Abstract
Background:
Recent studies report that the health benefits of physical activity differ depending on whether the activity is performed in the morning, afternoon or evening. The purpose of this systematic review was to examine whether the timing of physical activity within the 24-hour day is associated with health.
Methods:
Five databases were searched for English or French language peer-reviewed studies that examined whether the timing of physical activity within the day is associated with health. No limits were placed on publication year, study population, study design or health outcomes. Studies that examined acute effects of physical activity or timing of physical activity around food intake were excluded.
Results:
This systematic review examined 35 studies, with 17259 participants, and the following health outcomes: measures of sleep health, adiposity, fat-free mass and muscle size, cardiometabolic biomarkers, physical function and mobility, mental health, and risk of cardiovascular disease, cancer, and mortality. Heterogeneity across studies precluded meta-analyses, and we present our findings using narrative syntheses. Of the 35 studies, 11 reported that morning physical activity provides greater health benefits than afternoon/evening physical activity, while 12 found that morning physical activity provides fewer health benefits than afternoon/evening physical. In the remaining 12 studies, there was no clear difference in health benefits based on the timing of physical activity. The quality of evidence for the different health outcomes across study designs was very low.
Conclusion:
There is no consistent evidence that physical activity at one time of day provides more favourable health benefits than physical activity at a different time of day. (PROSPERO registration no.: CRD42021231088)
Keywords: physical activity, exercise, timing, health, systematic review
Highlights
This systematic review examined whether the timing of physical activity within the day is associated with health.
Thirty-five studies, with 17 259 participants, were included.
The results of 11 studies suggest that morning physical activity provides greater health benefits than afternoon or evening activity, the results of 12 studies suggest that morning physical activity provides smaller health benefits than afternoon or evening activity, and the results of 12 studies found no differences in health outcomes based on the timing of physical activity.
There is no consistent evidence that physical activity performed at one time of day provides more favourable health benefits than physical activity performed at a different time of day.
Introduction
The US Surgeon General’s 1996 report on physical activity and health provided the first national physical activity recommendations for public health1. The report recommended that adults get “a minimum of 30 minutes ofphysical activityof moderate intensity (such as brisk walking) on most, if not all, days of the week.”1p.6 That recommendation was informed by evidence that adults need to expend about 1000 kcal/week through moderate-to-vigorous physical activity (MVPA) to reduce morbidity and mortality risk1. This can be achieved with about 150 minutes of MVPA spread out in 30-minute bouts performed 5 days/week1. Canada’s Physical Activity Guide to Healthy Active Living2 made similar recommendations in 1998.
About a dozen years after the Surgeon General’s report was published, the United States3, Canada4 and the World Health Organization5 released updated physical activity recommendations. These recommendations removed the stipulation that MVPA takes place on most or all days of the week, and simply stated that adults need to accumulate 150 minutes/week of MVPA in bouts lasting at least 10 minutes.
In the past few years, the United States6, Canada7 and the World Health Organization8 released updated physical activity recommendations. These new recommendations no longer stipulate that MVPA needs to be accumulated in bouts of 10 minutes or longer. This stipulation was removed based on evidence that intermittent MVPA (<10 minutes) provides equivalent health benefits to regular bouts of MVPA6-8. Thus, although the amount and intensity of MVPA in public health recommendations have not changed since 1996, the significant changes to the components of the recommendations reflect the patterns in which the MVPA should be accumulated.
What has not been considered in the context of the recommendations is the time when physical activity is accumulated during the day (e.g. morning, afternoon or evening). Recent studies report that equivalent doses of physical activity in the morning, afternoon and evening may be differentially associated with adiposity9, cardiometabolic biomarkers10, cardiovascular disease11 and cancer12. These new studies, coupled with media interest13,14, have fuelled some individuals to prescribe exercise at specific times of the day because they believe it will optimize health benefits. However, the effects of the timing of physical activity has not been comprehensively examined.
The purpose of this systematic review was to examine whether the timing of physical activity during the 24-hour day is associated with health. The results could shed light as to whether the timing of physical activity should be considered in future public health recommendations and health promotion efforts.
Methods
Protocol and registration
This review is registered with the International Prospective Register of Systematic Reviews (PROSPERO; Registration no. CRD42021231088) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement15.
Eligibility criteria
We used the PICOS (Participants, Intervention/Exposure, Comparisons, Outcomes, Study design) framework to facilitate the search process and identify study concepts16.
Population
The population of interest were people, irrespective of age, sex, race/ethnicity or health status.
Intervention/exposure
The intervention or exposure was the timing of physical activity within the 24-hour day, irrespective of the intensity. We studied the effects of habitual physical activity or physical activity interventions. Studies looking at acute responses to a single bout of physical activity were excluded. We did not study the timing of physical activity relative to food or beverage intake, medication use or other therapeutics.
Comparison/control
The comparator was varying levels of the timing of physical activity. A non-exercise control group was not required for intervention studies.
Outcomes
All health outcomes were included (i.e. the search strategy was not limited to a specific health outcome or small number of health outcomes). We also included sleep and sedentary behaviour—the other movement behaviours that, in addition to physical activity, sum up to 24 hours in a day from a time-use perspective17—as potential outcomes. Physical fitness and athletic performance outcomes (e.g. maximal oxygen consumption [VO2max], muscle strength, sprinting speed) were not considered.
Study designs
All original primary research study designs were eligible except case studies, studies that only used qualitative data analysis and studies that only examined the acute effects of physical activity.
Information sources and search strategy
Five databases were searched: Ovid MEDLINE/PubMed, Ovid Embase, Ovid PsycINFO, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL) and EBSCO SPORTDiscus. Searches were conducted on 6 January 2021, with no limits placed on publication dates. Studies were eligible if they were published or in-press, in English or French, and were peer reviewed. Grey literature (e.g. book chapters, dissertations) and abstracts were excluded because this literature is difficult to search and is often not peer reviewed.
The following search terms were used: (1) “physical activity” OR “physical activities” OR “physically active” OR “physical exercise” OR “exercise” or “walk”; AND (2) “time of day” OR “timing.” More details on the search strategy are included in the supplementary materials (https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58).
To remove duplicates, records were imported into Covidence (Veritas Health Innovation, Melbourne, AU). During level 1 screening, two reviewers (IJ or JC or SZ) working independently, screened article titles and abstracts. Articles meeting initial screening criteria by either reviewer proceeded to level 2 screening. During level 2 screening, two reviewers (IJ or JC or SZ) examined full texts of the retrieved articles. Discrepancies were resolved via discussion until the reviewers reached consensus.
Data extraction
A reviewer (JC and SZ) extracted data from eligible studies into Microsoft Excel 365 (Microsoft Corp., Redmond, WA, US) worksheets, and the other (JC or SZ) verified their colleague’s results. Reviewers were not blinded to the article authors’ or journals’ names when extracting data. For each study, we extracted data on the results and important features such as the design, population examined, sample size, participants’ age, how the physical activity timing variables were measured and classified, and intervention characteristics. When the results of more than one regression model were reported, the results from the most fully adjusted model were extracted.
Risk of bias and study quality assessment
Risk of bias assessment was completed using methods described in the Cochrane Handbook18. These assessments were completed by one reviewer (JC or SZ) and verified by another (IJ). The quality of evidence for each health outcome was determined systematically using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework19. GRADE categorizes the quality of evidence into “high,” “moderate,” “low” and “very low.” The rating starts at “high” for randomized studies and at “low” for all other studies (e.g. observational studies, nonrandomized trials). The quality of evidence can be downgraded one or two levels if there are serious limitations across studies, for example, serious risk of bias, inconsistency of effects, indirectness or imprecision. The quality of evidence can be upgraded one level if there is no cause for downgrading, that is, there are no serious limitations, and there is a large magnitude of effect or evidence of a dose–response relationship19.
Results
Description of studies
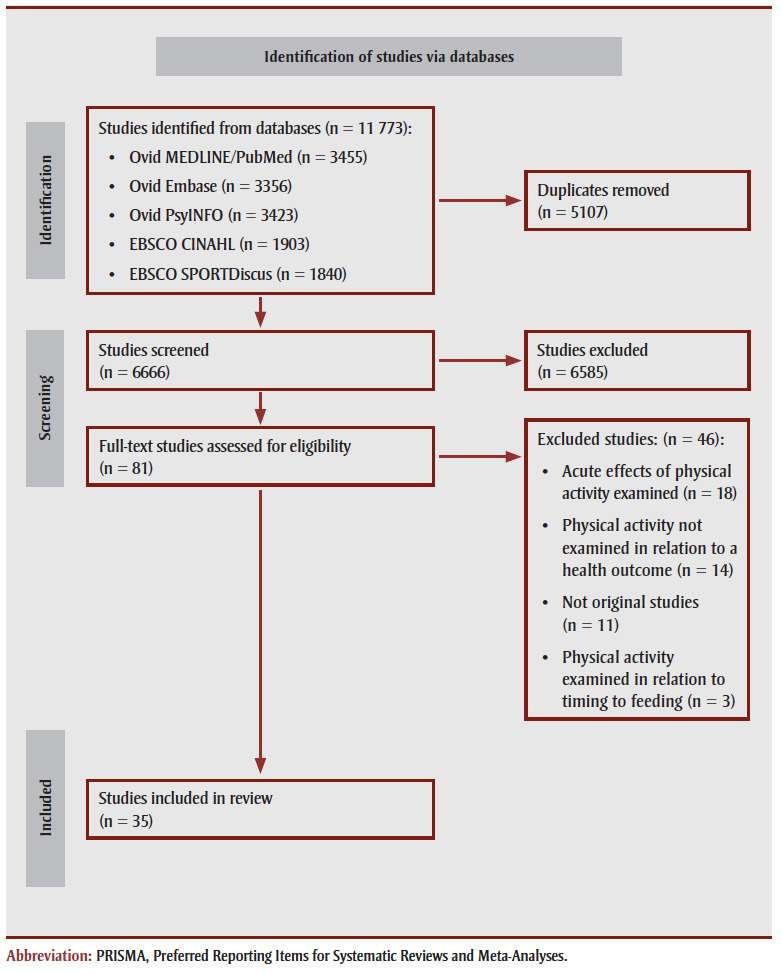
A total of 11773 studies were identified (PubMed, n=3455; EMBASE, n=3356; PsycINFO, n=3423; CINAHL, n=1903; SPORTDiscus, n=1840). After removal of duplicates, 6666 unique studies remained. Eighty-one studies passed level 1 screening and 35 passed level 2 screening for inclusion in the systematic review. Studies were excluded because they examined the acute effects of exercise (n=18); they did not examine the timing of physical activity in relation to a health outcome (n=14); they were not primary research studies (n=11); and they examined the timing of physical activity in relation to feeding (e.g. meal, dietary supplement) (n=3).
The PRISMA diagram15 is in Figure 1.
Figure 1. PRISMA statement 2020 flow diagram of the identification, screening, eligibility and inclusion of studies in this systematic review.
Characteristics and results of the 35 studies included in this review are in Supplementary Tables S1–S9, and risk of bias assessments of individual studies are in Tables S10–S18 (https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). Tables S1–S9 and S10–S18 are organized according to health outcome: sleep health (Tables S1 and S10), adiposity (Tables S2 and S11), fat-free mass and muscle size (Tables S3 and S12), cardiometabolic biomarkers (Tables S4 and S13), risk of cardiovascular disease (Tables S5 and S14), risk of cancer (Tables S6 and S15), physical function and mobility (Table S7 and S16), mental health (Tables S8 and S17) and risk of mortality (Tables S9 and S18). Several studies presented data for outcomes that covered two or more of these categories.
The samples in the 35 studies ranged from a small convenience sample of 11 to a large and diverse sample of 9952. Two studies examined children and youth while the remainder examined adults. Data across studies involved a total of 17 259 participants. Eight studies were randomized controlled trials (RCTs), 2 were randomized crossover studies, 5 were randomized studies without a control group, 4 were nonrandomized trials, 1used a prospective cohort design, 5 were case–control studies and 10 were cross-sectional studies.
In the 17 observational studies, physical activity timing was assessed using self-reported methods in 6 studies and device-based measures in 11 studies. Three approaches were used to assess or categorize physical activity timing: 8 studies measured the amount of physical activity accumulated during different time intervals (e.g. minutes of MVPA accumulated in the morning, afternoon and evening); 6studies categorized participants according to the time of day they typically exercised (e.g. non-exercisers or morning, afternoon or evening exercisers); and 3 studies looked at changes in MVPA patterns across the day (e.g. low activity in day but active in the evening, active in the day but inactive in the evening, etc.).
The exercise interventions lasted between 2 weeks and 10 months; 11/19 studies had a 12-week intervention. One study prescribed light-intensity physical activity and 1 study prescribed high-intensity interval training; the others prescribed MVPA. Seven interventions prescribed aerobic exercise, 3 prescribed resistance exercise, 3 prescribed both aerobic and resistance exercise and the remainder prescribed multimodal exercise programs. Six interventions compared morning versus afternoon exercise, 7 compared morning versus evening exercise, 2 compared morning versus afternoon versus evening exercise and 1 compared exercise completed either prior to or within 4 hours of bedtime.
Data synthesis
Meta-analyses could not be performed because of the heterogeneity in study design, measurement and classification of physical activity for observational studies, type and duration of exercise prescribed for interventions and type of statistical analyses used. We therefore present our results as narrative syntheses.
The process of conducting the narrative synthesis included: (1) constructing a method to abstract relevant study details and findings; (2) grouping studies based on health outcomes, study design and physical activity measures and timing classification; (3) tabulating positive, negative and null associations for these groupings; and (4) exploring whether study design, age or sex were moderator variables. Within the narrative syntheses, the term “mixed results” describes situations where there was a combination of null findings, findings that favoured morning physical activity and findings that favoured afternoon or evening physical activity. Results are consistent across age and sex unless otherwise stated.
Physical activity timing and sleep health
Five studies examined relationships between physical activity timing and measures of sleep health: a randomized crossover study20, a randomized trial without a control group21, a nonrandomized trial22 and 2cross-sectional studies23,24 (Table S1; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). Sleep health measures examined included sleep duration, sleep quality, sleep onset latency, wake after sleep onset, sleep efficiency, sleep fragmentation, sleep satisfaction and feeling refreshed or fatigued after awakening.
Mixed results were observed across these studies. One experimental study reported that evening exercise led to greater improvement in sleep onset latency and sleep satisfaction than morning exercise21. Conversely, 2 studies reported an association between physical activity in the morning23 or at least 4 hours before bedtime22 with better sleep health outcomes compared to when physical activity is performed later in the day. In 2 studies, physical activity timing was not associated with sleep health20,24.
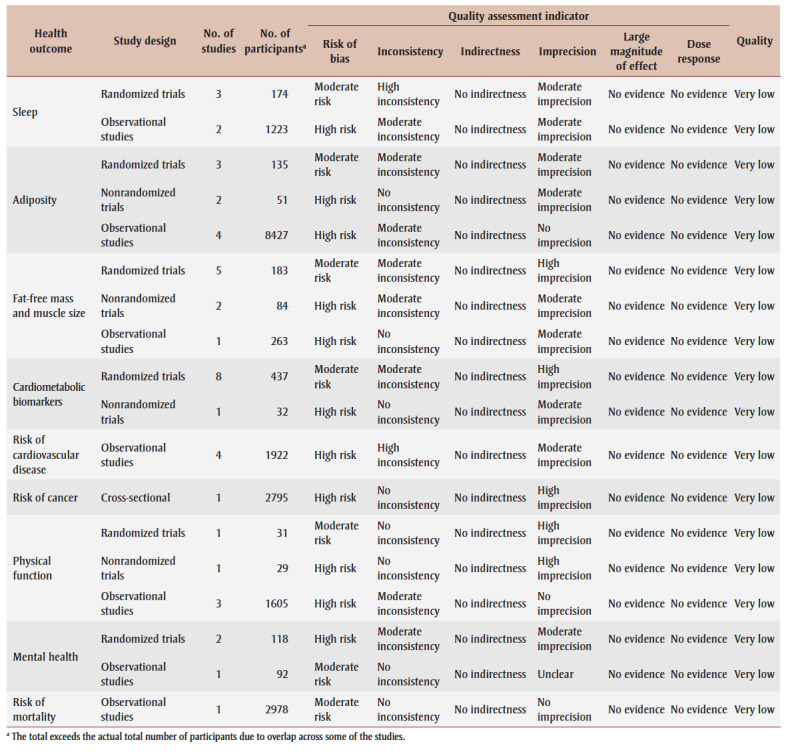
The quality of evidence was very low for all study designs (Table 1) because of concerns related to bias, inconsistency and imprecision, with no evidence of large effects or dose–response relationships.
Table 1. Quality assessment and quality of evidence rating based on studies examining the relationship between physical activity timing and health.
|
Physical activity timing and adiposity
Three RCTs25-27, 2 nonrandomized trials9,28 and 4 cross-sectional studies29-32 examined the relationship between physical activity timing and adiposity (Table S2; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). A variety of adiposity measures were examined including body mass index (BMI), waist circumference, body fat and trunk fat.
Results were mixed. One RCT reported that participants who performed more exercise in the morning had greater reductions in body fat than participants who performed more exercise in the late afternoon26. The other 2 RCTs reported no differences in adiposity in the morning and evening exercise groups25,27. The 2 nonrandomized trials reported that morning exercise resulted in smaller improvements to body fat than afternoon or evening exercise9,28. Two of the cross-sectional studies observed that morning physical activity was more strongly associated with obesity than physical activity at other times29,30. The other 2 cross-sectional studies mostly observed that the timing of physical activity was not associated with adiposity31,32.
The quality of evidence was very low across study designs (Table 1). There were concerns related to bias, inconsistency (randomized trials and observational studies only) and imprecision (randomized and nonrandomized trials only), with no evidence of large magnitude of effect or dose–response relationships.
Physical activity and fat-free mass and muscle size
Eight studies examined the relationship between physical activity timing and measures of fat-free mass and muscle size: 5RCTs25-27,33,34, 2 nonrandomized trials10,35 and 1 cross-sectional study31 (Table S3; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). The interventions consisted of resistance training27,33,34, aerobic training25,26 or a combination of resistance and aerobic training10,35. The results of the cross-sectional study31 and 5/7 experimental studies10,25,26,33,34 suggest that the timing of physical activity is not associated with measures of fat-free mass and muscle size. In the remaining 2/7 experimental studies, one reported that morning exercise results in greater changes in muscle size than evening exercise27, while the other reported the opposite35.
The quality of evidence was very low across study designs (Table 1). There were concerns related to bias, inconsistency (randomized and nonrandomized trials only) and imprecision, with no evidence of large effects or dose–response relationships.
Physical activity timing and cardiometabolic biomarkers
Eight studies examined the relationship between physical activity timing and cardiometabolic biomarkers10,25,27,36-40, including measures of glucose and insulin homeostasis, plasma lipids and lipoproteins, blood pressure, inflammatory markers and other hormones (e.g.testosterone, cortisol) (Table S4; https://osf.io/qcw6j/?view_onl y=4130e81638684feaa2dfa74f5e589d58). All 8 studies used an experimental design; 4 used either an RCT25,27,37 or randomized crossover36 design. Of these 8 studies, 7 prescribed a 12-week intervention10,25,27,37-40.
The studies examined a variety of exercise modalities: aerobic exercise25,37,38, resistance exercise27, combined aerobic and resistance exercise10,39,40 and high-intensity interval training36. Mixed results were observed across these 8 studies. Four reported that the timing of exercise training did not influence changes in cardiometabolic biomarkers25,27,39,40. Three reported that training in the morning resulted in less favourable changes in cardiometabolic biomarkers than training the evening36-38, while one study reported the opposite10.
The quality of evidence was very low for both the randomized trials and the nonrandomized trial (Table 1). For the randomized trials, there was a moderate concern of inconsistency and a high concern of imprecision. For the nonrandomized trial, there was a moderate concern of imprecision. There was no evidence of a large magnitude of effect or dose–response relationships.
Physical activity timing and risk of cardiovascular disease
Three case–control studies11,41,42 and 1 cross-sectional study43 examined the association between physical activity timing and cardiovascular disease (Table S5; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). One examined children and adolescents42, while the others examined adults11,41,43. One study examined high cardiovascular disease risk (i.e. 10-year cardiovascular disease risk prediction)43, while the others examined cardiovascular disease end points11,41,42.
Mixed results were observed across these 4 studies. The first case–control study reported that sports performed in the morning and evening but not afternoon were associated with a reduced odds of acute myocardial infarction11. The second case–control study reported that physical activity in the late afternoon (15:00–18:00), but not during the school day or evening, was associated with heart disease42. The third case–control study found that the reduced odds of coronary artery disease was similar irrespective of the typical time of day of exercise41. Finally, the cross-sectional study reported that higher cardiovascular risk was associated with a lack of physical activity in the afternoon and evening but in not the morning43.
The quality of evidence was rated as very low as there were high concerns related to bias and inconsistency and moderate concerns related to imprecision (Table 1).
Physical activity timing and risk of cancer
A single prospective cohort study examined the association between physical activity timing and cancer12 (Table S6; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). This study looked at prostate cancer in men and breast cancer in women. No significant protective effects were observed for early morning, late morning or afternoon physical activity.
The quality of evidence was rated as very low (Table 1) as there was a high risk of bias and imprecision with no evidence of a large magnitude of effect or dose–response relationship.
Physical activity timing and physical function and mobility
Five studies examined the relationship between physical activity timing and measures of physical function and mobility9,27,44-46 (Table S7; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). Three studies examined direct measures of function (e.g. walking speed, functional mobility)9,27,45, one examined falls44 and one examined frailty46. Four of these studies were cross-sectional27,44-46, and the fifth was a nonrandomized trial9.
The results of 3/5 studies suggest that while physical activity is associated with better physical function outcomes, the timing of physical activity is not relevant27,44,45. One cross-sectional study indicated that less physical activity in morning through afternoon, but not evening, was associated with frailty46. Conversely, walking test scores in the nonrandomized trial improved more in evening exercisers than morning exercisers9.
The quality of evidence was rated as very low across study designs (Table 1). There were concerns related to bias for all study designs, to inconsistency for observational studies and to imprecision for the randomized and nonrandomized trials. There was no evidence of a large magnitude of effect or dose–response relationships.
Physical activity timing and mental health
Three studies examined the association between physical activity timing and mental health47-49 (Table S9; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). In a 12-week RCT of older adults, afternoon exercise was more effective than morning exercise at improving cognitive function and mood47. In a 12-week nonrandomized trial of retired elite athletes with depression, morning and evening exercise led to comparable improvement in mood state48. In a case–control study of patients with Alzheimer disease and older adult controls, the altered physical activity levels in the patient group were most pronounced in the morning49.
The quality of evidence was rated as very low for both the randomized trials and case–control study (Table 1). For the randomized trials, there was a high concern of bias and a moderate concern of inconsistency and imprecision. For the case–control study there was a moderate concern of bias. There was no evidence of a large magnitude of effect or dose–responses relationships.
Physical activity timing and risk of mortality
A single study that used a prospective cohort design in 50- to 85-year-olds examined the association between physical activity timing and risk of mortality50 (Table S10; https://osf.io/qcw6j/?view_only=4130e81638684feaa2dfa74f5e589d58). The authors used accelerometers to measure physical activity over 7 days and examined whether average daily physical activity performed in each of 12 two-hour time intervals contributed to an all-cause mortality prediction model50. The prediction model also contained total physical activity and several sociodemographic, behavioural and health variables. None of the activity counts for the 12 two-hour time intervals reached statistical significance in the model.
The quality of evidence was rated as very low (Table 1). There was a moderate risk of bias without evidence of a large magnitude of effect or a dose–response relationship.
Discussion
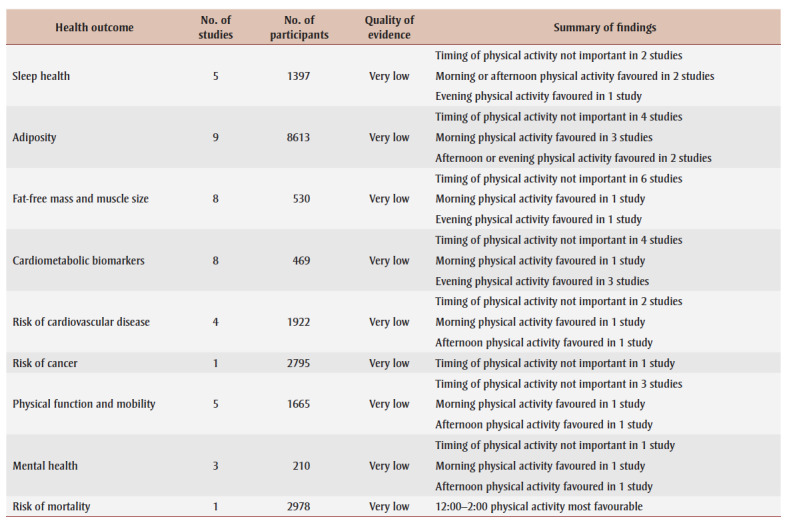
This review was prompted by evolving public health recommendations for physical activity and the desire to understand if the timing of physical activity within the day may be a consideration in future recommendations. We synthesized evidence from 35 peer-review studies of over 15000 unique participants that examined the relationship between the timing of physical activity within the day and indicators of health. Across the 35 studies, participant characteristics, study design and physical activity characteristics varied tremendously. A summary of findings is presented in Table 2.
Table 2. High-level summary of findings by health outcome.
|
There is no consistent evidence that physical activity performed at one time of day provides more favourable health benefits than that performed at a different time of day. Although the results of 11 of the reviewed studies (31%) suggest that physical activity in the morning provides greater health benefits than physical activity in the afternoon or evening, the results of 12 of the reviewed studies (37%) suggest that physical activity in the morning provides fewer health benefits than physical activity later in the day. The remaining studies found no clear difference in health outcomes based on the timing of physical activity. This pattern of mixed findings was observed for all 9 health outcome categories.
To the best of our knowledge, this is the first review to examine the relationship between physical activity timing within the 24-hour day and health outcomes. Previous systematic reviews have considered the timing of exercise around food intake (e.g. meals, nutritional supplements) as well as whether exercise performance varies according to the time of day when the exercise is performed. One recent systematic review concluded that exercise performed post-meal has a greater impact on postprandial glycemia than exercise performed pre-meal51. Another review and meta-analysis of protein timing around resistance exercise concluded that consuming adequate protein in combination with resistance exercise is key to maximizing gains in muscle size, but the timing of protein intake around the training session does not significantly influence these gains52. Other reviews concluded that performance in aerobic and anaerobic exercises is highest in the late afternoon to early evening53,54.
Strengths and limitations
Several gaps and limitations exist in the studies included in this review. Most studies focussed on MVPA and only one examined light physical activity21. The number of studies was small (1 to 9) for the 9 health outcomes where there was at least some evidence, and nonexistent for all other health outcomes. Almost half (47%) of the interventions lacked a control group and/or were nonrandomized. Furthermore, most interventions had small sample sizes (typically <20 per treatment arm) and were underpowered to detect small effects. Many of the observational studies also lacked precision.
It was difficult to examine dose–response relationships because the timing was often examined over broad time spans (e.g. all morning hours versus all evening hours). Studies in this topic area have also not considered whether the findings are modified by physical activity characteristics (e.g. type, intensity, dose) and sociodemographic characteristics (e.g. age, sex, race/ethnicity). Future studies should examine the timing of physical activity using a compositional data analysis approach that considers physical activity across the full 24-hour day17.
A notable strength of this systematic review is its comprehensive search strategy, with all study designs, all health outcomes and all populations included. A primary limitation is that the evidence was of very low quality because of concerns to do with bias, a lack of consistent findings and imprecise findings. Because this systematic review was limited to peer-reviewed studies, it is susceptible to publication bias because null findings are less likely to be published55. This review was also limited to English and French language papers; however, a recent analysis indicates that excluding non-English publications from evidence syntheses did not change the conclusions56.
Conclusion
The results of 35 studies examining the association between physical activity and health outcomes are mixed. The findings, which are based on very low quality evidence, do not consistently support that it would healthier to be physically active at one time of day over another. People should be encouraged to be active when it is most convenient for them.
Acknowledgements
This work was funded in part by a grant received from the Faculty of Health Sciences at the University of Ottawa.
Registration of the protocol
PROSPERO registration no. CRD42021231088, available from www.crd.york.ac.uk/PROSPERO/.
Conflicts of interest
The authors have no conflicts to declare.
Authors’ contributions and statement
JC came up with the idea for the systematic review with input from IJ, TJS and JT.
IJ designed the systematic review with input from all other authors.
IJ, JC and SZ performed literature searches, article screening, data abstraction, risk of bias assessments and quality of evidence assessments.
IJ wrote the first draft of the manuscript; this was edited for important intellectual content by all other authors.
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
References
- Centers for Disease Control and Prevention. Atlanta(GA): 1996. Physical activity and health: a report of the Surgeon General. [Google Scholar]
- Health Canada. Ottawa(ON): 1998. Canada’s Physical Activity Guide to Healthy Active Living. [Google Scholar]
- US Department of Health and Human Services. Washington(DC): 2008. Physical Activity Guidelines Advisory Committee Report, 2008. [DOI] [PubMed] [Google Scholar]
- Tremblay MS, Warburton DE, Janssen I, et al, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36((1)):36–46. doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva(CH): 2010. Global recommendations on physical activity for health. [PubMed] [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, et al, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320((19)):2020–8. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Chaput JP, Giangregorio LM, et al, et al. Canadian 24-hour movement guidelines for adults aged 18-64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45((10 (Suppl. 2))) doi: 10.1139/apnm-2020-0843. [DOI] [PubMed] [Google Scholar]
- Bull FC, Al-Ansari SS, Biddle S, et al, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54((24)):1451–62. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Donato F, Mastrodicasa M, et al, et al. Effects of the time of day of walking on dietary behaviour, body composition and aerobic fitness in post-menopausal women. J Sports Med Phys Fitness. 2010;50((2)):196–201. [PubMed] [Google Scholar]
- Mancilla R, Krook A, Schrauwen P, Hesselink MK, et al. Diurnal regulation of peripheral glucose metabolism: potential effects of exercise timing. Diurnal regulation of peripheral glucose metabolism: potential effects of exercise timing. Obesity (Silver Spring) 2020:S38–45. doi: 10.1002/oby.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhang Z, Long Q, et al, et al. Association between time of day of sports-related physical activity and the onset of acute myocardial infarction in a Chinese population. PLoS ONE. 2016;11((1)):e0146472–45. doi: 10.1371/journal.pone.0146472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzer J, o-Vinyals G, s N, et al, et al. Effect of time of day of recreational and household physical activity on prostate and breast cancer risk (MCC-Spain study) Int J Cancer. 2020;148((6)):1360–71. doi: 10.1002/ijc.33310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimons M, et al. What’s the best time of day to exercise, morning or evening. Washington Post. :Health Section–71. [Google Scholar]
- Reynolds G, et al. Late-day exercise had unique benefits for cholesterol levels and blood sugar control, a study of overweight men eating a high-fat diet found. The New York Times [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009:b2535–71. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7((1)):16–71. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuid D, Olds TS, et al. Integrating sleep, sedentary behaviour, and physical activity research in the emerging field of time-use epidemiology: definitions, concepts, statistical methods, theoretical frameworks, and future directions. Kinesiology. 2017;49((2)):1–18. [Google Scholar]
- Higgins JP, Green S, et al. Higgins JP, Green S, editors. London(UK): Cochrane handbook for systematic reviews of interventions. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, et al, et al. GRADE guidelines: 4. J Clin Epidemiol. 2011:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, et al, et al. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27((8)):1542–51. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- Seol J, Fujii Y, Inoue T, Kitano N, Tsunoda K, Okura T, et al. Effects of morning versus evening home-based exercise on subjective and objective sleep parameters in older adults: a randomized controlled trial. J Geriatr Psychiatry Neurol. 2021;34((3)):232–42. doi: 10.1177/0891988720924709. [DOI] [PubMed] [Google Scholar]
- Chen E, Viktorisson A, Danielsson A, Palstam A, Sunnerhagen KS, et al. Levels of physical activity in acute stroke patients treated at a stroke unit: a prospective, observational study. J Rehabil Med. 2020;52((4)):jrm00041–42. doi: 10.2340/16501977-2671. [DOI] [PubMed] [Google Scholar]
- Buman MP, Winkler EA, Kurka JM, et al, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. Buman MP, Winkler EA, Kurka JM, et al. 2014:323–34. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim SJ, Bang JW, Lee JH, et al. Relationship of the duration and timing of exercise with sleep quality in community-dwelling adults. Sleep Med Res. 2018;9((2)):83–91. [Google Scholar]
- Brooker PG, Gomersall SR, King NA, Leveritt MD, et al. The feasibility and acceptability of morning versus evening exercise for overweight and obese adults: a randomized controlled trial. Contemp Clin Trials Commun. 2019:100320–91. doi: 10.1016/j.conctc.2019.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis EA, Creasy SA, Honas JJ, Melanson EL, Donnelly JE, et al. The effects of exercise session timing on weight loss and components of energy balance: Midwest Exercise Trial 2. The effects of exercise session timing on weight loss and components of energy balance: Midwest Exercise Trial 2. Int J Obes (Lond) 2020:114–24. doi: 10.1038/s41366-019-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- r M, et al, et al. The effects of 12-week progressive strength training on strength, functional capacity, metabolic biomarkers, and serum hormone concentrations in healthy older women: morning versus evening training. Chronobiol Int. 2018;35((11)):1490–502. doi: 10.1080/07420528.2018.1493490. [DOI] [PubMed] [Google Scholar]
- Marinac CR, Quante M, Mariani S, et al, et al. Associations between timing of meals, physical activity, light exposure, and sleep with body mass index in free-living adults. J Phys Act Health. 2019;16((3)):214–21. doi: 10.1123/jpah.2017-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomistek AK, Shiroma EJ, Lee IM, et al. The relationship between time of day of physical activity and obesity in older women. J Phys Act Health. 2016;13((4)):416–8. doi: 10.1123/jpah.2015-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Mackay L, Chang K, et al, et al. Visualising combined time use patterns of children's activities and their association with weight status and neighbourhood context. Int J Environ Res Public Health. 2019:E897–8. doi: 10.3390/ijerph16050897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Pulakka A, Leskinen T, et al, et al. Daily physical activity patterns and their association with health-related physical fitness among aging workers - the Finnish Retirement and Aging study. J Gerontol A Biol Sci Med Sci. 2021;76((7)):1242–50. doi: 10.1093/gerona/glaa193. [DOI] [PubMed] [Google Scholar]
- Marinac CR, Quante M, Mariani S, et al, et al. Associations between timing of meals, physical activity, light exposure, and sleep with body mass index in free-living adults. J Phys Act Health. 2019;16((3)):214–21. doi: 10.1123/jpah.2017-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedliak M, Finni T, Peltonen J, kkinen K, et al. Effect of time-of-day-specific strength training on maximum strength and EMG activity of the leg extensors in men. J Sports Sci. 2008;26((10)):1005–14. doi: 10.1080/02640410801930150. [DOI] [PubMed] [Google Scholar]
- Sedliak M, Finni T, Cheng S, Lind M, kkinen K, et al. Effect of time-of-day-specific strength training on muscular hypertrophy in men. J Strength Cond Res. 2009;23((9)):2451–7. doi: 10.1519/JSC.0b013e3181bb7388. [DOI] [PubMed] [Google Scholar]
- smaa M, Schumann M, Sedliak M, et al, et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab. 2016;41((12)):1285–94. doi: 10.1139/apnm-2016-0271. [DOI] [PubMed] [Google Scholar]
- Savikj M, Gabriel BM, Alm PS, et al, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019:233–7. doi: 10.1007/s00125-018-4767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XQ, Zhao D, Zhu M, et al, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med. 2014;58((1)):64–9. doi: 10.1016/j.ypmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Heitkemper MM, et al. Effects of a 12-week moderate-intensity exercise training on blood glucose response in patients with type 2 diabetes: a prospective longitudinal study. Medicine (Baltimore) 2019;98((36)):e16860–9. doi: 10.1097/MD.0000000000016860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo SY, Kanaley JA, Guelfi KJ, Marston KJ, Fairchild TJ, et al. The effect of exercise timing on glycemic control: a randomized clinical trial. Med Sci Sports Exerc. 2020;52((2)):323–34. doi: 10.1249/MSS.0000000000002139. [DOI] [PubMed] [Google Scholar]
- Teo SY, Kanaley JA, Guelfi KJ, et al, et al. Exercise timing in type 2 diabetes mellitus: a systematic review. Med Sci Sports Exerc. 2018;50((12)):2387–97. doi: 10.1249/MSS.0000000000001732. [DOI] [PubMed] [Google Scholar]
- Zhao H, Chu XQ, Lian XQ, Wang ZM, Gao W, Wang LS, et al. Relationship between time of day physical exercise and the reduced risk of coronary artery disease in a Chinese population. Int J Sport Nutr Exerc Metab. 2014;24((2)):139–47. doi: 10.1123/ijsnem.2012-0226. [DOI] [PubMed] [Google Scholar]
- White DA, Willis EA, Panchangam C, et al, et al. Physical activity patterns in children and adolescents with heart disease. Pediatr Exerc Sci. 2020;32((4)):233–40. doi: 10.1123/pes.2020-0073. [DOI] [PubMed] [Google Scholar]
- Fenton SA, Ntoumanis N, Duda JL, et al, et al. Diurnal patterns of sedentary time in rheumatoid arthritis: associations with cardiovascular disease risk. RMD Open. 2020;6((2)):e001216–40. doi: 10.1136/rmdopen-2020-001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastasi AJ, Ahuja A, Zipunnikov V, Simonsick EM, Ferrucci L, Schrack JA, et al. Objectively measured physical activity and falls in well-functioning older adults: findings from the Baltimore Longitudinal Study of Aging. Am J Phys Med Rehabil. 2018:255–60. doi: 10.1097/PHM.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TF, Liao Y, Lin CY, Huang WC, Hsueh MC, Chan DC, et al. Moderate-to-vigorous physical activity duration is more important than timing for physical function in older adults. Sci Rep. 2020;10((1)):21344–60. doi: 10.1038/s41598-020-78072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, et al, et al. The relationship between physical activity and frailty among U.S. J Gerontol A Biol Sci Med Sci. 2018:622–9. doi: 10.1093/gerona/glx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Haitani T, Tanaka F, et al, ning vs, et al. Effects of the time-of-day (morning vs. Adv Gerontol. 2020;33((3)):595–9. [PubMed] [Google Scholar]
- Irandoust K, Taheri M, Chtourou H, Nikolaidis PT, Rosemann T, Knechtle B, et al. Effect of time-of-day-exercise in group settings on level of mood and depression of former elite male athletes. Int J Environ Res Public Health. 2019;16((19)):E3541–9. doi: 10.3390/ijerph16193541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Watts A, et al. Daily physical activity patterns during the early stage of Alzheimer’s disease. J Alzheimers Dis. 2017;55((2)):659–67. doi: 10.3233/JAD-160582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Leroux A, Cao Q, et al, et al. The predictive performance of objective measures of physical activity derived from accelerometry data for 5-year all-cause mortality in older adults: National Health and Nutritional Examination Survey 2003–2006. Smirnova E, Leroux A, Cao Q, et al. 2020:1779–85. doi: 10.1093/gerona/glz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeel M, Forster A, Richards EA, et al, et al. The effect of timing of exercise and eating on postprandial response in adults: a systematic review. Nutrients. 2020;12((1)):E221–85. doi: 10.3390/nu12010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld BJ, Aragon AA, Krieger JW, et al. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sports Nutr. 2013;10((1)):53–85. doi: 10.1186/1550-2783-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirizio GG, Vargas DA, Foster C, Vieira E, et al. Time-of-day effects on short-duration maximal exercise performance. Sci Rep. 2020;10((1)):9485–85. doi: 10.1038/s41598-020-66342-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappaert TA, et al. Time of day effect on athletic performance: an update. J Strength Cond Res. 1999;13((4)):412–21. [Google Scholar]
- Sterne JA, Egger M, Smith GD, et al. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323((7304)):101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020:42–54. doi: 10.1016/j.jclinepi.2019.10.011. [DOI] [PubMed] [Google Scholar]


