Pulmonary arterial hypertension (PAH), together with other forms of precapillary pulmonary hypertension (PH), is characterized by increases in pulmonary vascular resistance (PVR). Regardless of the initial pathogenic trigger(s), sustained pulmonary vasoconstriction, concentric pulmonary vascular remodeling, occlusive intimal lesions, in situ thrombosis, and pulmonary vascular wall stiffening are the major causes for the elevated PVR and pulmonary arterial pressure in patients with idiopathic PAH (IPAH). In addition to therapeutic targets on membrane receptors and soluble guanylate cyclase/phosphodiesterase (1), ion channels are potential therapeutic targets to slow down the progression or possibly reverse the progression of PAH/PH (2). Indeed, several types of ion channels, including voltage-gated K+ channels, two-pore domain K+ channels (e.g., KCNK3) (3), Ca2+-activated Cl− channels (e.g., TMEM16A) (4, 5), and transient receptor potential channels (e.g., TRPC6) are implicated in the development and progression of pulmonary vasoconstriction and vascular remodeling in PAH. Mutations in KCNK3 (6) and ABCC8 (7, 8) have been identified in patients with PAH. As reported in this issue of the Journal, the latter finding led to the study by Le Ribeuz and colleagues (pp. 539–554) on the role of ABCC8, the gene encoding SUR1 (sulfonylurea receptor 1), a regulatory subunit participating in forming the ATP-sensitive K+ (KATP) channel (3).
The KATP channel is inhibited by intracellular ATP and activated by a decrease in intracellular ATP or an increase in ADP, thus linking changes in membrane potential (Em) to metabolism. KATP channels are organized as octamers that include four inward-rectifier K+ (Kir) channels (Kir6.x) and four SUR subunits (Figures 1A and 1B) (9). ABCC8 encodes SUR1, which coassembles with Kir6 to form KATP channels and regulate the channel activity and sensitivity to ATP (10). Activity of K+ channels and Na+/K+ ATPase (Na+ pump) regulates Em and cell volume. Decreased K+ currents (IK) due to inhibited KATP channel activity by intracellular ATP and/or downregulated Kir6/SUR1 expression by genetic mutations and epigenetic regulation result in membrane depolarization that opens voltage-dependent Ca2+ channels, enhances Ca2+ influx through voltage-dependent Ca2+ channels, and increases cytosolic Ca2+ concentration ([Ca2+]cyt). A rise in [Ca2+]cyt triggers pulmonary artery (PA) smooth muscle cell (PASMC) contraction, migration, and proliferation (Figure 1C). K+ efflux through K+ channels is also involved in regulating the activity of intracellular caspases and the cell volume or apoptotic volume decrease (11). Activation of K+ efflux decreases cytosolic [K+] and relieves K+-mediated inhibition of caspase and nuclease activity and enhances PASMC apoptosis. Activation of K+ efflux also facilitates apoptotic volume decrease, an early hallmark of apoptosis, and induces PASMC apoptosis (Figure 1C).
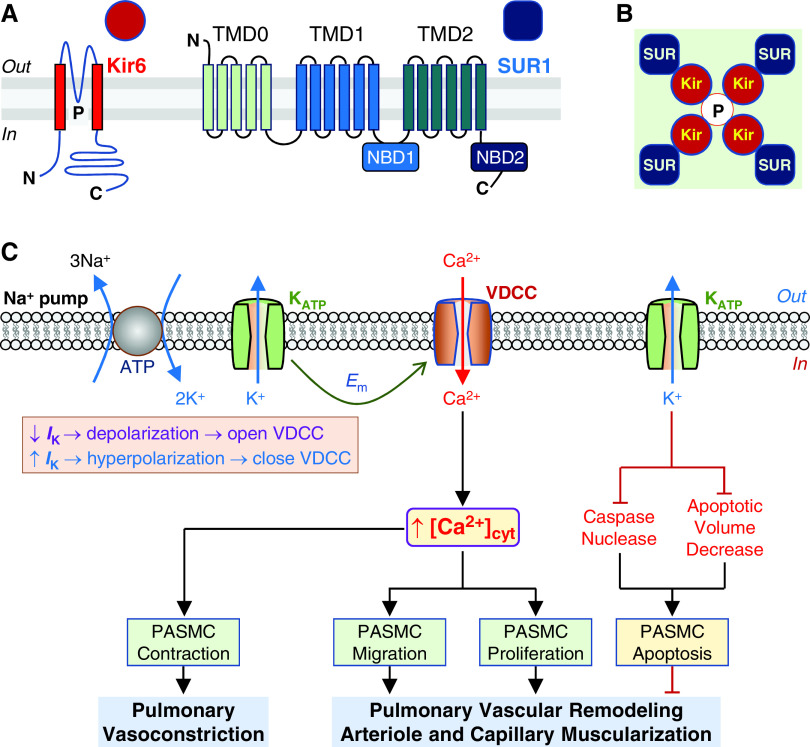
Figure 1.
Structure of ATP-sensitive K+ (KATP) channel and potential therapeutic role of KATP channel activators in pulmonary hypertension (PH). KATP channel, which is inhibited by intracellular ATP, is formed by the inward-rectifier K+ (Kir6.1 or Kir6.2) channel subunit and the sulfonylurea receptor (SUR1 or SUR2) subunit. (A and B) Topology of the Kir6 and SUR1 (A) and the KATP channel formed by Kir6 tetrameric core (red circles and the pore, P) and four peripheral SUR1 subunits (blue squares) (B). The Kir6 monomer contains two transmembrane spans with the pore region (P) located between the two transmembrane helices, and both N- and C-termini are in the intracellular site. SUR1 contains three transmembrane domains (TMD), TMD0 (with five transmembrane α-helices), TMD1 (with six transmembrane α-helices), and TMD2 (with six transmembrane α-helices). TMD0 is connected by a long cytosolic loop known as the CL3 linker. There are two nucleotide-binding domains (NBDs), NBD1 (located at the cytosolic loop between TMD1 and TMD2) and NBD2 (at the C-terminus) of TMD2. (C) Proposed mechanisms involved in the therapeutic effect of KATP channel activation on pulmonary vasoconstriction and vascular remodeling, the major causes for the elevated pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) in patients with PAH/PH. Membrane potential (Em) in pulmonary artery smooth muscle cells (PASMCs) is regulated by the activity of electrogenic Na+ pump and K+ channels in the plasma membrane. Decreased (↓) K+ currents (IK) due to inhibited KATP channel activity and/or downregulated Kir6/SUR1 expression result in membrane depolarization that subsequently opens voltage-dependent Ca2+ channels (VDCC), enhances Ca2+ influx through VDCC, and increases cytosolic Ca2+ concentration ([Ca2+]cyt) in PASMCs. A rise in [Ca2+]cyt causes PASMC contraction and thus pulmonary vasoconstriction and stimulates PASMC migration and proliferation that contributes to the development and progression of concentric pulmonary vascular remodeling and muscularization of pulmonary arteriole and capillary. Increased (↑) IK as a result, for example, of activation of KATP channels by cromakalim and diazoxide, causes membrane hyperpolarization or repolarization that subsequently closes VDCC. The resultant inhibition of Ca2+ influx through VDCC and decreases in [Ca2+]cyt lead to pulmonary vasodilation and regression of remodeled pulmonary arteries and arterioles. Furthermore, activation of K+ efflux through KATP channels (and other types of K+ channels) would relieve K+-mediated inhibition of caspase and nuclease activity and enhance PASMC apoptosis. Activation of K+ efflux through KATP channels would also facilitate apoptotic volume decrease, an early hallmark of apoptosis, and induce PASMC apoptosis. The inhibitory effects of KATP channel activation (via Kir6 and/or SUR1) on pulmonary vasoconstriction and vascular remodeling and the apoptotic effect on highly proliferated cells in the remodeled distal arteries all contribute to the potential therapeutic effects of the KATP channel activators.
Le Ribeuz and colleagues (3) explored the role of SUR1 and the potential activation of SUR1 as a therapeutic target for treating PAH/PH (12). They confirmed that SUR1 and Kir6.2 are expressed in lungs of a control subject, and expression is maintained in patients with IPAH with or without BMPR2 mutations. This is important, as they note that several voltage-gated K+ channels show decreased expression in PAH lungs, and KCNK3 mutation leads to a loss of function. SUR1 and Kir6.2 are expressed in PA endothelial cells (PAEC) and PASMCs. The SUR1 expression level was unchanged between control patients and patients with IPAH, but Kir6.2 was increased in PASMCs and decreased in PAECs from patients with IPAH.
Activation of SUR1 inhibited the proliferation of PAECs/PASMCs from control lung samples, but this response was blunted in IPAH cells. The KATP channels formed by Kir6.2/SUR1 are functional in normal rat/human PAs, and diazoxide activation of SUR1 induces PA relaxation. This effect was partially regulated by endothelial SUR1. SUR1 activation with diazoxide efficiently induced pulmonary vasodilation in PAs isolated from rats with monocrotaline (MCT)-induced PH rats and patients with IPAH.
The study then tested the potential of SUR1 activation to ameliorate PH in vivo using animals with MCT-induced PH, a model for severe PH, and chronic hypoxia-induced PH (HPH), a model for mild PH. Treatment with diazoxide in the prevention experiment slowed the progression of PH as evidenced by improvements in right ventricular (RV) systolic pressure (RVSP), cardiac output, PVR, RV hypertrophy, pulmonary wall thickness, and neomuscularization. Diazoxide treatment in the reversal experiment (Days 14–21 after initial MCT injection) also showed significant improvements in cardiac output, PVR, and pulmonary arterial neomuscularization but not RVSP, RV hypertrophy, or vessel wall thickening. In HPH animals, diazoxide administration in the third week of exposure improved RVSP and reduced vessel neomuscularization. The authors are clear that these studies are a proof of concept and that there are limitations to the use of diazoxide. Notably, reports have indicated that hypoglycemic infants develop PH secondary to diazoxide treatment (13). In the current study, experiments were also performed with additional SUR1 activators, VU0071063 and NN414, that were more selective and had greater potency, respectively (12), and revealed similar pulmonary vasodilative responses. NN414 was also tested in vivo in MCT-PH rats, with comparable ameliorations in pulmonary hemodynamics and vascular histological parameters as diazoxide treatment. The effects of 2 weeks of diazoxide treatment on cardiac function in healthy control rats showed only minor changes that would suggest that SUR1 activation is relatively safe; further carefully monitored safety studies are needed (3).
Overall, the well-designed and comprehensive study by Le Ribeuz and colleagues (3) sheds light on the potential for modulating KATP channels through the pharmacological activation of SUR1. It is interesting to note that, although the expression of SUR1 and Kir6.2 was not significantly changed in lung vascular cells in patients with IPAH, the expression amount and patten were different in rat PH models. In MCT-PH rats, SUR1 was increased and Kir6.2 decreased. In HPH rats, SUR1 was decreased and Kir6.2 was unchanged. This may reflect differences in the disease severity and/or the pathogenic mechanisms. It will be interesting to see if SUR1 activation is successful in attenuating PH in the other forms of PAH (e.g., heritable PAH) and precapillary PH. This is particularly true for patients with PH carrying gene mutations that could affect ion channel function and expression. In addition, KATP channels sense, and are modulated by, a decline in available ATP. Thus, one question is whether there is a link between the metabolic state in PAH and the activation or function of these channels. Metabolic dysregulation has been proposed to play a role in the hyperproliferative response of vascular endothelial cells and adventitial fibroblasts in PH (14, 15). In total, the observation that SUR1 is expressed at normal level in lung vascular cells in patients with IPAH and can be pharmacologically activated to modulate pulmonary vascular tone and slow the progression of PH makes it a very attractive candidate. Future studies to assess the specificity and safety of SUR1 activators are required, but this is a promising approach to potentially treat different types of PH.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2021-0549ED on March 3, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Montani D, Chaumais MC, Guignabert C, Günther S, Girerd B, Jaïs X, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther . 2014;141:172–191. doi: 10.1016/j.pharmthera.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 2. Lambert M, Capuano V, Olschewski A, Sabourin J, Nagaraj C, Girerd B, et al. Ion channels in pulmonary hypertension: a therapeutic interest? Int J Mol Sci . 2018;19:3162. doi: 10.3390/ijms19103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Ribeuz H, Masson B, Capuano V, Dutheil M, Gooroochurn H, Boët A, et al. SUR1 as a new therapeutic target for pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2022;66 doi: 10.1165/rcmb.2021-0180OC. [DOI] [PubMed] [Google Scholar]
- 4. Forrest AS, Joyce TC, Huebner ML, Ayon RJ, Wiwchar M, Joyce J, et al. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol . 2012;303:C1229–C1243. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papp R, Nagaraj C, Zabini D, Nagy BM, Lengyel M, Skofic Maurer D, et al. Targeting TMEM16A to reverse vasoconstriction and remodelling in idiopathic pulmonary arterial hypertension. Eur Respir J . 2019;53:1800965. doi: 10.1183/13993003.00965-2018. [DOI] [PubMed] [Google Scholar]
- 6. Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med . 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohnen MS, Ma L, Zhu N, Qi H, McClenaghan C, Gonzaga-Jauregui C, et al. Loss-of-function ABCC8 mutations in pulmonary arterial hypertension. Circ Genom Precis Med . 2018;11:e002087. doi: 10.1161/CIRCGEN.118.002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lago-Docampo M, Tenorio J, Hernández-González I, Pérez-Olivares C, Escribano-Subías P, Pousada G, et al. Characterization of rare ABCC8 variants identified in Spanish pulmonary arterial hypertension patients. Sci Rep . 2020;10:15135. doi: 10.1038/s41598-020-72089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tinker A, Aziz Q, Li Y, Specterman M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol . 2018;8:1463–1511. doi: 10.1002/cphy.c170048. [DOI] [PubMed] [Google Scholar]
- 10. Wheeler A, Wang C, Yang K, Fang K, Davis K, Styer AM, et al. Coassembly of different sulfonylurea receptor subtypes extends the phenotypic diversity of ATP-sensitive potassium (KATP) channels. Mol Pharmacol . 2008;74:1333–1344. doi: 10.1124/mol.108.048355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol . 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- 12. McClenaghan C, Woo KV, Nichols CG. Pulmonary hypertension and ATP-sensitive potassium channels. Hypertension . 2019;74:14–22. doi: 10.1161/HYPERTENSIONAHA.119.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen SC, Dastamani A, Pintus D, Yau D, Aftab S, Bath L, et al. Diazoxide-induced pulmonary hypertension in hyperinsulinaemic hypoglycaemia: recommendations from a multicentre study in the United Kingdom. Clin Endocrinol (Oxf) . 2019;91:770–775. doi: 10.1111/cen.14096. [DOI] [PubMed] [Google Scholar]
- 14. Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos C, Perez-Iratxeta C, et al. Identification of microRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation . 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation . 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]