Abstract
Irritable bowel syndrome (IBS) is a chronic collection of symptoms and lowers the quality of life. The management of such patients has always involved mitigating the symptoms produced by this disorder. This article reviews the role of probiotics in IBS by compiling various studies to deduce the possible symptomatic relief that probiotics may provide to IBS patients. Given the encouraging part of probiotics in abundant other gastrointestinal conditions, this article focuses on understanding the specific functional effects (if any) that are brought about by adding probiotics in patients with different types of IBS such as IBS with predominant constipation, IBS with predominant diarrhea, and even the unclassified type of IBS. The purpose of analyzing the role of probiotics is to study the changes brought about by them at the level of the gut microbiota in patients suffering from IBS, as this may prove to be of prime importance in managing such conditions with time. This article has also furnished an overview of the pathogenesis, diagnostic criteria, treatment modalities, sources of probiotics, and their therapeutic significance in IBS patients.
Keywords: fodmaps, rome iv criteria, gastrointestinal, bloating, flatulence, stool frequency, symptomatic relief, microbiome, irritable bowel syndrome, probiotics
Introduction and background
Irritable bowel syndrome (IBS) is a chronic disorder that results in a spectrum of gastrointestinal (GI) symptomatology, and this usually remains a diagnosis of exclusion [1,2]. Several attempts have been made to understand the close association between the increased longevity of individuals and their consumption of fermented dairy products. History claims that the effect of putrefaction (that led to diseases, again) by the gastrointestinal tract could be mitigated by lactobacilli [3-5]. Several studies claim that IBS can be found in 7%-15% of the general population [6,7]. It is found that the prevalence of IBS is the highest in South America (accounting for 21%) [8,9]. Among the geographic regions most studied, South Asia has the lowest prevalence, accounting for 7% of the cases [10-14]. Studies have shown a slight female predominance in the case of IBS compared to males [10].
Probiotics are living microorganisms that are nonpathogenic. They are known to produce several beneficial effects, such as altering the host's immune response in the gastrointestinal tract and lowering the growth of pathogenic organisms by enhancing the microbial balance. These can be consumed in the form of food and even dietary supplements. Several strains are found to be used as probiotics and these include Lactobacillus, Bifidobacterium, and even Saccharomyces. However, the exact count of various species to be used to bring about specific therapeutic gain is not known yet [15].
IBS is a term used to describe a collection of symptoms that results from several etiologies such as increased risk of infections, malabsorption from infectious diseases, small intestinal bacterial overgrowth (SIBO), antibiotic usage, changes in the gut microbiome, and exaggerated motor response leading to diarrhea- or constipation-like symptoms. Several factors leading to psychological stress, such as increased depression, anxiety-related conditions, can also contribute to IBS [16]. The various associations with IBS include psychological stress, smoking, frequent alcohol consumption, and younger age [14]. Evidence suggests that decreasing age also means a decreasing prevalence of IBS [11]. The Rome IV criteria classify patients based on symptoms as IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed bowel patterns (IBS-M) [12]. IBS results from pathophysiology that is broad and not yet entirely understood. The abnormal physiology can result in psychological stress and even motility disorders. The treatment goal for IBS is to make the patients feel symptomatically better by relieving symptoms of cramping, pain, diarrhea, constipation, and bloating, thereby helping them improve their quality of life [11]. Exercise can be encouraged to improve the peristaltic function of the intestine in the case of constipation and the use of laxatives, a high-fiber diet, and pro-kinetic drugs [11,13].
IBS is a collection of symptoms with a poorly understood etiology, thereby leading to lower quality of life. Out of the various available options, the use of probiotics in people suffering from IBS is still not clearly understood [15]. One must aim to understand the efficacy of their usage to improve patients' quality of life, thereby being one of the many solutions that relieve symptoms in patients. This article aims to review, from several studies, the efficacy of probiotics in people suffering from a chronic condition such as IBS and determine if modifying the gut's microbiome in such patients has an added benefit or if it instead further worsens the symptoms mentioned above that are already present in patients.
Review
Pathogenesis
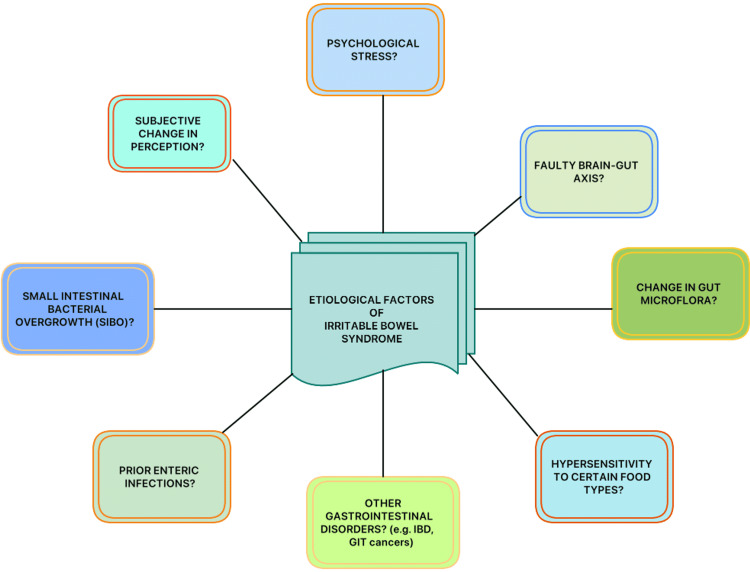
Although the pathogenesis and probable etiologies of IBS are still not clear, it is necessary to document the current understanding of IBS and its diagnostic and treatment modalities (Figure 1) [17]. Previous studies have shown that IBS can even be triggered by various factors ranging from psychological stress to enteric infections [18]. Another possible and unique mechanism mentioned is that the gut viscera is thought to be hypersensitive to different types of foods, the types differing from person to person. A large number of existing studies in the broader literature have also examined the female predominance in the case of IBS disorders.
Figure 1. Possible etiological factors of IBS.
IBS, irritable bowel syndrome; IBD, inflammatory bowel disease; GIT, gastrointestinal tract
Another relevant mechanism in evidence from the previously published literature is that IBS could also result from an alteration in the microflora of the gut, genetic predisposition to several other gut disorders like inflammatory bowel diseases (IBDs), or even a subjective change in the way people perceive their symptoms and severity, which often leads to complexities in which they are both diagnosed and managed [18].
Some have proposed that it could even involve the brain-gut axis. Several authors have recognized that IBS is a fault in the connection between the brain and the gut [18]. While understanding the cause of IBS is vital (which has been proven to be multifactorial as of today), this article aims to understand the effect that probiotics can have on these patients [19].
With the help of studies conducted to date, it is crucial to scrutinize and further summarize the role of modifying gut flora in IBS patients. Given that hypersensitivity could also be a possible etiology in IBS patients, answers to questions like "Are these patients hypersensitive to added probiotics?", "Do they tolerate them well in case of irritable bowel?" or "Do they worsen in the long run because of a possible dominance of certain bacterial species over the years despite using a broad spectrum of probiotics?" remain unanswered [20]. In the case of IBS patients, the enteric microbiota has become a target for therapeutic purposes, as proposed by several clinical and preclinical studies [21].
Pimentel and Lembo explained the reasons why a change in the gut microflora can result in the common symptoms, such as diarrhea, constipation, a combination of these two, bloating, and abdominal cramps, that patients complain of, all of which reduce their period of productivity during their active years of life as gut microbes exert effects on the host immune system and gut barrier function, as well as the brain-gut axis [22]. A positive hydrogen breath test would increase the bacterial overgrowth and present like a diarrhea-predominant IBS (IBS-D). On the other hand, a positive methane breath test would indicate an excess of methanogenic archaea in the gut presenting as constipation-predominant IBS because the intestinal contractility slows down due to the presence of the methane gas. Cytolethal distending toxin B (CdtB) is a common toxin produced by the microbes responsible for gastroenteritis and those leading to the IBS symptoms. A cross-reaction occurs between the antibodies raised against CdtB and vinculin, a cytoskeletal protein eventually impairing the function of gut motility and thereby further assisting in the overgrowth of bacteria [22].
Stasi et al. even proposed that the brain-gut axis can be affected by several psychological disorders [23]. These modify the motility of the digestive tract, various inflammatory pathways, and visceral sensitivity. This axis can be affected due to the release of the corticotropin-releasing hormone, which influences the mood in several autonomic and neuroendocrine ways. The serological adrenocorticotropic hormone and cortisol increase in cases of stress in IBS patients due to a rise in the pro-inflammatory cytokines [24]. Some studies have even attributed gastrointestinal motility to serotonin, reporting that its levels are increased in those IBS patients suffering from diarrhea. In contrast, those with constipation have a reduced concentration of plasma serotonin. Pharmacotherapy targeting these serotonin receptors might be of therapeutic importance [25,26]. Some studies also mention that abdominal pain can be linked to the hypersensitivity of the viscera [27].
Diagnosis of IBS
There are four major bowel patterns observed in IBS that are, namely, IBS-D (predominant diarrhea), IBS-C (predominant constipation), IBS-M (mixed diarrhea and constipation), and IBS-U (the unclassified type, symptoms of which cannot be categorized into any one of the three subtypes that have been mentioned) [28]. Although the Rome IV criteria have been formulated to diagnose IBS, it is crucial for physicians to clinically analyze the findings and exclude all possible causes before confirming them (Table 1) [29].
Table 1. Rome IV criteria for diagnosing IBS.
The patient should be meeting the criteria for the last three months, with the symptoms beginning at least six months before diagnosis [29].
IBS, irritable bowel syndrome
| Abdominal pain that is recurrent at least once a day or week in the last three months, along with a minimum of two of the following criteria: |
| Defecation-related conditions |
| Associated with a change in stool frequency |
| Associated with a change in form (appearance) of stool |
There is no individual-specific test to confirm IBS, and it is a combined approach involving factors mentioned in the Rome IV criteria (Table 1). These symptoms may seem atypical and may not be due to any other disorder [17]. Exacerbating factors like stress, other psychological disorders, and family history of other gastrointestinal diseases should be considered and this requires a physician's high index of suspicion after ruling out all other possibilities [17].
Treatment options for IBS
Given that IBS is usually a chronic condition, physicians must primarily focus on more sustainable therapeutic options, pharmacological or non-pharmacological. Currently, there are several non-pharmacological treatment options for IBS patients, the details of which will not be dealt with in this review. The benefits of lifestyle modifications, dietary changes, and probiotics must be analyzed with the help of evidence-based medicine so that clinicians navigate towards a holistic approach to treating patients. Given the benefits of probiotics for general gut health and improving the microflora, it is principal to scrutinize their possible role and side effects in IBS patients who are prone to not tolerating even small changes made to the enteric environment and to test the efficacy of probiotics concerning the common complaints (symptoms) mentioned by IBS patients as this condition significantly leads to a reduced quality of life [30].
A wide range of treatment options has been described in several studies. Dietary modifications for IBS patients include elimination diets (by eliminating foods that exacerbate symptoms), increased dietary fiber, a gluten-free diet, and exercise [31]. A diet with low fermentable oligo-di-mono-saccharides and polyols (FODMAPs) has been recommended to improve the global symptoms associated with IBS-D, such as pain and bloating [32,33]. A low FODMAP diet has also been recommended for patients with IBS as a first-line treatment in primary care [34]. The efficacy of probiotics in IBS has also been tested, and symptoms of bloating, abdominal pain, and flatulence showed significant improvements [35,36].
Probiotics are safe and effective in IBS patients, especially those used for a shorter duration such as for less than eight weeks; a higher dosage of a single probiotic strain seem to show greater benefits. The adverse events associated with probiotics are found to be safer in comparison to many other treatment options available today [37]. Probiotics have been shown to improve overall stool frequency, gut transit time, and stool consistency [38]. Bacillus coagulans strain LBSC (DSM17654) has been shown to be efficacious in alleviating IBS symptoms such as bloating, abdominal pain, constipation, diarrhea, nausea, vomiting, and stomach rumbling. It was found to be safe for human consumption and it helped improve the quality of life in IBS patients [39]. Thus, probiotics have an overall positive impact on IBS patients by improving their quality of life [40].
Medications to relieve the pain in IBS patients have been tested, but they come with noted side effects, e.g., antispasmodics (which caused more side effects such as blurred vision, dry mouth, constipation, and dizziness), peppermint oil (leading to symptoms of xerostomia, gastroesophageal reflux, and belching), antidepressants (with side effects like dry mouth and drowsiness), and loperamide (that reduced stool frequency, duration and intensity of pain yet an increased intensity of abdominal pain at night). Camilleri's observations concerning the efficacy of the drugs mentioned above did not show any significant improvement in symptoms [31]. However, some off-label drugs were tried by some studies, e.g., histamine H1 receptor agonist (ebastine) reduced overall IBS symptoms, abdominal pain, and visceral hypersensitivity [41]. GABAergic (gamma-aminobutyric acid) agents showed lower pain scores [42].
Medications for diarrhea include using 5-hydroxytryptamine or serotonin type 3 (5-HT3) receptor antagonists and have shown a significant effect on bloating, frequency, urgency, and stool consistency but no effect on pain sensation [43]. Bile acid sequestrants have also been tried for the same by using colestipol as an open-label treatment, and it showed an improvement in the severity scores of IBS patients and stool frequency [44]. Antibiotics like rifaximin showed an overall improvement in global symptoms and bloating but did not affect the frequency of bowel movements, urgency, or stool consistency [45].
A trial of lubiprostone (a chloride channel-related intestinal secretagogue) proved to improve the abdominal pain in IBS patients, although a common side effect of nausea was observed in them [46]. A 5-hydroxytryptamine or serotonin type 4 (5-HT4) receptor agonist like tegaserod has been shown to be effective in IBS-C patients and has improved productivity in work and quality of life [47].
The above-mentioned treatment modalities aid in appreciating the superiority of probiotics when their adverse events are compared to pharmacotherapeutic agents. Hence, we as researchers must continue analyzing the untapped benefits of probiotics in such chronic conditions to make more appropriate choices that are strain-specific to symptoms in IBS patients.
Sources of probiotics
The familiar sources of probiotics include kefir, yogurt, and some other fermented foods. All of these contain different microorganisms that potentially offer better gut health. The most commonly used organisms in these sources include Streptococcus thermophilus, Lactobacillus strains, Lactobacillus delbrueckii subsp. bulgaricus,and Bifidobacterium strains. Studies have proven that these enhance intestinal health and overall anti-inflammatory and immune responses [48].
Other than the usual Lactobacillus and Bifidobacterium species, some commonly used microorganisms in probiotic preparations include the Enterococcus and Streptococcus species. Different formulations of probiotic products are available, ranging from Bacillus species to yeasts (Saccharomyces cerevisiae and S. boulardii) and even Aspergillus oryzae, which is a filamentous fungus, all of which can be made available in the form of capsules, powders, pastes, tablets, or sprays depending on the feasibility. The use of probiotics is a more natural approach and has fewer side effects when compared to pharmacotherapeutic drugs [49].
A rising interest in tapping the benefits of probiotics is seen because of understanding what harmful effects can be brought about by the increased use of antibiotics that destroy the gut microbiota [50].
Therapeutic significance and prognostic value
Probiotics have long been indicated in several gastrointestinal conditions ranging from traveler's diarrhea to rotavirus enteritis and even in IBDs, colon cancer, acute pancreatitis, HIV-associated diarrhea, and IBS. Studies have shown that probiotics in the colonic mucosa help in producing essential nutrients, toxin elimination, improve intestinal immunity, prevent microbial translocation, and assist in the recovery of a disturbed gut mucosal barrier [51]. Many of the established effects of probiotics include their benefits in inflammatory diseases concerning the gastrointestinal tract, reduced unspecific complaints by healthy people, and improved stool consistency. Thus, when probiotics are consumed in sufficient amounts, they promote positive effects in the gut [52].
Dimidi et al. observed a decrease in Lactobacilli and Bifidobacterium and higher breath methane in adults complaining of functional constipation. Certain strains of probiotics can modify processes like secretion and motility of the gut and thereby alter the environment of the lumen [53]. The microbiota composition varies depending on the intestine's environmental conditions, an individual's diet, probiotic bacteria that are ingested, one's exposition to it, and how the host system permits the growth of new strains over a period dynamic [54,55].
A study conducted by Nobaek et al. observed the role of Lactobacillus plantarum in reducing the formation of gas in 60 IBS patients for four weeks. The two groups involved one receiving a plain rosehip drink and 400 ml of rosehip drink containing 5 × 107 CFU/ml of L. plantarum and 0.009 g/ml oat flour per day. A survey following the randomized control trial (RCT) revealed that it significantly reduced the complaint of flatulence in the test group and reduced abdominal pain in both groups. A better overall GI function had been reported at the 12-month follow-up by patients of the test group. However, there was no evidence that the L. plantatrum improved the bloating sensation in both groups [56].
To test the efficacy of probiotics that are commercially made available like VSL#3, a combination probiotic tablet consisting of bacterial strains of eight different types, namely, three strains of Bifidobacterium (B. breve, B. longum, B. infantis), four strains of Lactobacillus (L. acidophilus, L. plantarum, L. casei, and L. delbrueckii subsp. bulgaricus), and one strain of Streptococcus (S. salivarius subsp. thermophilus) on the various symptoms and colonic transit in patients with IBS. Kim et al. conducted a randomized, double-blind study in patients with IBS and bloating for a treatment duration of four and eight weeks. The study results mentioned that VSL#3 reduced flatulence and retarded the colonic transit without changing the function of the bowel in the same patients. For aspects of bloating, abdominal pain, and stool-related symptoms, the study did not observe satisfactory relief by the responders [57].
Dolin performed a randomized, double-blind, placebo-controlled clinical trial to test the role of probiotics in IBS-D and evaluate the effects of a probiotic that contained Bacillus coagulans GBI-30, 6086 in the same group of 52 patients over eight weeks during which they received either placebo or Bacillus coagulans GBI-30, 6086 each day. The study yielded a good outcome by significantly reducing the average number of bowel movements per day in comparison to the placebo group (P=0.042) [58].
Guglielmetti et al. conducted an RCT to analyze the therapeutic role of probiotics in IBS patients. They specifically tested the efficacy of Bifidobacterium bifidum MIMBb75 in 122 patients who were given either placebo (N=62) or MIMBb75 (N=60) once every day for four weeks. To assess the severity of the symptoms, they used a 7-point Likert scale, and the outcome involved a reduction in the global assessment of IBS symptoms. The IBS symptoms of pain (discomfort), distention (bloating), digestive disorder, and urgency significantly improved. Forty-seven percent of the patients given Bifidobacteria reported adequate relief. In comparison, only 11% of patients in the placebo group reported so (P<0.0001) [59].
Mezzasalma et al. carried out a randomized, double-double-blind parallel-group trial on 150 subjects with IBS-C to test the efficacy of two multispecies probiotic formulates. The subjects were divided into three groups (the third group being the placebo). For 60 days, probiotic formulations were administered to each group daily. This study concluded that the IBS-C patients responded well to each symptom, and a similar response was noticed even during the follow-up period when compared to the placebo group. It concluded that the supplementation of multispecies probiotics is effective in the IBS-C subjects [60].
The efficacy of Clostridium butyricum in treating IBS-D was tested by Sun et al. in 2018. It was a randomized, double-blind, prospective,multicentric, placebo-controlled trial and involved a trial of C. butyricum or placebo in a group of 200 patients for four weeks. The study revealed that the overall symptoms, stool frequency, and quality of life improved in IBS-D patients when treated with probiotics containing this particular species of the Clostridium group [61].
Yoon et al. conducted a study based on the Rome III criteria to analyze the efficacy of multispecies probiotics in patients suffering from IBS. It involved 49 IBS patients and was a randomized, double-blind, placebo-controlled trial. Patients were divided into two groups randomly and were either assigned placebo or multispecies probiotics twice a day for four weeks. The probiotics group was relieved substantially of the symptoms such as abdominal pain/discomfort, bloating, consistency, and stool frequency at the end of the four weeks. The multispecies probiotics were a mixture of B. longum, B. bifidum, B. lactis, L. acidophilus, L. rhamnosus, and S. thermophilus [62].
Saggioro et al. performed a clinical trial in 2004 that mentioned a possible role of the short short-term course of L. plantarum LP 01 and B. breve BR03 or L. plantarum LP 01 and LA 02 in treating the pain in different abdominal locations and severity associated with the various IBS symptoms. The trial based on the Rome II criteria was carried out for four weeks on 70 patients (31 males and 39 females) with a mean age of 40 years [63].
The effects of Escherichia coli strain Nissle 1917 (EcN) in IBS patients were studied by Kruis et al. through a prospective and double-blind study, using the Rome II criteria on 120 subjects for 12 weeks. The outcome was measured using the Integrative Medicine Patient Satisfaction Scale. They were randomized to either receive EcN (n=60) or a placebo (n=60). Although the responder rate was greater in the EcN group, there were significant differences only after weeks 10 and 11, with the best responses from that patient with the usage of antibiotics and gastroenteritis before the onset of IBS (prior alteration in the enteric microflora) [64].
The role of Medivac DS (consisting of Bacillus subtilis, Streptococcus faecium) was analyzed by Kim et al. on 40 patients with IBS. It was a double-blind, prospective study in which 20 subjects received a placebo while 20 of them received the Medivac DS probiotic for a treatment period of four weeks. The frequency and severity of abdominal pain decreased significantly in the Medivac DS group (Tables 2, 3). Thus, it proved its role in treating abdominal pain in IBS as it did not lead to any adverse events [65].
Table 2. Summary of included studies establishing the effect of probiotics in IBS patients.
GI, gastrointestinal; IBS, irritable bowel syndrome; IBS-C, IBS with predominant constipation; IBS-D, IBS with predominant diarrhea
| Author | Study design | Study population | Salient remarks |
| Nobaek et al., 2000 [56] | Randomized control trial | 60 IBS patients with a regular colonoscopy/barium enema | Flatulence reduced significantly in the test group. Abdominal pain reduced in both groups. Better overall GI function was observed during the follow-up. |
| Saggioro, 2004 [63] | Clinical trial | 70 IBS patients (31 males and 39 females) with a mean age of 40 years, including patients ranging between 26 and 64 years of age | Efficacious in reducing the severity of abdominal pain after a short course of probiotic therapy, responses that were checked on days 14 and 28 proved beneficial in IBS patients. |
| Kim et al., 2005 [57] | Randomized control, double-blind | IBS patients | Reduction in flatulence and colonic transit time without altering bowel function. |
| Kim et al., 2006 [65] | Double-blind, prospective study | 40 IBS patients | Marked reduction in the frequency and severity of abdominal pain in the subjects (P=0.044, P=0.038) but not in the placebo group. |
| Dolin et al., 2009 [58] | Randomized, double-blind | 52 IBS-D patients | Significantly reduced the average number of bowel movements (P=0.042). |
| Guglielmetti et al., 2011 [59] | Randomized control trial | 122 IBS patients | Improvement in pain, distention, urgency, and digestive disorder (P<0.0001). |
| Kruis et al., 2012 [64] | Double-blind, prospective study | 120 IBS patients | Significant improvement noted to be Δ20.0% points (95% CI 2.6, 37.4), P=0.01, and Δ18.3% points (95% CI 1.0, 35.7), P=0.02, after 10 and 11 weeks, respectively. Best responses were from those with prior alteration in enteric microflora (Δ45.7% points, P=0.029) and also showed greatest change in all groups. |
| Yoon et al., 2014 [62] | Randomized, double-blind trial | 49 IBS patients (probiotics: 25, placebo: 24) | Symptoms such as abdominal pain/discomfort, bloating, stool frequency, and consistency were relieved in the probiotics group compared to placebo (68% vs. 37.5%; P<0.05). |
| Mezzasalma et al. 2016, [60] | Randomized, double-blind, three-arm parallel-group trial | 150 IBS-C patients | Patients responded well to each symptom. Effectiveness proved well. |
| Sun et al., 2018 [61] | Randomized, double-blind, prospective study | 200 IBS-D patients | Overall symptoms, stool frequency, and quality of life improved. |
Table 3. Summary of included studies indicating prognostic significance.
IBS, irritable bowel syndrome; IBS-C, IBS with predominant constipation; IBS-D, IBS with predominant diarrhea
| Author | Prognostic outcomes |
| Nobaek et al., 2000 [56] | Reduced flatulence, abdominal pain, and bloating. Also, an overall improvement in gastrointestinal functions was observed. |
| Saggioro, 2004 [63] | Reduction in general pain at different abdominal locations and severity of various IBS symptoms. |
| Kim et al., 2005 [57] | Reduced flatulence and colonic transit time without altering bowel function. |
| Kim et al., 2006 [65] | The frequency and severity of abdominal pain reduced significantly. |
| Dolin et al., 2009 [58] | Reduced average number of bowel movements per day. |
| Guglielmetti et al., 2011 [59] | Reduction in the global assessment of IBS symptoms like pain, bloating, digestive disorder, urgency; adequate relief. |
| Kruis et al., 2012 [64] | Those with a prior history of gastroenteritis and antibiotics usage showed the best responses. Significant differences were recorded after about 10-11 weeks of probiotic use. |
| Yoon et al., 2014 [62] | Abdominal pain/discomfort, bloating were relieved substantially; stool frequency and consistency improved. |
| Mezzasalma et al., 2016 [60] | A better outcome was noticed in IBS-C subjects as it improved each symptom, including the follow-up period. |
| Sun et al., 2018 [61] | Overall symptoms, stool frequency, and quality of life improved in IBS-D patients. |
Hence, the prognostic role of probiotics in IBS has to be widely researched as they may have numerous potential benefits given the chronic course of the disorder and pharmacological management of the same population could lead to numerous side effects in a due period. It is critical to realize the above-mentioned utility benefits of probiotics in IBS and encourage their users to tap the several advantages they holds. Future investigations of probiotics in IBS patients should be aimed at identifying specific strains in relieving their symptoms.
Limitations
Given the pathophysiology of IBS, it is beyond the scope of this article to establish a confirmed etiology of the disorder as of today. Also, we have reviewed studies published over the years on only a few strains used in probiotic mixtures. Thus, the efficacies of the different species or their combinations used cannot be compared. This review has strictly limited its discussion to the usage of probiotics in IBS while not extending it to the role of lifestyle modifications and other supportive therapeutic interventions that complement a sooner recovery in the long course of IBS.
Conclusions
The primary objective of this review was to understand what role probiotics have (if any) in those suffering from IBS. It has been analyzed that several strains mentioned in the review have their functions of relieving and alleviating flatulence abdominal pain, including its frequency and severity, distention (bloating), digestive disorders, and urgency of the bowel. Probiotics have also proven beneficial in IBS patients by slowing down the transit time of the colon, reducing the average number of bowel movements per day, improving stool consistency, overall symptoms, and above all, the quality of life in these patients. It is noteworthy that adding probiotics to IBS patients' routine brings about symptomatic relief. A variety of strains in the probiotic mixtures have shown their benefits. Therefore, we conclude that probiotics have a beneficial role in chronic disorders like IBS. The future implications include encouraging researchers to be involved in more and more studies that can further help dictate management plans for IBS patients by possibly combining a few of these strains, given their benefits, and analyzing their competitiveness when used together in the enteric environment. Strain-specific probiotic usage targeting symptomatic management in IBS patients would be the next big leap in the field of gastroenterology.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Koloski NA, Talley NJ. Women and Health. Massachusetts, MA: Elsevier; 2012. Irritable bowel syndrome; pp. 1353–1366. [Google Scholar]
- 2.Drossman DA, Richter JE, Talley NJ, Thompson WG, Corazziari E, Whitehead WE. New York: Little, Brown; 1994. The Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology, and Treatment. A Multinational Consensus. [Google Scholar]
- 3.The epidemiology of irritable bowel syndrome. Canavan C, West J, Card T. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Hill C, Guarner F, Reid G, et al. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 5.Probiotics history. Gasbarrini G, Bonvicini F, Gramenzi A. J Clin Gastroenterol. 2016;50:0–9. doi: 10.1097/MCG.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 6.An evidence-based position statement on the management of irritable bowel syndrome. Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. https://pubmed.ncbi.nlm.nih.gov/19521341/ Am J Gastroenterol. 2009;104:0–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 7.Prevalence and demographics of irritable bowel syndrome: results from a large web-based survey. Andrews EB, Eaton SC, Hollis KA, et al. Aliment Pharmacol Ther. 2005;22:935–942. doi: 10.1111/j.1365-2036.2005.02671.x. [DOI] [PubMed] [Google Scholar]
- 8.Validation of symptom-based diagnostic criteria for irritable bowel syndrome: a critical review. Whitehead WE, Drossman DA. Am J Gastroenterol. 2010;105:814–820. doi: 10.1038/ajg.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiology of IBS. Choung RS, Locke GR III. Gastroenterol Clin North Am. 2011;40:1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.The epidemiology of irritable bowel syndrome in North America: a systematic review. Saito YA, Schoenfeld P, Locke GR III. https://pubmed.ncbi.nlm.nih.gov/12190153/ Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 11.Irritable bowel syndrome: a clinical review. Chey WD, Kurlander J, Eswaran S. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 12.Bowel habit subtypes and temporal patterns in irritable bowel syndrome: systematic review. Guilera M, Balboa A, Mearin F. Am J Gastroenterol. 2005;100:1174–1184. doi: 10.1111/j.1572-0241.2005.40674.x. [DOI] [PubMed] [Google Scholar]
- 13.Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Lovell RM, Ford AC. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Prevalence and risk factors of irritable bowel syndrome in healthy screenee undergoing colonoscopy and laboratory tests. Nam SY, Kim BC, Ryu KH, Park BJ. J Neurogastroenterol Motil. 2010;16:47–51. doi: 10.5056/jnm.2010.16.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Probiotics. Williams NT. Am J Health Syst Pharm. 2010;15:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 16.Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. Saha L. World J Gastroenterol. 2014;20:6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irritable bowel syndrome - from etiopathogenesis to therapy. Radovanovic-Dinic B, Tesic-Rajkovic S, Grgov S, Petrovic G, Zivkovic V. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162:1–9. doi: 10.5507/bp.2017.057. [DOI] [PubMed] [Google Scholar]
- 18.Irritable bowel syndrome. Ford AC, Sperber AD, Corsetti M, Camilleri M. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 19.The role of probiotics in digestive health. Ouwehand AC. Nutr Diet Suppl. 2015;7:103–109. [Google Scholar]
- 20.Latest insights on the pathogenesis of irritable bowel syndrome. Videlock EJ, Chang L. Gastroenterol Clin North Am. 2021;50:505–522. doi: 10.1016/j.gtc.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Mishima Y, Ishihara S. Int J Mol Sci. 2020;21:8664. doi: 10.3390/ijms21228664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Microbiome and its role in irritable bowel syndrome. Pimentel M, Lembo A. Dig Dis Sci. 2020;65:829–839. doi: 10.1007/s10620-020-06109-5. [DOI] [PubMed] [Google Scholar]
- 23.Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. J Gastroenterol. 2012;47:1177–1185. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 24.Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Dinan TG, Quigley EM, Ahmed SM, et al. Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Pharmacology of serotonin: what a clinician should know. De Ponti F. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alosetron and irritable bowel syndrome. Mayer EA, Bradesi S. Expert Opin Pharmacother. 2003;4:2089–2098. doi: 10.1517/14656566.4.11.2089. [DOI] [PubMed] [Google Scholar]
- 27.Visceral perception: inflammatory and non-inflammatory mediators. Bueno L, Fioramonti J. Gut. 2002;51:0–23. doi: 10.1136/gut.51.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Update on Rome IV criteria for colorectal disorders: implications for clinical practice. Simren M, Palsson OS, Whitehead WE. Curr Gastroenterol Rep. 2017;19:15. doi: 10.1007/s11894-017-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.What is new in Rome IV. Schmulson MJ, Drossman DA. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed K, Iswara K. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2022. Low-FODMAP diet. [PubMed] [Google Scholar]
- 31.Management options for irritable bowel syndrome. Camilleri M. Mayo Clin Proc. 2018;93:1858–1872. doi: 10.1016/j.mayocp.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 33.Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta-analysis. Didari T, Mozaffari S, Nikfar S, Abdollahi M. World J Gastroenterol. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diagnosis and management of irritable bowel syndrome in adults in primary care: summary of NICE guidance. Dalrymple J, Bullock I. BMJ. 2008;336:556. doi: 10.1136/bmj.39484.712616.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Ford AC, Quigley EM, Lacy BE, et al. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 36.Efficacy and safety of probiotics in irritable bowel syndrome: a systematic review and meta-analysis. Li B, Liang L, Deng H, Guo J, Shu H, Zhang L. Front Pharmacol. 2020;11:332. doi: 10.3389/fphar.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis based on seventeen randomized controlled trials. Wen Y, Li J, Long Q, Yue CC, He B, Tang XG. Int J Surg. 2020;79:111–119. doi: 10.1016/j.ijsu.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 38.Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: a prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT compliant] Gupta AK, Maity C. Medicine (Baltimore) 2021;100:0. doi: 10.1097/MD.0000000000023641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The effect of probiotics on quality of life, depression and anxiety in patients with irritable bowel syndrome: a systematic review and meta-analysis. Le Morvan de Sequeira C, Kaeber M, Cekin SE, Enck P, Mack I. J Clin Med. 2021;10:3497. doi: 10.3390/jcm10163497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A review of microbiota and irritable bowel syndrome: future in therapies. Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. Adv Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Wouters MM, Balemans D, Van Wanrooy S, et al. Gastroenterology. 2016;150:875–887. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 42.A placebo-controlled trial of pregabalin for irritable bowel syndrome. Saito YA, Almazar AE, Tilkes KE, Choung RS, Locke GR III, Zinsmeister A, Talley N. https://journals.lww.com/ajg/Fulltext/2016/10001/A_Placebo_Controlled_Trial_of_Pregabalin_for.521.aspx Am J Gastroenterol. 2016;111:0. [Google Scholar]
- 43.A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Garsed K, Chernova J, Hastings M, et al. Gut. 2014;63:1617–1625. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Gut. 2015;64:84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 45.The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Menees SB, Maneerattannaporn M, Kim HM, Chey WD. Am J Gastroenterol. 2012;107:28–35. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 46.Analysis of nausea in clinical studies of lubiprostone for the treatment of constipation disorders. Cryer B, Drossman DA, Chey WD, Webster L, Habibi S, Wang M. Dig Dis Sci. 2017;62:3568–3578. doi: 10.1007/s10620-017-4680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tegaserod in the treatment of irritable bowel syndrome (IBS) with constipation as the prime symptom. Layer P, Keller J, Loeffler H, Kreiss A. Ther Clin Risk Manag. 2007;3:107–118. doi: 10.2147/tcrm.2007.3.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yogurt and other fermented foods as sources of health-promoting bacteria. Kok CR, Hutkins R. Nutr Rev. 2018;76:4–15. doi: 10.1093/nutrit/nuy056. [DOI] [PubMed] [Google Scholar]
- 49.Probiotics and their fermented food products are beneficial for health. Parvez S, Malik KA, Ah Kang S, Kim HY. J Appl Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 50.Probiotics of diverse origin and their therapeutic applications: a review. Yadav M, Mandeep Mandeep, Shukla P. J Am Coll Nutr. 2020;39:469–479. doi: 10.1080/07315724.2019.1691957. [DOI] [PubMed] [Google Scholar]
- 51.Probiotics in gastroenterology. Fric P. Z Gastroenterol. 2002;40:197–201. doi: 10.1055/s-2002-22328. [DOI] [PubMed] [Google Scholar]
- 52.de Vrese M, Schrezenmeir J. Food Biotechnology. Advances in Biochemical Engineering/Biotechnology. Vol. 111. Berlin, Heidelberg: Springer; 2008. Probiotics, prebiotics, and synbiotics; pp. 1–66. [DOI] [PubMed] [Google Scholar]
- 53.Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Dimidi E, Christodoulides S, Scott SM, Whelan K. Adv Nutr. 2017;8:484–494. doi: 10.3945/an.116.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fate, activity, and impact of ingested bacteria within the human gut microbiota. Derrien M, van Hylckama Vlieg JE. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. Zhang C, Derrien M, Levenez F, et al. ISME J. 2016;10:2235–2245. doi: 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 57.A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Kim HJ, Vazquez Roque MI, Camilleri M, et al. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 58.Effects of a proprietary bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Dolin BJ. Methods Find Exp Clin Pharmacol. 2009;31:655–659. doi: 10.1358/mf.2009.31.10.1441078. [DOI] [PubMed] [Google Scholar]
- 59.Randomised clinical trial: bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life - a double-blind, placebo-controlled study. Guglielmetti S, Mora D, Gschwender M, Popp K. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 60.A randomized, double-blind, placebo-controlled trial: the efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. Mezzasalma V, Manfrini E, Ferri E, et al. Biomed Res Int. 2016;2016:4740907. doi: 10.1155/2016/4740907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sun YY, Li M, Li YY, et al. Sci Rep. 2018;8:2964. doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Yoon JS, Sohn W, Lee OY, et al. J Gastroenterol Hepatol. 2014;29:52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- 63.Probiotics in the treatment of irritable bowel syndrome. Saggioro A. J Clin Gastroenterol. 2004;38:0–6. doi: 10.1097/01.mcg.0000129271.98814.e2. [DOI] [PubMed] [Google Scholar]
- 64.A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. Int J Colorectal Dis. 2012;27:467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The effects of probiotics on symptoms of irritable bowel syndrome. (Article in Korean) Kim YG, Moon JT, Lee KM, Chon NR, Park H. https://pubmed.ncbi.nlm.nih.gov/16809947/ Korean J Gastroenterol. 2006;47:413–419. [PubMed] [Google Scholar]