Abstract
Introduction
Drug survival is an indirect measure of efficacy and safety and its post-marketing assessment using real-life data is invaluable.
Objectives
To investigate the survival of apremilast in a cohort of psoriasis patients treated with apremilast in a Greek hospital.
Methods
A retrospective cross-sectional study examined adult psoriasis patients receiving apremilast (March 2016 to January 2021). Primary endpoint was the cumulative survival probability at 52 weeks. Kaplan-Meier analysis was used to calculate survival probability. Cox regression analysis was performed to investigate potential risk factors for apremilast discontinuation.
Results
One hundred and two patients (29.4% females) with a mean age of 55.9 years (standard deviation 15.21) were included. Sixty-five patients (63.7%) had discontinued treatment by lock date: 19 (18.6%) due to lack of efficacy, 24 (23.5%) due to loss of efficacy, 15 (14.7%) due to adverse reactions, and 7 (6.9%) due to other reasons. Cumulative survival probability at 52 weeks was 52.1%. Median survival time for all reasons for discontinuation was 58 weeks (95% Confidence Interval 40.02, 75.98).
Conclusions
Approximately half of patients remained on apremilast after 1 year of treatment. Secondary drug failure was the most common reason for discontinuation.
Keywords: psoriasis, apremilast, survival, discontinuation, Greece
Take-home message.
Psoriasis treatment with biologics and small-molecule agents is of finite duration, most often due to efficacy-related reasons.
Apremilast survival was investigated in a real-life setting. Cumulative survival probability at 24, 52 and 208 weeks was 69.3%, 52.1% and 28.1%, respectively. Nineteen (18.6%), 24 (23.5%) and 15 (14.7%) patients discontinued apremilast due to lack of efficacy, loss of efficacy and adverse reactions, respectively.
Introduction
Apremilast (Otezla®, Amgen) is an orally administered PDE4 inhibitor, whose efficacy and tolerability for the treatment of moderate-to-severe plaque psoriasis was investigated in the ESTEEM phase III trials [1,2]. Psoriasis treatment with biologics and small-molecule agents is of finite duration, most often due to efficacy-related reasons [3] The probability that psoriasis patients will stay on a biologic treatment for ≥3 years is 53%-58% [4]. Anti-drug antibodies, genomic and transcriptomic parameters as well as non-compliance could possibly explain this phenomenon [4,5]. Forty-five of 382 (11.78%) ESTEEM 2 participants, who received apremilast, discontinued treatment due to lack of efficacy by week 52 [2]. Long-term (≥156 weeks) pooled data from the two ESTEEM trials showed that 34.7% of participants receiving apremilast discontinued treatment due to lack of efficacy, which was the most common reason for treatment cessation [6]. Drug survival – time from initiation to discontinuation of treatment – can be used as a surrogate measure for drug efficacy and tolerability [4]. As everyday practice may differ from the setting of clinical trials, real-world data is invaluable.
Objectives
This study was conducted to investigate the survival of apremilast in a cohort of patients treated for psoriasis in a real clinical setting.
Methods
A retrospective cross-sectional study was performed. Data was retrieved from the psoriasis archives of the 1st Dermatology Department, Aristotle University, Thessaloniki, Greece. All adult patients with any type of psoriasis, who had received at least one dose of apremilast from March 2016 until January 2021, were eligible for inclusion in the study. Dosing followed summary of product characteristics. Primary endpoint was cumulative survival probability at 52 weeks. Secondary endpoints were cumulative survival probability at weeks 24, 104, 156, and 208, percentage of patients achieving 75% reduction in their baseline Psoriasis Area Severity Index (PASI) score (PASI75) at weeks 16, 24, 52, 104 and 156 as well as mean/median drug survival for all reasons for discontinuation and stratified for specific reason (lack of and loss of efficacy, adverse events, other). Gender, age, body mass index (BMI, kg/m2), presence of scalp and nail psoriasis, presence of psoriatic arthritis, diabetes, hyperlipidaemia, hypertension and cardiovascular disease, as well as previous treatment with biologics were tested as potential predictors for drug discontinuation.
Treatment was considered discontinued if patients stopped receiving apremilast tablets. Reasons for discontinuation were primary drug failure (no ≥50% improvement in baseline PASI – PASI50 – by week 24), secondary drug failure (loss of achieved efficacy at two consecutive visits), adverse events and personal/other reasons. Patients lost to follow-up were considered to have discontinued treatment. Sequential recruitment of all eligible treated patients was performed to limit selection bias. The study protocol adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the First Dermatology Department, Aristotle University, Thessaloniki, Greece. Signed informed consent was obtained by all participating patients.
SPSS software version 25 (IBM Corp.) was used to perform all statistical analyses. Qualitative variables were described through relative frequencies. We used Shapiro-Wilk test to check for normal distribution of quantitative variables. Mean, standard deviation (SD) and 95% confidence interval (CI) were used in case of normal distribution, whereas median and interquartile range were used in the opposite case. A two-tailed significance level of <0.05 was set. Kaplan-Meier analysis was used to calculate survival probability. Study event was drug discontinuation due to any reason or loss to follow-up. Patients still on treatment were censored at the last available follow-up visit. Univariate and multivariate Cox regression analysis were performed to investigate potential risk factors for apremilast discontinuation (hazard ratio, significance level set at P ≤0.05). Akaike information criterion was used to choose the best-fitting model for survival prediction.
Results
Out of 2313 psoriasis patients registered in the psoriasis archives, 110 patients were potentially eligible for inclusion. Six patients did not give consent for study participation and 2 patients were excluded due to incompletely recorded data. We included 102 patients in our analysis (29.4% females, 70.6% males) with various types of psoriasis and a mean age of 55.94 years (SD 15.21). Patient baseline characteristics are presented in Table 1. Patients were followed-up for a total of 26,826 patient-weeks. Sixty-five patients (63.7%) had discontinued treatment by lock date: 19 (18.6%) due to lack of efficacy, 24 (23.5%) due to loss of efficacy, 15 (14.7%) due to adverse reactions, 3 (2.94%) due to other/personal reasons, while 4 (3.92%) were lost to follow-up. Cumulative survival probability at 24, 52, 104, 156, and 208 weeks was 69.3%, 52.1%, 39.8%, 31.2%, and 28.1%, respectively (Figure 1). Mean survival time per reason for discontinuation and PASI75 achievement rates are presented in Table 1. Median survival time for all reasons for discontinuation was 58 weeks (95% CI 40.02, 75.98). The only covariate found able to predict drug survival was the combination of cardiovascular disease and diabetes, which was associated with 78% less likelihood of apremilast discontinuation (hazard ratio 0.22, P = 0.035).
Table 1.
Patient Demographic Characteristics and Apremilast Survival
| Female | Male | ||||
|---|---|---|---|---|---|
| Sex†, n (%) | 30 (29.4) | 72 (70.6) | |||
| Age‡, years( | 57.1 (12.89, 57.1 – 52.29) | 55.17 (16.06, 51.37 – 58.97) | |||
| BMI§ (kg/m2) | 27.11 (8.01) | 27.68 (6.63) | |||
| Psoriasis duration§ (years) | 9 (14) | 13 (14) | |||
| Baseline PASI§ | 11 (5.4) | 11.7 (7.4) | |||
| Psoriatic arthritis† | 5 (16.7) | 15 (20.8) | |||
| Scalp psoriasis† | 28 (93.3) | 55 (76.4) | |||
| Nail psoriasis† | 13 (43.3) | 35 (48.6) | |||
| Smoking† | 15 (50.0) | 44 (61.1) | |||
| Comorbidities | |||||
| Hypertension† | 11 (36.7) | 23 (31.9) | |||
| Diabetes† | 6 (20.0) | 11 (15.3) | |||
| Hyperlipidemia† | 9 (30.0) | 18 (25.0) | |||
| Obesity† | 9 (30.0) | 24 (33.3) | |||
| Cardiovascular disease† | 5 (16.7) | 15 (20.8) | |||
| Median survival time (all reasons for discontinuation) 58 weeks 95%CI (40.02, 75.98) | |||||
| Mean survival time in weeks (95% Confidence Interval) | |||||
| All reasons for discontinuation | 96.75 (78.34, 115.15) | ||||
| Due to lack of efficacy | 178.06 (159.41, 196.72) | ||||
| Due to loss of efficacy | 152.35 (130.53, 174.17) | ||||
| Due to adverse events | 190.59 (174.45, 206.73) | ||||
| Due to other reasons (including loss to follow-up) | 211.06 (199.08, 223.05) | ||||
| Week 16 | Week 24 | Week 52 | Week 104 | Week 156 | |
| PASI75¶ | 20.80% | 46.00% | 45.74% | 30.33% | 13.20% |
number (percentage).
mean (standard deviation, 95% confidence interval).
BMI: Body Mass Index, median (interquartile range).
PASI75: percentage of patients having achieved 75% reduction in their baseline Psoriasis Area Severity Index score.
Figure 1.
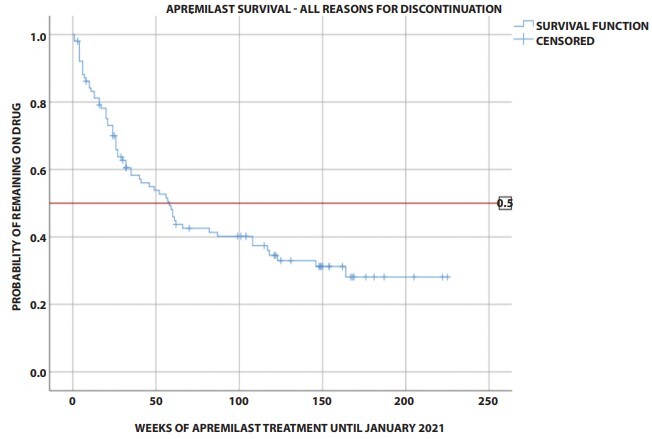
Cumulative survival probability of apremilast (Kaplan-Meier survival curve) for all reasons of drug discontinuation. Event: drug discontinuation due to any reason or loss to follow-up. Patients still on treatment were censored at the last available follow-up visit.
Conclusions
According to our results, approximately half of psoriasis patients treated with apremilast remained on treatment after one year and a little more than a quarter of them were still receiving the drug after 4 years of treatment. The most common reason for discontinuation was secondary drug failure. Patients suffering from both diabetes and cardiovascular disease were significantly less likely to discontinue apremilast. Limitations of our study are its retrospective nature, lack of a control group and absence of subgroup analysis of patients having received concurrent topical treatment.
According to real-world evidence, median apremilast survival ranges from 12.5 to 65 weeks, while 52-week survival probability ranges from 40.7% to 53.4% [7–11]. Two-year survival probability was 37.4% in a Japanese study [7]. Median time to drug discontinuation was 23 weeks for primary drug failure, 63 weeks for secondary failure and 8 weeks for adverse events [12]. Lunder et al found that apremilast had the lowest survival comparing to ustekinumab, adalimumab, etanercept, ixekizumab, infliximab and secukinumab, with ustekinumab having the longest duration in all examined psoriasis patients [3]. Patients on apremilast were more likely to discontinue treatment compared to patients on methotrexate in a nationwide French study [11]. In a cohort of patients with palmoplantar pustulosis, however, apremilast was associated with the longest survival (65 weeks) comparing to classic systemic treatments, such as cyclosporine, acitretin plus PUVA, methotrexate, acitretin, alitretinoin and fumaric acid esters [13]. Primary treatment failure was the most common reason for apremilast discontinuation in a USA study comparing various biologics and apremilast as well as in a smaller Austrian study [5,10]. Loss of efficacy was the main reason for apremilast cessation in a Japanese study (46.4%) [9]. Apremilast survival was significantly reduced in patients with scalp psoriasis (P = 0.001) in a small Spanish study [12]. The results of this study are comparable to other real-life apremilast survival data. Median apremilast survival at 52 weeks was found less than that of other systemic treatments for psoriasis, as presented in various real-life reports.
Footnotes
Funding: None.
Competing interests: None.
IRB approval: Ethics committee of The Hospital of Venereal and Dermatologic Diseases of Thessaloniki, Greece.
Authorship: All authors have contributed significantly to this publication
References
- 1.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 3.Lunder T, Zorko MS, Kolar NK, et al. Drug survival of biological therapy is showing class effect: updated results from Slovenian National Registry of psoriasis. Int J Dermatol. 2019;58(6):631–641. doi: 10.1111/ijd.14429. [DOI] [PubMed] [Google Scholar]
- 4.Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Br J Dermatol. 2020;183(2):294–302. doi: 10.1111/bjd.18981. [DOI] [PubMed] [Google Scholar]
- 5.Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34(2):e14826. doi: 10.1111/dth.14826.E. [DOI] [PubMed] [Google Scholar]
- 6.Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2) J Am Acad Dermatol. 2017;77(2):310–317.e1. doi: 10.1016/j.jaad.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 7.Saruwatari H. Real-world experiences of apremilast in clinics for Japanese patients with psoriasis. J Dermatol. 2019;46(12):1166–1169. doi: 10.1111/1346-8138.15104. [DOI] [PubMed] [Google Scholar]
- 8.Lee EB, Amin M, Wu JJ. Drug survival of apremilast in patients treated for psoriasis in a real-world setting. J Am Acad Dermatol. 2018;79(4):760–761. doi: 10.1016/j.jaad.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto M, Komine M, Kamiya K, Sugai J, Ohtsuki M. Drug survival of apremilast in a real-world setting. J Dermatol. 2019;46(7):615–617. doi: 10.1111/1346-8138.14943. [DOI] [PubMed] [Google Scholar]
- 10.Vujic I, Herman R, Sanlorenzo M, et al. Apremilast in psoriasis – a prospective real-world study. J Eur Acad Dermatology Venereol. 2018;32(2):254–259. doi: 10.1111/jdv.14598. [DOI] [PubMed] [Google Scholar]
- 11.Sbidian E, Billionnet C, Weill A, Maura G, Mezzarobba M. Persistence of apremilast in moderate-to-severe psoriasis: a real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br J Dermatol. 2020;182(3):690–697. doi: 10.1111/bjd.18047. [DOI] [PubMed] [Google Scholar]
- 12.Sahuquillo-Torralba A, de Unamuno Bustos B, Rodríguez Serna M, Monte Boquet E, Botella Estrada R. Treatment Persistence and Safety of Apremilast in Psoriasis: Experience With 30 Patients in Routine Clinical Practice. Actas Dermosifiliogr (Engl Ed) 2020;111(5):415–418. doi: 10.1016/j.ad.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Kromer C, Wilsmann-Theis D, Gerdes S, et al. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2019;17(5):503–516. doi: 10.1111/ddg.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]


