ABSTRACT
The use of antibodies in the treatment of lung diseases is of increasing interest especially as the search for COVID-19 therapies has unfolded. Historically, the use of antibody therapy was based on multiple targets including receptors involved in local hyper-reactivity in asthma, viruses and micro-organisms involved in a variety of pulmonary infectious disease. Generally, protein therapeutics pose challenges with respect to formulation and delivery to retain activity and assure therapy. The specificity of antibodies amplifies the need for attention to molecular integrity not only in formulation but also during aerosol delivery for pulmonary administration. Drug product development can be viewed from considerations of route of administration, dosage form, quality, and performance measures. Nebulizers and dry powder inhalers have been used to deliver protein therapeutics and each has its advantages that should be matched to the needs of the drug and the disease. This review offers insight into quality and performance barriers and the opportunities that arise from meeting them effectively.
KEYWORDS: Aerosols, antibodies, therapeutics, formulation, stability, lungs
Introduction
Knowledge regarding protein delivery to the lungs has been increasing since the end of the last Millennium as interest in the delivery of biotechnology products has grown.1,2 Initially the major focus and enormous intellectual output was on inhaled insulin for the treatment of diabetes. However, DNAse was a product of equal significance used to enhance mucociliary clearance in cystic fibrosis (Pulmozyme, Dornase Alpha, Genentech, SF, CA).3,4 The large body of knowledge generated by decades of research is a valuable outcome of the emphasis placed on protein formulation for inhalation that has value in considering approaches to the development of inhaled monoclonal antibody (mAb) formulations.5,6 A range of proteins have been studied with respect to pulmonary drug delivery.7 Passive uptake of proteins from the lungs is dictated primarily as a function of molecular weight and charge.8 The molecular weight of immunoglobulins predisposes them to retention in the lungs. However, if they are designed to bind to airway surface receptors active uptake can be achieved.9 Thorough reviews of recent developments in inhaled peptides and proteins, preclinical and clinical candidates are available.2,10
Antibodies and fragments have to be considered in terms of their applications (1. Mucosal; 2. Systemic; and 3. Fragments).11 Factors that impact on the efficacy of antibodies range from structural instability, fragmentation, at the level of primary and secondary disruption, to functional instability, with functional changes due to tertiary structure changes including aggregation or grafting.12,13
The use of mAb aerosol treatment of viral lung disease has shown promise.14 But, aside from biological target identification and respective protein formulation, there are other delivery considerations associated with inhalation compared to conventional routes of administration (i.e. intravenous). In particular, the methods of aerosol generation and sampling must be evaluated to assure product quality and performance before in vivo studies commence.15
The key parameters of interest in protein formulation are physico-chemical properties during manufacture, storage, handling and aerosol generation.16 The classical causes for concern are thermal, light or mechanical lability of the molecule itself that might give rise to either inactivation, degradation or both.16 Aggregation is a common issue in protein structural instability and may occur on storage, during handling and delivery.17 Figure 1 illustrates the drug product, contacts and handling elements that are subject to both extraneous environmental effects and intrinsic packaging, delivery, storage, and sampling phenomena that impact product quality and performance. Each represents a focus of attention during product development.
Figure 1.
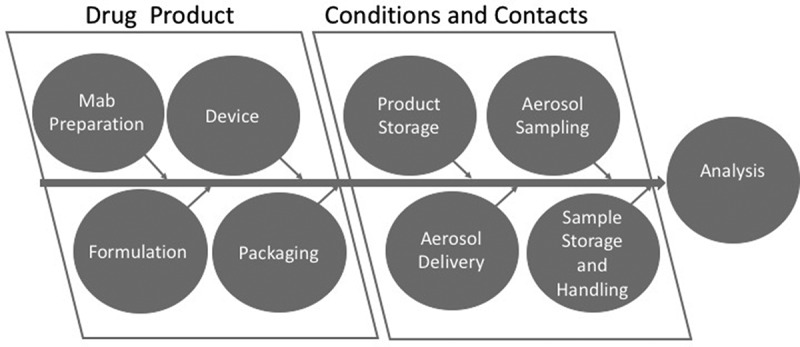
Diagram depicting the contributing factors from the drug product, conditions and contacts that contribute to the analytical quality and performance measures.
The drug product factors of mAb preparation, formulation with additives/excipients, combination with device and packaging are all defined by the manufacturer. The conditions and contacts relating to product storage, aerosol delivery, sampling and sample storage and handling contribute to quality and clinical performance as determined by analytical and bioanalytical chemistry, safety and efficacy testing.
There have been numerous reviews of the challenges presented in developing mAb therapeutic agents including formulation, delivery and analysis mostly in the context of parenteral administration.18,19 Inhaled mAb requires consideration of many of the same issues as parenteral products but this unique dosage form raises further challenges that must be addressed to guide properties of importance to quality, safety and efficacy.20 Antibodies have been considered for a variety of therapeutic purposes related to lung disease. The leading applications have been in the receptor targeting, pathogen binding or xenobiotic trapping.21
Interest in the use of inhaled mAb for the treatment of viral infections has, not surprisingly, raised the prospects of possibly treating COVID-19 with aerosol therapy.22 A review of recently registered/updated clinical trials supports this observation as shown in Table 1. Notably, Boehringer Ingelheim recently stopped a phase 2 clinical trial of its inhaled antibody, BI764918, due to lack of efficacy noted by its data monitoring group (Boehringer Ingelheim news release March 21st, 2021). There remains an urgent need for therapies that preserve the integrity of the lungs through local delivery thereby reducing morbidity and mortality.
Table 1.
Summary of inhaled antibody therapeutics noted primarily in clinicaltrials.gov
| Name Drug | Company | MM/YY |
|---|---|---|
| SARS-CoV2 Neutralizing Antibody DZIF-10 c by inhalation23 | University of Cologne | 11/20 |
| Phase 1A Safety Trial of Inhaled PK10571 (GB002)24 | Gossamer Bio | 06/20 |
| Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody in Subjects with Severe Conronavirus 2019 (COVID-19)25 | I-Mab Biopharma Co., Ltd | 12/20 |
| ACTIV-2: A study for outpatients with COVID-1926 | NIAID (with industry collaborators) |
04/21 |
| Inhaled antibody treatment for COVID-19 shows success in preclinical trials – AR71127 | Aridis | 10/20 |
| Monoclonal antibody for the treatment of inhalation anthrax – ETI-204 (Anthim)28 | NIAID | 08/10 |
The intent of this focused review is to address the interface between formulation, device and specialized analytical methods that may influence the accuracy and reproducibility of dose measurement with implications for quality, performance and anticipated safety, and efficacy assessment.
Routes of administration
Protein therapeutics do not survive oral ingestion to deliver the therapeutic agent to the systemic circulation and subsequently to the desired target, due to the harsh pH environment in the gastrointestinal tract.29 Historically, parenteral delivery of antibodies has been considered the most desirable route of administration.30 Injectable formulations deliver proteins and peptides directly to the systemic circulation avoiding degradation and instability that would arise by the oral route. However, high systemic concentrations of the antibody are required to achieve therapeutic levels at the site of action.31 Direct delivery to the lungs as a target organ system has the advantage that high local doses delivered as aerosol droplets or particles, with limited systemic exposure, can achieve therapeutic equivalency to much higher doses delivered systemically, by parenteral routes. In some cases, the required injectable dose may make therapy difficult if not impossible by systemic delivery.
While delivery of antibodies to the lungs is not directly invoking local immunity it should be noted that it is supplementing respiratory tract mucosal immunity. The nose and lungs are a primary sites of mucosal immunity and present the first line of immunological defense against airborne pathogens.32,33 Local induction of innate immunity, primarily through the action of macrophages and dendritic cells is followed by initiation of acquired immunity by recruitment of B and T cells. This leads to production of IgA and IgG specific to the mucosal environment. A strong mucosal immune response can prevent systemic exposure and infection. Communication between mucosal associated lymphoid tissues locally (nasal and bronchial) and elsewhere (gastro-intestinal, reproductive tract) can result in protective immunity being transferred between the site of initial pathogen exposure and other mucosal sites. Consequently, introducing inhaled antibody into this milieu would not only be directly therapeutic but would support and potentially lead to a more robust respiratory mucosal immune response.
Dosage form
Devices
There are three primary categories of aerosol delivery system, pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs) and nebulizers.34 The required dose and state of the therapeutic agent dictate the most suitable system to adopt. Historically, the organic propellants and limited dose (<1 mg) have mitigated against the use of pMDIs for the delivery of high dose drugs.35
Nebulizers have been favored for protein delivery due to the solubility of the protein and the relatively simple formulation due to constraints on additives.36 However, despite the lack of complexity the limited formulation options can be time-consuming to optimize. Stabilizing proteins in aqueous media may pose formulation challenges.36 A number of additives can be employed, as discussed below, and storing under low controlled temperature may be desirable.37
If the protein is fundamentally unstable in solution it can be formulated with stabilizing moieties and either freeze dried or spray dried for reconstitution. Either of these approaches would be suitable for nebulizer delivery. However, it should be noted that there are limitations on the additives that could be used as stabilizers for inhalation compared with those used for parenteral products.
Depending on the desired dose, dry powder inhaler delivery of spray dried material is a possibility.38 The advantage of dry powder formulation as would be the case for the reconstitutable nebulizer formulations is the potential to achieve room temperature stability and thereby make the product more readily available outside well-developed urban centers. This contrasts with the potential instability of mAb nebulizer solutions or degradation of reconstitutable formulations during freeze-thaw cycles.
Selection of device depends on dose required for effective therapy, quality and performance measures as defined by regulatory and compendial standards. These measures are described in detail below.
Formulation
Considerations in formulating and delivering proteins
Conventional structural stability with regard to primary, secondary and tertiary elements may all be addressed by formulation strategies. Assuming that all causes of primary structural instability may be avoided, the likely sources of instability, in-use or on storage, are the secondary and tertiary structural elements that are essential to the efficacy of an antibody. Figure 2 illustrates the environmental and product factors that can give rise to instability of the antibody at various stages during its preparation and characterization.
Figure 2.
Environmental and product factors that contribute to the quality and performance of inhaled monoclonal antibodies that are uses to assure efficacy and safety outcomes.
Parameters and conditions affecting stability include protein structure and concentration, temperature/humidity, pH, contact interfaces, light exposure, and the presence of excipients and contaminants.39 Physical stability assays are required to assess the impact of aggregation, fragmentation, primary, secondary and tertiary structural defects. Proteins formulated as high concentration liquids are susceptible to aggregation through a number of destabilizing mechanisms.40 Adsorption behavior of human mAbs at hydrophobic and hydrophilic surfaces can give rise to losses and instability.41
Metal ions, such as Cu2+, can bind to the mAb and undergo hydrolysis or oxidation, which may lead to cleavage of the molecule.42 Charge may contribute to stabilization of a specific molecular structure involved in hydrolysis leading to the possible formation of a copper binding pocket that causes increased susceptibility of the hinge region to degradation.
Instability of inhaled antibodies results from general susceptibility of proteins that require exquisite control of tertiary structure for activity and, as a consequence, require a protective formulation strategy. Unlike proteins intended for traditional parenteral administration, there are potential inhaler device interactions that may give rise to real-time instability or dosing variability. In addition, the way in which inhaled antibodies are collected, stored or manipulated for analysis may influence estimates of dose and dose uniformity.
Antibody formulations
Protein formulation strategy is largely focused on preventing aggregation and degradation in aqueous media and producing amorphous rather than crystalline structures in the solid state that would readily disperse either in aqueous media or in biological fluids.43
There are several categories of additives that can be added to antibody solutions to aid in stability.44 Common salts such as sodium chloride, ammonium sulfate, and calcium chloride can be used to impact protein aggregation and stability by altering the electrostatic environment around the antibody and/or creating an intermediate secondary structure. The solution pH and ion choice is important as, for example, below pH 4.5 antibody aggregation is induced to some extent; at pH 4.0 the cation and anion impact aggregation but only the anion has an effect at pH 3.0.45 Appropriate buffers such as citrate or histidine control pH to avoid antibody structural degradation.46 Surfactants including TWEEN-20, TWEEN-80, sodium dodecyl-sulfate, and Triton-X-100 influence surface tension, solubility, and aggregation of the antibody.47 The addition of sugars (glucose, sorbitol, sucrose, etc.) can also prevent aggregation where both the size of the sugar and it’s chemical interaction with the protein have an effect.48 Conformational stability and solubility enhancement can be achieved through co-solvents such as propylene and polyethylene glycol.49
When preparing the antibody as a solid dose by freeze drying or spray drying (as discussed below) many of the above excipients aid in stabilizing the antibody in the precursor solution. Processing stressors, such as shear and heat, become concerns however when generating a solid antibody product. Fortunately, many of the previously mentioned excipients such as sugars, glycols, and surfactants act as stabilizers; preventing protein degradation or unwanted crystallization.50,51 If heat is going to be essential to the manufacturing process (such as is required during spray drying) the impact of temperature on the antibody structure should be assessed through feasibility studies often in statistically designed experiments before committing to a specific technique.52
Physical and chemical stability of the antibody formulation can be assessed by a variety of instruments and assays. Crystallinity of a solid product can be confirmed via X-ray powder diffraction (XRPD).53 Circular dichroism uses polarized light to optically assess protein structure.54 Protein conformation can be assessed using gel permeation chromatography55 and gel electrophoresis39 which assay for homogeneity and peptide size differences respectively. Receptor-binding and neutralization assays ensure the antibody and respective formulation can suitably bind to or inactivate the intended target56 Mass spectroscopy is, among other things, useful for identifying structural features of the antibody.39,57 There are many other size and structure related assays and physical and chemical methods available that can provide supporting or complementary evidence when studying antibody stability.39 These techniques may be used to validate antibody stability throughout the development process: from formulation in solution, to manufacture (in the case of a dry product, to post-nebulization or post-actuation from dry powder inhaler, to collection methods). Table 2 identifies the methods employed to evaluate stability of proteins. Aggregation and concurrent loss or decrease in activity/function are the most common sources of instability during aerosolization or upon contact with collection surfaces and would be the focus of attention for inhaled Mabs.
Table 2.
Methods of assessing the stability of antibody therapeutics. *Aggregation and concurrent loss or decrease in activity/function are the most common sources of instability during aerosolization or upon contact with collection surfaces
| Sources of Instability | Assay/Test Methods |
|---|---|
| Primary Structure | Quantitative – Mass Spectrometry57 Qualitative – Western Blot58,59 |
| Secondary Structure | Nuclear Magnetic Resonance60,61 |
| Tertiary Structure | Circular Dichroism54 Optical Rotatory Dispersion |
| *Aggregation | Gel Permeation Chromatography55 Gel Electrophoresis62,63 Western Blot58,59 |
| *Activity/Function | Antigen Binding56 Neutralization (replicating antigens) |
Methods for determining the quality of nebulizer solutions are referenced in General Chapter <5> of the United States Pharmacopoeia alluding to specifications on microbial and foreign particulate limits.64
Microbials
The presence of microbes is a serious potential source of instability and subject of safety consideration. Enzyme catalyzed proteolysis will degrade the antibody while encouraging microbial growth. Approaches to measuring and setting limits on microbial burden are described in regulatory guidance documents.65 The guidance requires that attention is paid to USP compendial methods, General Chapter <610> Alternative Microbiological Sampling Methods for Nonsterile Inhaled and Nasal Products and General Chapter <1111> Microbiological Examination of Nonsterile Products Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use. The general approach to inhaled formulations is that coliform bacteria should be absent and that no more that 100 non-coliform micro-organisms/gram or mL can be cultivated from dry formulations (reconstitutable formulations for nebulization, dry powders).66
Foreign particulates
Foreign particulates present a safety consideration and often speak to the control of the manufacturing process and packaging components. Specifications on foreign particulate matter include numerical and size limits for parenteral products, as described in <788> Particulate Matter in Injections, which can be used as reference framework for aerosol products. Depending on the method employed no more than 3,000 particles ≥ 10 μm and 300 particles ≥ 25 μm by microscopy (or 6,000 and 600, respectively, by light obscuration) in low volume parenterals (≤100 mL) which are most relevant to inhaled products. For regulatory purposes it is recommended that limits be placed on foreign particulates in the ranges < 10 μm, 10–25 μm and >25 μm.65 Specific limits on inhalation are debated and it has been suggested that additionally particle size ranges should be considered where reasonable size ranges to include in acceptance criteria for quality control purposes could be 2–10 μm, 10–25 μm, and 25–100 μm.67 Since many of these particles originate in packaging it is important that their source is investigated. This approach cannot be understated given the susceptibility of monoclonal antibody formulations to aggregation which may be catalyzed by foreign particulates and, further, that they may undergo serious chemical degradation in the presence of metal ions.
Inhaled product handling and sampling consideration
Nebulizer solutions
Antibody delivery as liquid aerosol droplets atomized from a nebulizer is attractive given the high aqueous solubility of proteins. As such, many protein formulations are already in liquid form. Additionally, nebulized drug formulations do not involve a protein drying step and can deliver large doses.68 However, conformational distortions of the polypeptide structure are possible in solution, as an aerosol at the air-liquid interface, or during collection so care must be taken during drug product formulation. Table 3 lists the types of substrates and contacts for the mAb during delivery and collection that might be sources of instability.
Table 3.
Devices/components that serve a specific process in drug product performance and characterization and the components into which the drug (mAb) will come into contact
| Process | Device/Components | Composition |
|---|---|---|
| Aerosol Generation | Nebulizers Conducting Tubing Mouthpieces/Facemasks |
Polypropylene Polyethylene Polyethylene Teraphthalate Nickel Chrome Stainless-Steel |
| Sampling | Cascade Impactor Liquid Impinger |
Stainless-Steel Glass Aluminum Coating Substances (silicone oil, glycerol) |
| Handling and Storage | Filter Housing Filters Sample Transfer Instruments e.g. Pipets, injection needles Collection Vessels e.g. vials |
Polytetrafluorethylene (PTFE, Teflon) Stainless-Steel Glass fiber Polycarbonate Paper Plastics/Polymers Elastomers Glass Stainless-steel |
| Analysis | Chromatograph HPLC, UPLC, SEC, GPC |
Stainless-steel Packing material |
Common strategies for stabilizing liquid protein formulations employ surfactants, sugars, and salts to avoid aggregation and control surface tension, buffering agents to maintain pH, and other excipients to prevent oxidation or otherwise protect the antibody.44,69 As the bulk liquid is atomized, the air-liquid interface quickly increases causing the potential for protein adsorption, unfolding, or aggregation. This may be accompanied by foaming that gives rise to poor and/or variable dose delivery and irreproducible aerodynamic particle size characteristics and may be suppressed by surfactants. Excipients (such as sugars, polyols, and amino acids) can help reduce these outcomes by stabilizing the structure in solution. Nebulizer selection (thus, aerosol droplet generation method) is an important consideration as well to avoid possible heat or shear impacts on the antibody stability.16,70 Finally, the physical collection vessel used to assess the nebulized output can also impact binding affinity and aggregation of the antibody. The risks associated with sampling of nebulized proteins has been recognized for decades.71 Regardless of the collection substrate used (such as polypropylene, glass, or steel depending on assay) it is recommended that the substrate should be the same throughout development as protein stability can be dependent on either the physical collection material or the antibody itself.68 The choice of excipient(s), nebulizer, and aerosol collection substrate need to be screened for assurance of physical stability of the antibody and proper aerodynamic characterization. Figure 3 shows three common aerosol sampling devices used in aerodynamic particle characterization. Each has different sampling surface with respect to elemental composition, geometry and number.65,72 In addition each of the surfaces can be modified by coating (Figure 3(a) NGI and (b) ACI) or use of a collection medium (Figure 3(c) TSLI) that can be adjusted for compatibility with mAb sampling.
Figure 3.
Images of: (a) Next generation impactor showing collection cups (lower section); (b) assembled Andersen 8-stage non-viable impactor with collection plates (below) that are present on each stage and; (c) two-stage liquid impinger showing glass vessels in which samples are collected.
Dry powder inhalers
Drug delivery to the lungs can also be achieved using dry powder inhalers. In this case the therapeutic agent is formulated into a powder that, for high dose delivery (>1 mg) is delivered to the patient as an inhalable, low density aerosol.73,74 Unlike liquid antibody formulations that often need to be refrigerated, a solid-state dosage form, such as a dry powder, may be stable at room temperature.75 This makes dry powder inhalers a more patient friendly and portable option where special storage or electricity is unnecessary to receive the medication. The most common strategies of manufacturing protein powders are spray drying and spray-freeze drying.76 As with liquid formulations excipients are often added to the precursor formulation to enhance physicochemical stability and aerodynamic performance of the drug product. Spray drying is another widely available technique for synthesizing amorphous pharmaceutical powders. The drug and excipients are dissolved in solution and fed via peristaltic pump into a heated atomizing nozzle. An atomizing gas, typically nitrogen, disperses the liquid precursor solution into tiny droplets that quickly evaporate and leave behind microparticles. There are many synthetic parameters that can be adjusted to impact final particle size such as solids concentration, solvent, solid feed rate, atomizing gas pressure, and inlet temperature.38,77 In either method though there are high shear and/or temperature stressors applied to the antibody that may degrade the structure.
Quality and performance measures
Inhaled product performance measures
Aerosol characteristics
Aerodynamic performance needs to be assessed regardless of delivery platform. The United States Pharmacopoeia (USP) sections <601> and <1601> discuss in detail the various methods for characterizing dry powder and nebulized aerosols, respectively.72,78 Delivered dose uniformity (DDU) and mass median aerodynamic diameter (MMAD) are the most important performance metrics.79 They are not intended to be a substitute for in vivo experiments or predictive of lung deposition.80 Rather they are general quality measures of the drug product aerosol performance and can be used to justify further development and support safety and efficacy studies.81
Delivered dose for nebulizers is measured by actuating the nebulizer over a period time into a dose uniformity sampling apparatus (DUSA); a tube connected in line with the nebulizer and a vacuum pump. A breath profile simulator can be used to coordinate inhalation: exhalation ratio, tidal breathing volume, and breathing pattern with patient age or disease. The aerosol is collected over a period of time that allows for sufficient quantification of the drug product. Output rate is determined at the beginning, middle, and end of proposed dosing time to get an estimate of the total delivered dose. Dry powders are similarly sampled through a DUSA. The inhaler is actuated and vacuum is applied to collect the drug product on a filter. Vacuum flow rate is determined by the L/min necessary to produce a 4 kPa pressure drop across the inhaler. Vacuum is controlled via solenoid and operated for as long as it takes to pull 2 L of air at the prescribed flow rate.
The mass median aerodynamic diameter (MMAD) is a quality descriptor of the of the aerodynamic performance of an aerosol. Generally, a MMAD between 1–5 µm will result in a respirable product. Cascade impaction is the experimental technique employed to quantify the MMAD. Jet nebulizers generally produce MMADs in the range 1–2 µm with GSDs of approximately 2 while vibrating mesh nebulizers and ultrasonic nebulizers produce larger MMADs of 3–4 µm with narrower GSDs in the range 1.6–1.8.82 Doses of several hundreds of milligrams can be delivered from nebulizers the only limitation being the period of inhalation by the patient and the safety of the drug. Pentamidine, a drug used to treat Pneumocystis carinii pneumonia as secondary infection in patients infected with human immunodeficiency virus was given as a 300 mg dose.83 This method generates an aerodynamic particle size distribution (APSD) from which the MMAD and geometric standard deviation (GSD), the measure of the aerodynamic particle size range, can be calculated. USP <1601> dictates that the Next Generation Impactor (NGI) is operated at a flow rate of 15 L/min for nebulizers.78 Again, a breath profile simulator can be used to mimic patient breathing. For dry powders, the NGI should be operated at the same flow rate used for the DDU experiments above.72
For aqueous droplets where density approaches 1 g/mL the volume diameter is equivalent to aerodynamic diameter. Consequently, laser diffraction, a volume-based aerosol particle sizing method, may be employed to determine the particle size distribution as described in USP General Chapter <1601 > . The median diameter, D50, and Span, (D90-D10)/D50 are employed to describe the distributions obtained by this instrument and approximate to the MMAD and GSD used to describe the APSD. Laser diffraction has the advantage for nebulizer droplets that there are more data points describing the distribution than would be obtained by cascade impaction.
Stability
Nebulizers
There are three broad categories of nebulizer, air jet, ultrasonic and vibrating mesh.84 Nebulizer delivery has the advantage of presenting the therapeutic agent in an aqueous medium that avoids the need for dissolution following deposition and renders it available to penetrate to the airways surface. The airway surface presents an important barrier to foreign substances transported on air to the lungs. With respect to infectious agents it is the first line of innate and adaptive immunity.85 A therapeutic approach that facilitates delivery to the barrier without presenting safety concerns would be desirable.
Systemic delivery of antibodies has been shown to result in colitis, pneumonitis and nephritis.86,87 While serious adverse events are not anticipated in response to pulmonary delivery of mAbs the most likely sources would be immune responses to any foreign protein,88 e.g. synthetic component of the antibody construct,10 or to additives such as polyethylene glycol.89
The formulation may also impact the biological disposition of the aerosol. Generally, nebulized droplets are composed of isotonic solutions/suspensions that retain their droplet size in spray transit through the airways since they are in equilibrium with body fluids and atmospheric moisture in terms of water activity and vapor pressure.90,91 Figure 4 illustrates the potential behavior of nebulized droplets based on the colligative properties of the additives.
Figure 4.
Schematic of droplet behavior following aerosol generation in transit through the airways and upon deposition based on their colligative properties and water activity.
Hypertonic solutions have been employed as mucolytics in diseases where viscous mucus is present, notably the genetic disorder cystic fibrosis.92 The hypertonic droplets take on moisture in the airways and hydrate the mucus upon deposition which reduces the local viscosity and allows improvement in mucociliary clearance.
There is reason to believe that hypotonic fluids may have an advantage in the delivery of macromolecules, at mucosal surfaces including antibodies.93 Hypotonic solutions would give up moisture with respect to unsaturated air. However, at the high relative humidity of the lungs and given the rapid transit times for droplets to enter and deposit in the lungs it is unlikely that this phenomenon would be significant. Upon droplet deposition at the surface of the mucus the water would move to regions of high endogenous salt/solute concentration creating an osmotic gradient capable of transporting dissolved solutes from the droplet into the mucus facilitating migration to the airways’ epithelium. The association of hypotonicity with cough and bronchoconstriction may explain this oversight.94 However, individuals differ in their thresholds for cough. The use of hypotonic solutions at other mucosal surfaces has not been associated with safety concerns but this approach would require further exploration for lung delivery.95 Given the likely significance of an antibody therapy the risk of using this strategy to advance the delivery of macromolecules is worthy of further investigation but would have to be considered in the context of the overall fate of monoclonal antibodies in the lungs.96,97
Dry powder inhalers
The nature of the protein instability may be sufficient to require additives such as those employed in freeze dried parenteral products.98 If this is the case then knowledge required to prepare a powder suitable for reconstitution as a nebulizer solution would also be useful for dry powder inhaler formulation. Dry powder inhalers consist of a formulation, metering system (capsule, blister strip/disk or reservoir) and device.73 The dispersion principles from DPIs involve the use of the patient’s inspiratory flow to create a pressure drop through the device and shear of particles that are prepared in respirable size ranges to achieve adequate, rarely maximal, and reproducible dose delivery of the therapeutic agent as an aerosol suitable for deposition in the lungs.
It is unlikely that the traditional lactose carrier-based formulation would be employed as this requires potent, low dose therapeutic agents such as those used to treat asthma or chronic obstructive pulmonary diseases.73 Spray dried particles in which stabilizing additives are included in each particle allows high dose delivery of relevance to antibody therapies.77
The dispersion of dry powder requires sufficient applied energy to shear particles into near primary sizes. Shear is achieved through modulating linear velocity. Pressure drop across the device is correlated with shear since restriction of flow to achieve high linear velocities results in high pressure drop according to fundamental principles of fluid dynamics.99 The shear forces have to overcome those adhesive and cohesive forces causing particle aggregation, namely van der Waals, electrostatic, capillary and mechanical interlocking mechanisms.73,99 Where the cohesive forces are between drug-drug (mAb-mAb) or additive-additive (excipient-excipient) particle interactions and the adhesive forces would exist between drug and additives. In the case of a spray dried particle where the surface may be a heterogeneous mixture of antibody and excipient(s) both cohesive and adhesive forces may occur for single particle interactions. Generally, these forces are insufficient to cause instability on aerosolization but it is possible that the composition of the particle used to stabilize the mAb will require optimization to allow dispersion under the flow conditions imparted by the device.100 Moreover, in addition to the traditional concerns associated with chemical (structural and functional) stability of the antibody a further consideration of the physical stability of the powder is required since changes in particle size or dose delivered impact on potential safety and efficacy outcomes. The residual or ingress of moisture into dry powder formulations is an example of a source of instability.75,101
Manufacturing processes allow the manipulation of the aerodynamic properties of dry powders and the MMAD is usually in the range 2–4 µm with GSD of 1.5–1.8, recognizing that each product has its own specifications.82 The maximum dose delivered from a dry powder inhaler is 112 mg of Tobramycin, in a total powder, with excipients of 192 mg delivered from 4, #3 capsules (28 mg drug, 48 mg total).102
Target product profile
The dose of antibody required for therapy is dictated by the burden or prevalence of target moiety present. An endogenous target (host receptor or transporter) may require relatively low doses whereas an exogenous target (pathogen protein) might require high doses for neutralization. The regimen becomes complex where the target protein is a component of a replicating pathogen where early intervention is most likely to result in safe and efficacious treatment.
The accuracy and precision of delivery is required from the perspective of quality standards demanded by regulatory bodies described above for the drug product performance. However, it is also essential to correlating the dose to expected therapeutic outcomes. Dosage regimens may vary according to therapeutic need but the quality performance metrics must adhere to regulatory standards. Since some mAbs may have narrow therapeutic indices performance is critical to establishing the safety of the product.
The stability of the antibody or antibody fragment has to support the use of the product through a designated lifetime under nominal conditions of use that are commercially and clinically practical. Conventional storage life is up to 2 years. Proteins and peptides frequently require refrigeration (4°C) or freezing (−80°C). For reconstitutable products an additional in-use lifetime may be given to allow for a short period of storage and dispensing before the product is discarded.
Safety considerations are related to the drug and dose and are evaluated on a product-by-product basis. However, some generalizations can be made. For the same mAb with the same excipients delivered by nebulizer versus dry powder there may be feasibility limits on dose given the patients due to tolerance to a bolus (on a single breath) of powder by inhalation independent of the drug. Whereas the steady state delivery from a nebulizer and the concurrent delivery of water may more easily be tolerated. However, it is likely that different additives/excipients will be required which present another safety consideration. In general, as stated earlier in the text larger doses can be given by nebulizer than by dry powder inhaler. However, where dry powder doses are feasible and effective the ability to enhance room temperature stability and the ease of use and handling may be preferred by the patient.
Conclusion
Inhaled antibodies present an opportunity for local therapy for a variety of diseases, most notably infectious disease. The tertiary structure of the antibody is central to its action. Disruption of this structure, most notably through aggregation, has the potential to occur throughout the product performance and evaluation. Preparation, delivery and characterization of protein aerosols require consideration of the potential for instability based on the physical chemistry of the therapeutic agent and its compatibility with components of the formulation, device and sampling systems. Formulation strategies have evolved to prevent the degradation of the mAb in the dosage form. Consequently, the options of both nebulizer and dry powder inhaler delivery of solution droplets and particles, respectively, are now available. The potential for the mAb to interact with various surfaces of different composition during delivery and sampling must be considered. Verifying the structural and functional properties of the antibody after delivery and the efficiency of recovery from sampling devices and storage containers is essential to establishing the overall quality of the product, accuracy and reproducibility of dose delivery which underpin assessment of safety and efficacy.
Acknowledgements
The authors acknowledge funding support from NIH NIAID grant 2R44AI138728-02 and NC TraCS grant number C192014.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Fellner R, Terryah S, Tarran R.. Inhaled protein/peptide-based therapies for repsiratory disease. Mol Cell Peiatr. 2016;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews A, Ee P, Ge R. Developing inhaled protein therapeutics for lung diseases. Molecular Biomedicine. 2020;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett A. Exubera inhaled insulin: a review. Int J Clinical Practice. 2004;58:394–10. [DOI] [PubMed] [Google Scholar]
- 4.Pressler T. Review of recombinant human deoxyribonuclease (rhDNas) in management of patients with cycstic fibrosis. Biologics. 2008;2:611–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjei A, Gupta P. Inhalation delivery of therapeutic peptides and proteins. New York (NY): Marcel Dekker; 1997. [Google Scholar]
- 6.Kane C, O’Neil K, Conk M, Picha K. Inhalation delivery of protein therapeutics. Inflamm Allergy Drug Targets. 2013;12:81–87. [DOI] [PubMed] [Google Scholar]
- 7.Byron P, Patton J. Drug delivery via the respiratory tract. J Aerosol Med. 1994;7:49–75. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen N, Hickey A. Targeting inhaled therapy beyond the lungs. Respiration. 2014;88:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pera T, Penn R. Bronchoprotection and bronchorelaxation in asthma: new targets and new ways to target old ones. Pharmacol Ther. 2016;164:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohlich E, Salar-Behzadi S. Oral inhalation for the delivery of proteins and peptides to the lungs. Eur J Pharm Biopharm. 2021;163:198–211. [DOI] [PubMed] [Google Scholar]
- 11.Secher T, Mayor A, Heuze-Vourc’h N. Inhalation of immunotherapeutics/prophylactics to fight respiratory tract infections. An appropriate drug at the right place. Front Immunol. 2019;10:2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szleifer I. Protein adsorption on surfaces with grafted polymers: a theoretical approach. Biophys J. 1997;72:595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazquez-Rey M, Lang D. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 2011;108:1494–508. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari P, Vanover D, Lindsay K, Bawage S, Kirschman J, Bhosle S, Lifland A, Zurla A, Santangelo P. Engineered m-RNA-expressed antibodies prevent respiratory syncitial virus. Nature Commun. 2018;9:3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart I, Richardson Z, Lai S, Hickey A. Nebulized aerosol characterization toward reinforcement of airway mucosal defense against pathogens. In: Dalby R, Byron P, Peart J, Suman J, MYoung P, Traini D, editors. Respiratory drug delivery. River Grove (IL): DHI Publishing; 2018. p. 509–12. [Google Scholar]
- 16.Hertel S, Winter G, Friess W. Protein stability in pumonary drug delivery via nebulization. Adv Drug Del Rev. 2015;93:79–94. [DOI] [PubMed] [Google Scholar]
- 17.Frokjaer S, Otzen D. Protein drug stability: a formulation challenge. Nature Rev Drug Disc. 2005;4:298–306. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Mrsny R. Formulation and delivery issues for monoclonal antibody therapeutics. Adv Drug Del Rev. 2005;58:686–706. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Singh S, Zeng D, King K, Nema S. Antibody, structure, instability, and formulation. J Pharm Sci. 2007;96:1–26. [DOI] [PubMed] [Google Scholar]
- 20.Liang W, Pan H, Vllasaliu D, Lam J. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desoubeaux G, Reichert J, Sleeman M, Reckamp K, Ryffel B, Adamczewski J, Sweeney T, Vanbever R, Diot P, Owen C, Page C, Lerondel S, Le Pape A, Heuze-Vourc'h N. Therapeutic monoclonal antibodies for respiratory diseases: current challenges and perspectives. March 31-April 2nd, 2016. Vol. 8. Tours (France): Mabs; 2016:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Teran C, Tiruthani K, McSweeney M, Ma A, Pickles R, Lai S. Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy. Adv Drug Del Rev. 2021;169:100–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://clinicaltrials.gov/ct2/show/NCT04631705.
- 24.https://clinicaltrials.gov/ct2/show/NCT03473236.
- 25.https://clinicaltrials.gov/ct2/show/NCT04341116.
- 26.https://www.clinicaltrials.gov/ct2/show/NCT04518410.
- 27.Rees V. Inhaled antibody treatment for COVID-19 shows success in preclinical trials – AR711. Eur Pharm Rev. 2020;News. [Google Scholar]
- 28.[accessed 2021 May 04]. https://clinicaltrials.gov/ct2/show/NCT00138411.
- 29.Morishita M, Peppas N. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006;11:905–10. [DOI] [PubMed] [Google Scholar]
- 30.Awwad S, Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matucci A, Vultaggio A, Danesi R. The use of intravenous versus subcutaneous monoclonal antibodies in the treatment of severe asthma: a review. Respir Res. 2018;19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu D, Hickey A. Pulmonary vaccine delivery. Expert Rev Vaccines. 2007;6:213–26. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Thompson A, Hickey A, Staats H. Dry powder vaccines for mucosal administration: critical factors in manufacture and delivery. Curr Top Microbiol Immunol. 2012;354:121–56. [DOI] [PubMed] [Google Scholar]
- 34.Hickey A. Controlled delivery of inhaled therapeutic agents. J Controlled Rel. 2014;190:182–88. [DOI] [PubMed] [Google Scholar]
- 35.Hickey A. Emerging trends in inhaled drug delivery. Adv Drug Del Rev. 2020;157:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niven R. Delivery of biotherapeutics by inhalation aerosol. Crit Rev Ther Drug Carrier Syst. 1995;12:151–231. [DOI] [PubMed] [Google Scholar]
- 37.Laptos T, Omersel J. The importance of handling high-value biologicals: physico-chemical instability and immunogenicity of monoclonal antibodies. Exp Ther Med. 2018;15:3161–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ameri M, Maa Y-F. Spray drying of biopharmaceuticals: stability and process considerations. Drying Technology. 2006;26:763–68. [Google Scholar]
- 39.Basle YL, Chennell P, Tokhadze N, Astier A, Sautou V. Physico-chemical stability of monoclonal antibodies: a review. J Pharm Sci. 2020;109:169–90. [DOI] [PubMed] [Google Scholar]
- 40.Ripple DC, Dimitrova MN. Protein particles: what we know and what we do not know. J Pharm Sci. 2012;101:3568–79. [DOI] [PubMed] [Google Scholar]
- 41.Couston R, Skoda M, Uddin S, Cvd W. Adsorption behavior of human monoclonal antibody at hydrophobic and hydrophilic surfaces. Mabs. 2013. 126–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glover I, Basa L, Moore B, Laurence J, Sreedhara A. Metal ion interactions with mAbs: part 1. Mabs. 2015;7:901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chennamsetty N, Voynov V, Kayser V, Helk B, Trout B. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci USA. 2009;106:11937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narang A, Krause M, Pizzarro S, Yee J. Biologic drug substance and drug product manufacture. In: Hickey A, Rocha S, editors. Pharmaceutical Inhalation Aerosol Technology. Boca Raton (FL): CRC Press; 2019. p. 206–32. [Google Scholar]
- 45.Arosio P, Jaquet B, Wu H, Morbidelli M. On the role of salt type and concentration on the stability behavior of monoclonal antibody solution. Biophys Chem. 2012;168-169:19–27. [DOI] [PubMed] [Google Scholar]
- 46.Karow A, Bahrenburg S, Garidel P. Buffer capacity of biologics–from buffer salts to buffering by antibodies. Biotechnol Prog. 2013;29:480–92. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Wu G, Zhang X, Tian Z, Zhang N, Hu T, Dai W, Qian, F. Stabilizing two IgG1 monoclonal antibodies by surfactants: balance between aggregation prevention and structure perturbation. Eur J Pharm Biopharm. 2017;114:263–77. [DOI] [PubMed] [Google Scholar]
- 48.Nicoud L, Cohrs N, Arosio P, Norrant E, Morbidelli M. Effect of polyol sugars on the stabilization of monoclonal antibodies. Biophys Chem. 2015;197:40–46. [DOI] [PubMed] [Google Scholar]
- 49.Arakawa T, Timasheff S. Mechanism of polyethylene glycol interaction with proteins. Biochemistry. 1985;24:6756–62. [DOI] [PubMed] [Google Scholar]
- 50.Faghihi H, Najafabadi A, Vatanara A. Optimization and characterization of spray-dried IgG formulations: a design of experiment approach. DARU J Pharm Sci. 2017;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emami F, Vatanara A, Park E, Na D. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics. 2018;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickey A, Ganderton D. Statistical experimental design. Pharmaceutical Process Engineering. New York (NY): Informa Healthcare; 2010. 197–201. [Google Scholar]
- 53.Young A. Powder X-ray Diffraction and its application to biotherapeutic formulation development. American Pharm Rev. 2012;15:38371. [Google Scholar]
- 54.Greenfield N. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakrabarti A. Separation of Monoclonal Antibodies by Analytical Size Exclusion Chromatography. In: Böldicke T, editors. IntechOpen. 2018. 73321. [Google Scholar]
- 56.deJong L, Uges D, Frank J, Bischoff R. Receptor–ligand binding assays: technologies and applications. J Chromatography B. 2005;829:1–25. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Pan H, Chen X. Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spec Rev. 2008;28:147–76. [DOI] [PubMed] [Google Scholar]
- 58.Hnasko T, Hnasko R. The western blot. In: Hnasko R, editor. Elisa methods and protocols. New York (NY): Springer; 2015. p. 87–96. [Google Scholar]
- 59.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokunaga Y, Takeuchi K. Role of NMR in high ordered structure characterization of monoclonal antibodies. Int J Mol Sci. 2021;22:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bramham J, Podmore A, Davies S, Golovanov A. Comprehesive assessment of protein and excipient stability in biopharmaceutical formulations using 1H NMR spectroscopy. ACS Pharmacol Transl Sci. 2021;4:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakuma C, Yomioka Y, Li C, Nakagawa M, Kurosawa Y, Arakawa T, Akuta T. Analysis of protein denaturation, aggregation and post-transcriptional modification by agarose native gel electrophoresis. Int J Biol Macromol. 2021;172:589–96. [DOI] [PubMed] [Google Scholar]
- 63.Shaw M, Riederer B. Sample preparation for two-dimensional gel electrophoresis. Proteomics. 2003;3:1408–17. [DOI] [PubMed] [Google Scholar]
- 64.USP-NF . General Chapter <5> Inhalation and nasal drug products - general information and product quality tests. Rockville (MD): United States Pharmacopeia; 2020. [Google Scholar]
- 65.Fda(cder U. Metered dose inhaler (MDI) and dry powder inhaler (DPI) products - Quality Considerations. Silver Spring (MD): US Department of Health and Human Services; 2018. [Google Scholar]
- 66.Cundell T. Mould contamination in pharmaceutical drug products and medical devices. Eur Pharm Rev. 2013;18:67–75. [Google Scholar]
- 67.Blanchard J, Coleman J, Hayling CDA, Ghaderi R, Haeberlin B, Hart J, Jensen S, Malcomson R, Mittelman S, Nagao LM, Sekulic S, Snodgrass-Pilla C, Sundahl M, Thompson G, Wolff R. Foreign Particles Testing in Orally Inhaled and Nasal Drug Products. Pharm Res. 2004;21:2137–3147. [DOI] [PubMed] [Google Scholar]
- 68.Bodier-Montagutelli E, Respaud R, Perret G, Baptista L, Duquenne P, Heuzé-Vourc’h N,Vecellio L. Protein stability during nebulization: mind the collection step! Eur J Pharm Biopharm. 2020;152:23–34. [DOI] [PubMed] [Google Scholar]
- 69.Bodier-Montagutelli E, Mayor A, Vecellio L, Respaud R, Heuzé-Vourc’h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expert Opinion Drug Deliv. 2018;15:729–36. [DOI] [PubMed] [Google Scholar]
- 70.Respaud R, Vecellio L, Diot P, Heuzé-Vourc’h N. Nabulization as a delivery method for Mabs in respiratory diseases. Expert Opinion Drug Deliv. 2015;12:1027–39. [DOI] [PubMed] [Google Scholar]
- 71.Cipolla D, Gonda I. Method for collection of nebulized proteins. In: Cleland J, Langer R, editors. Formulation and Delivery of Proteins and Peptides. Washington (DC): Ameridcan Chemical Society; 1994. p. 343–52. [Google Scholar]
- 72.USP-NF . General Chapter <601> Inhalation and nasal drug products: aerosols, sprays, and powders - performance quality tests. Rockville (MD): United States Pharmacopeia; 2020. [Google Scholar]
- 73.Telko M, Hickey A. Dry powder inhaler formulation. Resp Care. 2005;50:1209–27. [PubMed] [Google Scholar]
- 74.Edwards D, Hanes J, Caponetti G, Hrkach J, Ben-Jebrai A, Eskew M, Mintzes J, Deaver D, Lotan N, Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–72. [DOI] [PubMed] [Google Scholar]
- 75.Maa Y-F, Nguyen P-A, Andya J, Dasovich N, Sweeney T, Shire S, Hsu CC. Effect of spray drying and subsequent processing conditions on residual moisture and physical/biochemical stability of protein inhalation powders. Pharm Res. 1998;15:768–75. [DOI] [PubMed] [Google Scholar]
- 76.Maa Y-F, Nguyen P-A, Sweeney T, Shire S, Hsu C. Protein inhalation powders: spray drying vs spray-freeze drying. Pharm Res. 1999;16:249–54. [DOI] [PubMed] [Google Scholar]
- 77.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25:999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.USP-NF . General Chapter <1601> Inhalation and nasal products - general information and product quality tests. Rockville (MD): United States Pharmacopeia; 2020. [Google Scholar]
- 79.Hickey A. Quality and performance tests. In: Hickey A, editor. Inhaled pharmaceutical product development perspectives. New York (NY): Elsevier/RTI; 2018. p. 33–51. [Google Scholar]
- 80.Apiou-Sbirlea G, Newman S, Fleming J, Siekmeier R, Ehrmann S, Scheuch G, Hochhaus G, Hickey A. Bioequivalence of inhaled drugs: fundamentals, challenges and perspectives. Ther Deliv. 2013;4:343–67. [DOI] [PubMed] [Google Scholar]
- 81.Hastedt J, Backman P, Clark A, Doub W, Hickey A, Hochhaus G, Kuehl PJ, Lehr C-M, Mauser P, McConville J, Niven R, Sakagami M, Weers JG. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP Workshop March 16-17th, 2015 in Baltimore, MD. AAPS Open. 2016;2:1. [Google Scholar]
- 82.Hickey A. Back to the future: inhaled drug products. J Pharm Sci. 2013;102:1165–72. [DOI] [PubMed] [Google Scholar]
- 83.Hickey A, Montgomery A. Aerosolized pentamidine for treatment and prophylaxis of Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. In: Hickey A, editor. Pharmaceutical Inhalation Aerosol Technology. New York (NY): Marcel Dekker; 2004. p. 459–72. [Google Scholar]
- 84.Pritchard J, Hollen D, Hatley R. Nebulizers. In: Hickey A, Rocha S, editors. Pharmaceutical Inhalation Aerosol Technology. Boca Raton (FL): CRC Press; 2019. p. 473–92. [Google Scholar]
- 85.Schaefer A, Lai S. Innate and adaptive barrier properties of airway mucus. In: Hickey A, Mansour H, editors. Inhalation aerosols, physical and biological basis for therapy. New York (NY): CRC Press; 2019. p. 257–74. [Google Scholar]
- 86.Blasco M, Ramos A, Malo C, Garcia-merino A. Acute pneumonitis and pericarditis related to alemtuzumab therapy in relapsing remitting multiple sclerosis. J Neurol. 2017;264:168–69. [DOI] [PubMed] [Google Scholar]
- 87.Hansel T, Kropshofer H, Singer T, Mitchell J, George A. The safety and side effects of monoclonal antibodies. Nature Rev Drug Discovery. 2010;9:325–38. [DOI] [PubMed] [Google Scholar]
- 88.Wolff R. Safety of inhaled protein for therapeutic use. J Aerosol Med. 1998;11:197–219. [DOI] [PubMed] [Google Scholar]
- 89.Guichard M-J, Leal T, Vanbever R. PEGylation an approach for improving the pulmonary delivery of biopharmaceuticals. Current Opinion in Colloid and Interface Sci. 2017;31:43–50. [Google Scholar]
- 90.Hickey A, Gonda I, Fildes F. Effect of hydrophobic coating on the behavior of a hygroscopic aerosol powder in an environment of controlled temperature and relative humidity. J Pharm Sci. 1990;79:1009–14. [DOI] [PubMed] [Google Scholar]
- 91.Hickey A, Martonen T. Behavior of hygroscopic pharmaceutical aerosols and influence of hydrophobic additives. Pharm Res. 1993;10:1–7. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Contreras L, Hickey A. Aerosol treatment of cystic fibrosis. Crit Rev Ther Drug Carrier Syst. 2003;20:317–56. [DOI] [PubMed] [Google Scholar]
- 93.Ensign L, Hoen T, Maisel K, Cone R, Hanes J. Enhanced vaginal drug delivery though use of hypotonic formulations that induse fluid uptake. Biomaterials. 2013;34:6922–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahakijpijarn S, Smyth H, Miller D, Weers J. Post-inhalation cough with therapeutic aerosols: formulation considerations. Adv Drug Del Rev. 2020;166:127–41. [DOI] [PubMed] [Google Scholar]
- 95.Ayehunie S, Wang -Y-Y, Landry T, Bogojevic S, Cone R. Hyperosmolal vaginal lubricant markedly reduce epithelial barrier properties in a three dimensional vaginal epithelium model. Toxicology Reports. 2018;5:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guillaminault L, Azzopardi N, Arnoult C, Sobilo J, Hervé V, Montharu J, Guillon A, Andres C, Herault O, Le Pape A, Diot P, Lemarie E, Paintaud G, Gouilleux-Gruart V, Heuze-Vourc'h N. Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J Controlled Rel. 2014;196:344–54. [DOI] [PubMed] [Google Scholar]
- 97.Guillon A, Pardessus J, Lhommet P, Parent C, Respaud R, Marchand D, Montharu J, De Monte M, Janiak P, Bioxel C, Audat H, Huille S, Guillot E, Heuze-Vourc'h N. Exploring the fate of inhaled monoclonal antibody in the lung parenchyma by microdialysis. Mabs. 2019;11:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang X, Pikal M. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21:191–200. [DOI] [PubMed] [Google Scholar]
- 99.Hickey A. Fundamentals of dry powder inhaler technology. In: Merkus H, Meesters G, Oostra W, editors. Particles and nanoparticles in pharmaceutical products. New York (NY): Springer; 2020. p. 213–32. [Google Scholar]
- 100.Hickey A. Complexity in pharmaceutical powders for inhalation: a perspective. KONA. 2018;35:3–13. [Google Scholar]
- 101.Shetty N, Cipolla D, Park H, Zhou Q. Physical stability of dry powder inhaler formulations. Expert Opinion Drug Deliv. 2020;17:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geller D, Heuerding S, Weers J. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphereTM technology. J Aerosol Med Pulm Drug Deliv. 2011;24:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


