Abstract
A thermophilic bacterium that can use O2, NO3−, Fe(III), and S0 as terminal electron acceptors for growth was isolated from groundwater sampled at a 3.2-km depth in a South African gold mine. This organism, designated SA-01, clustered most closely with members of the genus Thermus, as determined by 16S rRNA gene (rDNA) sequence analysis. The 16S rDNA sequence of SA-01 was >98% similar to that of Thermus strain NMX2 A.1, which was previously isolated by other investigators from a thermal spring in New Mexico. Strain NMX2 A.1 was also able to reduce Fe(III) and other electron acceptors. Neither SA-01 nor NMX2 A.1 grew fermentatively, i.e., addition of an external electron acceptor was required for anaerobic growth. Thermus strain SA-01 reduced soluble Fe(III) complexed with citrate or nitrilotriacetic acid (NTA); however, it could reduce only relatively small quantities (0.5 mM) of hydrous ferric oxide except when the humic acid analog 2,6-anthraquinone disulfonate was added as an electron shuttle, in which case 10 mM Fe(III) was reduced. Fe(III)-NTA was reduced quantitatively to Fe(II); reduction of Fe(III)-NTA was coupled to the oxidation of lactate and supported growth through three consecutive transfers. Suspensions of Thermus strain SA-01 cells also reduced Mn(IV), Co(III)-EDTA, Cr(VI), and U(VI). Mn(IV)-oxide was reduced in the presence of either lactate or H2. Both strains were also able to mineralize NTA to CO2 and to couple its oxidation to Fe(III) reduction and growth. The optimum temperature for growth and Fe(III) reduction by Thermus strains SA-01 and NMX2 A.1 is approximately 65°C; their optimum pH is 6.5 to 7.0. This is the first report of a Thermus sp. being able to couple the oxidation of organic compounds to the reduction of Fe, Mn, or S.
Dissimilatory iron-reducing bacteria (DIRB) have been isolated from a variety of anoxic environments, including the deep terrestrial subsurface, and are widely distributed among bacteria, as evidenced by 16S rRNA gene (rDNA) sequences (14, 22). Genera of DIRB include Geobacter (26, 29), Shewanella (36, 47), Pelobacter (31), Geovibrio (8), Geospirillum (19), Ferrimonas (44), “Geothrix” (22), Desulfuromusa (20), and Desulfuromonas (43). Several thermophilic DIRB have recently been described, including Bacillus infernus (6), Thermoterrabacterium (49), Deferribacter thermophilus (16), and Thermoanaerobacter spp. (21). Also, there are several reports of enrichment cultures of thermophilic bacteria that are capable of dissimilatory iron reduction (50, 57).
Most of the DIRB described to date are obligately anaerobic; exceptions include Shewanella spp. (36, 47) and Ferrimonas balearica (44). In this paper we describe the isolation and characterization of a facultatively anaerobic Thermus strain that is capable of dissimilatory iron reduction as well as growth with oxygen and nitrate as terminal electron acceptors. Although the physiology and genetics of the genus Thermus have been studied for three decades, strains showing this metabolic versatility have not previously been reported. Most strains of Thermus have been described as obligate aerobes (7), with a few being noted to reduce nitrate to nitrite (41, 42, 48, 56).
MATERIALS AND METHODS
Environmental sampling, enrichment culture, and strain isolation.
Rock and groundwater samples were collected from the Witwatersrand Supergroup at a 3.2-km depth in a South African gold mine operated by Western Deep Levels, Inc. The Witwatersrand Supergroup is a 2.9-billion-year-old formation of low-permeability sandstone and shale with minor volcanic units and conglomerates. The ambient temperature of the rock is approximately 60°C. Samples were collected from a freshly mined rock surface and from a water-producing bore hole that penetrated 121 m horizontally into the formation at a depth of 3,198 m. Groundwater was aseptically collected into sterile serum bottles, sealed without headspace with sterile butyl rubber closures, and then packed in ice chests and shipped to the Pacific Northwest National Laboratory in Richland, Wash. Sample material was used to inoculate enrichment cultures in various media, including one with H2 as the electron donor, intended to cultivate autotrophic iron-reducing bacteria. This enrichment medium contained 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (pH 7.0), 50 mM hydrous ferric oxide (HFO), 1.5 g of NH4Cl liter−1, 0.1 g of KCl liter−1, 0.6 g of NaH2PO4 liter−1, 0.1 g of CaCl2 · 2H2O liter−1, 1 g of yeast extract (Difco) liter−1, 10 ml of 10× Wolfe’s vitamin solution (4) liter−1, and 10 ml of 10× Wolfe’s mineral solution (4) liter−1; the headspace gas was 80% H2 and 20% CO2. The HFO was prepared as described by Lovley and Phillips (27). The 10× Wolfe’s vitamin solution contained (per liter of deionized water) 2.0 mg of biotin, 2.0 mg of folic acid, 10.0 mg of pyridoxine HCl, 5.0 mg of riboflavin, 5.0 mg of thiamine, 5.0 mg of nicotinic acid, 5.0 mg of pantothenic acid, 0.1 mg of cyanocobalamin, 5.0 mg of p-aminobenzoic acid, and 5.0 mg of thioctic acid. The 10× Wolfe’s mineral solution contained (per liter of deionized water) 2.14 g of nitrilotriacetic acid (NTA), 0.1 g of MnCl2 · 4H2O, 0.3 g of FeSO4 · 7H2O, 0.17 g of CoCl2 · H2O, 0.2 g of ZnSO4 · 7H2O, 0.03 g of CuCl2 · 2H2O, 5 mg of KAl(SO4)2 · 12H2O, 5 mg of H3BO4, 0.09 g of Na2MoO4, 0.11 g of NiSO4 · 6H2O, and 0.02 g of Na2WO4 · 2H2O. After incubation at 60°C with shaking for 60 days, the groundwater-inoculated medium showed significant Fe(III) reduction and growth of a rod-shaped bacterium. Subculturing of dilutions resulted in isolation of an axenic culture, designated strain SA-01. SA-01 was shown to grow aerobically in a complex organic medium, TYG (5.0 g of tryptone [Difco], 3.0 g of yeast extract [Difco], 1.0 g of glucose liter of H2O−1), or anaerobically in TYG containing 10 mM KNO3. Strain SA-01 was examined for purity by streaking it onto TYG medium solidified with 2% agar and by obtaining isolated colonies twice in succession. Frozen stocks were maintained in 16% glycerol at −80°C. A defined basal medium (formulated for cultivating Geobacter chapellii) (23) containing (per liter of deionized water) 0.42 g of KH2PO4, 0.22 g of K2HPO4, 0.2 g of NH4Cl, 0.38 g of KCl, 0.36 g of NaCl, 0.04 g of CaCl2 · H2O, 0.1 g of MgSO4 · 7H2O, 1.8 g of NaHCO3, 0.5 g of Na2CO3, 0.19 mg of Na2SeO4, 10 ml of 10× Wolfe’s trace element solution, and 15 ml of a 10× solution of Wolfe’s vitamins was used for all subsequent experiments. All solutions were made anaerobic by purging them with O2-free N2; they were sterilized by autoclaving or filtration. The basal medium was amended with various electron donors and electron acceptors, as indicated below. Cells were cultured under strictly anaerobic conditions in Balch tubes (Bellco, Vineland, N.J.) or serum vials fitted with butyl rubber stoppers and containing a mixture of 80% N2 and 20% CO2 in the headspace. In some cases, the basal medium was also amended with small amounts of TYG to enhance growth, as indicated below.
Identification and phylogeny.
The phylogeny of strain SA-01 was determined by 16S rDNA sequencing. DNA was extracted by a modified freeze-thaw procedure (38). Cells were cultured aerobically in TYG at 60°C, centrifuged, and resuspended in extraction buffer (2% sodium dodecyl sulfate, 0.2 M Na2HPO4 [pH 8.0]). Cells were frozen at −80°C, heat shocked for 10 min at 65°C, and then ballistically lysed with 0.1-mm-diameter glass beads and a beadbeater (Biospec Products, Bartlesville, Okla.). The supernatant was dialyzed against TE (10 mM Tris, 1 mM EDTA, [pH 7.8]) and ethanol precipitated. The 16S rDNA was amplified from the extracted DNA by PCR (Perkin-Elmer) with universal bacterial primers corresponding to Escherichia coli positions 7 to 27 and 1406 to 1392 (9). The PCR product was purified by agarose gel electrophoresis and with a GeneClean II kit (Bio 101, La Jolla, Calif.), and the 12-base uracil-DNA-glycosylase-generated 5′ overhang was annealed to the CloneAmp pAMP1 cloning vector (Gibco BRL). This construct, containing the 16S rDNA PCR insert, was then transformed into DH5α competent cells (Gibco BRL). The 16S rDNA sequences from 30 clones showed identical restriction fragment length polymorphism (RFLP) patterns when they were digested with the restriction endonuclease CfoI (Gibco BRL). Plasmid template DNA was prepared from one of these 30 clones and sequenced with an ABI Dye Terminator Cycle Sequencing Kit (Perkin-Elmer) with both plasmid and internal universal bacterial 16S rDNA primers. Sequence homology was determined with the BLAST program (2); phylogenetic analysis of 16S rDNA sequences was performed by the maximum-likelihood method (Genetic Data Environment program [32a]) with aligned E. coli positions 49 to 71, 102 to 180, 221 to 451, and 481 to 1259.
Electron donors and acceptors.
The abilities of strain SA-01 and Thermus strain NMX2 A.1 (provided by Hugh Morgan, University of Waikato, Hamilton, New Zealand) to grow with various combinations of electron donors and electron acceptors were tested in the basal medium at 60°C. Lactate (30 mM) was routinely used as the electron donor for testing nitrate, nitrite, Fe(III)-NTA, fumarate, sulfate, and thiosulfate (each was used at 10 mM, except nitrite, which was used at 1.0 mM) as terminal electron acceptors. Headspace gas was 80% N2 and 20% CO2. Fe(III)-NTA (100 mM) was prepared by sequentially dissolving 1.64 g of NaHCO3, 2.56 g of trisodium NTA (Sigma, St. Louis, Mo.), and 2.7 g of FeCl3 · H2O in water to a final volume of 100 ml. Control cultures lacking an electron acceptor or donor were also tested for growth and Fe(III) reduction. A zinc-acetate trap was placed in the headspace to trap H2S from media with sulfate or thiosulfate as the terminal electron acceptor to avoid potential sulfide toxicity (33). Growth was observed visually as turbidity in the culture tubes. Iron reduction was detected visually by color change as Fe(III) was reduced to colorless Fe(II). Uninoculated growth media served as controls. Cultures showing growth were subcultured (10% inoculum) in the same medium. Dissimilatory nitrate reduction by SA-01 and NMX2 A.1 was evaluated in anaerobic cultures grown at 60°C in TYG amended with 10 mM KNO3; nitrate and nitrite were quantified by ion exchange liquid chromatography (Dionex, Sunnyvale, Calif.) by using a model AG4A guard and a model AS4A separator column, with a mobile phase containing 1.75 mM NaHCO3 and 1.85 mM Na2CO3, and by suppressed conductivity detection.
Reduction of S0 by Thermus strains SA-01 and NMX2 A.1 was tested on an agar medium by methods described by Moser and Nealson (33). TYG medium containing 30 mM S0, 30 mM lactate, and 20 g of agar liter−1 was streaked with inoculum (grown aerobically in TYG broth) and incubated at 60°C anaerobically in a sealed canning jar containing 5% H2 and 95% N2. The jar also contained a trap with 0.1 M Zn-acetate to absorb sulfide. The elemental sulfur in the medium was added to the molten agar as polysulfide (a gift from Duane Moser, University of Wisconsin—Milwaukee). Sulfur reduction was evidenced by clearing of the S0 precipitate from the agar medium in the areas surrounding colonies and by testing the Zn-acetate traps for the presence of sulfide by the methylene blue method (3). Proliferation of cells in colonies was confirmed by phase-contrast microscopy. Controls consisted of uninoculated medium and medium inoculated with killed (autoclaved) cells.
Growth and reduction of Fe(III)-NTA by Thermus strains SA-01 and NMX2 A.1 were quantified in anaerobic basal medium containing 3 mM sodium lactate, 15 mM Fe(III)-NTA, 50 mg of tryptone liter−1, and 30 mg of yeast extract liter−1, with 50 ml each in 160-ml serum vials and with N2 and CO2 (80:20) as headspace gas. The basal medium was also amended to contain 3.7 mM NH4Cl. Thermus cells were grown aerobically in TYG broth (SA-01) or ATCC 697 medium (NMX2 A.1) and washed three times in 10 mM PIPES. Cells were resuspended in aerobic 10 mM PIPES at a density of 108 cells ml−1 and incubated at 65°C and 100 rpm for 48 h. Cells were then washed once in basal medium and inoculated into nine 50-ml precultures containing 10 mM Fe(III)-NTA, 10 mM lactate, 50 mg of tryptone liter−1, and 30 mg of yeast extract in basal medium liter−1 at a density of 5 × 106 cells ml−1. After inoculation, cultures were purged with filtered O2-free N2. Cultures were incubated at 65°C and 60 rpm until almost all of the Fe(III) was reduced: 4 days for SA-01 and 5 days for NMX2 A.1. The purpose of the precultures was to minimize intracellular reserves of storage products prior to inoculation into the Fe(III)-NTA reduction experiment. Precultures were harvested by centrifugation, while anaerobic conditions were maintained, and used to inoculate the experimental cultures at initial cell densities of 5.4 × 106 and 5.8 × 106 cells ml−1 for SA-01 and NMX2 A.1, respectively. As controls (i) the same growth medium but without lactate was inoculated with the same densities of cells and (ii) the same growth medium with lactate was not inoculated. Triplicate bottles were used for each treatment. Bottles were incubated at 65°C with shaking (60 rpm). Two consecutive transfers were made from the SA-01 cultures into fresh anaerobic media. Cells from the treatment containing Fe(III)-NTA and lactate were transferred into fresh medium containing Fe(III) and lactate; cells from the medium containing Fe(III)-NTA but not lactate were transferred into fresh medium containing Fe(III) but not lactate. One additional set of cultures identical to the treatment cultures, except lacking Fe(III)-NTA, was inoculated with cells from the first transfer of the treatment cultures to control for growth without Fe(III) reduction. Fe(II) was quantified by the ferrozine assay (28). Lactate and possible organic products (e.g., acetate) were quantified in filtered culture samples with a DX 500 ion chromatography system equipped with an Ion Pac AS 11 analytical column and a model CD 20 conductivity detector (Dionex). The eluent gradient was programmed to result in a 0.2 mM NaOH solution during equilibration and analysis and in a 35 mM NaOH solution during column regeneration. The flow rate was 1 ml min−1, and the injection volume was 50 μl. Cells were preserved in 3.5% formaldehyde and quantified by filtration, staining with acridine orange, and epifluorescence microscopy.
Oxidation of lactate to CO2 was also quantified in conjunction with the reduction of Fe(III)-NTA and, in cultures lacking Fe(III)-NTA, by using uniformly labeled Na [14C]lactic acid (99% radiopure; American Radiolabeled Chemicals, Inc., St. Louis, Mo.). Ethanol was removed from the radiolabeled lactate by purging with N2. The purged lactate solution was mixed with anaerobic sterile water and diluted in anaerobic basal medium prior to its addition to the cultures. Cultures used for measuring the oxidation of lactate to CO2 were 10 ml each and contained 4.6 × 107 cells ml−1, 3 mM Na-lactate (Sigma), and approximately 0.7 nM (0.4 μCi) 14C-labeled lactate in basal medium. Fe(III)-NTA (11 mM) also was present in the treatment cultures. The inoculum was grown aerobically in TYG broth and washed three times in anaerobic basal medium. Cultures were contained in 30-ml serum bottles with N2-CO2 (80:20) headspace gas and incubated at 65°C without shaking. An open, empty 2-ml cryovial (Nalgene) was placed inside the serum bottle to serve later as a trap. Duplicate cultures were sacrificed at each sampling time by adding 1.0 ml of 5.5 N HCl to each culture and 1.0 ml of 1 N KOH to the trap. One milliliter of 1.0 N KOH was calculated to be sufficient for trapping all of the CO2 in the headspace, all of the CO2 derived from acidification of the bicarbonate buffer, plus nearly all of the carbon in the [14C]lactate if it was all oxidized to 14CO2. Experimentally, it was determined that 1.0 ml of 1 N KOH could trap 87% of the C in the system when it was all released as CO2. Following acidification of the culture, 950 μl of the KOH was transferred to Opti-fluor scintillation fluid (Packard Instrument, Downers Grove, Ill.) for counting. These cultures were also analyzed for Fe(II).
Growth on and reduction of HFO by Thermus SA-01 were quantified with anaerobic basal medium containing 10 mM sodium lactate, 10 mM HFO, 50 mg of tryptone liter−1 and 30 mg of yeast extract liter−1, with 50 ml each in 100-ml serum vials and with N2-CO2 (80:20) headspace gas. A second treatment contained these components plus 0.1 mM 2,6-anthraquinone disulfonate (AQDS) (Aldrich, Milwaukee, Wis.). The inoculum was grown aerobically in TYG broth and washed three times in anaerobic 30 mM bicarbonate buffer (pH 7); cells were injected to obtain an initial density of 2.5 × 106 cells ml−1. Controls consisted of (i) growth medium to which the same density of killed (autoclaved) cells was added and (ii) uninoculated medium. Duplicate vials were used for each treatment. Vials were incubated at 60°C with shaking. Fe(II) and cell density were quantified as described above.
Reduction of the following electron acceptors by suspensions of Thermus strain SA-01 cells under nongrowth conditions was tested: 1 mM Fe(III)-NTA, 10 mM Fe(III)-citrate, 10 mM HFO, 125 μM Co(III)-EDTA, 125 μM Cr(VI), 125 μM U(VI), and 10 mM Mn(IV)-oxide. Cells were cultured either aerobically in TYG or anaerobically in TYG containing 10 mM KNO3. Cells were washed three times in anaerobic 30 mM bicarbonate buffer (pH 7.0) and resuspended in 10 ml of anaerobic 30 mM bicarbonate buffer (pH 7.0) in Balch tubes containing one or more electron donors, as described below. The headspace gas was 80% N2 and 20% CO2. For HFO, Fe(III)-citrate, and Fe(III)-NTA reduction assays, the cells were grown anaerobically and the potential electron donors were 1 mM acetate, 10 mM lactate, and 10 ml of H2 injected into the Balch tubes. For the Co(III)-EDTA, Cr(VI), U(VI), and Mn(IV)-oxide reduction assays, the cells were cultured aerobically, harvested by centrifugation, and then purged with O2-free N2. The electron donor for the Co(III)-EDTA, Cr(VI), and U(VI) assays was 10 mM lactate. For the Mn(IV) reduction assay, the bicarbonate buffer was adjusted to pH 7.4 by decreasing the percent CO2 in the headspace to 8%. The electron donors were 2 mM lactate and H2 (10 ml of H2 injected into the headspace in a separate treatment from lactate), and the electron acceptor was 10 mM MnO2, prepared as described by Lovley and Phillips (29). For all assays, controls consisted of tubes containing the same media without cells. Final cell densities were approximately 109 cells ml−1. Cell suspensions were incubated at 60°C with shaking. Fe(II) production was monitored by the ferrozine assay; Co(III)-EDTA was quantified by ion chromatography (15); Cr(VI) was quantified by reacting it with symdiphenylcarbazide reagent (0.25% in acetone) and measuring absorbance at 540 nm (51); and U(VI) was quantified with a kinetic phosphorescence analyzer (30). The Mn(II) product of MnO2 reduction was extracted in 0.5 N HCl for 15 min and filtered through a 0.2-μm-pore-size filter according to the method of Lovley and Phillips (29). The extracted Mn(II) was then reacted with 10 parts of a formaldoxamine-ammonium hydroxide solution and quantified spectrophotometrically at 450 nm, as described by Gorby et al. (15). All experiments were performed in triplicate.
Temperature and pH responses.
The effects of temperature on growth and Fe(III) reduction by SA-01 and NMX2 A.1 were quantified with basal medium containing 10 mM lactate, 10 mM Fe(III)-NTA, 5 mg of tryptone liter−1, 3 mg of yeast extract liter−1, and 1 mg of glucose liter−1. Cultures were incubated in serum vials with shaking at various temperatures. Growth and iron reduction rates were measured at various pHs in basal medium containing 10 mM lactate and 10 mM Fe(III)-NTA as described above, except that the medium was buffered with 50 mM sodium acetate at pH 5.0, 50 mM PIPES buffer at pH 6.0 and 6.5, 50 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES) buffer at pH 7 and 7.5, 50 mM 1,3-bis-Tris-propane buffer at pH 8.0 and 9.0, and 50 mM 3-(cyclohexylamino)propanesulfonic acid (CAPS) buffer at pH 10.0. Cultures were incubated at 60°C with shaking. Growth and Fe(II) were quantified as described above.
NTA biodegradation.
The abilities of strains SA-01 and NMX2 A.1 to mineralize NTA to CO2 were tested because it was noted that cells reduced Fe(III)-NTA to Fe(II), even when lactate or other potential electron acceptors were absent, albeit more slowly and to a lesser degree. A modification of the radiorespirometry method of Bolton and Girvin (5) was used for these experiments. Biodegradation was tested with cells suspended in a 10 mM HEPES (pH 7.0) buffer. Buffer solution (2.0 ml in each Balch tube) contained a 5.0 μM solution of [U-14C]Fe(III)-NTA (98% radiopure, 16.7 Bq ml−1). Cells were grown anaerobically in TYG containing 10 mM nitrate and then washed and resuspended in anaerobic HEPES buffer. Washed cells were added to the NTA-containing buffer solution to a final density of 2.5 × 107 cells ml−1 (SA-01) and 4.0 × 107 cells ml−1 (NMX2 A.1). Control tubes were not inoculated. The headspace gas was N2. The tubes were incubated without shaking at 60°C. An alkaline trap containing 0.2 ml of 0.6 N KOH (an amount more than adequate to trap all of the C within the tube as CO2) was attached to the underside of each rubber Balch tube stopper. Duplicate tubes were sacrificed after 5 and 12 days, at which times, the cultures were acidified with 0.4 ml of 1.0 N HNO3. After 24 to 48 h, the traps were removed and the radioactivity of subsamples (0.1 to 0.18 ml) was measured by liquid scintillation counting. Initial cell densities were 1.3 × 106 cells ml−1 for SA-01 and 3.6 × 106 cells ml−1 for NMX2 A.1. After 5 and 12 days of incubation, the cultures were acidified for 24 to 48 h and the label remaining in the culture fluid was quantified by liquid scintillation counting. Also, after 12 days of incubation, an alkaline trap was added to each tube immediately before acidification; the 14CO2 captured in the trap was measured by liquid scintillation counting to corroborate the data from the solution assay.
Nucleotide sequence accession number.
The 16S rDNA sequence of strain SA-01 has been deposited in GenBank under accession no. AF020205.
RESULTS
Identification and phylogeny.
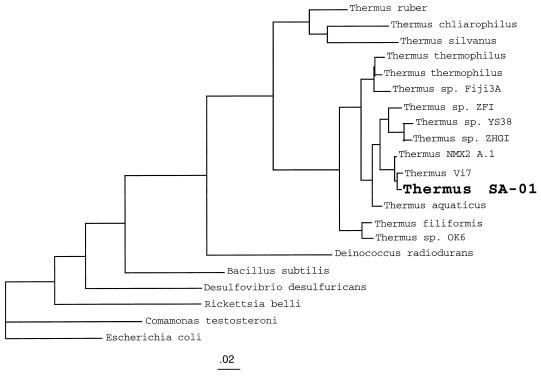
Comparison of the 16S rDNA sequence of strain SA-01 with gene sequences in GenBank, with BLAST (2), showed that this bacterium had >98% homology with the 16S rDNA sequence of Thermus strain NMX2 A.1 (45). Phylogenetic analysis of 16S rDNA sequences by the maximum-likelihood method showed SA-01 to be a member of the genus Thermus, closely related to strains NMX2 A.1 and Vi7 (55) (Fig. 1). After strain SA-01 had been subcultured several times in basal medium containing (i) lactate as the electron donor and nitrate as the electron acceptor, (ii) lactate as the electron donor and Fe(III)-NTA as the electron acceptor, and (iii) H2 as the electron donor and Fe(III)-NTA as the electron acceptor, the identities of these subcultures were confirmed to be the same as that of the original isolate by amplification of 16S rDNA, cloning, and comparison of RFLP patterns after digestion with CfoI (Gibco BRL). The restriction patterns of four clones from each of the subcultures matched the RFLP of the original isolate. Strain SA-01 also showed a filamentous morphology that is consistent with its placement within the genus Thermus.
FIG. 1.
16S rDNA-based molecular phylogeny (maximum-likelihood method) of various Thermus strains, including metal-reducing SA-01 and NMX2 A.1 and also various non-Thermus outgroup species. The phylogeny was constructed with sequences corresponding to E. coli positions 49 to 71, 102 to 180, 221 to 451, and 481 to 1259. The tree shows the close phylogenetic relationship of these metal-reducing strains within the genus Thermus. The scale bar shows the expected number of changes per sequence position.
Electron donors and electron acceptors.
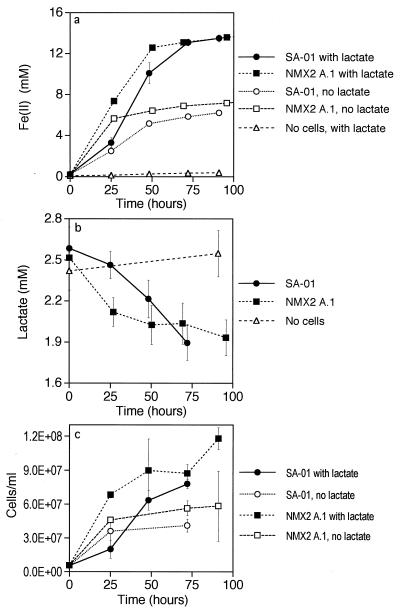
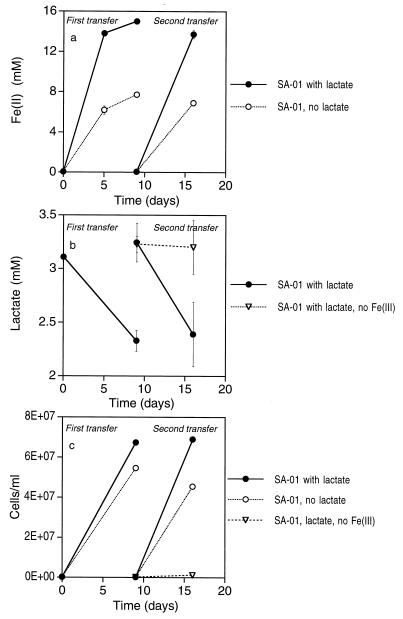
Thermus strains SA-01 and NMX2 A.1 grew in basal medium amended with lactate and any of the following terminal electron acceptors: O2, nitrate, and Fe(III)-NTA. Growth continued with repeated subculturing, regardless of the electron acceptor. Neither organism grew in the absence of an electron acceptor or with fumarate, nitrite, SO42−, or S2O32− as the terminal electron acceptor. Thermus strains SA-01 and NMX2 A1 were able to reduce Fe(III)-NTA coupled to lactate oxidation and growth (Fig. 2). Production of Fe(II) was concomitant with the disappearance of lactate and growth of cells. Other organic acids, such as acetate, were not detected in the medium by ion chromatography. Reduction of Fe(III)-NTA and cell reproduction also occurred in cultures that did not contain lactate; however, levels of iron reduction and cell growth in the absence of lactate were significantly lower than in the presence of lactate. When cells were transferred from this experiment into fresh medium twice, sequentially, reduction of Fe(III)-NTA to Fe(II) and growth of cells proceeded without diminution of the amount of iron reduced or the cell yield (Fig. 3). Iron reduction and cell growth proceeded through successive transfers in treatments with and without lactate; however, the levels of Fe(II) produced and the cell yields were again significantly lower in the treatments without lactate. When cells were transferred into medium containing lactate but without Fe(III)-NTA or another electron acceptor (second transfer), lactate utilization and cell growth were negligible (Fig. 3b and c).
FIG. 2.
Reduction of Fe(III)-NTA coupled to lactate oxidation and growth by Thermus strain SA-01. Fe(II) concentration (a), lactate concentration (b), and cell density (c) versus time are shown. Error bars show 1 standard deviation (n = 3).
FIG. 3.
Fe(II) concentrations (a) and cell densities (b) of Thermus strain SA-01 during repeated transfers into fresh basal medium. Error bars show 1 standard deviation (n = 3).
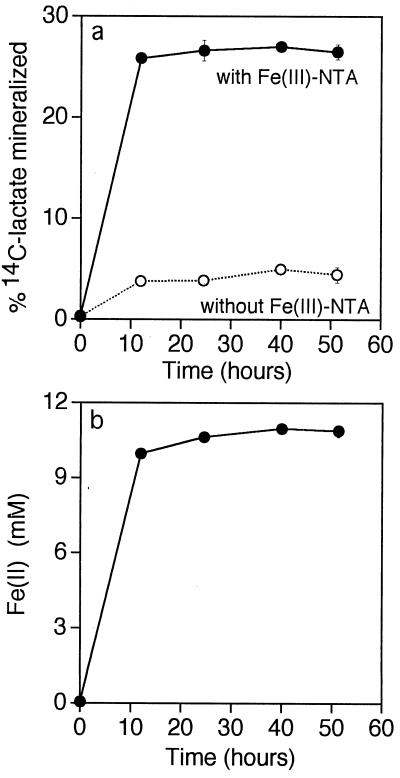
Oxidation of 14C-labeled lactate to 14CO2 occurred concomitantly with the reduction of Fe(III)-NTA to Fe(II) by Thermus strain SA-01; oxidation of [14C]lactate in the absence of Fe(III)-NTA was minimal (Fig. 4).
FIG. 4.
Mineralization of 14C-labeled lactate to 14CO2 by Thermus strain SA-01 in the presence and absence of Fe(III)-NTA (a) and concomitant reduction of Fe(III)-NTA to Fe(II) in the presence of lactate (b). Error bars show 1 standard deviation (n = 2).
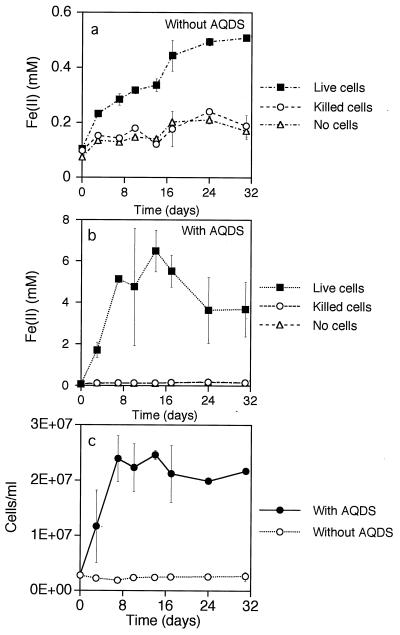
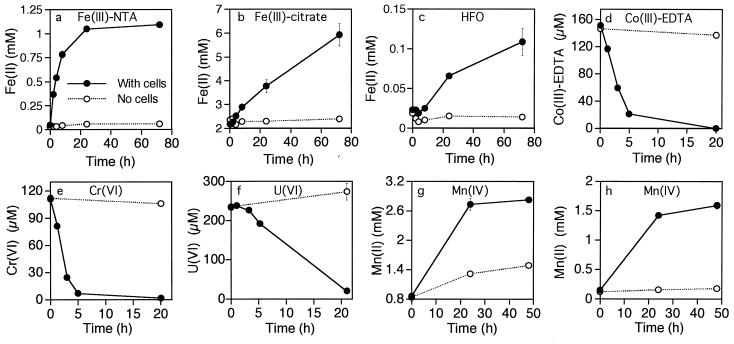
Thermus strain SA-01 reduced HFO to Fe(II); however, the rate of reduction was extremely low (Fig. 5). Rates of growth and HFO reduction were greatly accelerated in cultures containing a low concentration of the humic acid analog AQDS (Fig. 5b). Iron reduction was negligible in vials injected with dead cells and in uninoculated vials, regardless of whether AQDS was present. A black solid, presumably magnetite, was generated as a product of HFO reduction. The production of magnetite was indicated by the strong attraction of black particulates to a magnet after several days of incubation. The original HFO was reddish brown and was only weakly magnetic. Likewise, controls that were not inoculated or that were injected with killed cells did not form a black magnetic precipitate. Suspensions of Thermus strain SA-01 cells reduced Fe(III)-NTA, Fe(III)-citrate, and HFO (Fig. 6a to c); the cells also reduced Co(III)-EDTA, Cr(VI), and U(VI) (Fig. 6d to f). Strain SA-01 reduced Mn(IV) in the presence of either lactate or H2 (Fig. 6g and h). Lactate reduced MnO2 in the absence of cells, but the rate and level of Mn reduction were significantly lower than in the sample treated with cells.
FIG. 5.
Reduction of HFO and growth by Thermus strain SA-01 with lactate as the electron donor. Fe(II) concentrations without AQDS (a) and in the presence of 0.1 mM AQDS (b) and cell density (c) versus time are shown. Error bars show 1 standard deviation (n = 2).
FIG. 6.
Reduction of various electron acceptors by suspensions of Thermus strain SA-01 cells in media containing lactate, acetate, and/or H2 as the potential electron donor(s) (as described in Materials and Methods). Levels of reduction of Fe(III)-NTA (a), Fe(III)-citrate (b), HFO (c), Co(III)-EDTA (d), Cr(VI) (e), U(VI) (f), and Mn(IV) (g and h) are shown. Electron donors for Mn reduction were lactate (g) and H2 (h). Filled circles show results for experimental treatments (with cells); open circles show results for controls (no cells). Error bars show 1 standard deviation (n = 3).
Reduction of elemental sulfur by both strains SA-01 and NMX2 A.1 was evidenced by growth under anaerobic conditions with S0 as the sole electron acceptor and by clearing of S0 in TYG agar. Significant growth and clearing of S0 occurred within 24 h of inoculation, and sulfide was detected in the Zn-acetate traps. S0 was not cleared in control plates consisting of uninoculated growth medium and medium streaked with killed cells, and sulfide was not detected. Growth did not occur on TYG agar under anaerobic conditions without addition of an electron acceptor such as S0.
When Thermus strains SA-01 and NMX2 A.1 were grown anaerobically in TYG medium containing 10 mM nitrate, the nitrate was reduced quantitatively to nitrite. Growth did not occur in anaerobic TYG broth unless an electron acceptor such as nitrate was added.
Temperature and pH responses.
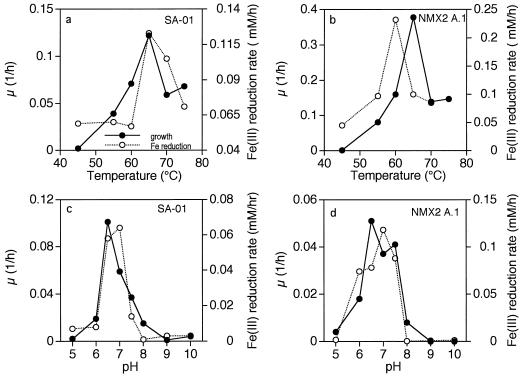
The optimum temperature for growth and Fe(III) reduction was approximately 65°C for both SA-01 and NMX2 A.1; the optimum pHs for growth and Fe(III) reduction were near neutrality for both strains (Fig. 7).
FIG. 7.
Growth rate (μ) and Fe(III) reduction rate versus temperature for Thermus strains SA-01 and NMX2 A.1 cultured with lactate as the electron donor and Fe(III)-NTA as the electron acceptor. Filled circles, μ; open circles, Fe(III)-reduction rate.
NTA biodegradation.
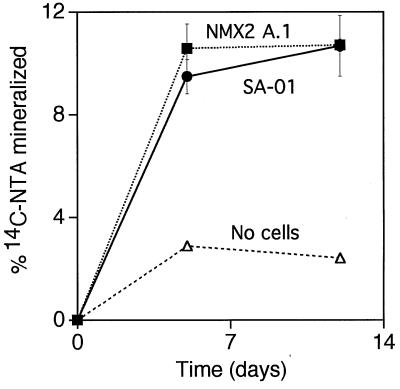
Both SA-01 and NMX2 A.1 mineralized approximately 10% of radiolabeled NTA in 5 days (Fig. 8). NTA incubated in the same solution, but without cells, resulted in less than 3% mineralization to 14CO2 in the same period.
FIG. 8.
Percent mineralization of 14C-labeled NTA by Thermus strains SA-01 and NMX2 A.1. Error bars show 1 standard deviation (n = 2).
DISCUSSION
Reduction of Fe(III) coupled to growth by Thermus strain SA-01 was demonstrated by (i) the disappearance of lactate as an electron donor and the production of Fe(II) from Fe(III) concomitant with cell growth, (ii) the lack of iron reduction or growth in the absence of live cells, and (iii) the fact that rates of Fe(III) reduction were optimal in the ranges of temperature and pH that are also optimal for growth of this and other species of Thermus. The identity and purity of the Thermus SA-01 strain was established by 16S rDNA cloning, sequencing, and phylogenetic analysis, performed shortly after isolation and also after growth and iron reduction in an iron-containing medium. Also, it was demonstrated that Thermus strain SA-01 grew, reduced Fe(III), and consumed lactate over three consecutive transfers into fresh medium.
Growth and reduction of iron by SA-01 was more rapid and extensive when Fe(III) was added in a soluble, chelated form, rather than as HFO, an amorphous iron oxide precipitate. Although this behavior is similar to that of some other DIRB (8, 24, 32, 37), the ability of SA-01 to reduce HFO was particularly poor in comparison to that of organisms such as Shewanella putrefaciens. Recent studies demonstrate that a subsurface S. putrefaciens strain, CN-32, could reduce approximately 40% of a 50 mM HFO suspension in bicarbonate-buffered lactate medium (12). Thermus strain SA-01 grew and reduced HFO at a much higher rate when a soluble humic acid analog, AQDS, was present in low concentration, as has also been shown for Shewanella and Geobacter (25). These findings are consistent with a model for bacterial reduction of iron oxides in which the likely rate-limiting step is the solubilization of Fe(III) or the transfer of electrons from cells to the surfaces of Fe(III)-oxide particles. In this model, metal reduction requires direct contact between cell surface-associated metal reductases (34) (or other cell surface components). Metal chelators can obviate the requirement for direct contact by maintaining Fe(III) in a soluble form that can then diffuse to the cell surface (32). Humic compounds, represented in our study by AQDS, can shuttle electrons between DIRB and iron-oxide minerals (25). Chelators and/or humic acids may enable some bacteria that are otherwise unable to reduce insoluble metal oxides to couple metal reduction to respiration. This inability to reduce iron oxides may be due to a lack of outer membrane-associated metal reductases (35, 36) or extracellular c-type cytochromes that function as ferric reductases, such as the one produced by Geobacter sulfurreducens (46).
Thermus strains SA-01 and NMX2 A.1 can use lactate and/or NTA as electron donors for growth and dissimilatory iron reduction. Because they appear to use both simultaneously when Fe(III) is chelated with NTA, it is difficult to determine the stoichiometry of lactate oxidation coupled to Fe(III) reduction. It appears that lactate was completely oxidized to CO2 by SA-01 since neither acetate nor other organic anions were detected by ion chromatography. At least a portion of the NTA was oxidized to CO2 as well; however, intermediate oxidation products may also occur. Anaerobic biodegradation of NTA in other genera has been reported previously (17). Further study is needed to determine the range of substrates that can serve as electron donors for dissimilatory iron reduction by Thermus strain SA-01 and related metal-reducing strains and to determine the biochemical pathways of substrate oxidation.
The finding of dissimilatory reduction of iron and other metals by Thermus strains was unexpected, given the long history of study of the physiologies of organisms within this genus. However, iron reduction in a variety of genera previously unknown to include metal-reducing species is now being reported. Examples of other such recent findings are found in reports on Bacillus infernus (6) Rhodobacter capsulatus (10), and Thermotoga maritima (52). However, the metal-reducing Thermus strains of this study are distinct from many other DIRB in that they are facultative anaerobes. The ability of Thermus strain SA-01 to use O2, nitrate, Fe(III), Mn(IV), and S0 as terminal electron acceptors is analogous to that of metal-reducing Shewanella strains, the only other organisms currently known to respire all of these electron acceptors and to reduce Mn(IV), Co(III), Cr(VI), and U(VI). With respect to metabolic versatility, Thermus strain SA-01 differs from Shewanella spp. only in that it appears not to use nitrite, sulfur oxyanions, or fumarate as terminal electron acceptors for growth. Thermus strains SA-01 and NMX A.1 differ also from metal-reducing Shewanella strains in that they reduce Fe(III) and other metals at lower rates.
Dissimilatory metal reduction by Thermus may be an important biogeochemical process in some thermic deep subsurface environments. Evidence that metal-reducing Thermus strains are also present in Witwatersrand rock was obtained by extraction, amplification, and cloning of DNAs from rock samples that were collected from the same mine as the groundwater from which SA-01 was cultured (13). One such clone from these directly extracted DNAs had a high degree of homology (>99% in its 16S rDNA) to SA-01. A highly organic seam, termed the carbon leader, is the major source of gold in the Witwatersrand Supergroup. The carbon leader also contains high concentrations of uranium and framboidal pyrite (39). Biological oxidation of organic matter coupled to reduction of Au(I), Au(III), U(VI), Fe(III), or S may have been involved in the concentrations of solid-phase elemental Au, U(IV), and pyrite within the carbon leader. Humic-like compounds in the carbon leader may have also served as electron acceptors and facilitated microbial reduction of solid-phase metal oxides, if present. The origin of the carbon leader has been debated, but one possibility that has been argued is that it is the fossilized remnant of an algal mat (11). Gold in the carbon leader commonly occurs in filamentous structures that are consistent with the sizes and morphologies of filamentous algae or bacteria. It is interesting to note that bacteria of the genus Thermus are commonly associated with algal-bacterial mats in many hot springs and that these mats are believed to be an important source of organic substrates for these organisms (1).
Dissimilatory metal-reducing strains of Thermus are not confined to the deep subsurface of South Africa, as shown by the related strain from New Mexico, Thermus strain NMX2 A.1, which also has this trait. We have recently determined that a strain isolated from Portugal, Thermus strain Vi7 (55), which is phylogenetically closely related to SA-01 and NMX2 A.1 (Fig. 1), is also able to reduce Fe(III)-NTA (18). It remains to be determined how widespread dissimilatory iron reduction is within the genus Thermus.
The genus Thermus represents one of the deep branches of the bacterial 16S rDNA phylogenetic tree (40). Other deeply branching bacterial genera, e.g., Aquifex and Thermotoga, are also thermophilic; however, these other genera are strict anaerobes that respire electron acceptors such as sulfur and Fe(III), which may have been used by the earliest forms of life (40, 52–54). This finding raises the question of whether early members of the genus Thermus reduced metal and/or sulfur and whether metal reduction has subsequently been lost from strictly aerobic species such as Thermus aquaticus. Alternatively, metal reduction may have been acquired more recently by aerobic members of the genus Thermus. Recently, it was shown that Thermus thermophilus HB8 could grow anaerobically in the presence of nitrate (41) and that this trait could be transferred via conjugation to an aerobic Thermus strain (42). Although it is currently unknown whether the factors involved in Fe(III) reduction in Thermus strains SA-01 and NMX2 A.1 are conjugative, their presence on a transmissible plasmid indicates that, in addition to nitrate respiration, Fe(III) respiration in Thermus can be horizontally transferred. Regardless of their evolutionary histories, the metabolic versatility evinced by these metal-reducing Thermus strains is remarkable and may enable growth in a wide variety of thermal environments, including those that are periodically or continuously anaerobic.
ACKNOWLEDGMENTS
This research was supported by the Subsurface Science Program, Office of Energy Research, U.S. Department of Energy (grants DE-FG03-93ER-61683 [T.L.K.] and DE-FG02-94ER61821 [T.C.O.]). Pacific Northwest National Laboratory is operated for the Department of Energy by the Battelle Memorial Institute under contract DE-AC06-76RLO1830. We also acknowledge support from the Department of Energy’s Natural and Accelerated Bioremediation (NABIR) Program.
REFERENCES
- 1.Alfredsson G A, Kristjansson J K. Ecology, distribution, and isolation of Thermus. In: Sharp R, Williams R, editors. Thermus species. New York, N.Y: Plenum Press; 1995. pp. 43–66. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.American Public Health Association. Standard methods for the examination of water and wastewater. 16th ed. Washington, D.C: American Public Health Association; 1985. [Google Scholar]
- 4.Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 5.Bolton H, Jr, Girvin D C. Effect of adsorption on the biodegradation of nitrilotriacetate by Chelobacter heintzii. Environ Sci Technol. 1996;30:2057–2065. [Google Scholar]
- 6.Boone D R, Liu Y, Zhao Z, Balkwill D L, Drake G R, Stevens T O, Aldrich H C. Bacillus infernus sp. nov. Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int J Syst Bacteriol. 1995;45:441–448. doi: 10.1099/00207713-45-3-441. [DOI] [PubMed] [Google Scholar]
- 7.Brock T D. Genus Thermus. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 333–337. [Google Scholar]
- 8.Caccavo F, Jr, Coates J D, Rosselo-Mora R A, Ludwig W, Schleifer K H, Lovley D R, McInerney M J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol. 1996;165:370–376. doi: 10.1007/s002030050340. [DOI] [PubMed] [Google Scholar]
- 9.Chandler D P, Fredrickson J K, Brockman F J. Effect of PCR template concentration on the composition and distribution of total community 16S rDNA clone libraries. Mol Ecol. 1997;6:475–482. doi: 10.1046/j.1365-294x.1997.00205.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobbin P S, Warren L H, Cook N J, McEwan A G, Powell A K, Richardson D J. Dissimilatory iron reduction by Rhodobacter capsulatus. Microbiology. 1996;142:765–774. doi: 10.1099/00221287-142-4-765. [DOI] [PubMed] [Google Scholar]
- 11.Dyer B D, Crumbing W E, Mossman D J. Nature and origin of stratiform kerogen seams in Lower Proterozoic Witwatersrand-type paleoplacers—the case for biogenicity. Geomicrobiol J. 1988;6:33–47. [Google Scholar]
- 12.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, H. Dong, T. C. Onstott, N. W. Hinman, and S. W. Li. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta, in press.
- 13.Fredrickson, J. K., and T. J. Bailey. Unpublished results.
- 14.Fredrickson J K, Gorby Y A. Environmental processes mediated by iron-reducing bacteria. Curr Opin Biotechnol. 1996;7:287–294. doi: 10.1016/s0958-1669(96)80032-2. [DOI] [PubMed] [Google Scholar]
- 15.Gorby Y A, Caccavo F, Jr, Bolton H., Jr Microbial reduction of cobaltIIIEDTA− in the presence and absence of manganese(IV) oxide. Environ Sci Technol. 1998;32:244–250. [Google Scholar]
- 16.Green A C, Patel B K C, Sheehy A J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol. 1997;47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 17.Jenal-Wanner U, Egli T. Anaerobic degradation of nitrilotriacetate (NTA) in a denitrifying bacterium: purification and characterization of the NTA dehydrogenase-nitrate reductase enzyme complex. Appl Environ Microbiol. 1993;59:3350–3359. doi: 10.1128/aem.59.10.3350-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieft, T. L. Unpublished results.
- 19.Laverman A M, Switzer Blum J, Schafer J K, Phillips E J P, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liesack W, Finster K. Phylogenetic analysis of five strains of gram-negative, obligately anaerobic, sulfur-reducing bacteria and description of Desulfuromusa gen. nov., including Desulfuromusa kysingii sp. nov., Desulfuromusa bakii sp. nov., and Desulfuromusa succinoxidans sp. nov. Int J Syst Bacteriol. 1994;44:753–758. [Google Scholar]
- 21.Liu S, Zhou J, Zhang C, Cole D R, Gajdarziska-Josifovska M, Phelps T J. Novel thermophilic Fe(III)-reducing bacteria from the deep subsurface: the evolutionary implications. Science. 1997;277:1106–1108. [Google Scholar]
- 22.Lonergan D J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovley, D. R. (University of Massachusetts). Personal communication to J. K. Fredrickson.
- 24.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature (London) 1996;382:445–448. [Google Scholar]
- 26.Lovley D R, Giavannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic matter to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 27.Lovley D R, Phillips E J P. Organic matter mineralization with the reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovley D R, Phillips E J P, Gorby Y A, Landa E R. Microbial reduction of uranium. Nature (London) 1991;350:413–416. [Google Scholar]
- 31.Lovley D R, Phillips E J P, Lonergan D J, Widman P K. Fe(III) and S0 reduction by Pelobacter carbinolicus. Appl Environ Microbiol. 1995;61:2132–2138. doi: 10.1128/aem.61.6.2132-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley D R, Woodward J C, Chapelle F H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature (London) 1994;370:128–131. doi: 10.1038/370128a0. [DOI] [PubMed] [Google Scholar]
- 32a.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser D P, Nealson K H. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl Environ Microbiol. 1996;62:2100–2105. doi: 10.1128/aem.62.6.2100-2105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers C R, Myers J M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers C R, Myers J M. Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol Lett. 1993;108:15–22. [Google Scholar]
- 36.Myers C R, Nealson K H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 37.Nealson K H, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 38.Ogram A, Sun W, Brockman F J, Fredrickson J K. Isolation and characterization of RNA from low-biomass deep-subsurface sediments. Appl Environ Microbiol. 1995;61:763–768. doi: 10.1128/aem.61.2.763-768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onstott T C, Tobin K, Dong H, DeFlaun M F, Fredrickson J K, Bailey T, Brockman F, Kieft T, Peacock A, White D C, Balkwill D, Phelps T J, Boone D R. The deep gold mines of South Africa: windows into the subsurface biosphere. Proc SPIE Int Soc Optical Eng. 1997;3111:344–357. [Google Scholar]
- 40.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 41.Ramírez-Arcos S, Fernández-Herrero L A, Berenguer J. A thermophilic nitrate reductase is responsible for strain specific anaerobic growth of Thermus thermophilus HB8. Biochim Biophys Acta. 1997;1396:215–227. doi: 10.1016/s0167-4781(97)00183-8. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez-Arcos S, Fernández-Herrero L A, Marín I, Berenguer J. Anaerobic growth, a property horizontally transferred by an Hfr-like mechanism among extreme thermophiles. J Bacteriol. 1998;180:3137–3143. doi: 10.1128/jb.180.12.3137-3143.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roden E E, Lovley D R. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetooxidans. Appl Environ Microbiol. 1993;59:734–742. doi: 10.1128/aem.59.3.734-742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossello-Mora R A, Ludwig W, Kempfer P, Amann R, Schleifer K H. Ferrimonas balearica gen. nov., sp. nov., a new marine facultative Fe(III)-reducing bacterium. Syst Appl Microbiol. 1995;61:196–202. [Google Scholar]
- 45.Saul D J, Rodrigo A G, Reeves R A, Williams L C, Borges K M, Morgan H W, Bergquist P L. Phylogeny of twenty Thermus isolates constructed from 16S rRNA gene sequence data. Int J Syst Bacteriol. 1993;43:754–760. doi: 10.1099/00207713-43-4-754. [DOI] [PubMed] [Google Scholar]
- 46.Seeliger S, Cord-Ruwisch R, Schink B. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J Bacteriol. 1998;180:3686–3691. doi: 10.1128/jb.180.14.3686-3691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semple K M, Westlake D W S. Characterization of iron-reducing Alteromonas putrefaciens strains from oilfield fluids. Can J Microbiol. 1987;33:366–371. [Google Scholar]
- 48.Sharp R, Cossar D, Williams R. Physiology and metabolism of Thermus. Biotechnol Handb. 1995;9:67–91. [Google Scholar]
- 49.Slobodkin A, Reysenbach A-L, Strutz N, Dreier M, Wiegel J. Thermoterrabacterium ferrireducens gen. nov., a thermophilic anaerobic dissimilatory Fe(III)-reducing bacterium from a continental hot spring. Int J Syst Bacteriol. 1997;47:541–547. doi: 10.1099/00207713-47-2-541. [DOI] [PubMed] [Google Scholar]
- 50.Slobodkin A, Wiegel J. Fe(III) as an electron acceptor for H2 oxidation in thermophilic anaerobic enrichment cultures from geothermal areas. Extremophiles. 1997;1:106–109. doi: 10.1007/s007920050022. [DOI] [PubMed] [Google Scholar]
- 51.Urone P F. Stability of colorimetric reagent for chromium: S-diphenylcarbazides in various solvents. Anal Chem. 1955;27:1354–1355. [Google Scholar]
- 52.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature (London) 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 53.Walker J C G. Iron and sulfur in the pre-biologic ocean. Precambrian Res. 1985;28:205–222. doi: 10.1016/0301-9268(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 54.Walker J C G. Was the Archaean biosphere upside down? Nature (London) 1987;329:205–222. doi: 10.1038/329710a0. [DOI] [PubMed] [Google Scholar]
- 55.Williams R, Sharp R. The taxonomy and identification of Thermus. Biotechnol Handb. 1995;9:1–42. [Google Scholar]
- 56.Williams R A D, Da Costa M S. The genus Thermus and related microorganisms. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3745–3753. [Google Scholar]
- 57.Zhang C, Liu S, Logan J, Mazumder R, Phelps T J. Enhancement of Fe(III), Co(III), and Cr(VI) reduction at elevated temperatures and by a thermophilic bacterium. Appl Biochem Biotechnol. 1996;57/58:923–932. [Google Scholar]