The mechanical excision of tumors by oncological surgery, as well as the effractions imposed on vascular and lymphatic vessels, can promote the dissemination of residual tumor cells to distant organs with the consequent formation of secondary metastatic lesions.1 Moreover, pain and inflammation, which are often elicited by surgical procedures, cause systemic adaptations such as the production of immunosuppressive glucocorticoids and vascular endothelial growth factor, thus affecting leukocyte chemotaxis and favoring neoangiogenesis, respectively. Local anesthetics that are currently used in clinical routine for their analgesic and anti-inflammatory properties can minimize the release of corticoids and catecholamines during surgical procedures. Moreover, several retrospective clinical trials recently suggested that the use of local anesthetics associated with general anesthesia during oncological surgery improves disease outcome and overall survival.2 Intrigued by these premises, we addressed the question if local anesthetics directly affect neoplastic cells and whether they promote an anti-tumor immune response that might explain their positive impact on cancer prognosis.
Accumulating preclinical evidence suggests that local anesthetics provoke direct cytotoxic effects, leading to the inhibition of proliferation, survival and migration of various malignant cell types. Several mechanisms have been implicated in this process such as the impairment of cyclin-dependent kinase activity, the suppression of DNA methylation, the induction of mitochondrial damage and dysfunctional ion transport.3 Driven by these findings, we studied the mode of cell death that is induced by local anesthetics, finding that apoptotic as well as necrotic signaling pathways are ignited in a dose- and time-dependent manner. Moreover, treatment with clinically relevant concentrations of the most frequently employed local anesthetics induced traits of immunogenic cell death (ICD) such as the release of ATP and the exodus of high-mobility group box 1 protein (HMGB1) into the extracellular space.4,5 This phenomenon was preceded by premortem cellular stress routines such as the induction of autophagy and the phosphorylation of eIF2alpha, which is known to be involved in immunogenic death as well as in the release of pro-inflammatory cytokines.6,7 In contrast to bona fide ICD-inducing chemotherapeutics such as mitoxantrone, oxaliplatin or microtubule-targeted poisons, local anesthetics were unable to induce the exposure of calreticulin at the plasma membrane, where it commonly acts as an “eat-me” signal for dendritic cells. Local anesthetics also failed to inhibit DNA-to-RNA transcription, which has recently be described as a prominent upstream signal causing ICD.8,9 Nevertheless, local anesthetics regulate adaptive immune response by promoting the phagocytosis of dying tumors by bone marrow-derived dendritic cells (BM-DCs) in vitro and by decreasing the growth of palpable tumors established in immunocompetent mice, if injected intratumorally.10 Interestingly, local anesthetics failed to promote anti-tumor effects in immunodeficient mice, as well as against tumors that were rendered unable to mount autophagic or ER stress responses due to the inactivation of ATG5 and EIF2AK3, respectively. Moreover, local treatment with local anesthetics induced the infiltration of tumors by CD4+ and CD8+ T cell effectors, but diminished the local recruitment of immunosuppressive T regulatory cells.4
As described previously, the efficacy of conventional antineoplastic treatments may be potentiated by immunostimulatory agents.11,12 Thus, we further investigated if the combination of local anesthetics with adjuvant stimulation may restore a full-blown ICD response and may potentiate antitumor immunity.13,14 Indeed, the local co-injection of recombinant calreticulin together with local anesthetics further improved the anticancer effect. The use of local anesthetics combined with sequential systemic immune checkpoint blockade even allowed to completely and durably eliminate some established cancers,4 in which case mice were protected from rechallenge with the same cancer, indicating that they had developed immunological memory.
Altogether, our preclinical findings highlight that the use of local anesthetics causes “immunogenic stress” rather than full-blown ICD. Thus, local anesthetics may be classified among the non-viral oncolytic therapies, which upon intra-tumoral injection elicit immunogenic physical and chemical damage.15–17 Irrespective of their classification, local anesthetics trigger the phagocytosis of cancer cells and the engulfment of tumor antigens by DC, followed by the recruitment of immune effectors into the tumor bed (Figure 1). Several strategies associating local anesthetics with immunostimulatory agents may be envisaged to further boost the cancer-immunity cycle.
Figure 1.
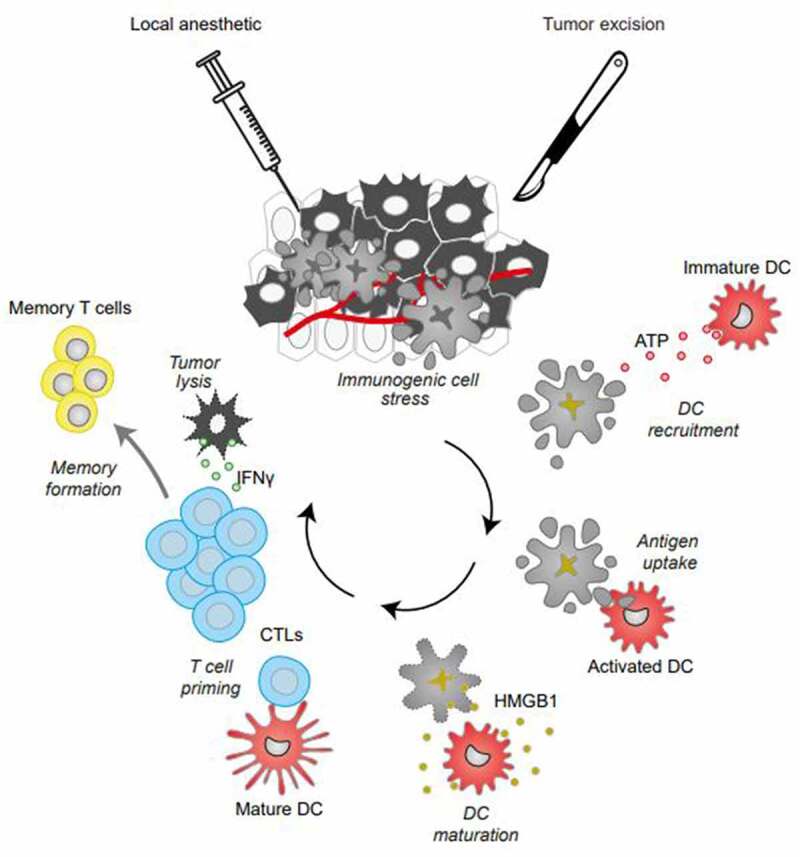
Oncolytic effects of local anesthetics. Local anesthetics employed at clinically relevant concentrations induce cell death. This effect is preceded by premortem autophagy and endoplasmic reticulum stress and accompanied by the release of danger-associated molecular patterns (DAMPs) such as ATP and high-mobility group box 1 protein (HMGB1), facilitating the recruitment of dendritic cells (DCs) for antigen presentation and T lymphocyte priming, altogether eliciting adaptive anticancer immune responses, tumor lysis by cytotoxic T lymphocytes and the generation of durable immune memory.
In sum, beyond their role in pain control, local anesthetics may be repurposed as antineoplastic co-treatments to avoid disease recurrence and improve overall survival because of their capacity to induce immunogenic stress. There is an urgent need to design randomized controlled trials that support this exciting hypothesis that ultimately would change clinical practice in onco-anesthesia.
Acknowledgments
OK is supported by Institut National du Cancer (INCa) and the DIM Elicit of the Ile-de-France. LB received a research grant by Bristol Myers Squibb Foundation France. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Program on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; INCa; Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM).
Funding Statement
This work was supported by the Labex Immuno-Oncology [ANR-18-IDEX-0001].
Data availability statement
The data that support the findings of this editorial are available at doi:10.1136/jitc-2021-004151. PMID:35483744. https://jitc.bmj.com/content/10/4/e004151.
Declaration of interest statement
OK is a scientific co-founder of Samsara Therapeutics. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Sotio, Vascage and Vasculox/Tioma. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of EverImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders.
References
- 1.Lambert AW, Pattabiraman DR, Weinberg RA.. Emerging biological principles of metastasis. Cell. 2017 Feb 9;168(4):670–3. doi: 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016 Mar 22;7(12):15262–15273. doi: 10.18632/oncotarget.7683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Chuang A, Kepp O, Kroemer G, Bezu L. Direct cytotoxic and indirect, immune-mediated effects of local anesthetics against cancer. Front Oncol. 2021;11:821785. doi: 10.3389/fonc.2021.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezu L, Wu Chuang A, Sauvat A, Humeau J, Xie W, Cerrato G, Liu P, Zhao L, Zhang S, Le Naour J, et al. Local anesthetics elicit immune-dependent anticancer effects. J Immunother Cancer. Apr 2022;10(4). doi: 10.1136/jitc-2021-004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012. Dec;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 6.Bezu L, Sauvat A, Humeau J, Gomes-da-Silva LC, Iribarren K, Forveille S, Garcia P, Zhao L, Liu P, Zitvogel L, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018. Aug;25(8):1375–1393. doi: 10.1038/s41418-017-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprooten J, Garg AD. Type I interferons and endoplasmic reticulum stress in health and disease. Int Rev Cell Mol Biol. 2020;350:63–118. doi: 10.1016/bs.ircmb.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020 May 8;125:e11622. doi: 10.15252/emmm.201911622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022. Apr;23(4):487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 10.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017. Apr;17(4):262–275. doi: 10.1038/nri.2017.9. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki T, Wennerberg E, Hensler M, Buque A, Kraynak J, Fucikova J, Zhou XK, Sveinbjørnsson B, Rekdal Ø, Demaria S, et al. LTX-315-enabled, radiotherapy-boosted immunotherapeutic control of breast cancer by NK cells. Oncoimmunology. 2021;10(1):1962592. doi: 10.1080/2162402X.2021.1962592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilones KA, Hensler M, Daviaud C, Kraynak J, Fucikova J, Galluzzi L, Demaria S, Formenti SC. Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: contribution of Vista blockade. Oncoimmunology. 2020 Oct 20;9(1):1830524. doi: 10.1080/2162402X.2020.1830524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020. Dec;17(12):725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 15.Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020. Jan;17(1):49–64. doi: 10.1038/s41571-019-0272-7. [DOI] [PubMed] [Google Scholar]
- 16.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020. Feb;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020. Feb;21(2):120–134. doi: 10.1038/s41590-019-0561-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this editorial are available at doi:10.1136/jitc-2021-004151. PMID:35483744. https://jitc.bmj.com/content/10/4/e004151.


