temperature, °C | |||||
| Community-level | 3,314 | 12.5 | 4.3 | 20.2 | 15.9 |
| Personal | 2,803 | 21.7 | 20.3 | 22.9 | 2.6 |
| Humidity, % | |||||
| Community-level | 3,314 | 65.9 | 54.9 | 78.4 | 23.5 |
| Personal | 2,803 | 41.9 | 30.4 | 53.2 | 22.7 |
Definition of abbreviations: IQR = interquartile range; n (obs) = number of observations; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter; ppb = parts per billion.
Table 3.
Associations of daily community-level exposure (by outdoor urban monitors) with personal daily exposure (by PAM)
| Community-Level Exposure | n (obs) | Difference (95% CI) in Personal Exposure | P Value |
|---|---|---|---|
| PM2.5, per 10 μg/m3 | 2,803 | 2.0 (0.9–3.2) | 0.0004 |
| O3, per 10 ppb | 2,789 | 0.6 (0.5–0.8) | <0.0001 |
| NO2, per 10 ppb | 2,803 | 1.0 (0.6–1.3) | <0.0001 |
| Temperature, per 10°C | 2,803 | 1.0 (0.9–1.1) | <0.0001 |
| Humidity, per 10% | 2,803 | 1.9 (1.7–2.1) | <0.0001 |
Definition of abbreviations: CI = confidence interval; n (obs) = number of observations; NO2 = nitrogen dioxide; O3 = ozone; PAM = personal air quality monitor; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter; ppb = parts per billion.
Results are from unadjusted linear mixed effects models of associations between daily averages of community-level exposure to each pollutant, temperature, or humidity with personal exposure to the corresponding pollutant, temperature, or humidity, scaled per 10-unit increase in community-level exposure.
Associations with Daily Lung Function
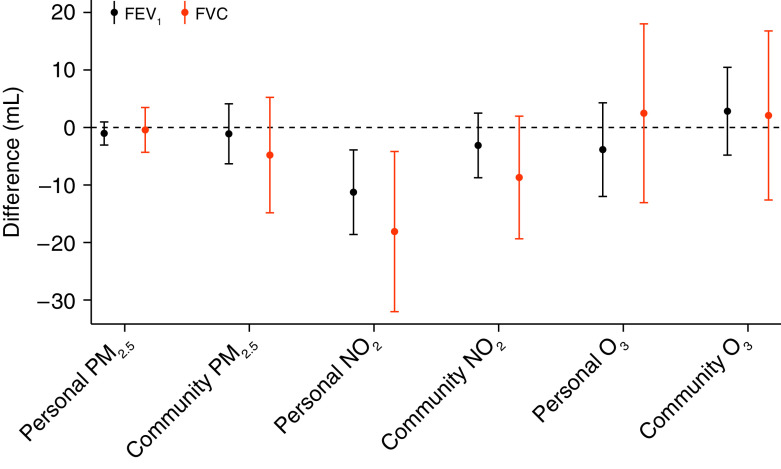
Associations between previous-day exposure to air pollutants and daily lung function are shown in Figure 1 and Table E2. In fully adjusted linear mixed-effects models, we found negative associations between previous-day personal exposure to NO2 measured by the PAMs and FEV1 (P = 0.002) and FVC (P = 0.01). Each IQR (5.1 ppb) higher previous-day personal exposure to NO2 was associated with an 11.3 ml (95% CI, −18.7 to −4.0) lower FEV1 and an 18.0 ml (95% CI, −32.0 to −4.2) lower FVC. There were no associations between previous-day personal and community-level exposure to PM2.5 and O3 and FEV1 and FVC overall. Secondary analyses of longer exposure averages (Table E3) demonstrate negative associations between the 1- to 7-day average personal NO2 and FEV1, although confidence intervals cross the null for all exposure averages longer than the previous day.
Figure 1.
Associations of previous-day pollutant exposures and lung function. Associations are scaled per interquartile range difference in previous-day pollutant exposure. All models are adjusted for previous-day temperature, relative humidity, age, sex, race, height, weight, total pack-year smoking history, season, income, and education. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter.
Effect Modification by Peripheral Eosinophil Level
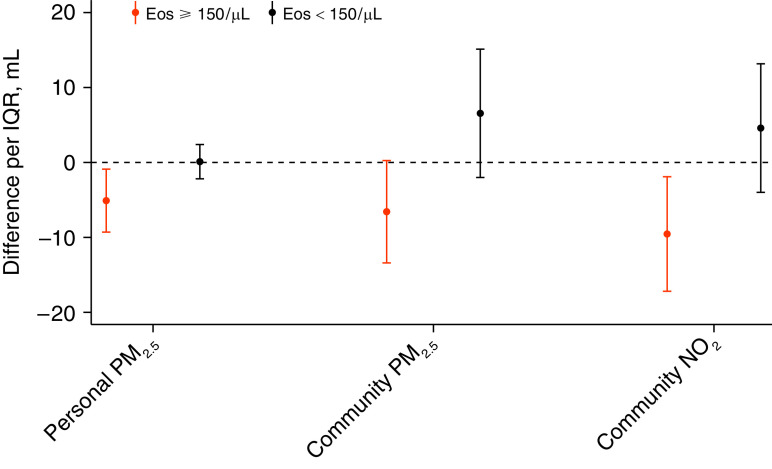
Associations of both PM2.5 and NO2 with FEV1 differed by peripheral eosinophil level (< vs. ⩾150 cells/μl) for personal and community-level PM2.5 and for community-level NO2 (Figure 2 and Table E4). Among the 55.2% of participants with eosinophils ⩾150/μl, each IQR (3.8 μg/m3) difference in daily personal PM2.5 exposure was associated with a 5.1 ml (95% CI, −9.3 to −0.8) lower FEV1, whereas personal PM2.5 was not associated with FEV1 in those with <150 eosinophils/μl (Pinteraction = 0.036). The same pattern was observed for community-level PM2.5: each IQR (3.6 μg/m3) higher community-level PM2.5 in the previous day was associated with a 6.6 ml (−13.4 to 0.2) lower FEV1 among those with eosinophil count ⩾150/μl (Pinteraction = 0.017). Community-level NO2 was negatively associated with FEV1 (9.5 ml lower FEV1 per IQR of 4.6 ppb; 95% CI, −17.1 to −1.9) among those with ⩾150 eosinophils/μl but not those without (Pinteraction = 0.017). Associations of personal NO2 and O3 with FEV1 did not differ by the eosinophil level (Pinteraction > 0.05).
Figure 2.
Associations of pollutants and FEV1 that were modified by eosinophil level (Pinteraction < 0.05). Stratum-specific associations are adjusted for previous-day temperature, relative humidity, age, sex, race, height, weight, total pack-year smoking history, season, income, and education. All associations are scaled per interquartile range difference in previous-day pollutant exposure: 3.8 μg/m3 for personal PM2.5, 3.6 μg/m3 for community-level PM2.5, and 4.6 ppb for community-level NO2. Eos = eosinophil count in cells/μl; NO2 = nitrogen dioxide; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter; ppb = parts per billion.
Sensitivity Analyses
Results of all sensitivity analyses are shown in the online supplement. Results were unchanged after adjusting for season as sine and cosine terms (Table E5). When we assessed associations between previous-day exposure to community-level O3 restricting to the O3 monitoring season and using the 8-hour maximum for O3, there were no associations with lung function (Table E6). There was no evidence of effect modification by cold versus warm season for personal pollutant exposures and FEV1 (Pinteraction > 0.05 for all exposures). Adjusting for the use of inhaler medications (Table E7) and excluding observations with worse-than-baseline symptoms or COPD exacerbations (Tables E8 and E9) did not change results.
Discussion
In this longitudinal study of 30 former smokers with moderate to severe COPD living in the Boston area, personal exposure to NO2 was associated with lower lung function. We also found that those with a higher eosinophil level appeared to be more susceptible to pollutants. Among those with a baseline eosinophil count ⩾150 cells/μl (approximately half the study population), personal and community-level PM2.5 and community-level NO2 were negatively associated with FEV1. Although effect sizes were small (e.g., an 11 ml lower FEV1 and 18 ml lower FVC per 5.1 ppb higher personal NO2), these findings are notable because they occurred at very low levels of pollutant exposure in a population whose lung function is already severely compromised. Associations with personal NO2 were not explained by differences in respiratory symptoms, suggesting a subclinical effect of NO2 on the airways.
A recently published study involving the use of personal monitors and daily symptom tracking among patients with COPD in London also found evidence that personal exposure to NO2 is harmful to daily respiratory health in COPD. The London study informed the present Boston study and had a very similar design with deployment of PAMs and daily health measures across 4 months, but it did not measure lung function by spirometry (28). The authors found a higher odds of COPD exacerbation in association with previous-day average personal exposure to NO2 but not PM2.5. The authors concluded that in their urban study area, gaseous pollutants might be more harmful than particles to the respiratory health of patients with COPD. Our lung function findings similarly suggest that personal exposure to NO2 is consistently harmful.
Despite a large literature on ambient pollution and lung function in the general population (9), a relatively small number of studies have examined gaseous and particulate matter pollution and lung function among people with COPD, whose lung function is already compromised (7, 29–33). A meta-analysis of panel studies involving patients with COPD dating back to 1993 concluded that on average, each 10 μg/m3 higher exposure to PM10 is associated with a 3.38 ml lower FEV1 (95% CI, −6.39 to −0.37), whereas findings for the gaseous pollutants were found to be less consistent (34). A randomized crossover study of traffic pollution exposure involving 40 former smokers with moderate to severe COPD found that both NO2 and PM2.5 exposure during city walks were associated with lower FEV1 and FVC (35). A recent study in the Boston area involving veterans with COPD found that previous-day levels of black carbon (a constituent of PM2.5 related to traffic) was associated with lower FEV1, but not indoor or ambient PM2.5, suggesting that the traffic-related component of indoor PM2.5 may be harmful to lung function in COPD (7).
Taken as a whole, our findings suggest a potential harmful effect of NO2 gas from both outdoor and indoor sources on lung function among people with COPD. NO2, like black carbon, is an indicator of traffic-related pollution when measured outdoors. Our finding that personal NO2 exposure was associated with lower lung function could be explained by local traffic-related NO2 entering the home. We found that community-level outdoor NO2 was associated with personal NO2 exposure in our study, suggesting a contribution from outdoor sources, although the magnitude of the association was modest (a 2 ppb higher personal NO2 per 10 ppb community-level NO2) and the shape of the association was not linear across the range of outdoor exposure. Local traffic pollution around the home, not captured by the Boston community monitors, likely further contributed to personal NO2 exposure. We also found evidence that indoor sources contribute to personal NO2 exposure. As noted in other studies (36, 37), we found that gas cooking and—to a lesser extent—gas heating were associated with higher personal exposure to NO2. We did not find statistical evidence of effect modification by season, suggesting that NO2 has a similar effect on lung function during the heating season, when the home is more tightly sealed, and during the warm season, when windows may be open.
Despite prior evidence linking increases in ambient O3 with COPD exacerbations and hospital visits (38–40), we did not find any associations between personal or community-level O3 and lung function in our study. This may be explained by the very low levels of personal O3 exposure in our study of older patients, who spent most of their time indoors. Indoor O3 is determined by both outdoor and indoor sources (41), although indoor levels are typically much lower than outdoor levels (42). In our study, median personal exposure to O3 was only 9.6 ppb, less than half the median outdoor level of 26.3 ppb. Controlled human exposure studies and cohort studies of generally healthy adults have found evidence of respiratory effects of 8- to 24-hour O3 exposure above 60 ppb (43). We tested if 8-hour ambient O3 exposure (median level, 33 ppb; IQR, 14 ppb) was associated with lung function and found no associations. However, we used a single community-level monitor as a proxy for outdoor exposure, which is not comparable to controlled human exposure studies, and our population was likely less exposed to outdoor O3 than generally healthy adults in cohort studies.
Although we did not find significant associations of PM2.5 (personal or community-level) or community-level NO2 with lung function overall, these associations were negative in the subset with higher eosinophil level. These findings must be interpreted with caution in this small study population. If confirmed to be true, then peripheral eosinophil count, which is used as a clinical biomarker to identify patients with COPD with inflammatory airways (11, 44) who are more likely to respond to anti-inflammatory steroid inhaler therapy (13, 45), may also help identify those who are more susceptible to ambient pollutants. Although eosinophil count has not been rigorously evaluated as a biomarker in air pollution research, atopy is a well-established risk factor for susceptibility to pollutants (46, 47). In atopic adults, traffic-related pollutants and allergens have been found to interact, causing eosinophilic airway inflammation (48–50). There is some evidence that patients with COPD with atopy are more susceptible to respiratory health effects of indoor PM2.5 (47). Additional studies are warranted to confirm if patients with eosinophilic COPD may be more susceptible to ambient PM2.5 and NO2.
Our study has a number of limitations. Because our study population consists of former smokers with COPD living in an urban environment, our findings may not be generalizable to current smokers or those living in other settings. Although we collected a large amount of longitudinal data (up to 120 observation-days per participant), our study only included 30 people. We used state-owned stationary monitors to measure day-to-day changes in outdoor exposure among participants living within a 50 km radius of Boston. Although this approach aligns with regulatory monitoring for health-based standards, it may not capture local changes in outdoor exposure to PM2.5, NO2, or O3 that may affect lung function. We did not collect activity information and therefore are unable to determine what proportion of the PAM measurements were from indoor versus outdoor exposures. Although we examined contributions of indoor sources and community-level concentrations to personal pollutant exposures, we did not measure the chemical composition of PM2.5 in the home or the outdoor environment. Therefore, we could not evaluate differential effects of PM mixtures or constituents on lung function, which others have found to be important drivers of heterogeneity (7, 51). We did not ascertain whether a participant ever left the home without the PAM, which likely resulted in some misclassification of the personal exposure estimates. We did not account for characteristics of participants’ homes, such as types of insulation used in the home or window-opening behavior of people living in the home, or cooking behaviors, which may influence participants’ personal exposure to pollutants (52).
Our study also has a number of strengths. Our unique longitudinal study design with daily personal measures of exposure and lung function allowed us to evaluate how day-to-day variability in individual-level exposures relate to daily lung function in a vulnerable population, while accounting for within-person correlation of measurements and adjusting for a robust list of potential individual-level and seasonal confounders. We used lightweight portable exposure monitors, calibrated to gold standard stationary monitors, to measure exposure at the individual level for a prolonged period (4 mo) spanning all four seasons. Most studies have relied on community-level monitors or brief sampling periods to estimate exposure to pollution, which may not capture personal exposure to pollutants, especially among patients with COPD who spend most of their time at home. Our study demonstrates that measuring personal pollution exposure using portable monitors is feasible for extended durations in a high-risk older population and may identify adverse health effects of pollution exposure that are not identified using outdoor EPA air quality monitors.
In this study of former smokers with COPD living in an urban environment, personal exposure to NO2 was associated with lower FEV1 and FVC, and NO2 and PM2.5 were both associated with lower lung function in those with higher eosinophil level. Our study highlights the need to address indoor and outdoor exposure to pollutants and suggests a potential role for personal air quality monitoring in targeted populations. Future work is needed to verify if eosinophil count is a biomarker for susceptibility to ambient pollutants in COPD.
Acknowledgments
Acknowledgment
The authors thank the SPACE study participants for volunteering their time and effort; the research staff who assisted in this study, especially Wendy Sun, whose hard work and patience was integral to the success of this study; and Drs. Ben Barratt, Lia Chatzidiakou, Rod Jones, and Mike Kellaway for their guidance on using the PAMs for respiratory health research. They also thank the staff of the Massachusetts Department of Environmental Protection for their assistance with the monitor calibrations.
Footnotes
Supported by National Institute of Environmental Health Sciences grants K23ES026204, R01ES031252, and P30ES000002 and the U.S. Environmental Protection Agency (USEPA) grant RD-835872. This work’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Furthermore, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Author Contributions: L.N. performed data collection, analysis, and interpretation and wrote the initial draft of the manuscript. B.A.C. provided expertise in statistical methodology and development of the analytical plan. C.-M.K. and P.K. guided personal and community-level exposure assessment, calibration of the monitors, and interpretation of the exposure data. M.B.R was responsible for designing the study and supervised all aspects of the project including data collection, analysis, and interpretation and writing of the manuscript. All authors contributed to the critical revision of the manuscript and approved of the final submitted version.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA . 2017;318:2446–2456. doi: 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA . 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, et al. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med . 2013;188:1351–1357. doi: 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S-H, Mortimer K, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, part 2: air pollution and organ systems. Chest . 2019;155:417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko FWS, Tam W, Wong TW, Chan DPS, Tung AH, Lai CKW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax . 2007;62:780–785. doi: 10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, et al. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest . 2016;149:447–458. doi: 10.1378/chest.15-0513. [DOI] [PubMed] [Google Scholar]
- 7. Hart JE, Grady ST, Laden F, Coull BA, Koutrakis P, Schwartz JD, et al. Effects of indoor and ambient black carbon and [formula: see text] on pulmonary function among individuals with COPD. Environ Health Perspect . 2018;126:127008. doi: 10.1289/EHP3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peacock JL, Anderson HR, Bremner SA, Marston L, Seemungal TA, Strachan DP, et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax . 2011;66:591–596. doi: 10.1136/thx.2010.155358. [DOI] [PubMed] [Google Scholar]
- 9. Paulin L, Hansel N. Particulate air pollution and impaired lung function. F1000 Res . 2016;5:F1000 Faculty Rev-201. doi: 10.12688/f1000research.7108.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;192:523–525. doi: 10.1164/rccm.201502-0235LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kolsum U, Damera G, Pham T-H, Southworth T, Mason S, Karur P, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol . 2017;140:1181–1184.e7. doi: 10.1016/j.jaci.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 12. Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2018;13:335–349. doi: 10.2147/COPD.S152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med . 2015;3:435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 14. Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med . 2017;377:1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 15. Leech JA, Smith-Doiron M. Exposure time and place: do COPD patients differ from the general population? J Expo Sci Environ Epidemiol . 2006;16:238–241. doi: 10.1038/sj.jea.7500452. [DOI] [PubMed] [Google Scholar]
- 16. Abt E, Suh HH, Allen G, Koutrakis P. Characterization of indoor particle sources: a study conducted in the metropolitan Boston area. Environ Health Perspect . 2000;108:35–44. doi: 10.1289/ehp.0010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18. Sun WY, Zhang C, Synn AJ, Nurhussien L, Coull BA, Rice MB. Change in inhaler use, lung function, and oxygenation in association with symptoms in COPD. Chronic Obstr Pulm Dis (Miami) . 2020;7:404–412. doi: 10.15326/jcopdf.7.4.2020.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatzidiakou L, Krause A, Popoola OAM, Di Antonio A, Kellaway M, Han Y, et al. Characterising low-cost sensors in highly portable platforms to quantify personal exposure in diverse environments. Atmos Meas Tech . 2019;12:4643–4657. doi: 10.5194/amt-12-1-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atmospheric Sensors. Digital Air Quality Monitoring Systems. http://atmosphericsensors.com/products/product-brochures/digital-air-quality-monitoring-systems/view
- 21. Chatzidiakou L, Krause A, Han Y, Chen W, Yan L, Popoola OAM, et al. AIRLESS team Using low-cost sensor technologies and advanced computational methods to improve dose estimations in health panel studies: results of the AIRLESS project. J Expo Sci Environ Epidemiol . 2020;30:981–989. doi: 10.1038/s41370-020-0259-6. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen JL, Schwartz J, Dockery DW. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air . 2014;24:103–112. doi: 10.1111/ina.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aaron SD, Donaldson GC, Whitmore GA, Hurst JR, Ramsay T, Wedzicha JA. Time course and pattern of COPD exacerbation onset. Thorax . 2012;67:238–243. doi: 10.1136/thoraxjnl-2011-200768. [DOI] [PubMed] [Google Scholar]
- 24. Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the medicare population. N Engl J Med . 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med . 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 26. Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med . 2019;7:745–756. doi: 10.1016/S2213-2600(19)30190-0. [DOI] [PubMed] [Google Scholar]
- 27. Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med . 2018;6:117–126. doi: 10.1016/S2213-2600(18)30006-7. [DOI] [PubMed] [Google Scholar]
- 28. Evangelopoulos D, Chatzidiakou L, Walton H, Katsouyanni K, Kelly FJ, Quint JK, et al. Personal exposure to air pollution and respiratory health of COPD patients in London. Eur Respir J . 2021;58:2003432. doi: 10.1183/13993003.03432-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, et al. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health . 2006;5:11. doi: 10.1186/1476-069X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJS, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest . 2006;129:1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- 31. Silkoff PE, Zhang L, Dutton S, Langmack EL, Vedal S, Murphy J, et al. Winter air pollution and disease parameters in advanced chronic obstructive pulmonary disease panels residing in Denver, Colorado. J Allergy Clin Immunol . 2005;115:337–344. doi: 10.1016/j.jaci.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 32. de Hartog JJ, Ayres JG, Karakatsani A, Analitis A, Brink HT, Hameri K, et al. Lung function and indicators of exposure to indoor and outdoor particulate matter among asthma and COPD patients. Occup Environ Med . 2010;67:2–10. doi: 10.1136/oem.2008.040857. [DOI] [PubMed] [Google Scholar]
- 33. Brauer M, Ebelt ST, Fisher TV, Brumm J, Petkau AJ, Vedal S. Exposure of chronic obstructive pulmonary disease patients to particles: respiratory and cardiovascular health effects. J Expo Anal Environ Epidemiol . 2001;11:490–500. doi: 10.1038/sj.jea.7500195. [DOI] [PubMed] [Google Scholar]
- 34. Bloemsma LD, Hoek G, Smit LAM. Panel studies of air pollution in patients with COPD: systematic review and meta-analysis. Environ Res . 2016;151:458–468. doi: 10.1016/j.envres.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 35. Sinharay R, Gong J, Barratt B, Ohman-Strickland P, Ernst S, Kelly FJ, et al. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. Lancet . 2018;391:339–349. doi: 10.1016/S0140-6736(17)32643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baxter LK, Clougherty JE, Laden F, Levy JI. Predictors of concentrations of nitrogen dioxide, fine particulate matter, and particle constituents inside of lower socioeconomic status urban homes. J Expo Sci Environ Epidemiol . 2007;17:433–444. doi: 10.1038/sj.jes.7500532. [DOI] [PubMed] [Google Scholar]
- 37. Zota A, Adamkiewicz G, Levy JI, Spengler JD. Ventilation in public housing: implications for indoor nitrogen dioxide concentrations. Indoor Air . 2005;15:393–401. doi: 10.1111/j.1600-0668.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 38. Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. COPD . 2016;13:372–379. doi: 10.3109/15412555.2015.1089846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol . 2006;163:579–588. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- 40. Li J, Sun S, Tang R, Qiu H, Huang Q, Mason TG, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis . 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tran VV, Park D, Lee Y-C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int J Environ Res Public Health . 2020;17:2927. doi: 10.3390/ijerph17082927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weschler CJ, Shields HC, Naik DV. Indoor ozone exposures. JAPCA . 1989;39:1562–1568. doi: 10.1080/08940630.1989.10466650. [DOI] [PubMed] [Google Scholar]
- 43. Rice MB, Guidotti TL, Cromar KR, ATS Environmental Health Policy Committee Scientific evidence supports stronger limits on ozone. Am J Respir Crit Care Med . 2015;191:501–503. doi: 10.1164/rccm.201411-1976ED. [DOI] [PubMed] [Google Scholar]
- 44. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med . 2016;193:965–974. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 45. Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med . 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neves MCLC, Neves YCS, Mendes CMC, Bastos MN, Camelier AA, Queiroz CF, et al. Evaluation of atopy in patients with COPD. J Bras Pneumol . 2013;39:296–305. doi: 10.1590/S1806-37132013000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaji DA, Belli AJ, McCormack MC, Matsui EC, Williams DL, Paulin L, et al. Indoor pollutant exposure is associated with heightened respiratory symptoms in atopic compared to non-atopic individuals with COPD. BMC Pulm Med . 2014;14:147. doi: 10.1186/1471-2466-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wooding D, Syberg-Olsen M, Yuen A, Carlsten C. Co-exposure to diesel exhaust and allergen impairs lung function and induces local and systemic inflammation. Am J Respir Crit Care Med . 2018;197:A6198. [Google Scholar]
- 49. Carlsten C, Blomberg A, Pui M, Sandstrom T, Wong SW, Alexis N, et al. Diesel exhaust augments allergen-induced lower airway inflammation in allergic individuals: a controlled human exposure study. Thorax . 2016;71:35–44. doi: 10.1136/thoraxjnl-2015-207399. [DOI] [PubMed] [Google Scholar]
- 50. Samuelsen M, Nygaard UC, Løvik M. Allergy adjuvant effect of particles from wood smoke and road traffic. Toxicology . 2008;246:124–131. doi: 10.1016/j.tox.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 51. Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, et al. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health . 2006;5:11. doi: 10.1186/1476-069X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor J, Mavrogianni A, Davies M, Das P, Shrubsole C, Biddulph P, et al. Understanding and mitigating overheating and indoor PM 2.5 risks using coupled temperature and indoor air quality models. Build Serv Eng Res Technol . 2015;36:275–289. [Google Scholar]