Abstract
Background
Racial/ethnic health inequities have been well-documented among youth and young adults with type 1 diabetes (T1D), yet little is known about how socioeconomic position (SEP) intersects with the risk marker of race/ethnicity to predict inequities in longitudinal glycemic control.
Purpose
To identify patterns of SEP, race/ethnicity, and clinical characteristics that differentiate hemoglobin A1c (HbA1c) trajectories among youth and young adults after T1D diagnosis.
Methods
The SEARCH for Diabetes in Youth cohort includes youth with diabetes diagnosed from 2002 to 2006 and 2008 who were followed through 2015. We analyzed data from 1,313 youth and young adults with T1D with ≥3 HbA1c measures. Classification tree analysis identified patterns of baseline demographic, SEP, and clinical characteristic that best predicted HbA1c trajectories over an average of 8.3 years using group-based trajectory modeling.
Results
Two HbA1c trajectories were identified: Trajectory 1 (77%) with lower baseline HbA1c and mild increases (from mean 7.4% to 8.4%) and Trajectory 2 (23%) with higher baseline HbA1c and major increases (from 8.5% to 11.2%). Race/ethnicity intersected with different SEP characteristics among non-Hispanic white (NHW) than in non-whites. Public health insurance predicted high-risk Trajectory 2 membership in non-whites, whereas parental education, household structure, diagnosis age and glucose checking frequency predicted membership for NHW youth and young adults. Two characteristics, race/ethnicity and parental education alone identified 80% of the Trajectory 2 members.
Conclusions
Race/ethnicity intersects with multiple SEP and clinical characteristics among youth and young adults with T1D, which is associated with particularly high risk of poor long-term glycemic control.
Keywords: Type 1 diabetes, Health inequities, Race, Ethnicity, Socioeconomic position, Intersectionality
Among youth and young adults with type 1 diabetes, different markers of socio-economic position predicted poor glycemic control over time in non-white compared to non-Hispanic white participants.
Introduction
Optimizing glycemic control is the overarching goal of type 1 diabetes mellitus (T1D) management based on evidence that normalizing blood glucose reduces the risk of chronic complications and premature mortality [1–3]. A majority of youth and young adults with T1D do not achieve glycemic target ranges, with recent data showing only 17% of youth and 21% of adults meeting current recommendations [4, 5]. Moreover, the qualitative patterns of glycemic control that youth may experience over time are heterogeneous, with increasing hemoglobin A1c (HbA1c) often observed in adolescence and young adulthood [5]. We have previously identified three HbA1c trajectories in a population-based study of youth and young adults with T1D, two of which were unfavorable, starting out from moderate levels at about 8% HbA1c and then worsening over time to about 10% HbA1c [6].
Racial/ethnic inequities in glycemic control among youth and young adults with T1D, including in longitudinal HbA1c measures, have been documented for decades [7–10]. Non-white youth and young adults with diabetes, particularly non-Hispanic black youth and young adults, have significantly higher average HbA1c and higher frequencies of very poor glycemic control than non-Hispanic white youth and young adults [4, 6, 8, 11]. These differences need to be interpreted bearing in mind that race/ethnicity is a complex social construct and a marker of risk, not a causal risk factor. Among the fundamental causes of racial/ethnic inequities are structural or institutional racism (i.e., societal and institutional practices, policies, and laws that differentially advantage white people and disadvantage people of color) [12–14] and differences in socioeconomic position (SEP), including both resources (i.e., material and social resources and assets) and prestige (i.e., rank or status in a social hierarchy) [15].
Moving beyond the description of race/ethnic health inequities in the United States (US) toward an understanding and quantification of potential actionable intervention points is complicated because SEP-related inequities in glycemic control manifest along multidimensional and related social categories (e.g., access to health insurance and health care, education, income, family composition, household structure, etc.) and macro-level characteristics (e.g., structural racism, neighborhood segregation, social cohesion, and deprivation) [7, 16–21]. Using multivariable regression models to parse out independent effects of race/ethnicity from SEP is problematic given the high degree and complexity of interrelationships. Whereas including statistical interaction terms in regression models is a step toward allowing for more complexity, fundamentally this method does not align well with the high complexity of interrelationships. Further, regression methods are not well-suited to detangle heterogeneity in patterns of health inequity across the population, for example, by identifying subgroups with different subsets of characteristics or experiences that intersect as risk factors for poorer health outcomes over time.
The intersectionality framework posits that multiple forces of social inequity intersect to affect the ways in which individuals experience oppression [22].This framework explicitly promotes the consideration of intersecting identities (e.g., race/ethnicity and sex), and thus multiple explanatory characteristics simultaneously in investigations of health inequities. Intersectionality research is well-established in the social sciences but less integrated in public health or medicine [23–25].Whereas intersectionality has primarily been applied to the study of the intersection of identities defined by race, ethnicity, and sex, a broader range of SEP characteristics (e.g., single parenthood, lack of health insurance, etc.) has been incorporated by some investigators as these characteristics can also compound structural inequalities [26].
Although a body of qualitative and ethnographic intersectional diabetes research exists, there are few quantitative intersectional diabetes studies [27, 28]. Given the multitude of influences on glycemic control, the understanding of inequities in glycemic control may be advanced by studying the combined influences of race/ethnicity and multiple other contributing characteristics as well as elucidating how the influences of different characteristics depend on each other.
Therefore, our aim was to use a classification tree-based method to the identification of patterns of race/ethnicity, SEP, and clinical characteristics early in the course of diabetes that predict and differentiate longitudinal patterns of glycemic control among youth and young adults with T1D over the first decade after diagnosis. Our intent was to advance the understanding of the previously observed racial and ethnic inequities in glycemic control to identify structural characteristics that place individuals at particularly high risk for long-term poor glycemic control and to advance our understanding of how different characteristics may work together with the ultimate goal of developing more effective intervention programs for youth with diabetes.
Methods
Study Design
The SEARCH for Diabetes in Youth study began in 2000 as a multicenter surveillance study of physician-diagnosed diabetes mellitus in youth younger than age 20 at diagnosis [29]. Across the subsequent funding cycles, the study evolved to support a prospective observational cohort. The SEARCH Cohort Study is comprised of cases identified through the surveillance effort (Online Supplemental Figure 1), including cases newly diagnosed in 2002–2005 (SEARCH Phase 1) and 2006 and 2008 (SEARCH Phase 2) who had a baseline research visit around the time of diagnosis and had diabetes for at least five years upon inclusion in the longitudinal cohort. Some cohort study participants diagnosed from 2002 to 2006 already had multiple visits because they were eligible for 12-, 24- and 60-month follow-up visits during SEARCH 2. The data collection sites were located in South Carolina, Ohio, Colorado (including Navajo Nation), Washington, and California. The study was approved by and followed procedures in accordance with the ethical standards of the respective local institutional review boards. Parents of participants under age 18 provided written informed consent while participants over age 8 provided assent; all participants aged 18 years or older provided written informed consent.
Sample Inclusions and Exclusions
The present analysis included youth and young adults with T1D. Diabetes type was based on the clinical diagnosis made by a physician or other health care professional within 6 months after diabetes diagnosis and was abstracted from medical records. To be consistent with a previous publication from this cohort on HbA1c trajectories [6], the inclusion criteria included having three or more measures of HbA1c which, in effect, limited the sample to those with diagnosis dates between 2002 and 2005 (as 2006 and 2008 incidence cases could not have accrued more than two measures of HbA1c by the end of the SEARCH Cohort Study). Thus, starting from the 1,931youth diagnosed with T1D between 2002 and 2005 and excluding 618 who had fewer than three measures of HbA1c during the ~9 years of follow-up through 2015 left 1,313 participants with data for this analysis.
Data Collection and Classification of Variables
At each study visit, participants completed questionnaires, had a variety of physical examinations, and had a fasting blood sample drawn by trained research staff. Whole blood samples were analyzed for HbA1c by the Northwest Lipid Metabolism and Diabetes Research Laboratories in Seattle, WA, using an automated nonporous ion-exchange high-performance liquid chromatography system (model G-7; Tosoh Bioscience, Montgomeryville, Pennsylvania) [3, 4, 30, 31].
Questionnaires included demographic questions on sex, race, and ethnicity modeled after the US Census [32, 33] and responses were re-categorized as non-Hispanic white (n = 1011) and non-white (n = 302). The non-white category included participants that self-identified as non-Hispanic African American (n = 128), Hispanic of any race (n = 140), and 34 participants of Asian-American, Native American, Asian Pacific Islander or Other race/ethnicity and those with unknown race and ethnicity. Collapsing race and ethnicity into a single binary variable was necessary to have sufficient sample sizes in subgroups to perform subsequent analyses. We conceptualize racial/ethnic groups as socially constructed categories.
At the time of the baseline visit, the parent/guardian reported their highest educational degree or level of schooling completed, as well as that of their child’s other parent/guardian, selecting from 16 different response categories, which were subsequently collapsed into a dichotomous variable as highest-educated parent having at least a college degree versus having less than a college degree [34, 35]. To assess household income, participants were presented with nine income ranges from “less than $5,000” to “$100,000 or greater.” Household income was categorized as <$25,000, $25,000–49,999, $50,000–$74,999, ≥$75,000. Current health insurance type was queried by asking about the kind of health insurance or health care plan, offering eight response choices which were subsequently grouped into private health insurance (i.e., insurance through employer, purchased independently, or from military) versus public (e.g., Medicaid, Medicare, state, tribal or other government-sponsored health plan, or Indian Health Service) [36]. Eight individuals indicating no health insurance or other types of insurance were excluded. If both private and public insurance were selected, participants were allocated to the private category [37]. Household structure and composition were queried and classified as single-parent versus two-parent or other structure, and as having more than 4 persons in the household versus 4 or fewer based on the variable’s distribution. Parental education, household income, health insurance type, household structure and composition were used to represent SEP in the analyses and were ascertained at the time of the baseline visit.
Information on age at diagnosis was computed based on the date of diagnosis and date of birth. Diabetes duration was calculated as the difference in months between date of each visit and date of diagnosis and used for the trajectory analyses. The duration of diabetes at the time of the baseline visit was used for the regression analyses.
Additional clinical variables from the baseline visit included self-reported information on insulin regimen (which was based on mode of insulin delivery and was classified as using a pump, using long-acting insulin in conjunction with rapid-acting insulin injections ≥3 times per day, and using long-acting with any other form of multiple daily injections) and self-monitoring of blood glucose (SMBG) which was reported by the participant and categorized as ≥4 times per day (in accordance with current guidelines) versus <4 times per day.
Statistical Methods
Outcome specification: groups based on HbA1c trajectories
In a first analytical step, we specified the outcome of interest, groups of HbA1c trajectories, which was needed to address the main research question. In this step, group-based trajectory modeling was used to identify groups of individuals in the T1D sample with similar underlying patterns in HbA1c values over time [38]. Models used a normal distribution for HbA1c values, and duration of diabetes in months was used as the time scale. The optimal number of trajectories was determined based on goodness-of-fit statistics, interpretability and class size [6]. Trajectory modeling uses all available data for each participant and is robust to data that are missing at random. Details about trajectory analysis have been described elsewhere [39, 40]. Models were fit using PROC TRAJ of SAS statistical software (v9.4, SAS Institute, Cary, NC). After determining the number of trajectories, we assigned each participant to her or his most likely trajectory group given their observed HbA1c pattern. We then examined patterns of SEP, race/ethnicity, and clinical characteristics that differentiate these HbA1c trajectories.
Classification tree analysis
We employed the conditional inference tree (CTree) method to identify patterns among the independent participant characteristics that classify youth and young adults with T1D into HbA1c trajectory groups. CTree is a method within the domain of decision trees which are a family of non-parametric statistical models whose goal is to identify subgroups of individuals defined by combinations of variables (e.g., SEP characteristics) that are homogenous with respect to the outcome of interest (e.g., HbA1c trajectory group, see below). A strength of decision trees is their ability to explore non-linear and complex relationships between predictors [41], making them ideally suited to the exploration of the intersecting impact of more than two variables. In contrast, the inclusion of many interaction terms in regression models leads to complex, difficult to fit and interpret models. Excellent illustrations of the classification tree methodology have been published [26, 42]. In brief, decision trees build a tree through recursive partitioning, so that the sample is split successively into homogenous subgroups. When the outcome is categorical, these trees are referred to as classification trees in contrast to regression trees for continuous outcomes. In this analysis, we use the CTree method to build the tree. This method uses a statistical hypothesis testing framework in building the decision tree in contrast to the original method for classification and regression trees. At each node, the CTree algorithm tests all predictors and selects the predictor and binary split that gives the best discrimination between the HbA1c trajectory groups. This discrimination is measured by the p-value corresponding to a test for the partial null hypothesis of independence between a single predictor and the outcome, i.e., the HbA1c trajectory group. Within each subgroup, the process repeats until no significant differentiation in the outcome groups is possible.
Random sampling without replacement was used to split the sample into a training (70%) and a testing sample (30%). The training dataset was used to generate the predictor models and the resulting tree. The test dataset was used to generate model performance, using the area under the receiver operating characteristic curve (AUC) to assess the accuracy of each tree in predicting the HbA1c trajectory group. We generated three unique trees by conducting three model runs: (a) demographic input variables only (age at diagnosis, sex, race/ethnicity), (b) demographic and SEP variables (parental education, household income, health insurance type, household structure and composition), and (c) demographic, SEP, and select clinical variables (insulin regimen, frequency of SMBG). This sequence was selected because from model 1 we hoped to get information on the key demographic predictors of the HbA1c trajectories that can be directly compared to the analysis of Kahkoska et al., [6] model 2 provided the answer to our research question as to which patterns of SEP characteristics exist that predict HbA1c trajectories, and model 3 allowed consideration of select clinical characteristics which are of interest to clinical care. Including both insulin regimen and frequency of SMBG resulted in severe collinearity and a slight AUC reduction and could thus only be assessed independently. Therefore, the more predictive clinical characteristic was retained in model 3, frequency of SMBG. CTree was implemented using the ctree package in R version 3.5.1. [43].
Results
Participant Characteristics
The final analysis included 1,313 youth and young adults with a provider diagnosis of T1D before the age of 20 years. About three quarters (77%) were non-Hispanic white, 49% were female, and the average age was 9.7 years (SD = 4.3, min = 1, max = 20) at the initial SEARCH research visit with a mean diabetes duration of 9.2 months (SD = 6.3) at baseline (Table 1). More than half (53%) were diagnosed before age 10. In terms of SEP characteristics, 24% of the youth and young adults lived in single-parent households and 62% had more than four persons in the household, 81% had private health insurance, 49% of the parents had a college degree or more education, 41% had a household income above $75,000. In terms of clinical characteristics, 87% conducted SMBG four or more times per day. The distribution of medication regimens included 8% using an insulin pump, 32% using long-acting insulin in combination with rapid-acting insulin injections 3 or more times a day and 60% utilized other combinations of insulin that did not include a long-acting insulin. Participants in our analytic sample were followed on average 8.3 years (SD = 2.0, range = 2–13 years) from time of diagnosis and had 3 or more hemoglobin HbA1c measures over that time span (37.4% had 3 measures, 37.8% 4 measures and 24.8% 5 measures).
Table 1.
Baseline SEP and clinical characteristics of youth and young adults with T1D participating in the SEARCH Cohort Study according to HbA1c trajectory group, N = 1,313
| Total Sample N = 1,313 |
Trajectory 1: Lower baseline HbA1c with minor increases N = 1,008 |
Trajectory 2: Higher baseline HbA1c with major increases N = 305 |
P-value | |
|---|---|---|---|---|
| Race and/or Ethnicity (N = 1313) | ||||
| Non-whitea | 302 (23.0) | 181 (18.0) | 121 (39.7) | <0.0001 |
| Non-Hispanic white | 1,011(77.0) | 827 (82.0) | 184 (60.3) | |
| Sex (N=1313) | ||||
| Female | 647 (49.3) | 487 (48.3) | 160 (52.5) | 0.2045 |
| Male | 666 (50.7) | 521 (51.7) | 145 (47.5) | |
| Age at Diagnosis < 10 years | 700 (53.3) | 574 (56.9) | 126 (41.3) | <0.0001 |
| Diabetes Duration, months (N = 1313) | 9.2 (6.3) | 9.0 (6.3) | 9.9 (6.3) | <0.0001 |
| Single Parent Household (N = 1,272) | ||||
| Yes | 311 (24.4) | 201 (20.4) | 110 (38.6) | <0.0001 |
| No | 961 (75.6) | 786 (79.6) | 175 (61.4) | |
| Number of People in the Household > 4 (N = 1,306) | 804 (61.6) | 616 (61.4) | 188 (62.1) | 0.8433 |
| Health Insurance (N = 1303) | ||||
| Private | 1,052 (80.7) | 852 (85.1) | 200 (66.2) | <0.0001 |
| Public | 251 (19.3) | 149 (14.9) | 102 (40.6) | |
| Parent Education (highest) (N = 1,305) | ||||
| College degree | 636 (48.7) | 544 (54.3) | 92 (30.4) | <0.0001 |
| Less than college degree | 669 (51.3) | 458 (45.7) | 211 (69.6) | |
| Household Income (N = 1227) | ||||
| < $25,000 | 168 (13.7) | 104 (11.0) | 64 (23.1) | <0.0001 |
| $25,000-49,000 | 272 (22.2) | 188 (19.8) | 84 (30.3) | |
| $50,000-74,000 | 282 (23.0) | 223 (23.5) | 59 (21.3) | |
| $75,000 or more | 505 (41.2) | 435 (45.8) | 70 (25.3) | |
| Insulin Regimen (N = 1303) | ||||
| Pump | 106 (8.1) | 92 (9.2) | 14 (4.7) | 0.0068 |
| Long-acting + rapid- acting 3+/day | 418 (32.1) | 331 (33.0) | 87 (28.9) | |
| All other insulin combos except long-acting | 779 (59.8) | 579 (57.8) | 200 (66.4) | |
| SMBG (N = 1306) | ||||
| 4 or more times/day | 1,134 (87.0) | 890 (88.8) | 244 (81.1) | 0.0004 |
| < 4 times/day | 169 (13.0) | 112 (11.2) | 57 (18.9) |
aThe non-white group includes 128 persons identifying as Black, 140 identifying as Hispanic and 35 persons identifying as Asian-American, Native American, Asian Pacific Islander, Other, or multiple race/ethnic groups.
HbA1c trajectories
In our previous work with this same sample [6], three HbA1c trajectories were identified and were named based on the shape of the trajectory over the follow up visits, characterized by: (1) low baseline HbA1c and mild increases (50.7%), (2) moderate baseline HbA1c and moderate increases (41.7%), and (3) moderate baseline HbA1c and major increases (7.5%). For the present analysis, however, we chose a more parsimonious two-group trajectory solution which was qualitatively similar to the three-group solution and aided in interpreting classification tree findings. The two-group solutation also mitigated concerns about the small size of the third group, which would be reduced further when splitting the sample into a training and testing dataset and would have limited power for identifying subgroups at risk for membership in this trajectory group.
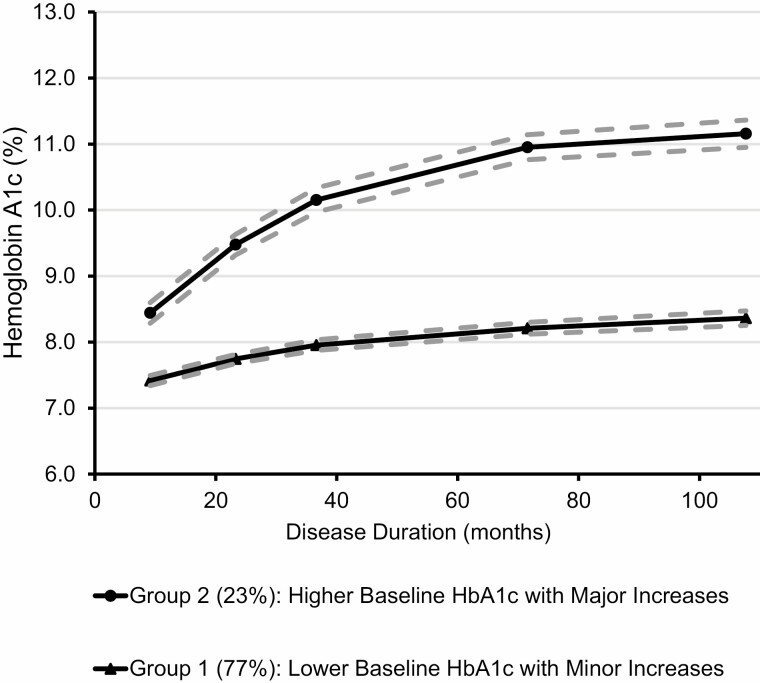
Trajectory 1 (77%) had a lower baseline HbA1c with minor increases over time (from mean 7.4% to 8.4% on average), and Trajectory 2 (23%) had a higher baseline HbA1c with major increases over time (from 8.5% to 11.2%, on average) (Fig. 1). Compared with Trajectory 1, participants with Trajectory 2 were significantly more likely to be of non-white race/ethnicity, to be diagnosed at an older age (age ≥10 years), have a parent with less than a college education, lack private health insurance at baseline, have a lower household income at baseline, use insulin combinations other than long acting and pump, and check their glucose less frequently (Table 1).
Fig. 1.
Trajectories of glycemic control based on HbA1c in 1,313 youth and young adults with T1D in the SEARCH for Diabetes in Youth Cohort Study.
SEP Patterns Determined by Classification Tree Analyses
CTree analysis first modeled patterns among demographic characteristics (earlier age of diagnosis, sex, and race/ethnicity (model 1). These demographic characteristics distinguished between the two trajectories with a prediction accuracy of 0.68 (95% CI 0.62, 0.74). Race/ethnicity was the best predictor of higher risk Trajectory 2 membership. Model 2 which featured the addition of SEP variables had an identical prediction accuracy (AUC = 0.68, 95% CI 0.62–0.74). The addition of the frequency of SMBG (Model 3) did not improve the predictive accuracy (AUC = 0.67, 95% CI 0.61-0.74) but led to changes in the interrelationships among segments of the non-Hispanic white population with a college-educated parent.
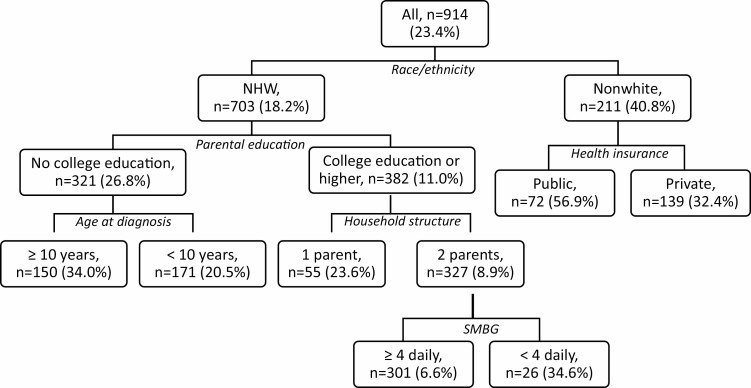
Fig. 2 visualizes the results of model 3 and additionally presents the probability of being in the high/increasing HbA1c trajectory (Trajectory 2) as predicted by the specific discriminating combinations of SEP, demographic, and clinical characteristics among T1D youth and young adults that were determined by the CTree analysis. Race/ethnicity was the most important predictor of risk group: 40.8% of non-white youth and young adults were in Trajectory 2 compared to 18.2% of non-Hispanic white youth and young adults. Moreover, among the non-white youth and young adults, those with public insurance were a particularly high-risk group, with 56.9% in Trajectory 2 compared to 32.4% of those with private health insurance.
Fig. 2.
Classification tree model for the probability of being in the high/increasing HbA1c trajectory (Trajectory 2) as predicted by SEP, demographic and clinical characteristics among T1D youth and young adults. 70% of the sample was used for model fitting (n = 914) and 30% for assessing model robustness (n = 391, see AUCs presented in results). Characteristics included age at diagnosis, gender, race/ethnicity, health insurance, parental college education, household income, household structure, household size, blood glucose checks. Each node depicts the total sample size and the percent in Trajectory 2.
Among non-Hispanic white youth and young adults without college-educated parents, older age at diagnosis (≥10 years) was associated with Trajectory 2 membership (34.0% versus 20.5% with diagnosis <10 years). Among non-Hispanic white youth and young adults with college-educated parents, living in a 1-parent household was associated with Trajectory 2 membership (23.6% versus 8.9% in a 2-parent household). Household income was not selected as a predictor by the CTree model.
The addition of the frequency of SMBG identified another high-risk subgroup among the non-Hispanic white youth and young adults with college-educated parents living in a 2-parent household, namely those that checked glucose fewer than 4 times a day had significantly greater Trajectory 2 membership (34.6%) compared to those who checked their glucose 4 or more times a day (with only 6.6% in Trajectory 2). Type of insulin regimen was not a significant predictor of trajectory.
Of all the individuals in Trajectory 2, 40.2% were characterized by being non-Hispanic white and not having college-educated parents (compared to only 35% of the total sample having these characteristics), and 40.2% were characterized by being non-white (compared to only 23.1% of the total sample) (Table 2). That is, more than 80% of individuals in Trajectory 2 were identifiable based on these two characteristics.
Table 2.
Patterns of SEP, demographic and clinical characteristics resulting from CTree in the SEARCH for Diabetes in Youth cohort (n = 914): Comparison of contribution of patterns to the total sample and to HbA1c Trajectory 2
| Race/ethnicity | Parental college education |
Age at diagnosis | Household structure | SMBG | Health insurance status |
Number with row-specific combination of characteristics N |
Percent of total sample with row-specific combination of characteristics % |
Number of high/increasing HbA1c trajectory members in row-specific combination of characteristics N |
Percent of high/increasing HbA1C trajectory members represented by row-specific combination of characteristics % |
|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | 703 | 76.9 | 128 | 59.8 | |||||
| No college | 321 | 35.1 | 86 | 40.2 | |||||
| No college | ≥ 10 years | 150 | 16.4 | 51 | 23.8 | ||||
| No college | <10 years | 171 | 18.7 | 35 | 16.3 | ||||
| College | 382 | 41.8 | 42 | 19.6 | |||||
| College | 1 parent | 55 | 6.0 | 13 | 6.1 | ||||
| College | 2 parents | 327 | 35.8 | 29 | 13.5 | ||||
| 4+ times/day | 301 | 32.9 | 20 | 9.3 | |||||
| <4 times/day | 26 | 2.8 | 9 | 4.2 | |||||
| Non-white | 211 | 23.1 | 86 | 40.2 | |||||
| Public | 72 | 7.9 | 41 | 19.1 | |||||
| Private | 139 | 15.1 | 45 | 21.0 |
Discussion
The intersectionality framework posits that multiple identities and social positions inhabited by individuals intersect to impact individual health, but this process reflects the systems of privilege and oppression that operate at the societal macro-level (e.g., racism, sexism). Even though the intersectional framework has much to offer population health research, applications to date are limited [23, 25–28]. Of the wide array of quantitative methods that could be used [25], we chose classification trees to accommodate complex combinations of characteristics, thereby identifying key differing patterns among the predictors by group [26, 41, 42]. Our findings reveal how between-group differences in characteristics interact to influence glycemic control trajectories that would otherwise not have been observed, such that race/ethnicity, which is a socially defined categorization, intersected differentially with select SEP characteristics for non-Hispanic whites compared to non-whites [22, 44].
For example, among non-white youth and young adults, having public health insurance played the most important role in predicting high-risk Trajectory 2 membership (i.e., higher baseline HbA1c with major increases over time). In contrast, among non-Hispanic white youth and young adults, parental education and household structure were the key SEP characteristics predictive of HbA1c trajectory. In addition, age of diagnosis and frequency of SMBG were informative in this subgroup. Non-white youth and young adults had the highest risk of being in Trajectory 2, particularly if they had public insurance at baseline. Other high-risk subgroups were non-Hispanic white youth and young adults who were diagnosed in adolescence and whose parent(s) did not have a college education and non-Hispanic white youth and young adults with college-educated parent(s) who were living in a two-parent household and monitoring their glucose less than four times daily. Two characteristics – race/ethnicity and parental education – together provided a sensitivity of 80% for identifying individuals in Trajectory 2. Classification into a trajectory is based on probabilities and is thus not perfect; nevertheless, this result suggests that few characteristics would be needed to identify a potentially high-risk trajectory group and reinforces the importance of addressing fundamental health inequity as a key strategy to improve clinical outcomes rather than focusing on self-management strategies in isolation.
We and others have previously shown the predictive importance of the risk marker non-white race/ethnicity in relation to HbA1c trajectories in relative risk terms, with non-whites exhibiting significantly elevated odds ratios ranging between 2 and 4.5 for membership in an unfavorable HbA1c trajectory [6, 8, 11]. Our study population was comprised of 77% non-Hispanic white youth and young adults and 23% non-white youth and young adults, yet the race/ethnic composition of Trajectory 2 was 59.8% non-Hispanic white versus 40.2% non-white. Moreover, the non-white sample had the highest proportion of members belonging to Trajectory 2 (higher/increasing HbA1c) at 40.8%, higher than the non-Hispanic white group as a whole or in any subgroup thereof. Among the non-white youth and young adults who relied on public insurance, 56.9% were in Trajectory 2. Thus, the current results highlight the absolute magnitude of the race/ethnicity-associated inequities.
Our findings additionally illustrate the importance of type of health insurance. We have recently shown that public health insurance is associated not just with a pattern of lower income and education but food insecurity and food assistance, which suggests that health insurance is an indicator for SEP [45]. These findings shed a new light on previous research on associations of health insurance with improved HbA1c [16, 46] and private health insurance and likelihood of preventive health care visits [46, 47]. In the present study, among both non-whites and non-Hispanic whites, the proportion of individuals with private health insurance among those in the low-risk HbA1c trajectory was higher than among those in the high-risk HbA1c trajectory. However, the differences were much more pronounced in non-whites. One potential explanation is that the higher diabetes-related out-of-pocket medical expenditures associated with having public insurance may have a more detrimental impact in individuals with more limited economic resources. None of the other predictors, including income, offered meaningful discriminatory power in determining SEP patterns in non-whites. This contrasts with findings by Naqvi et al. [28] who have shown that in adults with type 2 diabetes (T2D), black race and sex interacted with T2D health indicators. In totality, these findings suggest that the nuanced and layered disadvantage associated with non-white race/ethnicity, including but not limited to structural and interpersonal racism, is so strong that it overpowers the predictive value of all other demographic, SEP, and clinical characteristics [48].
In the non-Hispanic white population, complex patterns between SEP indicators emerged. First and foremost, parental educational attainment was a key differentiating characteristic, with youth and young adults whose parents did not have a college education constituting another particularly disadvantaged group that contributed 40.2% of the members of the high-risk Trajectory 2. In addition, household structure played an important role among non-Hispanic white youth and young adults with college-educated parent(s) in that youth and young adults living in a 2-parent household exhibited the lowest percent membership in the Trajectory 2 at 8.9%. Whereas this group comprised 35.5% of the total sample, it contributed only 13.6% to the membership of the Trajectory 2, which speaks to the protective role of this SEP characteristic in the non-Hispanic white population. Contrary to our expectations, household income did not contribute significantly to any of the models, suggesting that broader SEP characteristics are more predictive of ongoing glycemic control than household income alone.
Our findings are also informative in terms of the importance of frequency of SMBG, a characteristic that is often reinforced in clinical care. Inclusion of this variable did not improve the ability of the model to discriminate between HbA1c trajectory groups, but it did identify another high-risk subgroup – those checking blood glucose less than four times daily – among those who were seemingly advantaged on all characteristics, non-Hispanic white youth and young adults with college-educated parent(s) who were living in a two-parent household. While small in number, this subgroup had a significantly higher proportion of members in Trajectory 2, with 34.6% compared to 6.6% among those conducting SMBG more frequently.
In addition to structural or system-level interventions designed to address these needs, positive psychology may be explored as a target for future behavioral interventions. Similarly, future observational research should also consider including positive psychosocial attributes such as resilience and optimism, mental health, and indicators of health-related social needs such as food insecurity and lack of transportation as potential predictors, all of which have been linked to glycemic control [49–52]. This is particularly important for the non-white sample, among whom more than 59% had HbA1c values that resulted in their placement in the more advantageous trajectory, yet other than health insurance none of our measures of SEP differentiated this group from non-whites in the high-risk trajectory. This suggests that there are important differentiating factors that are yet to be discovered.
There are several limitations and strengths of this study. Due to our overall sample size, we combined race/ethnicity categories into non-Hispanic white individuals versus all non-white individuals and thus were unable to explore distinct forms of inequities that align with specific race/ethnicity categories [53]. We did not have baseline information on nativity or migration status, contextual factors, and structural inequality. All our predictor variables were measured at the baseline research visit and could have changed over time. Our baseline measure of health insurance status predated the active period of the 2010 Affordable Care Act. Over the roughly 9-year follow-up period, having only three to five HbA1c values for each participant is a further limitation, as is the fact that excluding those with two or fewer HbA1c values disproportionately removed those with the lowest SEP levels. Among the strengths of the study are the large number of SEP indicators and the large sample size. In addition, the CTree method allows for the identification of subgroups with different patterns of risk factors; these subgroups are data-driven in the sense that their characteristics are not defined a-priori and may thus uncover subgroups or patterns that were not known or pre-specified by the team.
In conclusion, we provide compelling evidence how the risk marker race/ethnicity interacts with indicators of SEP (as measured by health insurance status, parental educational attainment, household structure), age at diagnosis and SMBG to place youth and young adults with T1D at particularly high risk of poor long-term glycemic control. By considering multiple characteristics simultaneously, our work contributes to advancing the understanding of mechanisms underlying health inequity in T1D.
Our findings highlight the entanglement of several social and economic characteristics, including race/ethnic identity as part of a larger, lived experience for youth and young adults with T1D. In the long term, the structural inequities associated with race/ethnicity observed in this and other studies can only be addressed by eliminating existing racist policies and developing new policies in support of social justice and equity. In the shorter term, we hope that this research serves as a call to action for the development of programs targeting particularly high-risk groups, such as non-white youth and young adults with public health insurance or non-Hispanic white youth and young adults whose parents do not have a college education, particularly if these findings can be replicated in other samples. These findings can also inform the development of more tailored intervention efforts, targeting improvement of glycemic control among the subgroups of youth and young adults with T1D who are rendered most susceptible to adverse outcomes as a product of racialized and economic health inequity.
Supplementary Material
Compliance with Ethical Standards
Author Contributions: AD Liese, JM Lawrence, D Dabelea and JA Mendoza obtained funding for this study. BA Reboussin and AD Liese had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AD Liese conceptualized and designed the study, BA Reboussin and AR Kahkoska analyzed the data, AD Liese, A Bellatorre, JM Lawrence, and D Dabelea contributed to the acquisition of the data. AD Liese, AR Kahkoska and BA Reboussin drafted manuscript. All co-authors contributed to critical revisions of the manuscript for important intellectual content.
Additional Contributions: The SEARCH for Diabetes in Youth study is indebted to the youth, their families, and their health care professionals, whose participation made this study possible. We acknowledge the involvement of the South Carolina Clinical & Translational Research Institute at the Medical University of South Carolina, Seattle Children’s Hospital and the University of Washington, University of Colorado Pediatric Clinical and Translational Research Center, the Barbara Davis Center at the University of Colorado at Denver, the University of Cincinnati, and the Children with Medical Handicaps program managed by the Ohio Department of Health.
Source of Support: The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
SEARCH 3/4: The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California’s Marilyn Owsley Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group); the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062, UL1 Tr001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077, UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
Funding Grant Support (SEARCH 4): The SEARCH for Diabetes in Youth Cohort Study (1R01DK127208-01, 1UC4DK108173) is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and supported by the Centers for Disease Control and Prevention.
The Population Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, and U18DP006139) is funded by the Centers for Disease Control and Prevention (DP-15-002) and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Grant Support (SEARCH 1, 2, 3):
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati’s Children’s Hospital Medical Center (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U58/CCU019235-4, U01 DP000244, and U18DP002710-01] and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
Dr. Kahkoska was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (grant F30DK113728). This manuscript was additionally supported by R01DK117461 (MPI AD Liese and JA Mendoza).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis. The funders contributed to the interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
Conflict of interest (potentially here or on form): None of the authors have a conflict of interest. ARK has received financial support from Novo Nordisk for travel to present data in 2018.
References
- 1. Nathan DM, Cleary PA, Backlund JY, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Group UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 3. Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petitti DB, Klingensmith GJ, Bell RA, et al. ; SEARCH for Diabetes in Youth Study Group . Glycemic control in youth with diabetes: The SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668–72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1(5):e181851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV. Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes. Family and community contexts. Diabetes Care. 1997;20:1569–1575. [DOI] [PubMed] [Google Scholar]
- 8. Chalew SA, Gomez R, Butler A, et al. Predictors of glycemic control in children with type 1 diabetes: The importance of race. J Diabetes Complications. 2000;14:71–77. [DOI] [PubMed] [Google Scholar]
- 9. Hanson CL, Henggeler SW, Burghen GA. Race and sex differences in metabolic control of adolescents with IDDM: A function of psychosocial variables? Diabetes Care. 1987;10:313–318. [DOI] [PubMed] [Google Scholar]
- 10. Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care. 1991;14:20–25. [DOI] [PubMed] [Google Scholar]
- 11. Redondo MJ, Libman I, Cheng P, et al. Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care. 2018;41:dc172335. [DOI] [PubMed] [Google Scholar]
- 12. Gee GC, Ford CL. Structural racism and health inequities: Old issues, new directions. Du Bois Rev. 2011;8:115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Churchwell K, Elkind MSV, Benjamin RM, et al. ; American Heart Association . Call to action: Structural racism as a fundamental driver of health disparities: A presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 14. Williams DR, Lawrence JA, Davis BA. Racism and health: Evidence and needed research. Annu Rev Public Health. 2019;40:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. [DOI] [PubMed] [Google Scholar]
- 16. Liese AD, Ma X, Reid L, et al. Health care access and glycemic control in youth and young adults with type 1 and type 2 diabetes in South Carolina. Pediatr Diabetes. 2019;20:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim H, Elmi A, Henderson CL, Cogen FR, Kaplowitz PB. Characteristics of children with type 1 diabetes and persistent suboptimal glycemic control. J Clin Res Pediatr Endocrinol. 2012;4:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Apperley LJ, Ng SM. socioeconomic deprivation, household education, and employment are associated with increased hospital admissions and poor glycemic control in children with type 1 diabetes mellitus. Rev Diabet Stud. 2017;14:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Queen TL, Baucom KJW, Baker AC, Mello D, Berg CA, Wiebe DJ. Neighborhood disorder and glycemic control in late adolescents with Type 1 diabetes. Soc Sci Med. 2017;183:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuijdwijk CS, Cuerden M, Mahmud FH. Social determinants of health on glycemic control in pediatric type 1 diabetes. J Pediatr. 2013;162:730–735. [DOI] [PubMed] [Google Scholar]
- 21. Lloyd CE, Wing RR, Orchard TJ, Becker DJ. Psychosocial correlates of glycemic control: The Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. Diabetes Res Clin Pract. 1993;21:187–195. [DOI] [PubMed] [Google Scholar]
- 22. Crenshaw K. Mapping the margins: Intersectionality, identity politics, and violence against women of color. Stanford Law Rev. 1991;43:1299. [Google Scholar]
- 23. Bowleg L. The problem with the phrase women and minorities: Intersectionality-an important theoretical framework for public health. Am J Public Health. 2012;102:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapilashrami A, Hankivsky O. Intersectionality and why it matters to global health. Lancet. 2018;391:2589–2591. [DOI] [PubMed] [Google Scholar]
- 25. Bauer GR. Incorporating intersectionality theory into population health research methodology: Challenges and the potential to advance health equity. Soc Sci Med. 2014;110:10–17. [DOI] [PubMed] [Google Scholar]
- 26. Dey A, Hay K, Afroz B, et al. Understanding intersections of social determinants of maternal healthcare utilization in Uttar Pradesh, India. PLoS ONE. 2018;13( 10):e0204810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gagné T, Veenstra G. Inequalities in hypertension and diabetes in Canada: Intersections between racial identity, gender, and income. Ethn Dis. 2017;27:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naqvi JB, Helgeson VS, Gary-Webb TL, Korytkowski MT, Seltman HJ. Sex, race, and the role of relationships in diabetes health: Intersectionality matters. J Behav Med. 2020;43:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamman RF, Bell RA, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: Rationale, findings, and future directions. Diabetes Care. 2014;37:3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rewers MJ, Pillay K, de Beaufort C, et al. ; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15 Suppl 20:102–114. [DOI] [PubMed] [Google Scholar]
- 31. American Diabetes A. Standards of medical care in diabetes - 2020. Diabetes Care. 2020;43:224. [Google Scholar]
- 32. Grieco EM, Cassidy RC.. Overview of Race and Hispanic Origin: Census 2000 Brief, Vol. 8. Washington, DC, US: Department of Commerce, Economics and Statistics Administration; 2001. [Google Scholar]
- 33. Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;135:1–55. [PubMed] [Google Scholar]
- 34. Ferraro KF, Shippee TP. Aging and cumulative inequality: How does inequality get under the skin? Gerontologist. 2009;49:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsemann KM, Ailshire JA, Bell BA, Frongillo EA. Body mass index trajectories from adolescence to midlife: Differential effects of parental and respondent education by race/ethnicity and gender. Ethn Health. 2012;17:337–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. (CMS) CfMaMS, Published 2018.
- 37. Data Resource Center for Child and Adolescent H. National survey of children’s health. In: PsycTESTS Dataset: American Psychological Association (APA). Washington, DC: PsycTESTS Dataset Am Psychol Assoc; 2016. [Google Scholar]
- 38. Nagin DS. Group-based trajectory modeling: An overview. Ann Nutr Metab. 2014;65:205–210. [DOI] [PubMed] [Google Scholar]
- 39. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138:2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999;4:139. [DOI] [PubMed] [Google Scholar]
- 41. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181. [DOI] [PubMed] [Google Scholar]
- 42. Choi SK, Fram MS, Frongillo EA. Very low food security in US households is predicted by complex patterns of health, economics, and service participation. J Nutr. 2017;147:1992–2000. [DOI] [PubMed] [Google Scholar]
- 43. Hothorn T, Hornik K, Zeileis A. Ctree: Conditional Inference Trees. The Comprehensive R Archive Network; 2015. Available at https://rdrr.io/rforge/partykit/f/inst/doc/ctree.pdf [Google Scholar]
- 44. Dhamoon RK, Hankivsky O.. Why the Theory and Practice of Intersectionality Matter to Health Research and Policy (Health Ine Ed.). Vancouver, BC: UBC Press; 2011. [Google Scholar]
- 45. Sutherland MW, Ma X, Reboussin BA, et al. Socioeconomic position is associated with glycemic control in youth and young adults with type 1 diabetes. Pediatr Diabetes. 2020;21:1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Probst JC, Moore CG, Baxley EG. Update: Health insurance and utilization of care among rural adolescents. J Rural Health. 2005;21:279–287. [DOI] [PubMed] [Google Scholar]
- 47. Majidi S, Wadwa RP, Bishop FK, et al. The effect of insurance status and parental education on glycemic control and cardiovascular disease risk profile in youth with Type 1 Diabetes. J Diabetes Metab Disord. 2014;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health. Socio-economic status, stress and discrimination. J Health Psych. 1997;2:335–351. [DOI] [PubMed] [Google Scholar]
- 49. Wilson AL, McNaughton D, Meyer SB, Ward PR. Understanding the links between resilience and type-2 diabetes self-management: a qualitative study in South Australia. Arch Public Health. 2017;75:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson B, Eiser C, Young V, Brierley S, Heller S. Prevalence of depression among young people with Type 1 diabetes: A systematic review. Diabet Med. 2013;30:199–208. [DOI] [PubMed] [Google Scholar]
- 51. Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. [DOI] [PubMed] [Google Scholar]
- 52. Mendoza JA, Haaland W, D’Agostino RB, et al. Food insecurity is associated with high risk glycemic control and higher health care utilization among youth and young adults with type 1 diabetes. Diabetes Res Clin Pract. 2018;138:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valdez Z, Golash-Boza T. Towards an intersectionality of race and ethnicity. Ethn Racial Stud. 2017;40:2256–2261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.