Abstract
The hypothalamic paraventricular nucleus (PVN) plays a key role in hypertension, however the signaling pathways that contribute to the adaptability of the PVN during hypertension are uncertain. We present evidence that signaling at the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) GluA1 receptor subunit contributes to increased blood pressure in a model of neurogenic hypertension induced by 14-day slow-pressor angiotensin II (AngII) infusion in male mice. It was found that AngII hypertension was associated with an increase in plasma membrane affiliation of GluA1, but decreased GluA2, in dendritic profiles of PVN neurons. The increased plasma membrane GluA1 was paralleled by the results of whole-cell current clamping experiments, which showed heightened AMPA currents in PVN neurons from AngII-infused male mice. The inhibition of heightened AMPA currents was blocked by 1-Naphthyl acetyl spermine trihydrochloride, pointing to the involvement of GluA2-lacking GluA1 receptors in the elevated AMPA signaling in PVN neurons. A further functional role for GluA1 signaling in the PVN was demonstrated by the attenuated hypertensive response following silencing of GluA1 in the PVN of AngII-infused mice. In female mice, AngII-infusion did not impact blood pressure. In addition, AngII was not associated with alterations in transcription or plasma membrane localization of GluA1 in females. Posttranslational modifications that increase the plasma membrane localization of AMPA GluA1 and heighten the rapid signaling actions of glutamate in PVN neurons may serve as a molecular substrate underlying sex differences in hypertension.
Keywords: AMPA receptor, Blood pressure, GluA2, Sex difference, Naspm, Tumor necrosis factor alpha type 1 receptor
INTRODUCTION
Hypertension is a major risk factor for both stroke and dementia (Ungvari et al., 2021), and a leading global public health concern (Zhou et al., 2021), however the mechanisms subserving hypertension are still under active investigation (Brouwers et al., 2021). The elevated sympathetic nervous system activity prevalent in the leading form of hypertension, primary or essential hypertension, suggests a significant neurogenic component in the emergence of hypertension (Esler, 2000, Grassi and Ram, 2016). Models of neurogenic hypertension include continuous systemic administration of a subpressor dose of angiotensin II (AngII) which produces a gradual increase in blood pressure (“slow-pressor”) as previously described (Dickinson and Lawrence, 1963). A peptide that normally lacks blood brain barrier permeability, AngII is capable of influencing central cardiovascular regulatory pathways by acting on neural circuits spanning circumventricular organs and the hypothalamus (Young et al., 2012). This circuitry includes an excitatory pathway between the subfornical organ and sympathoexcitatory neurons in the paraventricular nucleus (PVN) of the hypothalamus (Bains and Ferguson, 1995, Llewellyn et al., 2012). However, the mechanisms by which glutamate signaling in this pathway contribute to hypertension are not well understood.
There is evidence that AngII-mediated hypertension is correlated with alterations in glutamate signaling within hypothalamic circuits (Li and Pan, 2017). Indeed, in different models of hypertension, increases in glutamatergic input to PVN pre-sympathetic neurons result in elevations of sympathetic outflow (Li and Pan, 2017). Significantly, AMPA-type glutamate receptors are found throughout the PVN (Eyigor et al., 2001). Additionally, it has also been shown that AMPA receptor signaling at post-synaptic sites in PVN pre-sympathetic neurons plays an important role in hypertension (Li and Pan, 2017).
The AMPA receptors are ligand-gated glutamate-activated ion channels that are responsible for most excitatory neurotransmision that occurs in the brain (Sheng and Kim, 2002). The AMPA receptors are multiunit proteins consisting of various combinations of GluA14 subunits that express distinct biophysical properties. Receptors expressing GluA1 and GluA2 are among the most abundant and mediate constitutive excitatory neurotransmission (Buonarati et al., 2019). AMPA receptors expressing GluA1 and lacking GluA2 are calcium-permeable, significantly involved in various forms of neural plasticity (Diering and Huganir, 2018), and are implicated in blood pressure regulation (Li et al., 2012). Interestingly, in diverse brain areas (Beattie et al., 2002, Ogoshi et al., 2005, Choi et al., 2010), GluA1 receptor signaling has been shown to be modulated by tumor necrosis factor alpha (TNFα), an inflammatory cytokine that has been shown to play an important role in hypertension by acting withing the PVN (Sriramula et al., 2013). However, it is unclear if GluA1 is present in PVN neurons also expressing the TNFα type 1 receptor (TNFR1), the predominant TNFα receptor in the PVN that is commonly expressed in PVN neurons projecting to the spinal cord (Woods et al., 2021).
Significantly, GluA1 has been reported to be expressed prominently in preautonomic regions of the PVN (Herman et al., 2000) and has also been shown to contribute to hyperactivity of PVN-spinal cord projection neurons in the spontaneously hypertensive rat (SHR) model (Li et al., 2012). The role of PVN GluA1 in AngII hypertension is less clear. Alterations in GluA1 signaling associated with hypertension may be mediated by changes in gene expression or posttranslational processes that regulate the subcellular localization of GluA1 as seen in various models of neural plasticity (Hanley, 2014) and in some forms of hypertension (Aicher et al., 2003, Hermes et al., 2008). Moreover, since gluamate receptor plasticity has been shown to differ in male and female mice (Ovalles et al., 2019, Milner et al., 2021), these events may be expected to be sex dependent. However, the role of PVN AMPA receptor subunits in hypertension induced by slow-pressor AngII in males and females is unclear. To investigate the role of PVN AMPA receptors in AngII hypertension, GluA1 gene expression was measured by in situ hybridization and plasma membrane localization in dendritic structures was measured by high-resolution immunohistochemical electron microscopy (EM), a method with the necessary resolution to detect subcellular protein localization in small structures like dendritic processes, a major site of functional AMPA receptors. In addition, the functional role of PVN GluA1 during AngII hypertension was assessed by whole-cell patch clamping and spatial-temporal gene silencing approaches.
EXPERIMENTAL PROCEDURES
Subjects.
The experimental subjects were 48 adult (postnatal > 90 days) male and cycling female C57BL/6 mice bred and maintained in a colony at Weill Cornell Medicine. Mice were housed in groups of at least two animals per cage. They were maintained on a 12-hr light/dark cycle (lights out 1800 hours) with unlimited access to water and rodent chow in their home cages. All experiments were approved by the Institutional Animal Care and Use Committees at Weill Cornell Medicine (2017–0016) in accordance with the 2011 Eighth Edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
AngII infusion and blood pressure recording.
Male and female mice were infused with AngII as described (Capone et al., 2012, Coleman et al., 2013). Briefly, following isoflurane anesthesia, mice were surgically implanted with subcutaneous osmotic mini-pumps (ALZET®, Cupertino, CA) filled with either vehicle [Veh: 0.1% bovine serum albumin (BSA)/0.9% saline] or AngII dissolved in Veh (600ng/kg−1/min−1; cat. # A9525, Sigma-Aldrich, St. Louis MO). Both Veh and AngII were infused for a 14-day period. Systolic blood pressure (SBP) was measured using a Hatteras MC-4000 tail-cuff blood pressure system (Cary, NC). During each blood pressure recording session, a total of 10–20 blood pressure measurements were made over a 10–20-minute period. Individual blood pressure values for each mouse were averaged over each session. These were then combined across mice to generate group mean SBP values in both treatment groups. Blood pressure recordings were made prior to and following the start of Veh and AngII infusion. To control for handling effects, in the in situ hybridization and immunohistochemical studies, mice received a final SBP recording session on day 13 after pump implantation and were perfused on day 14 (Milner et al., 2021).
Estrous cycle determination.
To assess estrous cycle stage, vaginal smear cytology (Turner and Bagnara, 1971) was performed prior to brain fixation. Female mice used in this study were in estrus or proestrus immediately prior to euthanasia.
In situ hybridization.
Levels of GluA1 and GluA2 mRNA in the PVN were assessed by in situ hybridization utilizing the RNAscope® 2.5 HD Brown Chromogenic Reagent Kit (ACD, Newark, CA) as previously described (Wang et al., 2012). Mice were first anesthetized with sodium pentobarbital (150 mg/kg, i.p.). Next, their brains were rapidly fixed by aortic arch perfusion sequentially with 5 ml of 1000 units/ml of heparin in 0.9% saline followed by 30 ml of 4% paraformaldehyde (PFA; Electron Microscopy Sciences (EMS), Fort Washington, PA, cat # 19208) in 0.1 M phosphate buffer (PB, pH 7.4). Solutions were delivered at a flow rate of 20 ml/minute. Following dissection, each brain was post-fixed in 4% PFA in PB/15% sucrose overnight and cryoprotected in a solution of 30% sucrose in PB for one or two days. After freezing, brain sections were cut on a cryostat (30 μm), collected in cryoprotectant solution (30% sucrose and 30% ethylene glycol in PB), and then stored at −20°C.
Prior to processing, brain sections from the caudal level of the PVN [~1.0 mm caudal to bregma (Paxinos and Franklin, 2000)], a region that contains a high density of sympathoexcitatory neurons (Sawchenko and Swanson, 1983), from each treatment group were mounted in 1x sterile phosphate buffered saline (PBS) on Superfrost® Plus slides (Cat. # 48311–703 Fischer Scientific) and allowed to air dry overnight. Slides were run in tandem to ensure that tissue form each animal was processed under identical labeling conditions so as to reduce variability in labeling between sections due to various experimental conditions. The slides containing the sections were baked for 1-hour at 60°C just prior to beginning in situ hybridization processing. After peroxidase quenching and incubation in target retrieval solution and protease, sections were then hybridized with probes (mouse-Gria1 ACD Cat # 426241; mouse-Gria2 ACD Cat # 416091) for 2-hours at 40°C. Controls were run during each experiment: 1). the positive housekeeping control probe Ppib (ACD Cat # 313911) and 2). the bacterial negative control probe DapB (ACD Cat # 310043). The hybridization signal was detected using the chromogen 3, 3’-diaminobenzidine (DAB) followed by counterstaining with 25% Gill’s Hematoxylin No. 1 (Cat. # GHS132; Sigma-Aldrich) and dehydrating and coverslipping from xylene with DPX (Cat # 06522, Sigma-Aldrich).
Images containing the PVN were collected using an Eclipse Nikon 80i microscope (Melville, NY) at 40x interfaced to a Micropublisher 5.0 digital camera (Q-imaging, BC, Canada) and IP Lab software (Scanalytics IPLab, RRID: SCR_002775). These images were analyzed using Halo® Software (Indica Labs, Albuquerque, New Mexico) and following manufacture’s guidelines (indicalab.com/wpcontent/uploads/2018/04/MK_51_103_RNAScope_data_analysis_guide_RevB.pdf). Using this software both hemispheres of the region of interest (ROI), i.e, the PVN, were traced manually, and standard staining color for the probe was entered into the program. Cell morphology parameters were manually set, and the program automatically analyzed each ROI. The number of brown particles overlying each cell, the number of probe-containing cells, and the pixel density of the particles were counted by the software system. Values for each hemisphere were averaged for each animal and these in turn were averaged to provide group means. An experimenter blind to the treatment conditions collected and analyzed the images.
Tissue preparation and dual labeling procedures for immunohistochemical EM.
After anesthesia with sodium pentobarbital (150 mg/kg), mouse brains were rapidly fixed via aortic arch perfusion at a flow rate of 20 ml/minute sequentially with: (a) 15 ml of 1000 units/ml of heparin in 0.9% saline, and (b) 40 ml of a mixture of 3.75% acrolein/2% PFA in 0.1 M PB. After dissection from the cranium, brains were post-fixed for 60-minutes in fixative. Then, the region of the brain containing the PVN was coronally sectioned (40 μm) using a vibratome. For dual labeling, GluA1 was labeled using an immunogold-silver (IGS) marker and TNFR1 was labeled using an immunoperoxidase marker (Milner et al., 2011). Forebrain sections containing the caudal PVN were punch coded and pooled into single containers. Excess aldehydes were removed by incubating brain sections in 1.0% sodium borohydride in PB, followed by a 30-minute incubation in 0.5% BSA in tris-buffered saline (TBS). Alternate brain sections were then incubated for 48-hours in a mixture diluted in 0.1% BSA of a goat anti-TNFR1 antiserum (1:100) along with one of the following antisera diluted at 1:100: 1). Rabbit anti-GluA1 antiserum; 2). Rabbit anti-GluA2 antiserum. Following primary antiserum incubation, sections were washed in TBS. Next, sections were incubated in anti-goat IgG conjugated to biotin, then incubated for 30-minutes in avidin-biotin-peroxidase complex (ABC, 1:100, Vectastain Elite Kit, Vector Laboratories, Burlingame, CA) in TBS. The bound peroxidase was visualized by reaction in DAB (Sigma-Aldrich, St. Louis, MO) and 0.003% hydrogen peroxide in TBS for 5–6 minutes. In preparation for IGS labeling, brain tissue was first rinsed in 0.01 M phosphate-buffered saline (PBS; pH 7.4). Then, to reduce non-specific binding of gold particles, brain sections were incubated for 10-minutes in a blocking solution consisting of 0.8% BSA and 0.1% gelatin in PBS. After this blocking step, sections were incubated for 2-hours in anti-rabbit 1 nm gold particle-conjugated IgG [1:50, EMS] diluted in the blocking solution. Following this, tissue was rinsed in the blocking solution followed by washing in PBS. Brain sections were then incubated in 2% glutaraldehyde in PBS for 10-minutes followed by rinsing in PBS. Next, the nano-gold particles were enlarged with a silver enhancement solution for 9–10 minutes (SEKL15 Silver enhancement kit, Prod # 15718 Ted Pella Inc., Redding, CA).
Electron Microscopy.
For electron microscopic processing, brain sections were post-fixed for 1-hour in a solution of 2% osmium tetroxide (EMS) in PB, followed by embedment in EM BED 812 between 2 sheets of Aclar plastic (Milner et al., 2011). Trapezoidal sections of the caudal PVN were ultrathin sectioned (60–80 nm) with a diamond knife using an ultramicrotome (Ultratome, NOVA, LKB, Bromma), collected on 400-mesh thin-bar copper grids (EMS). Ultrathin sections of the PVN were analyzed using a transmission electron microscope (Tecnai 12 BioTwin, FEI, Hillsboro, OR) interfaced to a digital camera (Advantage HR/HR-B CCD Camera System, Advanced Microscopy Techniques, Danvers, MA) that was used to collect images from the sampled tissue.
Ultrastructural analysis.
To ensure that tissue was sampled from regions of even reagent penetrance, electron micrographs were captured from the embedding media-tissue transition zone (Glass et al., 2015). The classification of profiles was based on well-established guidelines for the ultrastructural identification of neuronal and glial elements (Peters et al., 1991). Somata were distinguished by the presence of nuclei, Golgi bodies, as well as rough endoplasmic reticula. Profiles were defined as dendritic if they contained regular microtubule arrays, endomembranous organelles, and/or postsynaptic densities. Somata were distinguished by the presence of nuclei, Golgi bodies, as well as rough endoplasmic reticula. Structures that were at least 0.2 μm in diameter and that also contained numerous small synaptic vesicles were characterized as axon terminals, whereas profiles less than 0.2 μm and lacking small synaptic vesicles were designated as unmyelinated axons. Irregularly shaped profiles devoid of cytoplasmic organelles or containing arrays of filaments were considered to be glia. Profiles were determined to be immunogold-silver labeled if they contained at least one particle per small profile, or two particles for larger profiles, provided that structures not expected to be labeled for the primary antisera, such as myelin, were devoid of gold-silver deposits (Hara and Pickel, 2008).
An established procedure for the apportionment of particulate gold-silver labeling within subcellular compartments was used to estimate the distribution of GluA1 in cytoplasmic and plasma membrane compartments of dendritic profiles (Glass et al., 2015, Marques-Lopes et al., 2017). The partitioning ratio of GluA1 IGS particles were assessed to determine the extent to which IGS are partitioned between the plasma membrane or cytoplasm relative to total IGS as in (Van Kempen et al., 2015). A total of 30 fields per animal were imaged for subcellular analysis of IGS labeling in Veh and AngII-infused mice for a total of 15,606 μm2 of tissue equally sampled from each group.
Microcomputer Imaging Device software (MCID Imaging Research Inc., Ontario; RRID:SCR_014278) was used to determine surface area, cross-sectional area, average diameter, and major and minor axis lengths for all dendrites. Dendrites cut in cross-section expressing IGS labeling were sampled. Average diameter measures were used to classify dendrites as large (diameter ≥ 1 μm) or small (diameter <1 μm) that correspond to dendrites that are relatively closer or farther from the cell body (i.e. proximal versus distal dendrites), respectively (Harris and Spacek, 2016). The location IGS particles (i.e., on plasma membrane or within the cytoplasm) for each dendritic profile was counted by determining the number of IGS particles touching the dendrite plasma membrane and the number of IGS particles in cytoplasm (Ovalles et al., 2019). The partitioning ratio is calculated as the number of particles on the plasma membrane divided by the total number of particles per profile (Ovalles et al., 2019). These were calculated for each profile and then averaged for each animal in each treatment.
Primary Antisera. GluA1:
A rabbit polyclonal antiserum was used to label GluA1 (AB1504; Millipore, Burlington, MA). On Western blots this antibody recognizes one major band at about 106 kD on 10μg of mouse brain lysate (manufacturer’s data sheet, www.emdmillipore.com). Moreover, Western blotting, immunoprecipitation, and electron microscopy of anti-GluA1 in rat hippocampus demonstrate the expected locations in post-synaptic profiles (Hussain et al., 2015). GluA2: A rabbit polyclonal antiserum was used to label GluA2 (AB1768-I; Millipore). In mouse brain lysates, Western blotting yielded a band at ~108 kDa [manufacturer’s data sheet]. TNFR1: A goat polyclonal antiserum (SC1067, discontinued; Santa Cruz Biotechnology, Dallas, TX) was used to label TNFR1. Western blotting resulted in a band at ~55 kDa expected for TNFR1 [manufacturer’s data]. Labeling of this antisera by light and electron microscopy resulted in labeling of neuronal cell bodies and postsynaptic structures in the hypothalamus (Glass et al., 2017). GFP: A chicken polyclonal antiserum (GFP-1020; RRID: AB10000240; Aves, Davis, CA) was used to label EGFP (Marques-Lopes et al., 2015). On Western blots this antibody recognizes one major band at ∼27 kD and immunolabels cells in brain sections from transgenic mice expressing EGFP (manufacturer’s data sheet, www.aveslab.com). Also, immunolabeling for this antibody is absent in brain sections from mice not expressing EGFP (Volkmann et al., 2010, Milner et al., 2011).
Retrograde labeling of spinally projecting PVN neurons for electrophysiological recording.
As previously described (Wang et al., 2013) mice were anesthetized (2–4% isoflurane), and their spinal cords were exposed at the T2-T4 level through dorsal laminectomy. Under a surgical microscope, the tip of a glass pipette filled with fluorogold (FG 4%; Fluorochrome, Denver CO) which was pressure-ejected (100 nl) bilaterally into the intermediolateral nucleus (IML) region of the spinal cord and the incision was sutured after the injection.
Whole-cell voltage-clamp.
Whole-cell voltage-clamp recording of visually identified FG retrogradely-labeled PVN projection neurons was performed as previously described (Wang et al., 2013). Mice were anesthetized with 4% isoflurane and their brains were removed and immersed in sucrose-artificial cerebrospinal fluid (s-aCSF). Coronal brain slices (200 μm in thickness) containing the PVN were cut using a Leica VT1000s vibratome with s-aCSF composed of (in mmol/L): 125 sucrose, 26 NaHCO3, 5 KCl, 1 NaH2PO4, 5 MgSO4, 1 CaCl2, 10 glucose, 4.5 lactic acid, at pH 7.4. The brain slices were stored in a custom-designed chamber containing lactic acid-artificial cerebrospinal fluid (l-aCSF) with 95% O2 and 5% CO2 at 32°C for 1-hour. The l-aCSF was composed of (in mmol/L): 124 NaCl, 26 NaHCO3, 5 KCl, 1 NaH2PO4, 2 MgSO4, 2 CaCl2, 10 glucose, 4.5 lactic acid, 95% O2 and 5% CO2, at pH 7.4. The PVN was identified using the lateral ventricle, fornix, and optic chiasm as landmarks. The coronal slices containing the PVN were then transferred to the recording chamber and continuously perfused with the l-aCSF. The FG-labeled spinal PVN neurons in brain slices were briefly identified under an E600 epifluorescence microscope (Nikon, Tokyo) using a lucifer yellow filter (Fig. 5A). The FG-labeled neurons located in the medial one-third of the PVN area between the third ventricle and the fornix were patched for whole-cell voltage-clamp (Li et al., 2002) using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). To block the voltage-gated Na+ channels and non-AMPA receptor-mediated cation channels, 1 μmol/L tetrodotoxin (TTX) and 5 μmol/L (2R)-amino-5-phosphonovaleric acid (AP5) were added to the l-aCSF buffer. The holding potential was at −60 mV. Control and AMPA (30 μmol/L)-containing buffers were separately perfused toward the patched neuron for 30-seconds using a double-barrel microtubing located on the other side of the patch recording electrode (Coleman et al., 2010, Suh et al., 2010). An ALA-VM4 valve-controlled gravity valve system was used to control the local perfusion. The patch recording pipette tip resistances were 5–8 MΩ as filled with an intracellular solution (in mmol/L): 130 K-gluconate; 10 NaCl, 1.6 MgCl2, 1 EGTA, 10 HEPES, 2 Mg-ATP, adjusted to pH 7.3. After formation of a GigaΩ seal, the electrode capacitance was nullified. After breaking the plasma membrane, the cell membrane capacitance (Cm) was read directly from Membrane Test of Window pClamp 8.2 (Axon Instruments, Union City, CA). Cell membrane capacitance and series resistance were monitored throughout the recording, with series resistance generally compensated >80%. Signals were low-pass filtered at 2 kHz and acquired at a sampling rate of 5–10 kHz. The peak amplitude, rather than the area under the current curve or its decay kinetics, of AMPA-induced currents were measured.
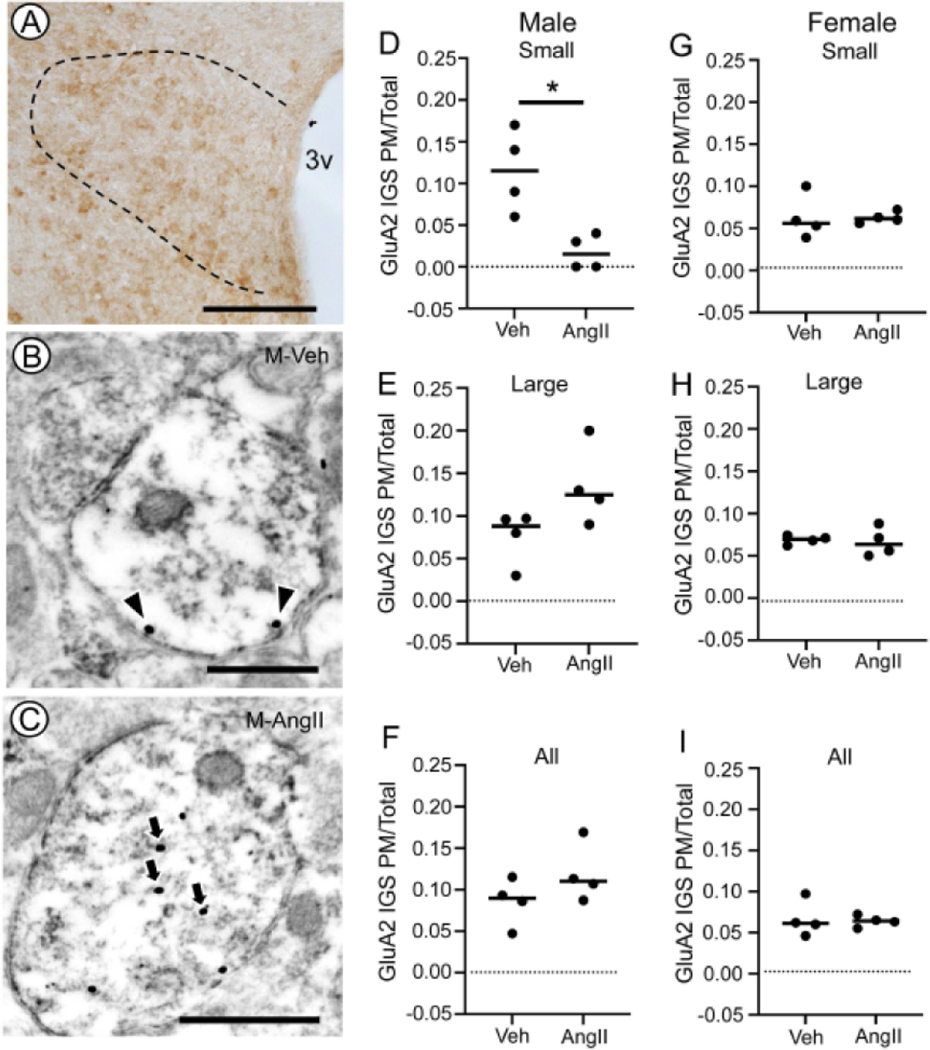
Fig. 5. Plasma membrane GluA2 IGS in TNFR1-labeled dendritic profiles of PVN neurons after AngII infusion in male and female mice.
Light micrograph illustrating GluA2 labeling in the dorsal hypothalamus of a vehicle-infused male mouse (A). The PVN is indicated by the bounded region. Electron micrographs illustrating GluA2 immunogold-silver (IGS) labeling in the cytoplasm (arrows) and on the plasma membrane (arrowheads) of immunoperoxidase TNFR1 labeled dendritic profiles of PVN neurons from male mice infused with vehicle (M-Veh, B) or AngII (M-AngII; C). Male mice infused with AngII showed a lower partitioning ratio for plasma membrane GluA2 IGS in small-size dendritic profiles compared to Veh-infused mice (D). There were no differences in the plasma membrane partitioning ratio of GluA2 IGS in large (E) profiles or profiles collapses across all sizes (F). In female mice, there were no differences in the plasma membrane partitioning ratio of GluA2 in small (G) or large (H) dendritic profiles. There also was no difference in plasma membrane GluA2 in dendrites collapsed across size (I). 3v: Third ventricle; M-AngII: Male AngII treatment; M-Veh: Male Veh treatment; PM/Total: Plasma membrane labeling/Total labeling. D: * p< 0.05 GluA2 IGS PM/Total Veh versus AngII in small dendrites in males. Lines in D-I are group medians overlain with data points representing means for each animal. Scale bars: A: 500 μm; B and C: 500 nm
Spatial-temporal gene silencing.
Knockdown of GluA1 was achieved using a neurotropic serotype 2 recombinant adeno-associated virus (AAV; ~4.7 kb). The vector (AAV-GluA1) expressed a GluA1 short hairpin RNA (targeting sequence: 5’-GGAAGCTCTCATTAGCATTAT-3’) driven by a U6 promoter, and an enhanced green fluorescent protein (EGFP) reporter driven by a CMV promoter (cat #: shAAV-260749; Vector Biolabs, Philadelphia PA). The titer of the rAAV-GluA1 vector was 3.7 × 1012 GC/ml. The shRNA is validated for ~90% knockdown of mRNA in NIH/3T3 cells. A vector expressing EGFP was used as a control (Control vector. cat #: 7004, Vector Biolabs). Viral vectors were bilaterally microinjected into the PVN using stereotaxic surgical procedures as previously described (Glass et al., 2015). Under 2–4% isoflurane anesthesia, vectors (~100 nl/hemisphere,) were injected into the PVN approximately 1.0 mm posterior and 0.2 mm lateral to bregma, at a depth of 4.8 mm (Paxinos and Franklin, 2000). Microinjections were made by interfacing a picospritzer (Picospritzer II, General Valve Corp., Fairfield, NJ) to a glass pipette (WPI, Sarasota, FL) via a pipette holder and plastic tubing. Injections were made over a 10-minute interval, and to prevent leakage, the pipette was left in place for an additional 10-minutes. Bone wax was used to cover the bore-holes, and the mice were allowed to recover in their home cages. Mice were allowed to recover for 21-days to allow for maximal gene knockdown before testing.
Light microscopic immunohistochemistry.
After induction of deep anesthesia with sodium pentobarbital (150mg/kg), mouse brains were perfusion-fixed with 4% PFA in PB. Following dissection, brains were post-fixed in 4% PFA in PB overnight and sectioned (40 μm) with a vibratome (VT1000X Leica Microsystems, Buffalo Grove, IL). In preparation for processing, forebrain sections were punch coded and pooled into single vials so that brain sections had similar exposure to all reagents (Milner et al., 2011). Next, to minimize nonspecific labeling sections were incubated in 0.5% BSA in 0.1 M TBS for 30-minutes. Following this step, brain sections were incubated in primary chicken anti-EGFP (1:1000), rabbit anti-GluA1 (1:100), or rabbit GluA2 (1:100) antisera. Next, sections were incubated in anti-chicken or anti-rabbit IgG conjugated to biotin, then incubated for 30-minutes in ABC in TBS. The bound peroxidase was visualized by reaction in DAB and 0.003% hydrogen peroxide in TBS for 5–6 minutes. Sections were mounted on glass slides in 0.05 M PB, dehydrated through an ascending series of alcohol through xylene, and coverslipped with DPX (catalogue #:06522, Sigma-Aldrich). For measurement of GluA1 and GluA2, images containing the PVN were collected using an Eclipse Nikon 80i microscope at 20x interfaced to a Micropublisher 5.0 digital camera and IP Lab software. Using ImageJ software (RRID: SCR_003070), the PVN was traced out and the average pixel density within the region was calculated in each hemisphere (Pierce et al., 1999). Values for each hemisphere in each brain were averaged and these values were averaged across animals to achieve group means.
Drugs and reagents.
AMPA (cat. # A6816), AP5 (cat. # 8054), AngII (cat. # A9525), TTX (cat. # 554412), and Naspm (cat. # N193) were obtained from Sigma-Aldrich.
Statistical analyses.
Data were tested for normality and in cases where normality was breached distributions were evaluated using a non-parametric test. Blood pressure results were analyzed by factorial and repeated measures ANOVA followed by post-hoc testing (Tukey’s test for multiple comparisons). In situ hybridization, immunoelectron microscopic, and electrophysiological data were analyzed by t-tests or factorial ANOVA followed by post-hoc testing (Tukey’s test for multiple comparisons). In cases where distributions deviated from equality of variance data were analyzed by Welch’s t-test, Welch’s ANOVA, or the Mann-Whitney U test as indicated in the text. Data analyzed using a fixed-effect model are applicable only to the animals sampled in the present studies. Statistical analyses were conducted using Prism 8 (GraphPad) software.
Image preparation.
Light and electron micrographs were prepared by adjusting images for contrast, sharpness, and/or brightness using Photoshop 11 software (Adobe, San Jose, CA). These images were then imported into PowerPoint to add lettering and symbols. Adjustments were made to the entire image without alterations to labeling. Prism 6 software was used to produce the graphical figures (GraphPad Software, La Jolla, CA).
RESULTS
GluA1 and GluA2 mRNA levels in the PVN of male mice infused with slow-pressor AngII
The relationship between slow-pressor AngII administration and PVN AMPA receptor subunit transcription was examined in male mice given AngII (N=6) or Veh (N=6) for 14-days and subject to blood pressure recording. With respect to SBP, there was a main effect of treatment (F1, 10 = 8.3, p< 0.02, Factorial ANOVA), session (F4, 40 = 28, p≤ 0.04, Repeated Measure ANOVA), and a treatment by session interaction (F4, 40 = 3.9, p≤ 0.01, Repeated Measure ANOVA). Compared to Veh-treated animals, male mice treated with AngII showed an increase in SBP, with significant increases relative to baseline at days 11 and 13 (p≤ 0.05, Tukey’s test; Fig. 1A).
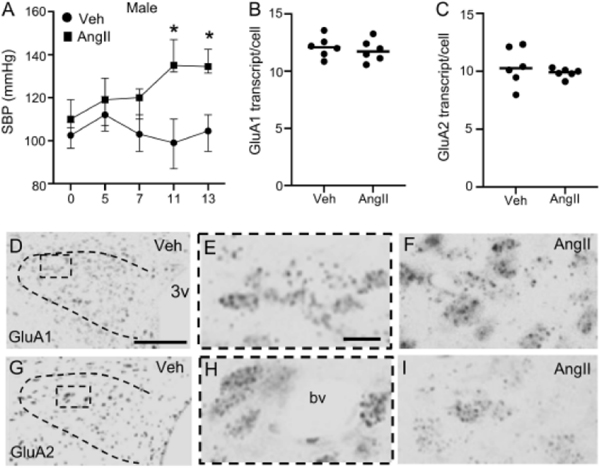
Fig. 1. Blood pressure and AMPA receptor subunit mRNA levels in the PVN of male mice following AngII infusion.
Systolic blood pressure (SBP) was elevated in male mice infused with AngII versus vehicle (Veh; A). There were no differences in GluA1 (B) or GluA2 (C) transcript in Veh and AngII-infused male mice. Light micrographs from PVN (bounded area) showing GluA1 at low (D) and high (E, area from the bounded box in D) magnifications in a Veh-infused mouse. GluA1 mRNA from an AngII-infused mouse is shown at high magnification in F. Light micrographs showing GluA2 mRNA in the PVN of a male Veh-infused mouse are shown at low (G) and high (H, area in the bounded box from G) magnifications. A micrograph illustrating GluA2 mRNA from a male AngII-infused mouse is shown in (I). 3v: Third ventricle; bv: blood vessel. A: * p< 0.05 SBP AngII versus Veh Days 11 and 13. Points on the line graph in A are presented as medians ± 25th and 75th quartiles as error bars. Lines in B and C are group medians overlain with data points representing means for each animal. Scale bars: 400 μm (D, G), 25 μm (E, F, H, I).
In male mice infused with Veh or AngII, GluA1 and GluA2 mRNA was measured in the caudal PVN, a region with a high proportion of spinally projecting sympathetic neurons (Sawchenko and Swanson, 1983). There was no difference in the total number of GluA1 transcripts per PVN cell in Veh and AngII-infused male mice (t10 = 0.6, p≥ 0.5; Fig. 1B). Similarly, there was no difference in GluA2 transcript/cell in both groups (t5.6 = 0.78, p≥ 0.4, Welch’s Unpaired t-test; Fig. 1C). Further, Veh and AngII-infused mice did not show a difference in the pixel density of either GluA1 (t10 = 0.2, p≥ 0.8, Unpaired t-test, not shown) or GluA2 (t10 = 1.3, p≥ 0.2, Unpaired t-test, not shown). Light micrographs illustrating PVN GluA1 and GluA2 mRNA expression are shown in Figs. 1D–F and Figs. 1G–I, respectively.
These results demonstrate that male mice infused AngII show an increase in SBP, but not GluA1 or GluA2 mRNA expression in the PVN.
GluA1 and GluA2 mRNA levels in the PVN of female mice infused with slow-pressor AngII
The effect of AngII on blood pressure was also investigated in female mice. In contrast to males, and consistent with prior reports (Marques-Lopes et al., 2014), there was no treatment (F1, 8 = 1.4, p≥ 0.2, Factorial ANOVA), session (F4, 32 = 1.2, p≥ 0.3, Repeated Measure ANOVA), or treatment by session interaction (F4, 32 = 1.4, p≥ 0.2, Repeated Measure ANOVA) with respect to SBP. Female mice infused with either Veh (N=5) or AngII (N=5) did not show significant differences in blood pressure at any day of SBP recording (Fig. 2A).
Fig. 2. Blood pressure and AMPA receptor subunit mRNA levels in the PVN of female mice following AngII infusion.
In contrast to males, female mice infused with Veh or AngII did not differ with respect to SBP (A). Similar to males, there were no differences in GluA1 (B) or GluA2 (C) mRNA in female mice infused with Veh or AngII. Light micrographs illustrating GluA1 mRNA in the PVN (bounded area) of a female mouse infused with Veh are shown at low (D) and high magnification (E, from the area in the bounded box in D). Also shown is GluA1 mRNA from a female mouse infused with AngII at high magnification (F). GluA2 mRNA from the PVN of a female mouse infused with Veh is shown at low (G) and high (H, area in the bounded box in G) magnification. Micrograph of GluA2 mRNA in an AngII-infused mouse is also shown at high magnification (I). 3v: Third ventricle; bv: blood vessel. Points on the line graph in A are presented as medians ± 25th and 75th quartiles as error bars. Lines in B and C are group medians overlain with data points representing means for each animal. Scale bars: 400 μm (D, G), 25 μm (E, F, H, I).
Expression of PVN GluA1 and GluA2 mRNA was further measured in female mice infused with Veh or AngII. Similar to males, there were no differences in the number of GluA1 transcripts/cell (t8 = 0.86, p≥ 0.4, Unpaired t-test; Fig. 2B) or pixel density (t8 = 1.27, p≥ 0.2, Unpaired t-test; not shown) in Veh versus AngII-infused females. Interestingly, levels of GluA1 transcript overall were lower in females compared to males. This is consistent with prior reports of sex differences in glutamate receptor localization in the PVN (Marques-Lopes et al., 2014). Levels of GluA2 mRNA were also assessed in the PVN of Veh and AngII-infused female mice. No difference in either GluA2 transcript per cell (t8 = 0.5, p≥ 0.6, Unpaired t-test; Fig. 2C) or pixel density (t8 = 0.06, p≥ 0.9, Unpaired t-test; not shown) was seen when comparing Veh and AngII-infused females. Examples of GluA1 mRNA in both groups of female mice are shown in Figs. 2D–F. Light micrographs illustrating GluA2 mRNA in Veh and AngII treated females are presented in Figs. 2G–I.
These results demonstrate that in contrast to males, AngII did not affect blood pressure in female mice. However, similar to males, PVN AMPA receptor subunit gene expression was unaltered in females infused with AngII.
Subcellular distribution of GluA1 in TNFR1 expressing neurons following AngII infusion in male mice
To investigate the effect of hypertension on GluA1 localization in caudal PVN neurons, dual labeling immunohistochemical EM was used to assess the effect of 14-day administration of Veh (N=4) or AngII (N=4) on the subcellular distribution of GluA1 in dendrites of PVN neurons of male mice. Blood pressure was measured prior to AngII infusion and 13 days later. There was an effect of treatment (F1, 6 = 12.1, p≤ 0.02, Factorial ANOVA) session (F1, 6 = 55, p≤ 0.0003, Repeated Measure ANOVA) and a treatment by session interaction (F1, 6 = 30.4, p≤ 0.002, Repeated Measure ANOVA) on SBP. Systolic blood pressure was increased on the last day of testing in male mice infused with AngII (p≤ 0.05, Tukey’s test; Fig. 3A).
Fig. 3. Blood pressure and plasma membrane GluA1 IGS labeling in TNFR1-labeled dendritic profiles of PVN neurons following AngII infusion in male mice.
Male mice infused with either Veh or AngII did not differ in SBP prior to pump implantation (Day 0). In contrast to mice treated with vehicle, mice receiving AngII showed increased SBP on the last day of blood pressure recording (A). The partitioning ratio of plasma membrane GluA1 IGS in small-size dendritic profiles of PVN neurons was higher in male mice infused with AngII compared to Veh (B). In contrast, there were no differences in the partitioning ratio of plasma membrane GluA1 in large dendritic profiles (C) or in dendrites collapsed across all profile sizes (D) in male mice from each treatment group. Light micrograph illustrating GluA1 labeling in the dorsal hypothalamus of a vehicle-infused mouse (E). The location of the PVN is indicated in the dashed area. Electron micrograph illustrating size-regions in a longitudinally-sectioned dendritic profile from a male mouse infused with vehicle (Veh, F) where GluA1 immunogold-silver (IGS) labeling is present in the cytosol (arrows). Large (double chevron) and small (single chevron) regions of the dendritic profile are illustrated. Cross-sectionally labeled dendrites from mice infused with Veh (G) or AngII (H). In the Veh condition, IGS labeling for GluA1 is present in the cytoplasm (arrows). Labeling for GluA1 is shown near the plasma membrane (arrowhead) of a dendritic profile from an AngII-treated mouse. PM/Total: Plasma membrane labeling/Total labeling. A: * p< 0.05 SBP Veh versus AngII day 13; B: * p< 0.05 Veh versus AngII GluA1 IGS PM/Total in small dendrites. Lines in A-D are medians which are overlain with data points representing means for each animal. Scale bars: E: 250 μm; F: 1 μm; G-H: 500 nm
It has been reported that TNFR1 is highly expressed in caudal PVN neurons projecting to the spinal cord and also implicated in hypertension, therefore TNFR1 was used to identify putative PVN projection neurons (Woods et al., 2021). A total of 556 dual GluA1 SIG and TNFR1 immunoperoxidase labeled dendritic profiles from caudal PVN neurons were counted in Veh (n= 252) and AngII (n= 304) infused mice. The diameter of dendrites decrease the more distal they are from the cell body (Harris and Spacek, 2016). Since distal dendritic processes receive extensive input from glutamatergic axon terminals and play an essential role in excitatory neurotransmission (London and Hausser, 2005), the partitioning ratio of GluA1 was measured in small (< 1 μm) dendritic profiles in the PVN of Veh and AngII-infused mice (Ovalles et al., 2019). Small dendritic profiles (Veh: n= 111; AngII: n=112) from AngII-infused mice had a higher partitioning ratio of GluA1 on the plasma membrane compared to Veh treated mice (t6 = 2.6, p≤ 0.05, Unpaired t-test; Fig. 3B). There were no differences in the plasma membrane partitioning ratio of GluA1 in large (≥ 1 μm; Veh: n= 141; AngII: n= 192) dendritic profiles (t6 = 0.17, p≥ 0.8, Unpaired t-test; Fig 3C). Collapsed across all dendrites there was a trending increase in plasma membrane GluA1 in dendritic profiles of AngII versus Veh-infused mice (t6 = 2.2, p≥ 0.06, Unpaired t-test; Fig. 3D). There was no difference in the density of GluA1 (total particles/area) between the two treatments (not shown). A light micrographic example of GluA1 labeling in cell bodies in the dorsal hypothalamus from a Veh-infused mouse is shown in Fig. 3E and EM illustrations of GluA1 SIG labeling in dendritic profiles of PVN neurons from vehicle and AngII infused mice are shown in Figs 3F–H.
These results demonstrate that the increase in blood pressure in response to AngII is associated with an increase in plasma membrane-affiliated GluA1 in small TNFR1-labeled dendritic profiles of PVN neurons in male mice.
Subcellular distribution of GluA1 in TNFR1 expressing neurons following AngII infusion in female mice
The effect of AngII infusion on GluA1 localization in the caudal PVN was also investigated in female mice treated with either Veh (n=4) or AngII (n=4). In contrast to male mice, there was no effect of treatment (F1, 6 = 3.7, p≥ 0.1, Factorial ANOVA) session (F1, 6 = 0.1, p≥ 0.7, Repeated Measure ANOVA) or a session by treatment interaction (F1, 8 = 1.8, p≥ 0.2. Repeated Measure ANOVA) with respect to SBP in female mice (Fig. 4A).
Fig. 4. Blood pressure and plasma membrane GluA1 IGS labeling in TNFR1-labeled dendritic profiles of PVN neurons from female mice infused with AngII.
Female mice infused with either Veh or AngII did not differ in SBP at baseline (Day 0), or on the last day of blood pressure recording following pump implantation (A). There were no differences in plasma membrane GluA1 immunogold-silver (IGS) labeling in dendritic profiles segregated by small (B) and large (C) sizes, or when collapsed across dendrites of all sizes (D) in Veh and AngII-infused female mice. PM/Total: Plasma membrane labeling/Total labeling. Lines in A-D are group medians overlain with data points representing means for each animal
A total of 482 dual labeled dendritic profiles were counted in the PVN of females (Veh= 244; AngII= 238). Unlike male mice, there was no difference in the partitioning ratio of plasma membrane GluA1 (t6 = 0.8, p≥ 0.4, Unpaired t-test; Fig. 4B) in small dendritic profiles (Veh: n= 44; AngII: n= 49) in females. There were also no differences in plasma membrane GluA1 in large (Veh: n= 200; AngII: n= 189) dendritic profiles (t6 = 0.9, p≥ 0.3, Unpaired t-test; Fig. 4C) or dendrites collapsed across all sizes (t6 = 1.0, p≥ 0.3, Unpaired t-test; Fig. 4D).
These results demonstrate AngII did not increase blood pressure and also did not impact the distribution of PVN GluA1 in female mice
Subcellular distribution of GluA2 in TNFR1 expressing neurons following AngII infusion in male and female mice
Given the increase in plasma membrane GluA1 in males, we also assessed if the GluA2 subunit was altered in Veh or AngII treated male mice (same mice as above). An example of GluA2 immunolabeling in the dorsal PVN of a Veh-infused male mouse is shown in Fig. 5A. Dendritic profiles in the PVN of Veh and AngII-infused males showed GluA2 IGS labeling and discrete TNFR1 immunoperoxidase labeling. It was found that labeling for GluA2 was affiliated with the plasma membrane, in addition to the cytoplasm in dendritic profiles of PVN neurons from both groups of male mice (Fig. 5B, C).
A total of 707 GluA2-labeled profiles were counted in male mice treated with Veh (n= 356) or AngII (n= 351). In small dendritic profiles (Veh: n= 125; AngII: n= 98), the partitioning ratio of GluA2 on the plasma membrane was lower in AngII compared to vehicle treated mice (t6 = 2.8, p≤ 0.05, Unpaired t-test; Fig. 5D). There was no difference in plasma membrane GluA2 in large (Veh: n= 231, AngII n= 253) dendritic profiles of AngII versus Veh infused mice (t6 = 2.1, p≥ 0.07, Unpaired t-test; Fig. 5E). Collapsed across all dendritic profiles there was also no difference in plasma membrane GluA2 between the two groups (t6 = 1.5, p≥ 0.1, Unpaired t-test, Fig. 5F). In addition, there was no difference in the total density of GluA2 between Veh and AngII-infused animals (not shown).
In females, a total of 649 dendritic profiles were counted in mice infused with Veh (n= 326) or AngII (n= 323). When comparing a total of 206 small dendritic profiles following treatment with Veh (n= 89) or AngII (n= 117) no significant differences were found in the GluA2 partitioning ratio (t6= 0.3, p> 0.7 Unpaired t-test; Fig. 5G). There also were no differences in large profiles (n= 443) in Veh (n= 237) or AngII (n= 206) infused female mice (t6= 0, p≥ 0.99, Unpaired t-test; Fig. 5H). Collapsed across all profiles, there was no difference in the GluA2 partitioning ratio when comparing Veh and AngII-infused female mice (t6= 0.2, p≥ 0.8 Unpaired t-test; Fig. 5I). In addition, there was no difference in the total density of GluA2 between Veh and AngII-infused animals (not shown).
These results show that the slow-pressor response to AngII in male, but not female, mice is associated with a decrease in plasmalemmal GluA2 in small TNFR1-labeled dendritic profiles of PVN neurons.
Heightened AMPA-mediated currents in PVN sympathoexcitatory neurons of male mice after AngII infusion
To assess the effect of AngII on rapid AMPA signaling in the PVN of sympathoexcitatory neurons of male mice, we measured AMPA currents in PVN neurons projecting to the spinal cord of Veh (N=6) and AngII (N=6) infused mice. To record from PVN neurons projecting to the spinal cord, male mice were microinjected with FG in the spinal cord and 7-days later received osmotic minipumps containing vehicle or AngII. In vehicle-infused mice systolic blood pressure did not differ from baseline to 14 days after infusion (97.6±4.5 versus 98.2±4.8, p≥ 0.05, t-test) whereas in Ang-infused mice it increased over this time (108.7±1.7 versus 131±4.8, p≤ 0.001, ttest).
Currents induced by AMPA (30 μM) were measured in slices containing the PVN obtained from Veh (Fig. 6A) and AngII (Fig. 6B) infused male mice by whole-cell recording of visually identified FG-labeled PVN neurons. To identify AMPA currents from GluA2-lacking AMPA receptors, recordings were made in the presence or absence of the GluA2-lacking AMPA receptor antagonist Naspm (Tsubokawa et al., 1995). There were main effects of AngII (F1, 22 = 6.9, p≤ 0.02), Naspm (F1, 22 = 25.4, p≤ 0.0001), and an AngII by Naspm interaction (F1, 22 = 9.7, p≤ 0.005) with respect to AMPA currents in PVN-spinal cord projection neurons. Currents were significantly increased in PVN neurons from AngII (n= 9 neurons, 3 mice) compared to Veh-infused (n= 8 neurons, 3 mice) mice (p≤ 0.0006, Tukey test; Fig. 6C). In addition, AMPA currents were reduced in PVN neurons from AngII-infused mice when slices were pre-treated with Naspm (n= 4 neurons, 3 mice) versus vehicle (p≤ 0. 0003, Tukey test; Fig. 6C). In Veh-infused mice, there were no differences in AMPA currents in PVN neurons when treated with Naspm (n= 5 neurons, 3 mice) or vehicle (p≥ 0.3, Tukey test; Fig. 6C). An example of a FG-labeled PVN neuron and recording electrode is shown in Fig. 6D.
Fig. 6. GluA2-lacking AMPA receptor currents are elevated in PVN-spinal cord projection neurons from male mice infused with AngII.
Whole-cell current traces in PVN neurons from mice infused with vehicle (Veh; A) or AngII (B). In the absence of 1-Naphthylacetyl spermine (Naspm), AMPA-induced currents are significantly higher in patched-PVN neurons from mice infused with AngII compared to those infused with Veh (C). The heightened AMPA currents in PVN neurons from AngII-infused mice are reduced by pre-treatment with the GluA2-lacking AMPA receptor blocker (C). In PVN neurons from Veh-infused mice, Naspm does not significantly reduce AMPA currents (C). An example of a patched retrogradely-labeled PVN neuron is shown in D. C: V/V: Veh/Veh; V/N: Veh/Naspm; A/V: AngII/Veh; A/N: AngII/Naspm. * p< 0.05 AMPA currents V/V versus A/V and A/V versus A/N. Lines in C are group medians overlain with data points representing means for each animal. D: Scale bar: 30 μm
These results demonstrate that GluA2-lacking AMPA receptor currents are elevated in PVN neurons projecting to the spinal cord in AngII-infused male mice. Together with the high-resolution immunoelectron microscopic findings, these results suggest a heightening of rapid GluA1 signaling involving increased plasma membrane receptors in PVN sympathoexcitatory neurons during AngII hypertension in males.
Effect of GluA1 silencing in PVN neurons of male mice on blood pressure following slow-pressor AngII
To investigate the functional role of PVN GluA1 in hypertension, GluA1 was silenced in the PVN via bilateral microinjections of a neurotropic AAV shRNA expressing vector in male mice treated with 14-day slow-pressor administration of AngII. There was no difference in SBP at baseline versus 21-days post-vector injection in mice receiving the GluA1 shRNA vector or a control GFP-expressing vector (F1, 11 = 0.7, p≥ 0.4, Repeated Measure ANOVA; not shown). With respect to SBP following AngII, there were effects of vector (F1, 11 = 5.3, p≤ 0.05, Factorial ANOVA), session, (F5, 55 = 8.7, p≤ 0.001, Repeated Measure ANOVA) and a vector by session interaction (F5, 55 = 3.2, p≤ 0.02, Repeated Measure ANOVA) with regard to SBP. Following AngII infusion, control vector injected male mice showed higher SBP compared with GluA1 shRNA injected mice at days 11 and 14 (Fig. 7A). Expression of GluA1 protein immunoreactivity was reduced in mice receiving the GluA1 shRNA compared to those receiving the control vector (t11 = 5.9, p≤ 0.001, Unpaired t-test; Fig 7B). The specificity of the shRNA for GluA1 knockdown was also investigated by measuring GluA2 in the injection site. There was no difference in GluA2 protein immunoreactivity in mice microinjected with control and GluA1 shRNA vectors (Control: 19.7±2.6 pixel density versus GluA1: 18.1±1.0 pixel density; t11 = 0.6, p≥ 0.5, Unpaired t-test; not shown). Examples of labeling for GFP and GluA1 in the PVN of AAV-GFP and AAV-GluA1 shRNA microinjected mice are presented in Fig. 7 C–D and Fig. 7E–F, respectively.
Fig. 7. Attenuation of AngII-induced hypertension following bilateral PVN GluA1 silencing in male mice.
Mice receiving the AAV-GFP vector show an increase in systolic blood pressure (SBP) at days 11 and 14 (A). Relative to controls, SBP is lower in mice following AAV-shRNA vector microinjections at days 11 and 14 (A). Expression of PVN GluA1 is significantly reduced in mice microinjected with GluA1 shRNA compared to mice microinjected with the control vector (B). Light micrographs showing GFP (C) and GluA1 (D) expression in the PVN following local microinjection of the AAV-GFP vector. In addition, micrographs showing GFP (E) and GluA1 (F) in the PVN following local microinjection of AAV- shRNA against GluA1 are also shown. In A: * p< 0.05 SBP at Days 11 and 14 versus Day 0 in AAV-GFP mice; #: p< 0.05 SBP at days 11 and 14 in AAV-shRNA versus AAV-GFP mice; @: p< 0.05 SBP Day 14 versus Day 0 in AAV-shRNA mice. In B: *p< 0.05 GluA1 pixel density AAV-GFP versus AAV-shRNA mice. 3v: Third ventricle. Points on the line graph in A are presented as medians ± 25th and 75th quartiles as error bars. Lines in B are group medians overlain with data points representing means for each animal. C-F: Scale bars: 500 μm.
DISCUSSION
We report that GluA1 signaling in the PVN significantly contributes to the slow-pressor response to AngII in adult male mice. Specifically, it was shown that slow-pressor AngII infusion was associated with an increase of plasma membrane GluA1 in dendritic profiles of neurons expressing TNFR1, a marker of PVN-spinal cord projection neurons. It was also found that AngII-infused mice showed elevated AMPA currents in PVN neurons projecting to the spinal cord. Further, hypertension induced by AngII was attenuated in animals following silencing of GluA1 in PVN neurons. In contrast, in female mice, AngII infusion did not impact blood pressure or plasma membrane localization of GluA1. These results point to an important role for GluA1 signaling in the PVN during AngII hypertension in male mice.
Altered glutamate-dependent signaling in the PVN has been reliably observed following hypertension induced by AngII and other forms of hypertension (Li et al., 2003, Coleman et al., 2010, Glass et al., 2015, Ma et al., 2018, Zhou et al., 2019). Activity-related changes in the amount, subunit composition, post-translational state, and subcellular location of AMPA-type glutamate receptors are among the factors that are implicated in various forms of glutamate-dependent synaptic plasticity (Diering and Huganir, 2018). The AMPA receptors have varied subunit combinations, and, based on studies in the hippocampus, heteromers formed by GluA1 and GluA2 or GluA2 and GluA3 subunits constitute the major variants (Henley and Wilkinson, 2016). Variants expressing GluA2 have a linear current-voltage relationship and are calcium-impermeable, whereas GluA1 expressing GluA2-lacking variants show high conductance, rapid decay kinetics, calcium permeability, and are highly implicated in neural plasticity (Henley and Wilkinson, 2016). GluA2, when unedited at the Q/R site (Sommer et al., 1991) at position 607, is calcium-permeable. In the adult rodent brain the vast majority of GluA2 are edited at this residue and are thus calcium impermeable (Paschen and Djuricic, 1995, Carlson et al., 2000). However, data suggests that decreased editing efficiency in adult brain may occur in certain disease contexts, including neurodegenerative diseases (Kawahara et al., 2004, Gaisler-Salomon et al., 2014), suggesting that alterations in GluA2 editing may contribute to certain severe brain disorders, although, to our knowledge, this has never been described in the hypothalamus during hypertension.
In the present study we examined the effect of AngII on AMPA subunit gene expression in the caudal PVN, an important locus of preautonomic neurons (Sawchenko and Swanson, 1983). It was shown that AngII administration was not associated with altered GluA1 mRNA levels in the PVN of male or female mice. In addition, there was also no change in GluA2 transcription following hypertension in either sex. Similarly, with respect to both GluA1 and GluA2 immunoreactivity, there were no differences in the density of labeling in dendritic profiles of PVN neurons, also indicating that protein levels of GluA1 and GluA2 subunits were not elevated following AngII. In sum, the present results indicate that transcriptional regulation of two major AMPA receptor subunits is not significantly altered in the PVN by slow-pressor AngII treatment in males or females.
Beyond protein abundance, the plasma membrane incorporation of AMPA receptors is critical for glutamate-dependent long-term changes in neural signaling (Hanley, 2014). In this study, we investigated the effect of AngII on the subcellular distribution of AMPA subunits in the caudal PVN. It is important to note that glutamate inputs preferentially target dendritic regions distal from the cell body and glutamate release probability is higher at distal dendritic areas (Jensen et al., 2021). The attenuation of currents from distal dendrites can be countered by increased density of receptors and probability of glutamate release (Jensen et al., 2021). Therefore, in the present study we investigated the effect of AngII on AMPA subunit localization in dendrites of small and large size that correspond to distal versus proximal dendritic regions, respectively. It was found that in AngII-infused male mice there was an increase in plasma membrane GluA1 limited to small-diameter dendritic profiles, indicating that increased plasma membrane GluA1 is restricted to distal areas of the dendritic tree in PVN neurons of AngII-infused male mice.
Interestingly, it was found that the elevated plasma membrane GluA1 occurred in dendritic profiles also labeled for TNFR1, a protein that has been shown to be highly expressed in PVN sympathoexcitatory neurons (Woods et al., 2021). An increase in plasma membrane GluA1 in TNFR1 PVN neurons from AngII-infused mice is consistent with evidence that TNFα in the PVN can influence local neural signaling (Kang et al., 2008, Yu et al., 2015), sympathetic activity (Dange et al., 2015, Yu et al., 2015), and systemic blood pressure (Sriramula et al., 2013, Dai et al., 2015, Dange et al., 2015) (Shi et al., 2010, Yu et al., 2015) in hypertension models. It is also in agreement with prior evidence that TNFR1 signaling in the PVN plays an influential role in local glutamate-mediated transmission and AngII hypertension in male mice (Woods et al., 2021). The present results expand on these prior reports by showing that changes in AMPA receptor localization during AngII hypertension are compartmentalized to subregions of the dendritic tree of PVN neurons that are most intensively targeted by glutamate inputs and that are also are receptive to TNFα.
The increase in plasma membrane GluA1 coupled with a decrease in plasmalemmal GluA2 indicates that GluA2-lacking AMPA receptors are recruited to the surface membrane in PVN neurons of hypertensive male mice. The latter suggestion is consistent with a prior report on AMPA receptor subunit subcellular distributions in subcellular fractions showing a decrease in the ratio of surface to cytoplasmic GluA2 in the PVN of SHR rats (Li et al., 2012). However, the present results contrast with another report (Ovalles et al., 2019) that the subcellular localization of GluA1 in estrogen receptor beta (ERß) expressing neurons was not affected in male mice infused with AngII. These discrepancies may be related to measuring GluA1 in TNFR1 versus ERß-containing PVN neurons.
At the cellular level, the functional nature of the increase in plasma membrane GluA1 was supported by whole-cell current clamping experiments. In AngII-infused mice, it was shown that there was a marked increase in AMPA currents in PVN-spinal projection neurons that was reversible by the GluA2-lacking AMPA receptor antagonist Naspm. A further functional role for PVN GluA1 signaling at the whole-animal level was demonstrated by the attenuated hypertensive response to AngII following silencing of GluA1 in PVN neurons. Taken together, the immunoelectron microscopic, electrophysiological, and gene silencing studies suggest that an increase in the pool of distal dendritic GluA1-expressing GluA2-lacking AMPA receptors available for activation by extracellular glutamate may contribute to increased excitatory signaling in PVN neurons during AngII hypertension in male mice (Qi et al., 2013, Kang et al., 2014).
In contrast to males, female mice did not show an increase in blood pressure in response to AngII. The lack of a pressor response in female mice is consistent with other findings that female mice are protected from the hypertensive actions of AngII (Xue et al., 2005, Girouard et al., 2009, Marques-Lopes et al., 2017). In terms of AMPA receptor subunits, AngII-infused female mice did not differ from Veh-treated mice with respect to GluA1 or GluA2 mRNA levels in the PVN. Interestingly, levels of GluA1 transcript overall were higher in males compared to females. Differences in brain glutamate systems have been shown in females and males (Wickens et al., 2018), including AMPA receptors in hippocampus (Monfort et al., 2015) and hypothalamus (Diano et al., 1997, Marques-Lopes et al., 2014). In terms of protein subcellular distribution, GluA1 and GluA2 localization did not differ in Veh and AngII-infused female mice. The immobility of GluA1 following AngII observed in the present study corresponds with a prior report showing that the subcellular distribution of GluA1 in dendritic profiles of estrogen receptor expressing PVN neurons was not impacted in cycling female mice infused with AngII (Ovalles et al., 2019). The basis for the difference between males and cycling female in AngII hypertension is uncertain, but it may involve the actions of gonadal hormones including estrogen. This contention is supported by evidence that ovariectomy (Xue et al., 2005), aging (Marques-Lopes et al., 2014), or accelerated ovarian failure (AOF) induced by 4vinylcyclohexene diepoxide (Milner et al., 2021) sensitizes female mice to the hypertensive actions of AngII. In addition, it has been shown that only female mice at the late stage of AOF comparable to menopause show an increase in plasma membrane GluA1 in ERβ-expressing dendritic profiles of PVN neurons (Ovalles et al., 2019). These results suggest that estrogen contributes to sex differences in blood pressure and GluA1 distribution in PVN neurons following AngII.
In summary, the present results demonstrate that GluA1 signaling in PVN neurons contributes to hypertension in male mice. The lack of a role for GluA1 in cycling-female mice, but not in mice with advanced AOF (Ovalles et al., 2019), suggests that estrogen signaling may account for sex differences in hypertension sensitivity.
Highlights.
AMPA GluA1 and GluA2 gene expression in the PVN are unaltered by angiotensin II (AngII) in male and female mice
GluA1 is increased and GluA2 is decreased on the plasma membrane of PVN neurons in AngII-infused male, but not female, mice
Heightened AMPA currents in PVN neurons from AngII-infused males are dependent on calcium-permeable AMPA GluA1 receptors
The increase in blood pressure induced by AngII is inhibited by AMPA GluA1 silencing in PVN neurons
Increased plasma membrane AMPA GluA1 in PVN neurons may serve as a molecular substrate of hypertension in male mice
Acknowledgement:
This research was supported by NIH grants: HL135498 (MJG), HL136520 (MJG, TAM)
ABBREVIATIONS
- AAV
adeno-associated virus
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AngII
angiotensin II
- BSA
bovine serum albumin
- CSF
cerebrospinal fluid
- DAB
diaminobenzidine
- EGFP
enhanced green fluorescent protein
- EM
electron microscopic
- FG
fluorogold
- IGS
immunogold-silver
- IML
intermediolateral region of the spinal cord
- Naspm
1-Naphthylacetyl spermine
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PVN
paraventricular nucleus of the hypothalamus
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
- shRNA
short hair-pin RNA
- TBS
tris-buffered saline
- TNFα
tumor necrosis factor alpha
- TNFR1
tumor necrosis factor alpha receptor 1
- TTX
tetrodotoxin
- Veh
vehicle
Footnotes
Conflicts: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aicher SA, Sharma S, Mitchell JL (2003) Structural Changes in AMPA-Receptive Neurons in the Nucleus of the Solitary Tract of Spontaneously Hypertensive Rats. Hypertension 41:1246–1252. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV (1995) Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol 268:R625–R633. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295:22822285. [DOI] [PubMed] [Google Scholar]
- Brouwers S, Sudano I, Kokubo Y, Sulaica EM (2021) Arterial hypertension. Lancet. [DOI] [PubMed] [Google Scholar]
- Buonarati OR, Hammes EA, Watson JF, Greger IH, Hell JW (2019) Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci Signal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C (2012) Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32:4878–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NG, Howard J, Gahring LC, Rogers SW (2000) RNA editing (Q/R site) and flop/flip splicing of AMPA receptor transcripts in young and old brains. Neurobiol Aging 21:599606. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS (2010) Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 149:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, Milner TA, Iadecola C, Pickel VM (2013) Membrane trafficking of NADPH oxidase p47(phox) in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J Neurosci 33:4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM (2010) Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30:12103–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SY, Peng W, Zhang YP, Li JD, Shen Y, Sun XF (2015) Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflamm 12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dange RB, Agarwal D, Teruyama R, Francis J (2015) Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflamm 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Horvath TL (1997) Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology 138:778–789. [DOI] [PubMed] [Google Scholar]
- Dickinson CJ, Lawrence JR (1963) A slowly developing pressor response to small concentrations of angiotensin. Its bearing on the pathogenesis of chronic renal hypertension. Lancet 1:1354–1356. [DOI] [PubMed] [Google Scholar]
- Diering GH, Huganir RL (2018) The AMPA receptor code of synaptic plasticity. Neuron 100:314329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M (2000) The sympathetic system and hypertension. Am J Hypertens 13(6 Pt 2):99S–105S. [DOI] [PubMed] [Google Scholar]
- Eyigor O, Centers A, Jennes L (2001) Distribution of ionotropic glutamate receptor subunit mRNAs in the rat hypothalamus. J Comp Neurol 434:101–124. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Kravitz E, Feiler Y, Safran M, Biegon A, Amariglio N, Rechavi G (2014) Hippocampus-specific deficiency in RNA editing of GluA2 in Alzheimer’s disease. Neurobiol Aging 35:1785–1791. [DOI] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C (2009) NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Chan J, Pickel VM (2017) Ultrastructural characterization of tumor necrosis factor alpha receptor type 1 distribution in the hypothalamic paraventricular nucleus of the mouse. Neuroscience 352:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, Milner TA, Butler SD, Young CN, Davisson RL, Iadecola C, Pickel VM (2015) NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J Neurosci 35:9558–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Ram VS (2016) Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10:457–466 [DOI] [PubMed] [Google Scholar]
- Hanley JG (2014) Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca(2+)-permeable AMPA receptors. Semin Cell Dev Biol 27:14–22. [DOI] [PubMed] [Google Scholar]
- Hara Y, Pickel VM (2008) Preferential relocation of the N-methyl-D-aspartate receptor NR1 subunit in nucleus accumbens neurons that contain dopamine D1 receptors in rats showing an apomorphine-induced sensorimotor gating deficit. Neuroscience 154:965977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Spacek J (2016) Dendrite structure. In: Dendrites (Stuart G. et al. , eds): Oxford Scholarship; Online. DOI: 10.1093/acprof:oso/9780198745273.003.0001 [DOI] [Google Scholar]
- Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17:337–350. [DOI] [PubMed] [Google Scholar]
- Herman JP, Eyigor O, Ziegler DR, Jennes L (2000) Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol 422:352–362. [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Silverman MB, Lynch PJ, McKee BL, Bailey TW, Andresen MC, Aicher SA (2008) Sustained hypertension increases the density of AMPA receptor subunit, GluR1, in baroreceptive regions of the nucleus tractus solitarii of the rat. Brain research 1187:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Thomas GM, Luo J, Huganir RL (2015) Regulation of AMPA receptor subunit GluA1 surface expression by PAK3 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America 112:E5883–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TP, Kopach O, Reynolds JP, Savtchenko LP, Rusakov DA (2021) Release probability increases towards distal dendrites boosting high-frequency signal transfer in the rodent hippocampus. Elife 10:e62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su Q, Suo YP, Yue LY, Zhu GQ, Qin DN (2014) Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol 274:436–444 [DOI] [PubMed] [Google Scholar]
- Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB (2008) Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol 295:H227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S (2004) Glutamate receptors: RNA editing and death of motor neurons. Nature 427:801. [DOI] [PubMed] [Google Scholar]
- Li DP, Byan HS, Pan HL (2012) Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci 32:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL (2002) Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol 88:2664–2674. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL (2017) Glutamatergic Regulation of Hypothalamic Presympathetic Neurons in Hypertension. Curr Hypertens Rep 19:78. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG, Patel KP (2003) Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circulation Res 93:990–997. [DOI] [PubMed] [Google Scholar]
- Llewellyn T, Zheng H, Liu X, Xu B, Patel KP (2012) Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol 302:R424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Hausser M (2005) Dendritic computation. Annual Rev Neurosci 28:503–532. [DOI] [PubMed] [Google Scholar]
- Ma HJ, Chen SR, Chen H, Li L, Li DP, Zhou JJ, Pan HL (2018) α2δ−1 Is Essential for Sympathetic Output and NMDA Receptor Activity Potentiated by Angiotensin II in the Hypothalamus. J Neurosci 38:6388–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Lynch MK, Van Kempen TA, Waters EM, Wang G, Iadecola C, Pickel VM, Milner TA (2015) Female protection from slow-pressor effects of angiotensin II involves prevention of ROS production independent of NMDA receptor trafficking in hypothalamic neurons expressing angiotensin 1A receptors. Synapse 69:148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Tesfaye E, Israilov S, Van Kempen TA, Wang G, Glass MJ, Pickel VM, Iadecola C, Waters EM, Milner TA (2017) Redistribution of NMDA Receptors in Estrogen-Receptor-beta-Containing Paraventricular Hypothalamic Neurons following Slow-Pressor Angiotensin II Hypertension in Female Mice with Accelerated Ovarian Failure. Neuroendocrinology 104:239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Lopes J, Van Kempen T, Waters EM, Pickel VM, Iadecola C, Milner TA (2014) Slow-Pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta–containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. J Comp Neurol 522:3075–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Contoreggi NH, Yu F, Johnson MA, Wang G, Woods C, Mazid S, Van Kempen TA, Waters EM, McEwen BS, Korach KS, Glass MJ (2021) Estrogen receptor beta contributes to both hypertension and hypothlamic plasticity in a mouse model of perimenopause. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP (2011) Degenerating processes identified by electron microscopic immunocytochemical methods. Methods in molecular biology 793:23–59. [DOI] [PubMed] [Google Scholar]
- Monfort P, Gomez-Gimenez B, Llansola M, Felipo V (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem Neurosci 6:1420–1427. [DOI] [PubMed] [Google Scholar]
- Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH (2005) Tumor necrosis-factoralpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol 193:384–393. [DOI] [PubMed] [Google Scholar]
- Ovalles AC, Contoreggi N, Wang G, Marques-Lopes J, Van Kempen TA, Iadecola C., Waters EM, Glass MJ, Milner TA (2019) Plasma membrane affiliated AMPA GluA1 in estrogen receptor ß-containing paraventricular hypothalamic neurons increases following hypertension in a mouse model of postmenopause. Neuroscience 423:192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Djuricic B (1995) Regional differences in the extent of RNA editing of the glutamate receptor subunits GluR2 and GluR6 in rat brain. J Neurosci Methods 56:21–29. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB (2000) The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Peters A, Palay SL, Webster H (1991) The fine structure of the nervous system. New York: Oxford University Press. [Google Scholar]
- Pierce JP, Kurucz O, Milner TA (1999) The morphometry of a peptidergic transmitter system before and after seizure. I. Dynorphin B-like immunoreactivity in the hippocampal mossy fiber system. Hippocampus 9:255–276. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM (2013) Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 13:48–54. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ (2002) Postsynaptic signaling and plasticity mechanisms. Science 298:776780. [DOI] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK (2010) Brain microglial cytokines in neurogenic hypertension. Hypertension 56:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH (1991) RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell Immunol 67:11–19. [DOI] [PubMed] [Google Scholar]
- Sriramula S, Cardinale JP, Francis J (2013) Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS ONE 8:e63847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA (2010) A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci 13:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa H, Oguro K, Masuzawa T, Nakaima T, Kawai N (1995) Effects of a spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia. J Neurophysiol 74:218–225. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT (1971) General Endocrinology. Philadelphia: W.B. Saunders. [Google Scholar]
- Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A (2021) Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen TA, Dodos M, Woods C, Marques-Lopes J, Justice NJ, Iadecola C, Pickel VM, Glass MJ, Milner TA (2015) Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neurosci 307:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann K, Chen YY, Harris MP, Wullimann MF, Koster RW (2010) The zebrafish cerebellar upper rhombic lip generates tegmental hindbrain nuclei by long-distance migration in an evolutionary conserved manner. The Journal of Comparative Neurology 518:2794–2817. [DOI] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, Guruju MR, Anrather J, Davisson RL, Iadecola C, Pickel VM (2013) Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol 304:R1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens MM, Bangasser DA, Briand LA (2018) Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front Mol Neurosci 11:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Marques-Lopes J, Contoreggi NH, Milner TA, Pickel VM, Wang G, Glass MJ (2021) Tumor necrosis factor alpha-receptor type 1 activation in the hypothalamic paraventricular nucleus contributes to glutamate signaling and angiotensin II-dependent hypertension. J Neurosci 41:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Pamidimukkala J, Hay M (2005) Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol 288:H2177–H2184. [DOI] [PubMed] [Google Scholar]
- Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL (2012) ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122:3960–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Xue BJ, Wei SG, Zhang ZH, Beltz TG, Guo F, Johnson AK, Felder RB (2015) Activation of central PPAR-γ attenuates angiotensin II-induced hypertension. Hypertension 66:403411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Perel P, Mensah GA, Ezzati M (2021) Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol 28:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP (2019) Impaired Hypothalamic Regulation of Sympathetic Outflow in Primary Hypertension. Neurosci Bull 35:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]