Abstract
Introduction
Minimal knowledge exists regarding skin cancers in Black individuals, which may adversely affect patient care.
Objectives
To describe clinical features and risk factors of skin cancers in Black individuals.
Methods
Retrospective study of Black individuals diagnosed with skin cancer between January 2000 and January 2020 at our institution.
Results
38,589 patients were diagnosed with skin cancer, of which 165 were Black individuals. One-hundred-thirteen of these Black individuals were diagnosed with melanoma, 35 with squamous cell carcinoma (SCC), and 17 with basal cell carcinoma (BCC). Most melanomas (80.0%, n = 90) were of the acral subtype; 75% (6 of 8 cases with dermoscopic images) displayed a parallel ridge pattern (PRP). The surrounding uninvolved background skin was visible in 7 cases, all demonstrating a PRP. This disappeared adjacent to most of the melanoma lesions (n = 4, 57.1%). creating a peripheral hypopigmented “halo”. The nonmelanoma skin cancers were pigmented and had similar dermoscopic features as reported in predominantly White populations. Most SCCs (n = 5, 71.4%) had a hypopigmented “halo” and most BCCs (n = 10, 55.6%) had an accentuated reticular network adjacent to the lesions.
Conclusions
Skin cancers are pigmented in Black individuals. In both acral melanomas and SCCs, we noted a peripheral rim of hypopigmentation between the lesions and the surrounding uninvolved background skin, while BCCs had accentuation of the background pigmentation adjacent to the lesions. Most acral melanomas displayed a PRP, which was also seen in surrounding uninvolved background skin.
Keywords: skin cancer, skin of color, ethnic skin, dermoscopy
Introduction
Skin cancer is the most common malignancy worldwide and its incidence is expected to increase [1,2]. Although much is known about the presentation of skin cancer in fair skin individuals, there is a paucity of data regarding disease morphology and contributing risk factors among those with darker skin [2–5]. This is likely secondary to the relatively higher incidence of skin cancer in those with fair skin, as well as disparities related to systemic racism in healthcare systems.
It is essential for healthcare providers to assess skin lesions with consideration of skin types. Experience has shown that skin cancer risk factors and morphology are not the same across all skin types, but there have been few studies specifically examining Black individuals [6,7]. This void increases the risk of missed diagnoses, and simultaneously may increase morbidity through the unnecessary biopsies of benign skin lesions.
Objectives
To address these knowledge gaps, we sought to describe the clinical and dermoscopic features of skin cancers in Black patients that were seen at Memorial Sloan Kettering Cancer Center (MSKCC). Additionally, we identified skin cancer risk factors and features of early disease.
Methods
This study was approved by the Institutional Review Board at MSKCC and adhered to the Helsinki declaration. We conducted a retrospective review of Black patients with biopsy-confirmed basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma seen at MSKCC between January 1st, 2000, and January 1st 2020. We identified patients with billing record ICD diagnostic codes for these skin cancers (n = 38,948). At our institution, race/ethnicity information is collected from all patients, and we selected those who self-identified as Black (n = 359). Racial information was missing from 344 cases and these were excluded. We screened medical records and included Black patients who received treatment for skin cancers at our institution (n = 165). We conducted a review of medical records through January 2020 and recorded demographic information, medical history, and skin cancer risk factors, including a history of cancer genetic syndromes, large congenital nevi, HPV infection, trauma, burns, and immunocompromised states. Additionally, we recorded the pathology diagnosis and anatomic location of each cancer. We excluded those who did not receive treatment at our institution (n = 194) since they did not have complete reports within our medical record system.
All lesions selected for biopsy by the dermatology service undergo clinical and dermoscopic imaging. Thus, we cross-referenced all 165 cases with our image database to conduct a subset analysis on available images. Most patients (n = 146) were referred to MSKCC after a biopsy was performed by a community-based practitioner, and therefore did not have images in our database. There were clinical and dermoscopic images of 37 lesions from 19 patients (11 melanomas from 11 patients, 8 SCCs from 3 patients, and 18 BCCs from 5 patients). We evaluated images for standard dermoscopic features [8–13]. We also evaluated the surrounding uninvolved background skin to identify dermoscopic structures or patterns present in this surrounding skin.
To analyze melanoma cases, we utilized the World Health Organization melanoma classification algorithm as a basis for subtype classification [14].
Frequencies, relative frequencies, means, standard deviations (SD), and ranges were used to describe the distribution of characteristics and dermoscopic features of skin cancers.
Results
Overall Results
Our search yielded a total of 165 Black patients diagnosed with BCC, SCC, or melanoma between January 1st, 2000 and January 1st, 2020. The average age at diagnosis was 58.9 years (SD = 3.2) and 59% (n = 96) were female. One hundred and thirteen patients (68.5%) were diagnosed with melanoma, 35 (21.2%) with SCC, and 17 (10.3%) with BCC.
Acral Lentiginous Melanomas (ALM)
The majority of melanomas were acral (80.0% of all melanoma cases, n = 90), with 83.4% (n = 75) occurring on plantar surfaces, 13.3% (n = 12) in nail units, and 3.3% (n = 3) on palmar surfaces. The average age at diagnosis was 61.7 years (SD = 13.0) and 52.2% (n = 47) were male.
Most plantar ALMs were invasive (Table 1) and half of these cases were classified as AJCC 8th edition stage IV melanoma at the time of initial biopsy. All dermoscopic images (n = 8) had structureless areas with multiple colors and most had a parallel ridge pattern (PRP) (Table 2). The surrounding uninvolved background skin was visible in 7 of these cases, all demonstrating a PRP and most also demonstrating a fibrillar pattern (Figure 1). Four of these cases (57.1%) demonstrated a peripheral hypopigmented “halo” (Figure 1). Additionally, diffuse plantar lentigines were found in 5 of these cases (71.4%).
Table 1.
Melanoma Characteristics
| Melanoma subtype | In-situ versus Invasive | Depth of invasion (mm) Mean (median, range) |
Ulceration Y/N | Mitotic index (mit/mm2) Mean (SD) | ||
|---|---|---|---|---|---|---|
| n (%) in-situ | n (%) invasive | n (%) yes | n (%) no | |||
| Plantar ALM (n=75) | 27 (36.00) | 48 (64.00) | 5.84 (4, 0.75–55) | 37 (49.33) | 38 (50.67) | 5.03 (5.91) |
| Nail-unit ALM (n=12) | 6 (50.00) | 6 (50.00) | 3.15 (2.9, 1.6–5) | 4 (33.33) | 8 (66.67) | 4.00 (4.30) |
| Palmar ALM (n=3) | 1 (33.33) | 2 (66.67) | 5.25 (5.25, 3.5–7) | 1 (33.33) | 2 (66.67) | 1.50 (0.71) |
| Other melanomas on non-acral and non-mucosal surfaces (n=19) | 1 (5.26) | 18 (94.74) | 7.31 (4.5, 0.4–20) | 3 (15.79) | 16 (84.21) | 5.25 (6.70) |
The pathologic characteristics of the melanomas observed in our Black patient population. ALM = acral lentiginous melanoma
Table 2.
Dermoscopic Features of Melanomas
| Dermoscopic Features | Prevalence: n (%) |
|---|---|
| Acral lentiginous melanoma cases on the soles (n=8) | |
| Structureless areas with multiple shades of brown, blue, black, and pink colors | 8 (100.00) |
| Parallel ridge pattern | 6 (75.00) |
| Ulceration | 2 (25.00) |
| Atypical fibrillar pattern | 2 (25.00) |
| Parallel ridge pattern of surrounding skin* | 7 (100.00) |
| Fibrillar pattern of surrounding skin* | 4 (57.14) |
| Peripheral hypopigmentation* | 4 (57.14) |
| Diffuse plantar lentigines* | 5 (71.43) |
| Acral lentiginous melanoma cases on the nail-unit (n=3) | |
| Hutchinson sign (pigmentation of the nail fold) | 3 (100.00) |
| Brown-to-black parallel lines on the nail plate with irregular spacing, thickness, and disruption of parallelism | 3 (100.00) |
| Band involving more than 2/3 of the nail plate | 2 (66.67) |
| Nail dystrophy | 1 (33.33) |
Figure 1.
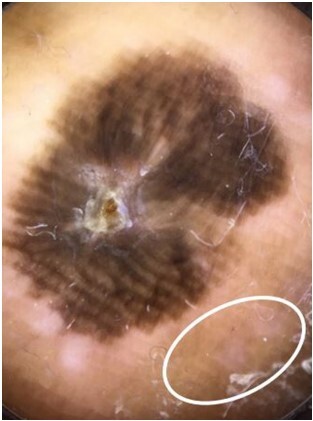
Acral melanoma on the sole of the foot. The normal background skin surrounding the acral melanomas had noticeable pigmentation demonstrating a parallel ridge pattern (solid oval). There was loss of the pigmentation and dermoscopic patterns surrounding the lesion itself, generating a hypopigmented “halo” around the acral lentiginous melanoma on the plantar surface. This “halo” was seen in 57.1% (n = 5) of plantar melanoma cases and was most evident in cases with the most heavily pigmented surrounding skin.
Half of the nail unit ALMs were invasive (Table) and two-thirds (n = 8) were classified as AJCC 8th edition stage IV melanoma at time of initial biopsy. There were dermoscopic images of 3 nail-unit melanomas, all of which were heavily pigmented (Table 2). The melanoma-in-situs (n = 2) demonstrated micro-Hutchinson sign and brown to black parallel lines on the nail plate with irregular spacing, thickness, and disruption of parallelism (Figure 2). The invasive melanoma (n = 1) demonstrated Hutchinson sign, pigmentation of the entire nail plate, and nail dystrophy (Figure 2).
Figure 2.
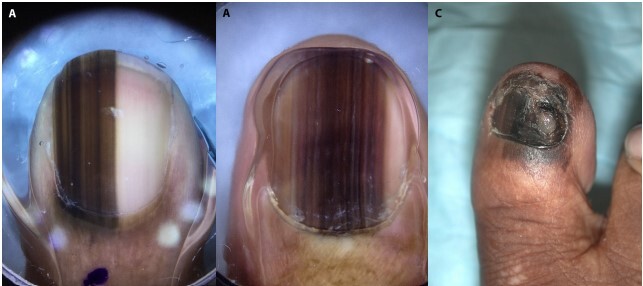
Nail melanoma cases. (A,B) The dermoscopic images of two melanoma-in-situ cases that demonstrate micro-Hutchinson sign and brown to black parallel lines on the nail plate with irregular spacing, thickness, and disruption of parallelism. (C) The clinical image of an invasive melanoma case that demonstrates and easily visible Hutchinson sign, diffuse pigmentation of the whole nail plate, and nail dystrophy.
There were only 3 palmar ALMs, 2 of which were invasive (Table 1) and classified as AJCC 8th edition stage IV melanoma at the time of initial biopsy. There were no dermoscopic images of these ALMs.
None of these patients had a family history of melanoma or had received testing for germline mutations increasing melanoma risk.
Melanomas, Not Otherwise Specified
Nineteen (16.8%) melanoma cases were on non-acral cutaneous surfaces, and the average age at diagnosis was 54.7 years (SD = 18.2). Twelve (63.2%) of these patients were female. Two-thirds of these cases presented as AJCC 8th edition stage IV melanoma (Table 1).
Most cases occurred on the lower extremities (31.6%, n = 6) and back (26.3%, n = 5). The most common subtype was spindle cell and/or epithelioid (36.8%, n = 7). One patient developed a dermal primary melanoma within a giant congenital nevus and no other risk factors were identified. There were no clinical or dermoscopic images.
Other melanoma subtypes included mucosal (n = 8, 7.1%), ocular (n = 4, 3.5%), and metastatic with unknown primary lesion (n = 6, 5.3%). Mucosal melanomas were located on the anus (n = 3), nasopharynx (n = 2), vagina (n = 2), and vulva (n = 1). No clinical or dermoscopic images were available.
Squamous Cell Carcinomas
There were 29 Black individuals with 35 SCCs, and the average age at diagnosis was 56 years (SD = 16). Most cases (71.4%, n = 25) were invasive. Most SCCs were located on anogenital surfaces (n = 11), lower extremities (n = 7), or the head and neck (n = 6). Known SCC risk factors were identified in 80% of patients (n = 28), including active HIV infection (n = 11), chronic wounds (n = 4), prior radiation to the area (n = 2), psoriasis (n = 2), hidradenitis suppurativa (n = 1), and lichen simplex chronicus (n = 1). HPV testing was conducted in 8 total cases, of which 7 were positive for high-risk HPV, located on the anogenital region (n = 3) and nail unit (n = 4).
There were dermoscopic images of 8 SCCs, many of which were heavily pigmented (Table 3). In the surrounding uninvolved background skin, half had a pigmented reticular network and an associated “halo” effect (Figure 3); the other half had patchy hyperpigmentation.
Table 3.
Dermoscopic Features of Non-Melanoma Skin Cancers (Basal Cell Carcinomas And Squamous Cell Carcinomas).
| DERMOSCOPIC FEATURES | PREVALENCE: n (%) |
|---|---|
| Pigmented squamous cell carcinoma cases (n=3) | |
| Adherent scales with pigment | 3 (100.00) |
| Peripheral hypopigmentation, loss of pigmented network seen in surrounding skin | 3 (100.00) |
| White circles | 1 (33.33) |
| Milky-red areas | 1 (33.33) |
| Non-pigmented squamous cell carcinoma cases (n=4) | |
| Adherent white scale | 4 (100.00) |
| White circles | 3 (75.00) |
| Ulceration | 3 (75.00) |
| Peripheral hypopigmentation, loss of pigmented network seen in surrounding skin | 2 (50.00) |
| Dotted vessels | 2 (50.00) |
| Serpentine vessels | 1 (25.00) |
| Shiny white strands | 1 (25.00) |
| Peripheral islands of hyperpigmentation | 1 (25.00) |
| Nail unit squamous cell carcinoma case (n=1) | |
| Heavy pigmentation of the nail plate | 1 (100.00) |
| Nail dystrophy | 1 (100.00) |
| Subungual hyperkeratosis | 1 (100.00) |
| Basal cell carcinoma cases (n=18) | |
| Loss of normal background pigmentation and network | 13 (72.22) |
| Milky red area | 12 (66.67) |
| Accentuated normal background pigmentation network surrounding the lesion itself | 10 (55.56) |
| Leaf-like areas | 9 (50.00) |
| Blue-gray ovoid nests | 9 (50.00) |
| Multiple blue-gray dots and globules | 9 (50.00) |
| Shiny white blotches and strands | 9 (50.00) |
| Central hypopigmentation | 6 (33.33) |
| Spoke-wheel like structures | 4/18 (22.22) |
| Scale and/or crust | 2/18 (11.11) |
| MAY globules | 1/18 (5.56) |
| Milky white areas | 1/18 (5.56) |
| Ulceration | 1/18 (5.56) |
| Peripheral hypopigmentation | 1/18 (5.56) |
Figure 3.
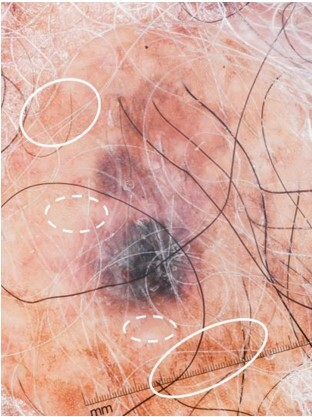
Loss of normal pigmented network surrounding the squamous cell carcinoma lesion. Note the pigmented network seen in the normal skin surrounding the pigmented squamous cell carcinoma (solid ovals) and the loss of network just adjacent to the lesion (dashed ovals). This was observed in 4 cases (57.1%).
Basal Cell Carcinomas
There were 15 Black individuals with 17 BCCs that were diagnosed and treated at our institution and the average age at diagnosis was 54.4 years (SD = 21.6). Most lesions were located on the head and neck (n = 12). Pigmentation was noted on the pathology report of 6 cases (35.3%). Pathologic subtypes included combined nodular and infiltrative (n = 6), micronodular (n = 3), nodular (n = 3), superficial (n = 1), and combined superficial and nodular (n = 1). Two patients had Gorlin syndrome, and no other risk factors were observed.
Dermoscopic images were available for 18 BCCs (from 5 patients), all of which were pigmented (Table 3). The surrounding uninvolved background skin was assessed in 15 cases. All cases had a pigmented reticular network, and 11 cases (73.3%) had an accentuated network adjacent to the lesion (Figure 4).
Figure 4.
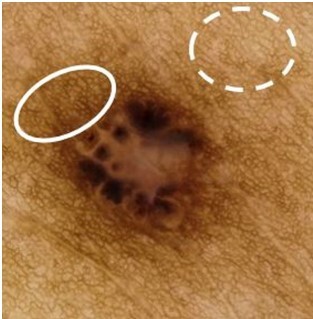
Accentuated pigmented network surrounding the basal cell carcinoma lesion. The pigmentation network observed in the normal skin (dashed oval) was accentuated surrounding the basal cell carcinoma lesion itself (solid oval). This was observed in 55.6% (n = 10) of the basal cell carcinoma cases.
Conclusions
Over a 20-year period, only a small proportion of our skin cancer patients were Black individuals; this is likely due to the overall low incidence of skin cancer in this population and also secondary to disparities in access to healthcare facilities [15]. Reports have suggested that skin cancers are found at more advanced stages in Black people compared to white individuals – possibly due to differences in screening, skin cancer knowledge, healthcare access, or cancer types across populations [16,17]. However, before recommending for or against screening or public education efforts to possibly address these disparities, it is imperative to discern the features of skin cancers in Black patients and determine whether these features can differentiate benign from malignant lesions in asymptomatic Black individuals.
It has been reported that melanomas in Black individuals are diagnosed at locally advanced stages with higher rates of metastases [18]. While overarching gross statistics reveal that melanoma thickness is greater in Blacks compared to Whites, the difference narrows when the comparison focuses on ALMs, the most common subtype in Blacks [18,19]. An analysis of the SEER database revealed that the average depth across all subtypes for Caucasians and African Americans was 1.09 mm and 2.02 mm, respectively; when stratifying for subtype, the average depth of ALMs was 2.05 mm and 2.28 mm, respectively [19]. Our review revealed a high proportion of advanced cases, but it is important to underscore that MSKCC is a tertiary cancer center and therefore it is more likely to see advanced cases.
It is interesting to note that 90.6% (n = 102) of melanomas in our population were ALM, mucosal, or ocular subtypes, all of which have not been associated with ultraviolet radiation (UVR) exposure. Superficial spreading and lentigo maligna melanomas are most closely associated with UVR exposure; these subtypes accounted for only 2 of our cases (1.8%), suggesting that UVR likely plays little to no role in the pathogenesis of melanoma in Black individuals [20–22]. As suggested by a recent systematic review, photoprotection will likely provide minimal benefit [20].
We were unable to identify any major risk factors in Black individuals. Benign pigmented macules and mottled hyperpigmentation on acral surfaces are suggested risk factors, but approximately 50% of Black individuals have these features [23–25]. While many of our patients had diffuse pigmented macules on the plantar surfaces, it is unlikely that this is a risk factor given the high prevalence of this finding and the low prevalence of melanoma in Black individuals [23,25].
The PRP has been suggested to have high sensitivity and specificity for early ALM in Caucasian and Asian populations, but it has not been validated in individuals of other races/ethnicities [26]. While the majority of our plantar melanoma cases demonstrated the PRP, these individuals also had the PRP in surrounding uninvolved background skin. A limitation of this finding is that the clinically uninvolved skin displaying the PRP was not biopsied. Therefore, it is possible that this PRP may represent subclinical extension of the melanomas, though we believe this is unlikely since the PRP was diffusely present in the surrounding uninvolved background skin in the majority of our cases. Additionally, the PRP has been described in benign ethnic pigmented macules [27,28]. Therefore, this knowledge adds credence that the PRP may lack discriminatory power in Black individuals; if used, it may lead to an escalation of unnecessary biopsies [29]. Future studies analyzing the PRP in Black individuals may benefit from acquiring dermoscopy images of contralateral volar surfaces. Regarding other dermoscopic features observed in our ALM cases, the presence of structureless areas with multiple colors proved most useful, but this was not helpful in differentiation of in-situ and invasive disease, since cases of all stages exhibited this feature.
A novel feature we identified was a hypopigmented “halo” surrounding 57.1% (n = 4) of the plantar melanoma lesions, all of which were melanoma in-situ cases. Further studies with larger sample sizes would be helpful in identifying whether this is a sensitive and specific feature for early diagnosis.
Early detection also remains a challenge for nail-matrix melanomas, since patients of color often have benign pigmented nail bands [30]. To better understand how to differentiate these benign from malignant lesions, we need data comparing ethnic pigmented nail bands and nail-matrix melanomas. While we await such studies, reassuring factors include the involvement of multiple nails and stability over time.
Regarding the other melanoma subtypes, no identifiable risk factors were found. One patient developed a dermal melanoma within a giant congenital nevus; dermoscopy will not be useful in the early identification of these dermal lesions.
Regarding squamous cell carcinomas (SCC), UVR exposure is the most common risk factor for the development of SCC in those with fair skin, but trauma and inflammatory processes predominate the pathogenesis in Black populations, a trend also observed in our population [1,2,31–33]. Most Black patients had SCCs on non-sun exposed areas, which is consistent with other literature reports, suggesting photoprotection will likely have little to no benefit [31,32].
SCCs in Black individuals are often superficial, discrete, hard lesions arising from an indurated, rounded, elevated base [31,32]. The dermoscopic features observed in our images were similar to those described in white individuals, but there was a higher incidence of pigmented variants [8,10,31,33]. We observed a similar “halo” pattern as was described for plantar ALMs, but a larger study is needed to validate the predictive importance of this result.
Finally, in terms of our basal cell carcinomas (BCC), The majority of BCCs (70.6%) occurred on the head and neck region, suggesting UVR may play some role in the pathogenesis [34]. The low incidence of BCC in Black individuals suggests that other factors must predispose certain individuals to be more sensitive to UVR. There is a known association between skin tone and BCC risk, of which Black individuals with lighter complexions are more likely to develop BCCs [35]. Two of our patients had Gorlin syndrome, but no other risk factors were observed.
Pigmented BCCs are quite rare overall but are the most prevalent type in Black populations [34,36]. All of our dermoscopy images were heavily pigmented, and the features were consistent with those known for pigmented BCCs. We observed reticular pigmentation in the surrounding uninvolved background skin, and 55.6% (n = 10) of cases had a hyperpigmented network adjacent to the lesion. Central hypopigmentation was seen in 33.3% (n = 6) of cases. These features are not to be confused with those of a dermatofibroma, a pigment network and central scar-like white patch [37]. It is important to note that no cases were associated with significant morbidity.
Our study is limited since it is a retrospective analysis of a single tertiary cancer center. Referral bias is likely the reason for the high proportion of advanced disease cases. It is also important to consider other socioeconomic limitations that may hinder the ability of some Black patients to seek care at MSKCC. We observed that BCCs and SCCs are pigmented and demonstrate similar dermoscopic features to those reported in predominately White populations. Our study brings into question the ability of the PRP to distinguish ALM from benign acral lesions. It has been reported that the PRP can be present in benign pigmented macules on volar surfaces, and we also observed this pattern in the surrounding uninvolved background skin, brining into question the reliability of this feature for differentiating benign and malignant lesions in Black patients. Future research is needed to elucidate whether the rim of peripheral hypopigmentation that we observed around the ALMs can assist in the differentiation of ALMs and benign pigmented macules.
Black individuals deserve equal care, which does not necessarily mean the same care. Instead, it means that Black individuals deserve customized care based on the features most useful at discriminating benign from malignant lesions. Towards this end, a better understanding of the clinical, morphologic, and dermoscopic features of skin cancers in those with darker skin is required.
Footnotes
Funding: Supported in part by Memorial Sloan Kettering Cancer Center’s NIH/National Cancer Institute Cancer Center support grant P30 CA008748.
Competing interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Gordon R. Skin cancer: an overview of epidemiology and risk factors. Semin Oncol Nurs. 2013;29(3):160–169. doi: 10.1016/j.soncn.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Gloster HM, Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741–764. doi: 10.1016/j.jaad.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 3.Buster KJ, Stevens EI, Eelmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30(1):53-viii. doi: 10.1016/j.det.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin and hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 Suppl):S12–S37. doi: 10.1016/j.ijwd.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80(5):387–394. [PubMed] [Google Scholar]
- 6.Hogue L, Harvey VM. Basal Cell Carcinoma, Squamous Cell Carcinoma, and Cutaneous Melanoma in Skin of Color Patients. Dermatol Clin. 2019;37(4):519–526. doi: 10.1016/j.det.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Ezenwa E, Stein JA, Krueger L. Dermoscopic features of neoplasms in skin of color: A review. Int J Women Dermatol. 2021;7(2):145–151. doi: 10.1016/j.ijwd.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato J, Horimoto K, Sato S, Minowa T, Uhara H. Dermoscopy of Melanoma and Non-melanoma Skin Cancers. Front Med (Lausanne) 2019;6:180. doi: 10.3389/fmed.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basal cell carcinoma. dermoscopedia. Vol. 11. UTC; Jun 8, 2019. [Accessed January 25, 2021]. dermoscopedia contributors – Ash Marghoob, Natalia Jaimes; p. 10. Available from https://dermoscopedia.org/w/index.php?title=Basal_cell_carcinoma&oldid=16531. [Google Scholar]
- 10.Squamous cell carcinoma. dermoscopedia. Vol. 17. UTC; Nov 27, 2020. [Accessed January 25, 2021]. dermoscopedia contributors - Florentina Dimitriou, Theresa Deinlein, Iris Zalaudek; p. 18. Available from https://dermoscopedia.org/w/index.php?title=Squamous_cell_carcinoma&oldid=17536. [Google Scholar]
- 11.SCC. dermoscopedia. Vol. 10. UTC; Sep 19, 2018. [Accessed January 12, 2021]. dermoscopedia contributors – Luc Thomas, Amelie Boespflug; p. 32. Available from https://dermoscopedia.org/w/index.php?title=SCC&ol-did=13642. [Google Scholar]
- 12.Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173(4):1041–1049. doi: 10.1111/bjd.14045. [DOI] [PubMed] [Google Scholar]
- 13.Starace M, Alessandrini A, Brandi N, Piraccini BM. Use of Nail Dermoscopy in the Management of Melanonychia: Review. Dermatol Pract Concept. 2019;9(1):38–43. doi: 10.5826/dpc.0901a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch Pathol Lab Med. 2020;144(4):500–522. doi: 10.5858/arpa.2019-0561-RA. [DOI] [PubMed] [Google Scholar]
- 15.Manuel JI. Racial/Ethnic and Gender Disparities in Health Care Use and Access. Health Serv Res. 2018;53(3):1407–1429. doi: 10.1111/1475-6773.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75(2 Suppl):667–673. doi: 10.1002/1097-0142(19950115)75:2+<667::aid-cn-cr2820751409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photo-protection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748–762. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166(17):1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 19.Mahendraraj K, Sidhu K, Lau CSM, McRoy GJ, Chamberlain RS, Smith FO. Malignant Melanoma in African-Americans: A Population-Based Clinical Outcomes Study Involving 1106 African-American Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1988–2011) Medicine (Baltimore) 2017;96(15):e6258. doi: 10.1097/MD.0000000000006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes FCPS, Sleiman MG, Sebastian K, Bogucka R, Jacobs EA, Adamson AS. UV Exposure and the Risk of Cutaneous Melanoma in Skin of Color: A Systematic Review. JAMA Dermatol. 2021;157(2):213–219. doi: 10.1001/jamadermatol.2020.4616. [DOI] [PubMed] [Google Scholar]
- 21.Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res. 2011;24(5):879–897. doi: 10.1111/j.1755-148X.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward N, Wilmott J, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 23.Coleman WP, 3rd, Gately LE, 3rd, Krementz AB, Reed RJ, Krementz ET. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116(5):548–551. [PubMed] [Google Scholar]
- 24.Lewis MG. Malignant melanoma in Uganda. (The relationship between pigmentation and malignant melanoma on the soles of the feet) Br J Cancer. 1967;2(3):483–495. doi: 10.1038/bjc.1967.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palicka GA, Rhodes AR. Acral Melanocytic Nevi: Prevalence and Distribution of Gross Morphologic Features in White and Black Adults. Arch Dermatol. 2010;146(10):1085–1094. doi: 10.1001/archdermatol.2010.299. [DOI] [PubMed] [Google Scholar]
- 26.Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol. 2010;162(4):765–71. doi: 10.1111/j.1365-2133.2009.09594.x. [DOI] [PubMed] [Google Scholar]
- 27.Phan A, Dalle S, Marcilly MC, Bergues JP, Thomas L. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147(5):634. doi: 10.1001/archdermatol.2011.47. [DOI] [PubMed] [Google Scholar]
- 28.Saida T, Miyazaki A, Oguchi S, et al. Significance of Dermoscopic Patterns in Detecting Malignant Melanoma on Acral Volar Skin: Results of a Multicenter Study in Japan. Arch Dermatol. 2004;140(10):1233–1238. doi: 10.1001/archderm.140.10.1233. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28(1):21–27. doi: 10.1097/01.dad.0000187931.05030.a0. [DOI] [PubMed] [Google Scholar]
- 30.Ruben BS. Pigmented lesions of the nail unit: clinical and histopathologic features. Semin Cutan Med Surg. 2010;29(3):148–158. doi: 10.1016/j.sder.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21(4):170–7. 206. quiz 178. [PMC free article] [PubMed] [Google Scholar]
- 32.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 33.Morais PM, Schettini APM, Rocha JA, Silva RCD., Júnior Pigmented squamous cell carcinoma: case report and importance of differential diagnosis. An Bras Dermatol. 2018;93(1):96–98. doi: 10.1590/abd1806-4841.20186757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta R, Gordon SL, Council ML, Hurst EA. Clinical Characteristics of Basal Cell Carcinoma in African Americans: A 10-Year Retrospective Review at a Single Academic Institution. Dermatol Surg. 2019;45(5):660–665. doi: 10.1097/DSS.0000000000001744. [DOI] [PubMed] [Google Scholar]
- 35.Beckenstein MS, Windle BH. Basal cell carcinoma in black patients: the need to include it in the differential diagnosis. Ann Plast Surg. 1995;35(5):546–548. doi: 10.1097/00000637-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Nouri K. Skin cancer. New York, NY, USA: McGraw-Hill Medical; 2008. [Google Scholar]
- 37.Kelati A, Aqil N, Baybay H, Gallouj S, Mernissi FZ. Beyond classic dermoscopic patterns of dermatofibromas: a prospective research study. J Med Case Rep. 2017;11(1):266. doi: 10.1186/s13256-017-1429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


