INTRODUCTION
Myofascial pain is a common cause of acute and chronic pain. The term “myofascial pain” encompasses many different painful conditions and can exist independently of other pain generators, known as primary myofascial pain. Common primary myofascial pain diagnoses include piriformis syndrome, iliopsoas-related pain, and pain related to compression of the brachial plexus by the scalene muscles (neurogenic thoracic outlet syndrome). Frequently, myofascial pain coexists with or is secondary to other acute and chronic painful musculoskeletal conditions including (1) head and neck disorders (temporomandibular disorders, cervical degenerative disc disease, cervical facet arthropathy, neck pain after whiplash injury, cervicobrachial syndrome, and cervicogenic or chronic tension-type headache); (2) thoracolumbar back disorders (degenerative disc disease, kyphosis, scoliosis, and lumbar facet arthropathy); (3) pelvic pain; and (4) upper and lower extremity pain disorders. Myofascial pain is most effectively treated with a multimodal treatment plan including injection therapy (known as trigger point injections [TPIs]), physical therapy, postural or ergonomic correction, and treatment of underlying musculoskeletal pain generators.
The objectives of this review are to describe the known pathophysiology of myofascial pain and trigger points (TrPs), discuss the clinical presentation of myofascial pain and piriformis syndrome, an extremely common primary myofascial pain disorder, describe best practices for TPI and piriformis muscle injection therapy, and outline the current data for TPI therapy with local anesthetic and/or steroids and botulinum toxin.
NATURE OF THE PROBLEM: THE PATHOPHYSIOLOGY OF MYOFASCIAL PAIN AND TrPs
Although much remains to be discovered about the pathophysiology of myofascial pain, several mechanistic theories have been advanced in recent years. Certainly, as with most chronic pain conditions, the biopsychosocial model of pain pathophysiology applies.1 Underlying biomechanical and postural factors may interact with neurologic factors, psychological elements including depression and anxiety, and hormonal and nutritional imbalances. These factors, in total or in part, may lead to peripheral sensitization, autonomic dysregulation, and central sensitization, which then amplifies the pain experienced by patients with myofascial pain. Vasoactive mediators, pronociceptive neurotransmitters, and inflammatory mediators including bradykinin, norepinephrine, serotonin, calcitonin gene–related peptide, substance P, tumor necrosis factor α, and interleukin-1β have all been identified in the hypersensitive loci of TrPs.2–4 These substances are pronociceptive and sensitize peripheral nociceptors. In a sensitized state, nociceptors spontaneously discharge with a lower threshold to painful stimulation and also exhibit discharge to nonpainful stimuli.5 Over time, this heightened abnormal peripheral sensory input creates a state of central neuronal sensitization.6 The hypothalamus-pituitary-adrenocortical and sympathetic-adrenal-medullary system responses to experimentally induced stress in patients with myofascial pain has shown that plasma concentrations of cortisol, epinephrine, and norepinephrine were found to be significantly increased in myofascial pain patients than in healthy controls.7
CLINICAL PRESENTATION AND DIAGNOSIS
Myofascial Pain
A careful history and physical examination remain the keystone of diagnosis. As discussed previously, TrPs are localized painful areas of skeletal muscle containing taut bands that can be exquisitely sensitive to digital pressure (Fig. 1). TrPs may be active or latent. Active TrPs are present in patients with painful regional conditions. Latent TrPs are asymptomatic but may be revealed by deep palpation on physical examination. Latent TrPs are very common and have been identified in the shoulder girdle muscles of 45% to 55% of healthy young adults.8 TrPs are different from tender points, which are defined as a localized area of tenderness in a muscle, muscletendon junction, fat pad, or bursal region.9 Myofascial pain may occur after an injury, with chronic strain from repetitive microtrauma, or without any clear precipitating event. Aberrant body mechanics or postural abnormality may initiate or further maintain the problem. The quality of pain tends to be a deep “aching” of variable intensity and the pain is generally confined to a specific anatomic region. Characteristic referred pain patterns are associated with specific muscles, although these referral patterns are often unreliable.10 Commonly involved TrP musculature include the trapezius, splenii, cervical and lumbar paraspinal, piriformis, and quadratus lumborum.
Fig. 1.
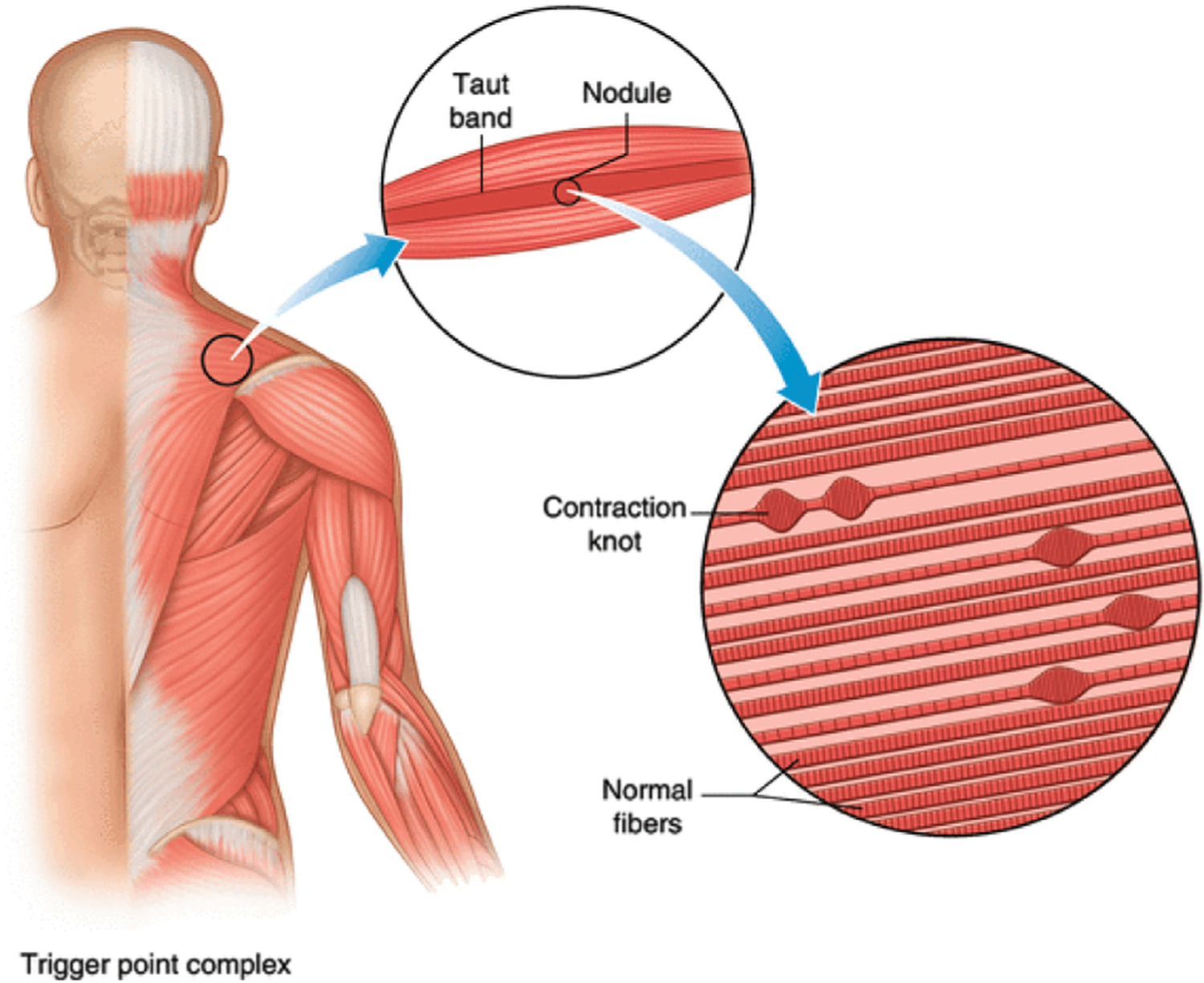
Schematic of the anatomy of a taut band and trigger point.
Simons and colleagues have developed a set of diagnostic criteria that are often referenced when describing the features of TrPs including presence of taut band, tenderness from taut band, reproducibility of pain, local twitch response, restricted range of motion, autonomic symptoms, and referred pain.11 Furthermore, palpation of an active TrP can cause referred pain through activation of the central nervous system along the distribution of the nerve innervating the muscle that is activated.12
It is essential to have hands-on training in the physical examination of myofascial pain and TrPs.13 Musculoskeletal examination should be performed with the objective of identifying possible orthopedic or neurologic pathologies that could have a role in generating secondary myofascial pain and dysfunction. A distinct pattern of TrP findings may reveal itself in myofascial pain syndrome after a given insult.14 These painful TrPs limit full range of passive motion in the afflicted muscle group. Although these findings have been suggested as diagnostic criteria,15–17 investigators have found it problematic to demonstrate consistent agreement in the presence or absence of TrPs among examiners in blinded studies with control groups.18–20 Discrepancies in diagnosis may be attributed in part to a lack of a standardized examination technique as well as variability in the interpretation of examination findings. Furthermore, variations in muscle anatomy, physical conditioning, and deconditioning can pose obstacles to proper diagnosis as well. The most reproducible diagnostic findings on physical examination include identification of a TrP in an affected muscle, referral of pain to a zone of reference, and reproduction of the patient’s regular pain on physical examination.21
Differential diagnosis of myofascial pain should include (1) musculoskeletal and neuropathic disorders such as arthritis, degenerative disk disease, radiculopathy, bursitis, and tendonitis; (2) autoimmune or infectious etiologies; (3) metabolic and endocrine dysfunction; (4) psychiatric disorders including depression and anxiety; and (5) fibromyalgia or diffuse amplified musculoskeletal pain.
Imaging studies have only recently demonstrated anatomic changes associated with TrPs. Ultrasound (US) examination in combination with Doppler blood flow has been reported to allow visualization of TrPs, and US imaging can help direct muscle injection techniques. Recently developed techniques using magnetic resonance and US elastography purport to reveal changes in intramuscular signal consistent with TrPs, but the use of this technology in clinical practice has not yet been validated.22,23
Piriformis Syndrome
Piriformis syndrome consists of pain in the buttock with or without radiation in the distribution of the ipsilateral sciatic nerve. It is considered the principal pain generator in approximately 8% of patients presenting with the buttock as the origin of pain. The syndrome can be a consequence of an abnormal relationship between the sciatic nerve and the piriformis muscle that results in irritation of the sciatic nerve. A hypertrophic muscle, infection, or invasion of the muscle by tumor can cause pressure or irritation on the nerve.24,25 In 78% to 84% of the population, the sciatic nerve passes in front of the muscle. In 12% to 21% of individuals, the divided nerve passes through or posterior to the piriformis and is exposed to muscle contractions, which trigger sciatic symptoms.24
There is no consensus set of diagnostic criteria and is often diagnosed from a history indicative of this condition with confirmatory physical examination with an often normal electrodiagnostic testing and imaging.26 The syndrome should be considered in patients who have buttock pain, tenderness to palpation over the piriformis muscle, and possible symptoms of sciatic nerve pain. In addition, positive response to provocative maneuvers may indicate the diagnosis, including the following: specific confirmatory physical examination testing for piriformis syndrome includes the active piriformis test, the Beatty test, the FAIR test, and the Pace test.26
PATIENT SELECTION
Trigger Point Injection
TPI is a widely used invasive therapy wherein a needle is guided directly into a TrP that has been previously identified on physical examination. TPI is best used as part of a comprehensive multimodal treatment plan. This strategy can be particularly beneficial when TPI is initially used to reduce pain in patients otherwise intolerant of physical therapy or stretching, allowing the physical modalities to be more effective.27 TPIs should be considered in patients once a thorough evaluation is completed to rule out other causes of back pain including muscle strain, axial back pain, structural causes of pain, discogenic back pain, vertebrogenic back pain, spinal stenosis, vertebral body disease (including fracture), and radicular back pain.
Piriformis Muscle Injection
As discussed previously, piriformis syndrome is a condition associated with low back pain where the traversing sciatic nerve is compressed by the sciatic nerve. Once diagnosed, patients can be treated with conservative management first, including pharmacologic therapy and physical therapy. If pain persists after conservative management, it is reasonable to trial a piriformis muscle injection.
Risks and Contraindications
The following conditions are contraindications to TPI and piriformis muscle injection: (1) infection, systemic or localized (absolute); (2) coagulopathy (relative); (3) distorted or complicated anatomy (relative); and (4) patient refusal (absolute).28 According to the anticoagulation guidelines from the American Society of Regional Anesthesia and Pain Medicine, TPIs classify as a low-risk procedure.28
TPIs are commonly performed as an outpatient procedure. Although serious complications are rare, there have been case reports of complications particularly in the cervical and thoracic region.
The most commonly reported serious complication is pneumothorax.29–31 A case series of 38 patients developed pneumothorax after acupuncture or acupoint insertion with one death from pneumothorax in the series.32 Specific attention to TrP technique and use of ultrasound guidance can help avoid this complication in the cervical and thoracic injections.33,34 Management of iatrogenic pneumothorax depends on size of the pneumothorax and symptoms and can range from observation, oxygen supplementation and close monitoring to ultrasound or CT-guided aspiration and tube thoracotomy.35,36
Rare possibility of intrathecal injection has been reported in a 28-year-old woman after superficial trapezius TPI.37 This patient developed respiratory depression and pneumocephalus requiring emergency tracheal intubation and ventilatory support and fully recovered over the course of 24 hours. There has been another case report of cervical epidural abscess that has been reported.38
Skeletal muscle myotoxicity has been demonstrated in experimental studies with the use of local anesthetic agents as they cause reversible myonecrosis. Histologic changes include hypercontracted myofibrils followed by lytic degeneration of striated muscle sarcoplasmic reticulum and myocyte edema and necrosis over 1 to 2 days. Muscle regeneration occurs within the next 3 to 4 weeks as myoblasts, basal laminae, and connective tissue elements remain intact. This effect has been seen in only a few case reports of myotoxic complications after local anesthetic administrations causing clinically relevant myopathy and myonecrosis after TPIs.39 In experimental studies, procaine produces the least and bupivacaine causes the most severe muscle injury.
There has been one case report of severe hypokalemic paralysis after left iliopsoas muscle injection with ultrasound guidance that highlights the importance that high index of suspicion is warranted for prompt diagnosis and management.40 It was postulated in this case that the cause of the hypokalemia was due to transcellular shifting due to epinephrine component of the TPI injectate.
Other complications that can occur include vasovagal syncope, allergic reaction, skin infection, and hematoma formation.41 Proper preparation, taking adequate sterile precautions, and close monitoring of the patient during and after the procedure can minimize long-term effects.
TECHNIQUE FOR TPI (EXCLUDING PIRIFORMIS MUSCLE)
Technique for TPI
Preparation
TPIs should be performed by skilled professionals with adequate anticipation and preparation for complications such as vasovagal reaction and allergic reaction. After reviewing the contraindications as described earlier and after discussing the risks of the procedure including, but not limited to increased pain, infection, bleeding, allergic reaction, soft tissue injury, pneumothorax, cutaneous atrophy, bleaching of the skin (if steroid is administered), written informed consent is obtained.
Technique
The targeted areas are then identified and marked by the presence of discrete TrPs with localized tenderness, hypertonicity, and taut bands. Using the nondominant hand, the skin and underlying tissue is pinched between the index finger and thumb, and the needle attached to the syringe with the injectate is inserted using the dominant hand at a 30° angle until the taut band is reached. The needle is then advanced and retracted to various sites within the muscle until relaxation is achieved. At each site, after aspiration is performed to ensure negative return of blood or fluid, 0.2 to 0.5 mL of solution is injected. The needle is then withdrawn after all the injectate is administered in a fanlike distribution in the muscle and a sterile band-aid is applied.
EVIDENCE FOR THE USE OF IMAGE GUIDANCE FOR TPI (EXCLUDING PIRIFORMIS MUSCLE)
Traditionally, TPIs have been performed using blind technique without image guidance by palpating the TrPs and inserting the needle with or without injecting a solution. However, palpation of TrPs can be technically difficult in obese patients that can result in ineffective injection if placed in the soft tissues and can cause complications if inadvertently placed in other tissues. Several diagnostic methods including electromyography, magnetic resonance elastography, and ultrasonography have been studied to determine the location and characteristics of myofascial TrPs.22,42,43 All these methods were shown to have limitations in proper identification. However, subsequent studies have supported the use of ultrasound, which is considered to be safe, portable, and inexpensive imaging modality for identification and evaluation of the effectiveness of therapeutic interventions in myofascial TrPs.23,44
The technique for using ultrasound guidance for TPIs was first described by Botwin and colleagues.33 The authors described the muscle to have hyperechoic marbled appearance while adipose tissue had mixed echogenicity, using a 13–6 MHz 38 mm broadband linear array transducer. They demonstrated clear visualization of the injectate using a 25g 1.5-inch needle under direct ultrasound guidance that was further confirmed with color mode. A subsequent study also demonstrated ultrasound can differentiate myofascial tissue with and without active TrPs.23 Several studies have shown that ultrasound-guided injections are extremely effective modality in various musculoskeletal locations and maximize injection accuracy and minimize potential complications.45–48 Ultrasound may be considered by practitioners for guiding musculoskeletal injections to avoid complications from blind injections.
However, there are very limited number of studies on the effectiveness of ultrasound-guided injections for myofascial pain syndrome. Ultrasound-guided interfascial block with lidocaine between the rhomboids major and trapezius showed statistically significant improvement in pain and quality of life similar to pulsed radiofrequency treatment.49 Ultrasound-guided deep injection using 12 to 18-MHz US transducer of the rhomboid major muscle was more effective than superficial injection of trapezius muscle for parameters of pain, disability, and quality of life in 61 patients with myofascial pain syndrome in a prospective randomized double-blinded study at 2 and 4 weeks post-treatment.50 The author’s findings corroborated with other studies that deep injections were superior to superficial injections and they concluded that ultrasound guidance can help to minimize the complications of blind injections such as pneumothorax, air embolism, inadvertent intrathecal injection, peripheral nerve injuries, and muscle injuries that are all rare reported complications, which is discussed in detail in the following section.31
Shear wave elastography using ultrasound has emerged as a quantitative method of measuring the mechanical properties of the soft tissue including skeletal muscle through external induction of the shear wave or with the use of the radiation force of the ultrasound.51–53 A recent pilot feasibility study on 41 patients compared ultrasound-guided myofascial injections and blind injections with the use of shear wave elastography and showed statistically significant improvement in the pain (VAS) scores, neck disability scores (NDI), and shoulder pain disability score (SPADI) at baseline and at 4 weeks with significantly higher efficacy in the ultrasound group.48
Limitations of these studies comparing ultrasound-guided myofascial injections with blind injections are small sample size and lack of placebo intervention and short follow-up period.50 Other efforts to characterize neuromuscular activation using surface electromyography signals using machine learning have been studied but have not been evaluated in clinical outcome studies.54,55
TECHNIQUE FOR PIRIFORMIS MUSCLE INJECTION AND EVIDENCE FOR THE USE OF IMAGE GUIDANCE
The use of imaging devices in the performance of piriformis injection procedures can help increase accuracy and reduce complications. It is important to select the appropriate image guidance to increase the success rate of procedures. Fluoroscopy and ultrasound are the most commonly used imaging techniques to perform piriformis injections. Ultrasound has been shown to provide higher accuracy in needle placement. In a study by Finnoff and colleagues, ultrasound-guided piriformis injections were found to be significantly more accurate than fluoroscopically guided contrast-controlled injections.56 The authors concluded that despite the use of bony landmarks and contrast, most of the fluoroscopically attempted piriformis injections were placed superficially within the gluteus maximus.
The use of electromyography for localization of the piriformis muscle or other TrPs has been reported with mixed results. In a 2016 study, it was reported that there was no correlation between the location of a TrP and the position of peak EMG amplitude.57 Fluoroscopic guidance relies on identification of bony target points that guide the practitioner to the piriformis muscle, whereas sonography can directly identify the piriformis muscles and provide a real-time image of surrounding soft tissues (nerves, muscles, vessels, etc.), an image of needle tip advancement relevant to surrounding structures, and visualization of injectate spread.58 In addition, neither the patients nor clinicians are exposed to radiation during an ultrasound-guided procedure and therefore present as a much safer option long-term with the absence of cumulative doses of radiation associated with repeat procedures.59
For the fluoroscopically guided injection with EMG guidance, the patient is placed prone on a fluoroscopy table, and the inferior margin of the sacroiliac joint is imaged and marked. The needle insertion site is 1 to 2 cm caudal and 1 to 2 cm lateral to the inferior margin of the sacroiliac joint. After sterile preparation and infiltration of local anesthetic, a 7-to 10-cm insulated needle is inserted and advanced with the nerve stimulator turned on (1 mA, 2 Hz, 0.1 msec) until an evoked motor response of the sciatic nerve is achieved (dorsiflexion, plantar flexion, eversion, inversion) at 0.4 to 0.6 mA. The needle is then withdrawn until the sciatic stimulation disappears to avoid intraneural injection and 1 to 2 mL of contrast agent is injected. The contrast agent should outline the piriformis muscle belly with no sign of spillage (Fig. 2). After the characteristic spread of dye is achieved, a local anesthetic solution with steroid is administered.
Fig. 2.
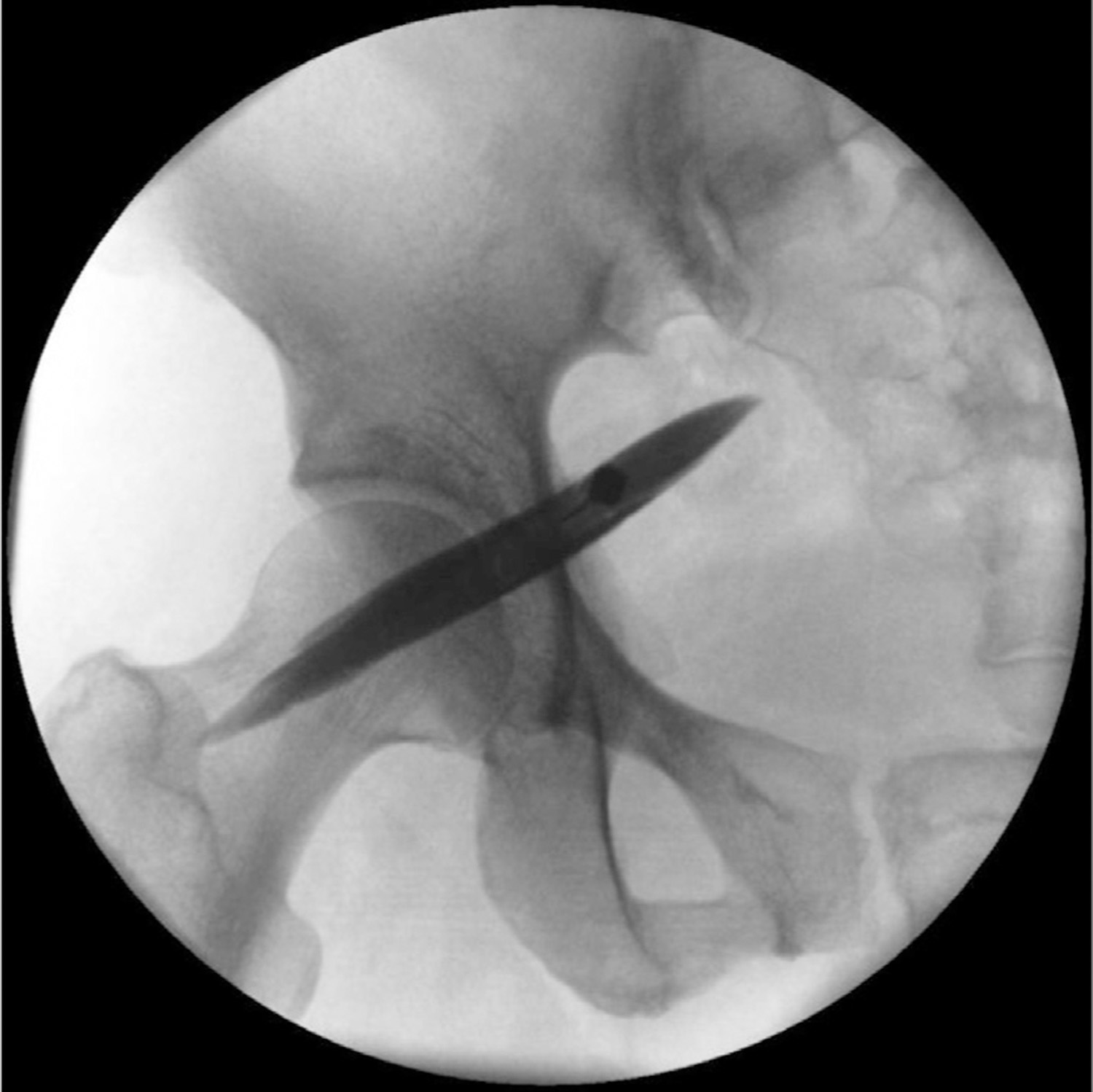
Fluoroscopic-guided piriformis muscle injection.
For the ultrasonography-guided procedure, the piriformis is identified in long axis with the transducer in the oblique axial plane on the body just inferior to the sacroiliac joint and greater sciatic notch (Fig. 3). It is important to maintain visualization of the piriformis musculature, lateral edge of the sacrum, and sciatic nerve to avoid needle contact with the nerve itself.60 Although the muscle is being visualized in the long axis, the nerve will be in the short axis and is typically seen deep to the piriformis when in the prone position. During this preprocedure scan, Doppler imaging should be used to locate vessels that will need to be avoided.61 While maintaining visualization of the needle, the needle can then be inserted in plane with the transducer until it enters the piriformis muscle tissue at the targeted site. The needle trajectory can be adjusted as the needle is inserted to reach the desired location and to avoid the sciatic nerve. When the needle is in the correct position at the muscle, the medication can be injected and visualized entering the tissue.62 Local anesthetic is visualized as anechoic, whereas corticosteroid may be hyperechoic with particulate steroids or anechoic in nonparticulate steroids.
Fig. 3.
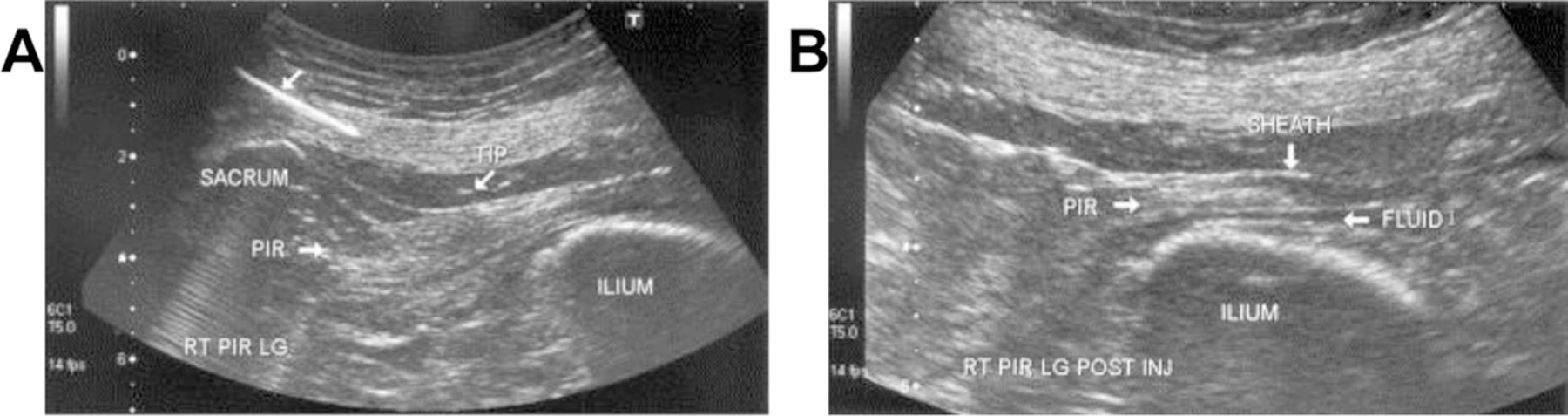
(A) Longitudinal US view of the piriformis during needle placement using a medial-to-lateral approach parallel to the long axis of the transducer. The proximal end of the needle has been digitally enhanced to highlight the needle trajectory. (B) Postinjection tenogram at the level of the greater sciatic foramen. Anechoic injectate (FLUID) within the piriformis tendon sheath lies superficial and deep to the hyperechoic tendon. RT PIR LG, right side, piriformis, longitudinal view; TIP, needle tip. (Reproduced with permission from Archives of Physical Medicine and Rehabilitation, Authors Jay Smith, Mark-Friedrich Hurdle, Adam J. Locketz, Steven J. Wisniewski. December 2006. Copyright © 2006 American Congress of Rehabilitation Medicine and the American Academy of Physical Medicine and Rehabilitation. Published by Elsevier Inc. All rights reserved. (PERMISSIONS HAVE BEEN OBTAINED BY ALC).)
Injectate Therapeutic Options
Saline, corticosteroids, a variety of local anesthetics including lidocaine and bupivacaine, botulinum toxin serotype A (BoNT-A), and dry needling have all been used and studied. Stimulation of the local twitch response in direct needling of the TrP is valuable in achieving immediate effect.63 There is good evidence to suggest that there is no advantage of one injection therapy over another, or of any drug injectate over dry needling.64 In a systemic review of 23 randomized controlled trials (RCTs), Cummings and White concluded that any effect derived from TPI is likely derived from the needle itself, rather than any specific substance injected, as there was no difference in therapeutic benefit of “wet” needling versus “dry” needling.64 Their review also suggested that pain reduction with saline TPI is equal to pain reduction with local anesthetic TPI, both being significant.
Although adding corticosteroid preparation to local anesthetic is a common practice, it has not been reliably shown to reduce pain more than TPI with local anesthetic alone.64,65 Botulinum toxin type A (BoNT-A) produces sustained and prolonged relaxation of muscles by inhibiting release of Ach at the motor endplate and is itself an analgesic inhibiting central sensitization.66 Commercially prepared BoNT-A is expensive and should be used with care by a well-trained physician. Despite the widespread practice of TPI for myofascial pain, there is no consensus regarding the number of injection points, frequency of administration, and volume or type of injectate. Controlled studies are needed to evaluate the comparative efficacy of TPIs and their potential benefits in long-term pain reduction, if any.
OUTCOMES DATA AND DISCUSSION FOR TPIs WITH LOCAL ANESTHETIC ± STEROID
Over the years, few well-designed RCTs regarding TPIs have been performed with even fewer published in the last decade. Available literature investigated the efficacy of the injection for myofascial pain with diverse medications (Table 1). Initial studies evaluated the efficacy of TPI with various types of local anesthetics, concentrations of local anesthetics, and a variety of steroids.65,67–70 The majority of these studies evaluated the injection techniques on the cervical neck, shoulder, masseter, abdominal/pelvic, and trapezius muscles. Regardless of medication used, the key component involved placing the needle into a taut band, which resulted in improved pain compared with baseline. Recent studies largely in the emergency medicine literature within the last 5 years have reaffirmed findings from earlier studies. In a study where patients presented to the emergency department with lumbar myofascial pain, patients were randomized to either intravenous (IV) nonsteroidal anti-inflammatory drugs (NSAIDs; 50 mg dexketoprofen) or TPI with 1.0% lidocaine. Of 54 patients enrolled in this RCT, TPIs were found to have superior analgesic effect compared with IV NSAIDs at all studied time points up to 60 minutes after intervention.71 Though superior to pharmacologic, the type of injectate did not seem to make a substantial difference in most studies. Roldan and colleagues compared patients treated with either TPIs containing normal saline or lidocaine 1% with 40 mg triamcinolone who presented to the emergency department for management of lumbar myofascial pain. They found that there was no statistically significant difference between the two groups immediately after injection or at 2 weeks follow-up.72
Table 1.
Outcomes data for trigger point injections with local anesthestic/steroid
| Study Author | Study Type | Study Size | Metrics | Study Question | Outcomes |
|---|---|---|---|---|---|
| Hong CZ et al,63 1994 | RCT | 58 | PS, Cervical ROM | Comparing 0.5% lidocaine TPI to dry needling in upper trapezius muscle MPS. | Lidocaine TPI resulted in more immediate soreness than dry needling. However, dry needling resulted in greater intensity and longer duration of soreness after procedure than lidocaine TPI |
| Tschopp KP et al,67 1996 | RCT | 107 | PS | Comparing 0.25% bupivacaine to 1.0% lidocaine to saline TPI for MPS. | No difference in relief between groups so long as needle hits muscle belly. |
| Hameroff SR et al,68 1981 | Crossover double-blind RCT | 15 | PS | Comparing bupivacaine to etidocaine to saline for TPI evaluating relief 7 days after injection for MPS. | Local preferred to saline alone. |
| Iwama H et al,69 2007 | RCT | 20 | PS | Comparing 0.25% to 1.0% lidocaine for TPI for MPS. | 0.25% lidocaine had less injection pain and better efficacy (14 d relief compared to 7 d) |
| Zaral idou AT et al,70 2009 | RCT | 68 | PS | Comparing ropivacaine to levobupivacaine for TPI for MPS. | No significant differences were found between groups at 2 wk out. |
| Garvey TA et al,65 1989 | Double-blind RCT | 63 | NRS | Comparing local anesthetic TPI, local anesthetic with steroid TPI, acupuncture, and cool spray with acupuncture for MPS. | No difference between types of procedural techniques was noted. Did not matter if medication was injected for procedure, both resulted in pain relief. |
| Kocak AO et al,71 2019 | RCT | 54 | VAS | Comparing NSAID and TPI for low back MPS. | TPI was superior to NSAIDs when assessed with pain relief within the first hour of intervention. |
| Roldan CJ et al,72 2020 | RCT | 48 | NRS | Comparing local anesthetic and steroid TPI to saline TPI in ED patients. | Resulted in similar change in pain relief in both groups. |
| Iwama H et al,73 2001 | RCT | 21 | PS | Testing injection pain with dilute local anesthetic in volunteers as well as using dilute local anesthetic doses in patients with MPS. | Less pain with dilute local injections. Duration relief in MPS patients not affected by using dilute local at low enough doses. |
| Krishnan SK et al,74 2000 | RCT | 30 | VAS | Comparing injection pain of bupivacaine, ropivacaine, bupivacaine with steroids, ropivacaine with steroids, and just needle insertion. | Ropivacaine was less painful (alone) compared to bupivacaine or either local anesthetic in combination with steroids. |
| Yoon SH et al,75 2007 | RCT | 77 | VAS, NDI, SF-36 | Comparing needle sizes on injection pain. | No difference was noted between sizes of needles used. |
| Ga H et al,76 2009 | RCT | 39 | VAS, FACES, PPI, GDS-SF | Comparing TPI with 0.5% lidocaine with acupuncture for MPS. | No difference between groups. |
| Mitidieri AMS et al,77 2020 | RCT | 35 | VAS, NCS, MPQ | Comparing acupuncture to TPI (local anesthetic) for pelvic pain from abdominal MPS. | No difference between outcomes when analyzed at 1 wk, 1 mo, 3 mo, and 6 mo out except for MPQ differences at 1 wk. |
Abbreviations: FACES, Wong-Baker FACES Paine Scale; GDS-SF, Geriatric Depression Scale-Short Form; MPQ, McGill Pain Questionnaire; MPS, myofascial pain syndrome; NCS, Numeric Categorical Scale; NDI, Neck Disability Index; NPAD, Neck Pain and Disability Scale; NPQ, Neck Pain Questionnaire; NRS, numeric rating score; NSAID, nonsteroid anti-inflammatory drugs; PFDI-20, Pelvic Floor Distress Inventory - 20; PPI, Pressure Pain Intensity Scores; PS, pain score (internal system); RCT, randomized controlled trial; ROM, range of motion; SF-36, Medical Outcomes Study 36 Item Short Form Health Survey; TPI, trigger point injection; VAS, visual analog score.
Aside from comparing efficacy of the injection for myofascial pain relief, studies have been performed evaluating if the concentrations of local anesthetic used affected the presence of pain on injection or its efficacy of relief.69,73 Iwama and colleagues compared 0.25% lidocaine to 1.0% lidocaine TPI in patients with bilateral shoulder myofascial pain. It was found that injection pain was statistically significantly less with injection of 0.25% lidocaine compared with 1.0% with overall improved pain relief at 7 and 14 days postinjection.69 A follow-up study performed by Iwama and colleagues compared water diluted 0.25% lidocaine TPI to water diluted 0.20% lidocaine TPI in patients with shoulder and cervical myofascial pain found that both patient cohorts reported statically significant pain relief for the same duration of time.73 The same study evaluated diluted mepivacaine and bupivacaine along with diluted lidocaine to evaluate its effect on injection pain. The study found that diluted mepivacaine resulted in the least amount of pain with injection.
Other studies have also studied this phenomenon, finding that pain on injection varies among local anesthetics. Krishnan and colleagues studied intramuscular injection pain in healthy volunteers comparing the amount of injection pain when bupivacaine, ropivacaine, bupivacaine with steroids, and ropivacaine with steroids were used. In the study, bupivacaine injections were noted to be more painful than ropivacaine in intensity that was deemed not associated with the differences in the local anesthetic pH.74 Needle size affecting the pain upon injection was investigated by Yoon and colleagues comparing 21-gauge to 23-gauge needles for TPIs in 77 patients with trapezius myofascial pain found no statistical difference in pain scores at the time of injection or visual analog scores at follow-up.75 Injection pain aside, only one study in 1981 found indicate benefit of local anesthetic compared with normal saline for TPI 7 days after injection in a cohort of only 15 patients.68 Ga and colleagues also found this similar trend when acupuncture was compared to TPI with 0.5% lidocaine in patients with myofascial pain. When both modalities were used resulting in a twitch response, there was no statistically significant difference in the amount of pain improvement in patients, up to 1 month out.76 This was further validated by Mitideri and colleagues when Ashi acupuncture was compared to local anesthetic TPI in patients with pelvic pain from abdominal myofascial pain. When the taut band was intervened upon, they found equal efficacy in pain relief between both intervention groups.77
OUTCOMES DATA AND DISCUSSION FOR TPIs WITH BOTULINUM TOXIN
Similar to the studies evaluating local anesthetic and steroids for TPI, there is a paucity of well-designed RCTs evaluating the efficacy of botulinum toxin in the management of myofascial pain (Table 2). One of the earlier studies was performed by Wheeler and colleagues and was a double-blind RCT where 33 participants were randomized to receive either 50 or 100 units of BoNT-A or normal saline injection into symptomatic TrPs of the cervicothoracic area. Their findings showed significant improvement in pain scores, neck disability, and an increase in pressure pain threshold testing by algometer; however, there was no significant difference between BoNT-A groups and saline control groups.78 Another study by Qerama and colleagues was a double-blind RCT in patients with chronic myofascial pain comparing TPI performed with 50 U BoNT-A to normal saline. This study found that there was no statistically significant difference in pain outcomes between the 2 groups at 28 days after injection.79 Kwanchuay and colleagues compared the efficacy of BoNT-A TPI in patients for upper trapezius myofascial pain to normal saline TPI. Their study found no difference in efficacy in VAS reduction; however, BoNT-A did increase the pressure pain threshold at 6 weeks after the injection in a statistically significant manner.80 Dessie and colleagues also compared BoNT-A TPI to normal saline TPI in patients with pelvic myofascial pain syndrome where no statistically significant difference was found between the 2 groups at 4 and 12 weeks postprocedure.81
Table 2.
Outcomes data for trigger point injections with botulinum toxin
| Study Author | Study Type | Study Size | Metrics | Study Question | Outcomes |
|---|---|---|---|---|---|
| Wheeler AH et al,78 1998 | Double-blind RCT | 33 | NPAD, PS | Comparing 50 U BoNT-A, 100 U BoNT-A, and normal saline TPI for MPS. | All 3 groups improved pain. No statistically significant benefit between injection types. |
| Qerama E et al,79 2006 | Double-blind RCT | 30 | NRS, PPDT, PPTT | Comparing BoNT-A TPI to saline TPI for MPS. | No difference in pain relief between groups at 28 d, but BoNT-A caused decreased EMG activity. |
| Kwanchuay P et al80 2015 | Double-blind RCT | 33 | VAS, PPT | Comparing BoNT-A to saline TPI for MPS. | No difference between groups 6 wk out though BoNT-A TPI resulted statistically significant increase in pain threshold 6 wk out. |
| Dessie SG et al,81 2019 | Double-blind RCT | 59 | VAS, PFDI-20 | Comparing saline or BoNT-A TPI for abdominal MPS in pelvic pain for MPS. | No statistically significant difference between groups at 4 and 12 wk after injection. |
| Gobel H et al,82 2006 | Double-blind RCT | 145 | PS | Comparing BoNT-A to saline TPI for MPS. | BoNT-A provided better relief between weeks 5 and 8. |
| Kamanli A et al,83 2005 | Single-blind RCT | 29 | PPT, PS, VAS, NHP | Comparing BoNT-A TPI to dry needling to bupivacaine TPI for MPS. | TPI in general found to have better benefit than dry needling. Authors noted bupivacaine was best for TPI as it was fast acting meanwhile BoNT-A TPI should be used in medically refractory cases. |
| Ferrante FM et al,84 2005 | Double-blind RCT | 132 | VAS, PPT | Comparing saline to 10, 25, or 50 U BoNT-A in up to 5 active TrPs for cervicothoracic MPS | No significant differences occurred between placebo and BoNT-A groups among all outcomes. |
| Harden RN et al,85 | Double-blind RCT | 23 | PPT, Cervical ROM, MPQ, BDI, HSES, STAI, PDI, VAS | Comparing BoNT-A 25 U per trigger point (max 4 TrPs) to saline TPI for cervical MPS and chronic tension type headache | Participants in the BoNT-A group reported greater reductions in headache frequency compared with placebo. |
| Graboski et al,86 | Double-blind RCT | 18 | VAS | Comparing BoNT-A 25 U per TrP to 0.5% bupivacaine for MPS | Both treatments were effective in reducing pain significantly but no difference between BoNT-A and bupivacaine groups. |
| Venancio et al87 2009 | Double-blind RCT | 45 | VAS | Comparing dry needling, 0.25% lidocaine, and BoNT-A for MPS | All groups showed favorable reductions in pain; however, the BoNT-A group demonstrated less use of rescue medication, and less postinjection local sensitivity |
| Nicol et al,88 2005 | Double-blind RCT | 114 | NRS, SF-36, BPI, NDI | Comparing BoNT-A to saline TPI for cervical and shoulder girdle MPS | BoNT-A provided improved pain scores, headaches, and physical function parameters compared to saline TPI |
| Benecke et al,89 2011 | Double-blind RCT | 154 | NRS | Comparing BoNT-A to saline TPI for cervical and shoulder MPS | BoNT-A provided improved pain relief compared to saline at 8 wk after TPI |
| Miller et al,90 2009 | Double-blind RCT | 47 | VAS, MOPQ | Comparing BoNT-A to saline TPI for cervical and shoulder MPS | BoNT-A TPI led to improved pain intensity scores compared to saline TPI |
BDI, Beck Depression Inventory; BoNT-A, botulinum toxin type A; HSES, Headache Specific Self-Efficacy Scale; MOPQ, Modified Oswestry Pain Questionnaire; MPQ, McGill Pain Questionnaire; MPS, myofascial pain syndrome; NDI, Neck Disability Index; NHP, Nottingham Health Profile; NRS, numeric rating score; PDI, Pain Disability Index; PPDT, pressure pain detection thresholds; PPT, pressure pain threshold; PPTT, pressure pain tolerance thresholds; PS, pain score (internal system); RCT, randomized controlled trial; ROM, range of motion; SF-36, Medical Outcomes Study 36 Item Short Form Health Survey; STAI, State-Trait Anxiety Inventory; TPI, trigger point injection; VAS, visual analog score.
Other studies have found that BoNT-A may provide durable relief compared with other medications upon injection. In a double-blind RCT by Gobel and colleagues, patients with myofascial pain were randomized to receive either BoNT-A injections (10 sites, 40 U each) to saline TPI. The study found that BoNT-A did result in statistically significant pain control at 5 weeks with fewer days of pain between 5 and 12 weeks.82 Kamanil and colleagues compared lidocaine TPI, BoNT-A TPI, and dry needling in patients with myofascial pain. At 1 month follow-up, patients receiving a TPI had statistically improved VAS compared with dry needling. However, all 3 modalities resulted in decreased VAS after treatment. Furthermore, in the study, it was argued by the authors that lidocaine TPI was less disruptive than dry needling and more cost-effective than BoNT-A TPI though BoNT-A may be useful for patients with MPS resistant to conventional treatment.83
Ferrante and colleagues found no statistically significant improvement compared to placebo with BoNT-A injection when injected directly into painful TrPs for cervicothoracic MP.84 They concluded that although it is intuitive for the clinician to consider therapeutic injection of BoNT-A as a treatment for MP (given its a priori similarity to TPI), peculiarities inherent to the use of toxin in lieu of dry needling or local anesthetic must be accounted for (ie, toxin spread through fascial planes), including the effects of dosing of toxin, volume of injectate, muscles chosen to inject, postural relations and abnormalities, and injection technique. Harden and colleagues were able to identify a short-term (12-week) reduction in MP of chronic tension-type headache with BoNT-A injection as compared with placebo.85 Graboski and colleagues found no significant difference in BoNT-A versus 0.5% bupivacaine injected into TPs of patients with MPS, though both were effective in reducing pain below the baseline level.86 Venancio and colleagues studied 45 MP patients who were assigned randomly to 1 of the following 3 groups: dry needling, 0.25% lidocaine TPI, and BoNT–A TPI, and assessed over a 12-week period.87 Although all 3 groups showed favorable response to treatment, the BoNT-A group demonstrated less use of rescue medication, and less postinjection local sensitivity.87 In another study, Nicol and colleagues reported that BoNT-A injected directly into painful muscle groups using a “follow the pain” and pattern injection technique in lieu of TrPs led to reduced average numerical pain scores, reduced number of headaches per week, and improvement in general activity and sleep quality of life measures.88 Similar positive findings were seen in the studies by Benecke and colleagues89 and Miller and colleagues90 wherein patients with cervical myofascial pain received BoNT-A using a fixed-location injection technique.
OUTCOMES DATA AND DISCUSSION FOR PIRIFORMIS INJECTION WITH LOCAL ANESTHETICS ± STEROID
In patients who fail to respond to conservative treatment of piriformis syndrome that includes activity modification, rest, mobilizing soft tissue restrictions, medical management, physical therapy, and alternative therapies such as acupuncture or dry needling, invasive options have been suggested.91,92 Injection interventions for piriformis TrPs with local anesthetics with or without steroids have been studied (Table 3). Early studies involving the analysis of 279 patients found that about 79% of the patients had at least 50% improvement when steroid (triamcinolone) injections to the piriformis were added to the physical therapy protocol suggesting the added benefit of injection treatment.93 In a later study, 239 patients were injected with local anesthetic of bupivacaine and betamethasone demonstrated significant reduction of pain in 45% of patients for 2 to 4 months and 15% having 8 months or longer with significant improvement of pain.94
Table 3.
Outcomes data for piriformis injection with local anesthestic ± steroid
| Author/Year | Sample Size | Methods | Image guidance/Additional Treatment | Injectate Used | Main findings/Outcome | Follow-up |
|---|---|---|---|---|---|---|
| Fishman LM et al,93 2002 | 279 | Before-after trial of cohort of consecutive patients identified by operational definition | No image guidance/received PT/serially reported pain and disability assessments | Lidocaine and triamcinolone | 79% had >50% relief at 10 mo | 48 mo |
| Filler AG et al94 2005 | 162 | 239 consecutive patients failed treatment of sciatica | MRI | Marcaine | 15% complete relief, 16% no relief, recurrence in 69% at 4 mo | 4 mo |
| Masala S et al,95 2012 | 23 (cases-13/control-10) | Case-control study | CT | Methyl prednisone and lidocaine | Improvement in VAS at 5–7 d, 2,3,6, and 12 mo | 12 mo |
| Ozisik PA et al,96 2014 | 10 | Case series | CT | Depomedrol and Marcaine | 9/10 patients had full recovery, no pain or VAS score of 1–3 | 2 y |
| Nazlikul H et al,98 2018 | 102 | Prospective randomized control study | No image guidance/stretching exercises (n 5 51), injections in neural therapy group (n 5 51) | 6 sessions of lidocaine injections with T11-S2 segmental injections and sacral canal injections | Improvement in VAS and ODI, more improvement in the neural therapy group | None |
| Terlemez et al,99 2019 | 30 | Prospective cohort | Ultrasound | Lidocaine and betamethasone | Improvement in pain score from baseline at 1 wk and 1 mo | 1 mo |
| Rosales J et al,100 2015 | 49 | Prospective | Ultrasound | Lidocaine + saline + methyl-prednisone acetate | 73.7% had some relief VAS-pre-8.3/post-2.8, relief lasted 5.3 wk | 6 wk |
| Misirlioglu et al,101 2015 | 50 | Prospective double-blinded, RCT | Ultrasound | Lidocaine only vs lidocaine and betamethasone | Pain reduction (NRS and LAS scale) from baseline but no difference between LA vs steroid group at 1 and 3 mo | 3 mo |
| Burke CJ et al,102 2019 | 38 | Consecutive patients | Ultrasound/hydrodissection | LA and steroid (17-betamethasone, 21-triamcinolone) | 84% immediate relief (pre-4.7/post-0.5), 47% continued relief at 33.6 d | 1 mo |
A subsequent study involving 162 patients, using MRI guidance and injection of local anesthetic provided complete relief without recurrence in 15% of patients and short-term relief up to 4 months with recurrence in 69% of patients.94 Sixteen percent had no relief with injection. Another small study of 13 patients and 10 control subjects showed the potential benefit of CT-guided piriformis injection with local anesthetic and steroid with statistically significant improvement in VAS score at 5 to 7 days, 2,3,6, and 12 months.95 A small case series showed 9 of 10 patients who received CT-guided piriformis injection had full and sustained recovery after piriformis injection.96 In a study by Jeong and colleagues, 63 patients underwent ultrasound-guided piriformis injection of 40 mg triamcinolone, in which 40.5% of enrolled participants showed significant improvement with injection and 18.9% had partial improvement.97
As part of neural therapy to reduce pain and improve function, 51 patients with piriformis syndrome received 6 sessions of lidocaine injections that involved piriformis muscle injections, T11-S2 segmental injections and sacral canal injections along with stretching exercises.98 Both the control group and the treatment group received stretching exercises as part of treatment and the study showed statistically significant improvement in pain and functional level in both groups with changes from baseline noticeably larger in the neural therapy group. This study showed that adding lidocaine injections can play a conjunctive role with other treatment options.
Steroid injections with local anesthetic have been studied in a recent cohort of 32 patients receiving injection therapy in the piriformis muscle using ultrasound guidance and showed statistically significant improvement in pain scale from baseline and at 1 month as well as 1 week to 1 month.99 In another study of 49 patients with deep gluteal syndrome of which piriformis syndrome is one of the subtypes, ultrasound-guided injection of a mixture of 20 mLs of normal saline, 4 mLs of 2% lidocaine, and 1 mL of corticosteroid (40 mg of methyl-prednisone acetate) in the perisciatic region between the gluteus maximus and pelvic trochanteric muscles provided some level of pain relief in 73.7% with reduction in pain score from 8.3 preinjection to 2.8 postinjection. Recurrence of pain was reported in 50% of the patients and the effect lasted for 5.3 weeks.100
Although these previous studies have shown that piriformis injection with image guidance with local anesthetic and steroid had improvement in pain level at varying degrees, the effect of local anesthetic alone versus local anesthetic with a steroid was studied in a randomized controlled double-blinded study of 50 patients. This study failed to show a statistically significant difference in the pain level in 2 groups with and without steroid.101 After a test injection and diagnosis of piriformis syndrome, one group (n = 22) received 5 mL of 2% lidocaine and other group (n = 25) received 4 mLs of 2% lidocaine and 1 mL of betamethasone. Both groups had a significant reduction in pain compared with baseline, there was no difference between the groups at rest, in motion, 1 month, or 3 months, suggesting that the addition of steroids may not have an added benefit over local anesthetic injections.
Another study has studied the effect of hydrodissection before injection of corticosteroid in piriformis syndrome and showed promising results. In this study, hydrodissection was performed by injecting fluid in the perineural tissue using ultrasound guidance to help reduce the adhesions and broaden the tissue space to deliver local anesthetic and steroid. Of the 38 patients studied, 17 received betamethasone and 21 received triamcinolone. Thirty-two patients (84%) received immediate pain relief with a reduction in pain score from 4.7/10 to 0.5/10. Of the 19 patients followed up at 33.6 days, 9 patients (47%) reported continuous pain relief.102 Further large-scale studies are needed to compare the effect of hydrodissection before injection treatment.91
OUTCOMES DATA AND DISCUSSION FOR PIRIFORMIS INJECTION WITH BoNT
Piriformis muscle injection with BoNT-A is an increasingly common injection when the duration of effect from local anesthetic/corticosteroid injections is insufficient. A typical dose would be 100 units in a 2-mL volume.103 Owing to its paralytic effect on the muscle, it causes atrophy and fatty degeneration of the muscle over time as evidenced by MRI.104 This reduction in muscular volume would decrease pressure on the sciatic nerve and is the mechanism of analgesia93,105–112 but is a more profoundly effective treatment when combined with physical therapy.93,107,110 Several uncontrolled studies have evaluated the use of BoNT-A, often in combination with physical therapy, and reported high rates of success that lasted for months (Table 4).103,106–111 A controlled study demonstrated superiority of BoNT-A over placebo injection for 10 weeks.109 Other controlled studies have reported that the efficacy of botulinum toxin is superior to that of local anesthetic/steroid or normal saline injections for the treatment of piriformis syndrome.110,113
Table 4.
Outcomes data for piriformis injection with botulinum toxin
| Study (Year) | Intervention | Results |
|---|---|---|
| Porta,103 2000 | BoNT-A (dose not reported), 0.5% bupivacaine, methylprednisolone | BoNT-A and steroid group had no significant differences in pain reduction at 1 mo; however, at 2 mo postinjection, there was a significant reduction in the BoNT-A group compared with the steroid group |
| Lang,106 2004 | 5000 u BoNT-B | Significant reduction in hip and buttock pain at 4, 12, and 16 wk |
| Fishman et al,107 2004 | 12,500 u BoNT-B | Combined with PT, 88.9% of patients injected had 50% improvement on the VAS |
| Yoon et al,108 2007 | 150 u BoNT-A vs 5 mg dexamethasone/1% lidocaine | Pain significantly lower in Dysport group at 4, 8, and 12 wk vs baseline P < .0001 |
| Childers et al,109 2002 | 100 u BoNT-A | Significant improvement in pain, spasm, distress, and interference with activities |
| Fishman et al,110 2002 | 200 u BoNT-A vs 1.5 mL 2% lidocaine/20 mg triamcinolone, vs placebo | 50% improvement at last 2 visits following 20 mg TL vs placebo injection: P = .001; BoNT-A vs TL: P = .044; BoNT-A vs placebo: P = .001 |
| Fishman et al,111 2017 | 300 u BoNT-A vs saline | VAS score decreased significantly at 2,4,6,8,10, and 12 wk compared with placebo |
| Rodriguez-Pinero et al,112 2018 | 100 U BoNT-A | VAS scores and quality of life scores statistically significantly reduced at 1 and 6 mo after injection |
| Yan,113 2021 | 100 u BoNT-A/1% lidocaine/0.5% bupivacaine vs 1% lidocaine/0.5% bupivacaine/4 mg dexamethasone | Median pain-free days were 30 d for the BoNT-A group and 1 d for the non–BoNT-A group |
Dr Chadwick has served as a consultant for Swing Therapeutics and receives research funding from the National Institutes of Health, National Institute of General Medical Sciences, Grant # K23GM123320.
SUMMARY
Myofascial pain and myofascial pain syndromes are among some of the most common acute and chronic pain conditions. The pathophysiology of myofascial pain includes biomechanical and postural factors that likely interact with neurologic factors, psychological elements including depression and anxiety, and hormonal and nutritional imbalances. These factors (in total or in part) may create peripheral sensitization, autonomic dysregulation, and ultimately, central spinal cord sensitization, which can amplify the symptoms experienced by patients with myofascial pain. Many interventional procedures can be performed in both an acute and chronic pain setting to address myofascial pain syndromes. Injections can be achieved with or without imaging guidance such as fluoroscopy and ultrasound; however, the use of imaging in years past has been recommended to improve safety and accuracy of needle placement. Injections can be performed using no injectate (dry needling), or can involve the administration of local anesthetics, botulinum toxin, or corticosteroids, with the evidence suggesting that most injectates have minimal or no superiority over one another. A proper history and physical examination of the patient and imaging studies may prove to be helpful in identifying the correct myofascial pain syndrome and aiding in developing an appropriate treatment strategy for these very common conditions.
KEY POINTS.
Trigger points are localized painful areas of skeletal muscle containing taut bands that can be exquisitely sensitive to digital pressure.
Conservative treatment of myofascial pain is recommended; however, if this fails to relieve pain, then trigger point injections can be considered.
Trigger point injections should be part of a multidisciplinary treatment program that includes physical therapy.
Trigger point injections are commonly done with local anesthetic with or without corticosteroid, botulinum toxin, or without injectate (dry needling).
The growing accessibility of imaging, including ultrasound and fluoroscopy, has improved available options for safe injection technique to myofascial trigger points.
CLINICS CARE POINTS.
A trigger point is the hallmark of myofascial pain syndromes, which may be treated with physical therapy and trigger point injections.
Common injectates used for trigger point injections include local anesthetics, corticosteroids, and botulinum toxins.
The growing accessibility of ultrasound has improved available options for imaging guidance for trigger point injections and piriformis muscle injection.
Ultrasound guidance may be used to improve safety and in the case of piriformis syndrome has been shown to improve accuracy of needle placement in the piriformis muscle.
Outcomes data for trigger point injections have shown analgesic benefit with the use of local anesthetics, corticosteroids, and botulinum toxins for myofascial pain syndromes, with no evidence of superiority of any injectate.
Outcomes data for piriformis muscle injections have shown that local anesthetic, steroid, and botulinum toxins are efficacious in reducing pain and improving symptoms. Botulinum toxins may provide superior analgesia and may be considered when local anesthetics/steroids are helpful but not providing long-term relief.
DISCLOSURE
Dr Chadwick receives research funding from the National Institutes of Health, National Institute of General Medical Sciences, grant # K23GM123320. Dr Chadwick has served as a consultant for Swing Therapeutics.
REFERENCES
- 1.Koukoulithras I, Plexousakis M, Kolokotsios S, et al. A Biopsychosocial Model-Based Clinical Approach in Myofascial Pain Syndrome: A Narrative Review. Cureus 2021;13(4):e14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuan TS. Current studies on myofascial pain syndrome. Curr Pain Headache Rep 2009;13(5):365–9. [DOI] [PubMed] [Google Scholar]
- 3.Shah JP, Phillips TM, Danoff JV, et al. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985) 2005;99(5):1977–84. [DOI] [PubMed] [Google Scholar]
- 4.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr Pain Headache Rep 2004;8(6):468–75. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-de-las-Peñas C, Cuadrado ML, Arendt-Nielsen L, et al. Myofascial trigger points and sensitization: an updated pain model for tension-type headache. Cephalalgia 2007;27(5):383–93. [DOI] [PubMed] [Google Scholar]
- 6.Mendell LM, Wall PD. RESPONSES OF SINGLE DORSAL CORD CELLS TO PERIPHERAL CUTANEOUS UNMYELINATED FIBRES. Nature 1965;206:97–9. [DOI] [PubMed] [Google Scholar]
- 7.Yoshihara T, Shigeta K, Hasegawa H, et al. Neuroendocrine responses to psychological stress in patients with myofascial pain. J Orofac Pain 2005;19(3): 202–8. [PubMed] [Google Scholar]
- 8.Russell IJ, Bieber CS. Myofascial pain and fibromyalgia syndrome. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s textbook of pain. 5th edition. Elsevier; 2006. p. 669–81. [Google Scholar]
- 9.Borg-Stein J, Stein J. Trigger points and tender points: one and the same? Does injection treatment help? Rheum Dis Clin North Am 1996;22(2):305–22. [DOI] [PubMed] [Google Scholar]
- 10.Hua NK, Van der Does E. The occurrence and inter-rater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: a prospective study in non-specific low back pain patients and controls in general practice. Pain 1994;58(3):317–23. [DOI] [PubMed] [Google Scholar]
- 11.Simons DGTJ, Simons LS. Myofasical pain and dysfunction: the trigger point manual. Wiliams & Wilkins; 1999. [Google Scholar]
- 12.Central SM. nervous system mechanisms of muscle pain: ascending pathways, central sensitization, and pain-modulating systems. In: Mense SGR, editor. Muscle pain: Understanding the mechanisms. Springer; 2010. p. 105–76. [Google Scholar]
- 13.Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol 2007;21(2):367–87. [DOI] [PubMed] [Google Scholar]
- 14.Ettlin T, Schuster C, Stoffel R, et al. A distinct pattern of myofascial findings in patients after whiplash injury. Arch Phys Med Rehabil 2008;89(7):1290–3. [DOI] [PubMed] [Google Scholar]
- 15.Simons GD, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain 1998;75(1):1–17. [DOI] [PubMed] [Google Scholar]
- 16.Rivner MH. The neurophysiology of myofascial pain syndrome. Curr Pain Headache Rep 2001;5(5):432–40. [DOI] [PubMed] [Google Scholar]
- 17.Travell JG, Simons DG. Lower half of the body. Myofascial pain and dysfunction: the trigger point manual. Williams and Wilkins; 1999. [Google Scholar]
- 18.Gerwin RD, Shannon S, Hong CZ, et al. Interrater reliability in myofascial trigger point examination. Pain 1997;69(1–2):65–73. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CY, Hong CZ, Adams AH, et al. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Arch Phys Med Rehabil 2000; 81(3):258–64. [DOI] [PubMed] [Google Scholar]
- 20.Nice DA, Riddle DL, Lamb RL, et al. Intertester reliability of judgments of the presence of trigger points in patients with low back pain. Arch Phys Med Rehabil 1992;73(10):893–8. [PubMed] [Google Scholar]
- 21.Tantanatip A, Chang KV. Myofascial Pain Syndrome. StatPearls 2021. [PubMed] [Google Scholar]
- 22.Chen Q, Basford J, An KN. Ability of magnetic resonance elastography to assess taut bands. Clin Biomech (Bristol, Avon) 2008;23(5):623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil 2009;90(11):1829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benzon HT, Katz JA, Benzon HA, et al. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology 2003;98(6):1442–8. [DOI] [PubMed] [Google Scholar]
- 25.Wun-Schen C Bipartite piriformis muscle: an unusual cause of sciatic nerve entrapment. Pain 1994;58(2):269–72. [DOI] [PubMed] [Google Scholar]
- 26.Probst D, Stout A, Hunt D. Piriformis Syndrome: A Narrative Review of the Anatomy, Diagnosis, and Treatment. PM R 2019;11(Suppl 1):S54–63. [DOI] [PubMed] [Google Scholar]
- 27.Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil Mar 2002;83(3 Suppl 1):S40–7, s48-S47. [DOI] [PubMed] [Google Scholar]
- 28.Narouze S, Benzon HT, Provenzano D, et al. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): Guidelines From the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med 2018;43(3):225–62. [DOI] [PubMed] [Google Scholar]
- 29.Shafer N Pneumothorax following “trigger point” injection. JAMA 1970;213(7): 1193. [PubMed] [Google Scholar]
- 30.Fitzgibbon DR, Posner KL, Domino KB, et al. Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology 2004; 100(1):98–105. [DOI] [PubMed] [Google Scholar]
- 31.Cheng J, Abdi S. Complications of Joint, Tendon, and Muscle Injections. Tech Reg Anesth Pain Manag 2007;11(3):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao DY, Zhang GL. [Clinical analysis on 38 cases of pneumothorax induced by acupuncture or acupoint injection]. Zhongguo Zhen Jiu 2009;29(3):239–42. [PubMed] [Google Scholar]
- 33.Botwin KP, Sharma K, Saliba R, et al. Ultrasound-guided trigger point injections in the cervicothoracic musculature: a new and unreported technique. Pain Physician 2008;11(6):885–9. [PubMed] [Google Scholar]
- 34.Seol SJ, Cho H, Yoon DH, et al. Appropriate depth of needle insertion during rhomboid major trigger point block. Ann Rehabil Med 2014;38(1):72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician 2002;65(4):653–60. [PubMed] [Google Scholar]
- 36.Park JS, Kim YH, Jeong SA, et al. Ultrasound-guided Aspiration of the Iatrogenic Pneumothorax Caused by Paravertebral Block -A Case Report. Korean J Pain 2012;25(1):33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson LS, Hoffman RS. Intrathecal injection: unusual complication of trigger-point injection therapy. Ann Emerg Med 1998;32(4):506–8. [DOI] [PubMed] [Google Scholar]
- 38.Elias M Cervical epidural abscess following trigger point injection. J Pain Symptom Manage 1994;9(2):71–2. [DOI] [PubMed] [Google Scholar]
- 39.Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med 2004; 29(4):333–40. [DOI] [PubMed] [Google Scholar]
- 40.Soriano PK, Bhattarai M, Vogler CN, et al. A Case of Trigger-Point Injection-Induced Hypokalemic Paralysis. Am J Case Rep 2017;18:454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammi C, Schroeder JD, Yeung B. Trigger Point Injection. StatPearls 2021. [PubMed] [Google Scholar]
- 42.Ge HY, Fernandez-de-Las-Penas C, Yue SW. Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chin Med 2011;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas K, Shankar H. Targeting myofascial taut bands by ultrasound. Curr Pain Headache Rep 2013;17(7):349. [DOI] [PubMed] [Google Scholar]
- 44.Rha DW, Shin JC, Kim YK, et al. Detecting local twitch responses of myofascial trigger points in the lower-back muscles using ultrasonography. Arch Phys Med Rehabil 2011;92(10):1576–1580 e1. [DOI] [PubMed] [Google Scholar]
- 45.Muir JJ, Curtiss HM, Hollman J, et al. The accuracy of ultrasound-guided and palpation-guided peroneal tendon sheath injections. Am J Phys Med Rehabil 2011;90(7):564–71. [DOI] [PubMed] [Google Scholar]
- 46.Daniels EW, Cole D, Jacobs B, et al. Existing Evidence on Ultrasound-Guided Injections in Sports Medicine. Orthop J Sports Med 2018;6(2). 2325967118756576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh SH, Lee SC, Lee WY, et al. Ultrasound-guided intra-articular injection of hyaluronic acid and ketorolac for osteoarthritis of the carpometacarpal joint of the thumb: A retrospective comparative study. Medicine (Baltimore) 2019;98(19): e15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang JJ, Kim J, Park S, et al. Feasibility of Ultrasound-Guided Trigger Point Injection in Patients with Myofascial Pain Syndrome. Healthcare (Basel) 2019;7(4). 10.3390/healthcare7040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho IT, Cho YW, Kwak SG, et al. Comparison between ultrasound-guided interfascial pulsed radiofrequency and ultrasound-guided interfascial block with local anesthetic in myofascial pain syndrome of trapezius muscle. Medicine (Baltimore) 2017;96(5):e6019. 10.1097/MD.0000000000006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metin Okmen B, Okmen K, Altan L. Comparison of the Efficiency of Ultrasound-Guided Injections of the Rhomboid Major and Trapezius Muscles in Myofascial Pain Syndrome: A Prospective Randomized Controlled Double-blind Study. J Ultrasound Med 2018;37(5):1151–7. [DOI] [PubMed] [Google Scholar]
- 51.Ballyns JJ, Turo D, Otto P, et al. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med 2012;31(8): 1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Q, Bensamoun S, Basford JR, et al. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil 2007;88(12):1658–61. [DOI] [PubMed] [Google Scholar]
- 53.Nightingale K, Soo MS, Nightingale R, et al. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol 2002; 28(2):227–35. [DOI] [PubMed] [Google Scholar]
- 54.Lin YC, Yu NY, Jiang CF, et al. Characterizing the SEMG patterns with myofascial pain using a multi-scale wavelet model through machine learning approaches. J Electromyogr Kinesiol 2018;41:147–53. [DOI] [PubMed] [Google Scholar]
- 55.Jiang CF, Lin YC, Yu NY. Multi-scale surface electromyography modeling to identify changes in neuromuscular activation with myofascial pain. IEEE Trans Neural Syst Rehabil Eng 2013;21(1):88–95. [DOI] [PubMed] [Google Scholar]
- 56.Finnoff JT, Hurdle MF, Smith J. Accuracy of ultrasound-guided versus fluoroscopically guided contrast-controlled piriformis injections: a cadaveric study. J Ultrasound Med 2008;27(8):1157–63. [DOI] [PubMed] [Google Scholar]
- 57.Barbero M, Falla D, Mafodda L, et al. The Location of Peak Upper Trapezius Muscle Activity During Submaximal Contractions is not Associated With the Location of Myofascial Trigger Points: New Insights Revealed by High-density Surface EMG. Clin J Pain 2016;32(12):1044–52. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, Lee MS, Lee S, et al. A Prospective Randomized Comparison of the Efficacy of Ultrasound- vs Fluoroscopy-Guided Genicular Nerve Block for Chronic Knee Osteoarthritis. Pain Physician 2019;22(2):139–46. [PubMed] [Google Scholar]
- 59.Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol 1994;5(1):71–84. [DOI] [PubMed] [Google Scholar]
- 60.Chang KV, Wu WT, Lew HL, et al. Ultrasound Imaging and Guided Injection for the Lateral and Posterior Hip. Am J Phys Med Rehabil 2018;97(4):285–91. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson J Interventional techniques. Fundamentals of musculoskeletal ultrasound. 3rd ed. Elsevier; 2013. p. 357–8. [Google Scholar]
- 62.Bardowski EA, Byrd JWT. Piriformis Injection: An Ultrasound-Guided Technique. Arthrosc Tech 2019;8(12):e1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 1994; 73(4):256–63. [DOI] [PubMed] [Google Scholar]
- 64.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil 2001;82(7): 986–92. [DOI] [PubMed] [Google Scholar]
- 65.Garvey TA, Marks MR, Wiesel SW. A prospective, randomized, double-blind evaluation of trigger-point injection therapy for low-back pain. Spine (Phila Pa 1976) 1989;14(9):962–4. [DOI] [PubMed] [Google Scholar]
- 66.Cui M, Khanijou S, Rubino J, et al. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004;107(1–2):125–33. [DOI] [PubMed] [Google Scholar]
- 67.Tschopp KP, Gysin C. Local injection therapy in 107 patients with myofascial pain syndrome of the head and neck. ORL J Otorhinolaryngol Relat Spec 1996;58(6):306–10. [DOI] [PubMed] [Google Scholar]
- 68.Hameroff SR, Crago BR, Blitt CD, et al. Comparison of bupivacaine, etidocaine, and saline for trigger-point therapy. Anesth Analg 1981;60(10):752–5. [PubMed] [Google Scholar]
- 69.Iwama H, Akama Y. The superiority of water-diluted 0.25% to neat 1% lidocaine for trigger-point injections in myofascial pain syndrome: a prospective, randomized, double-blinded trial. Anesth Analg 2000;91(2):408–9. [DOI] [PubMed] [Google Scholar]
- 70.Zaralidou AT, Amaniti EN, Maidatsi PG, et al. Comparison between newer local anesthetics for myofascial pain syndrome management. Methods Find Exp Clin Pharmacol 2007;29(5):353–7. [DOI] [PubMed] [Google Scholar]
- 71.Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: A prospective randomized study. Am J Emerg Med 2019;37(10):1927–31. [DOI] [PubMed] [Google Scholar]
- 72.Roldan CJ, Osuagwu U, Cardenas-Turanzas M, et al. Normal Saline Trigger Point Injections vs Conventional Active Drug Mix for Myofascial Pain Syndromes. Am J Emerg Med 2020;38(2):311–6. [DOI] [PubMed] [Google Scholar]
- 73.Iwama H, Ohmori S, Kaneko T, et al. Water-diluted local anesthetic for trigger-point injection in chronic myofascial pain syndrome: evaluation of types of local anesthetic and concentrations in water. Reg Anesth Pain Med 2001;26(4): 333–6. [DOI] [PubMed] [Google Scholar]
- 74.Krishnan SK, Benzon HT, Siddiqui T, et al. Pain on intramuscular injection of bupivacaine, ropivacaine, with and without dexamethasone. Reg Anesth Pain Med 2000;25(6):615–9. [DOI] [PubMed] [Google Scholar]
- 75.Yoon SH, Rah UW, Sheen SS, et al. Comparison of 3 needle sizes for trigger point injection in myofascial pain syndrome of upper- and middle-trapezius muscle: a randomized controlled trial. Arch Phys Med Rehabil 2009;90(8): 1332–9. [DOI] [PubMed] [Google Scholar]
- 76.Ga H, Choi JH, Park CH, et al. Acupuncture needling versus lidocaine injection of trigger points in myofascial pain syndrome in elderly patients–a randomised trial. Acupunct Med 2007;25(4):130–6. [DOI] [PubMed] [Google Scholar]
- 77.Mitidieri AMS, Baltazar M, da Silva APM, et al. Ashi Acupuncture Versus Local Anesthetic Trigger Point Injections in the Treatment of Abdominal Myofascial Pain Syndrome: A Randomized Clinical Trial. Pain Physician 2020;23(5):507–18. [PubMed] [Google Scholar]
- 78.Wheeler AH, Goolkasian P, Gretz SS. A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine (Phila Pa 1976) 1998;23(15): 1662–6; discussion 1667. [DOI] [PubMed] [Google Scholar]
- 79.Qerama E, Fuglsang-Frederiksen A, Kasch H, et al. A double-blind, controlled study of botulinum toxin A in chronic myofascial pain. Neurology 2006;67(2): 241–5. [DOI] [PubMed] [Google Scholar]
- 80.Kwanchuay P, Petchnumsin T, Yiemsiri P, et al. Efficacy and Safety of Single Botulinum Toxin Type A (Botox(R)) Injection for Relief of Upper Trapezius Myofascial Trigger Point: A Randomized, Double-Blind, Placebo-Controlled Study. J Med Assoc Thai 2015;98(12):1231–6. [PubMed] [Google Scholar]
- 81.Dessie SG, Von Bargen E, Hacker MR, et al. A randomized, double-blind, placebo-controlled trial of onabotulinumtoxin A trigger point injections for myofascial pelvic pain. Am J Obstet Gynecol 2019;221(5):517 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gobel H, Heinze A, Reichel G, et al. Dysport myofascial pain study g. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain 2006;125(1–2):82–8. [DOI] [PubMed] [Google Scholar]
- 83.Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 2005;25(8):604–11. [DOI] [PubMed] [Google Scholar]
- 84.Ferrante FM, Bearn L, Rothrock R, et al. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology 2005;103(2):377–83. [DOI] [PubMed] [Google Scholar]
- 85.Harden RN, Cottrill J, Gagnon CM, et al. Botulinum toxin a in the treatment of chronic tension-type headache with cervical myofascial trigger points: a randomized, double-blind, placebo-controlled pilot study. Headache 2009;49(5): 732–43. [DOI] [PubMed] [Google Scholar]
- 86.Graboski CL, Gray DS, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomised double blind crossover study. Pain 2005;118(1–2):170–5. [DOI] [PubMed] [Google Scholar]
- 87.Venancio Rde A, Alencar FG Jr, Zamperini C. Botulinum toxin, lidocaine, and dry-needling injections in patients with myofascial pain and headaches. Cranio 2009;27(1):46–53. [DOI] [PubMed] [Google Scholar]
- 88.Nicol AL, Wu II, Ferrante FM. Botulinum toxin type a injections for cervical and shoulder girdle myofascial pain using an enriched protocol design. Anesth Analg 2014;118(6):1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benecke R, Heinze A, Reichel G, et al. Botulinum type A toxin complex for the relief of upper back myofascial pain syndrome: how do fixed-location injections compare with trigger point-focused injections? Pain Med 2011;12(11):1607–14. [DOI] [PubMed] [Google Scholar]
- 90.Miller D, Richardson D, Eisa M, et al. Botulinum neurotoxin-A for treatment of refractory neck pain: a randomized, double-blind study. Pain Med 2009;10(6): 1012–7. [DOI] [PubMed] [Google Scholar]
- 91.Vij N, Kiernan H, Bisht R, et al. Surgical and Non-surgical Treatment Options for Piriformis Syndrome: A Literature Review. Anesth Pain Med 2021;11(1): e112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michel F, Decavel P, Toussirot E, et al. Piriformis muscle syndrome: diagnostic criteria and treatment of a monocentric series of 250 patients. Ann Phys Rehabil Med 2013;56(5):371–83. [DOI] [PubMed] [Google Scholar]
- 93.Fishman LM, Dombi GW, Michaelsen C, et al. Piriformis syndrome: diagnosis, treatment, and outcome–a 10-year study. Arch Phys Med Rehabil 2002;83(3): 295–301. [DOI] [PubMed] [Google Scholar]
- 94.Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine 2005;2(2):99–115. [DOI] [PubMed] [Google Scholar]
- 95.Masala S, Crusco S, Meschini A, et al. Piriformis syndrome: long-term follow-up in patients treated with percutaneous injection of anesthetic and corticosteroid under CT guidance. Cardiovasc Intervent Radiol 2012;35(2):375–82. [DOI] [PubMed] [Google Scholar]
- 96.Ozisik PA, Toru M, Denk CC, et al. CT-guided piriformis muscle injection for the treatment of piriformis syndrome. Turk Neurosurg 2014;24(4):471–7. [DOI] [PubMed] [Google Scholar]
- 97.Jeong HS, Lee GY, Lee EG, et al. Long-term assessment of clinical outcomes of ultrasound-guided steroid injections in patients with piriformis syndrome. Ultrasonography 2015;34(3):206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nazlikul H, Ural FG, Ozturk GT, et al. Evaluation of neural therapy effect in patients with piriformis syndrome. J Back Musculoskelet Rehabil 2018;31(6): 1105–10. [DOI] [PubMed] [Google Scholar]
- 99.Terlemez R, Ercalik T. Effect of piriformis injection on neuropathic pain. Agri Nov 2019;31(4):178–82. [DOI] [PubMed] [Google Scholar]
- 100.Rosales J, Garcia N, Rafols C, et al. Perisciatic Ultrasound-Guided Infiltration for Treatment of Deep Gluteal Syndrome: Description of Technique and Preliminary Results. J Ultrasound Med 2015;34(11):2093–7. [DOI] [PubMed] [Google Scholar]
- 101.Misirlioglu TO, Akgun K, Palamar D, et al. Piriformis syndrome: comparison of the effectiveness of local anesthetic and corticosteroid injections: a double-blinded, randomized controlled study. Pain Physician 2015;18(2):163–71. [PubMed] [Google Scholar]
- 102.Burke CJ, Walter WR, Adler RS. Targeted Ultrasound-Guided Perineural Hydrodissection of the Sciatic Nerve for the Treatment of Piriformis Syndrome. Ultrasound Q 2019;35(2):125–9. [DOI] [PubMed] [Google Scholar]
- 103.Porta M A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of myofascial pain syndrome and pain from chronic muscle spasm. Pain 2000;85(1–2):101–5. [DOI] [PubMed] [Google Scholar]
- 104.Al-Al-Shaikh M, Michel F, Parratte B, et al. An MRI evaluation of changes in piriformis muscle morphology induced by botulinum toxin injections in the treatment of piriformis syndrome. Diagn Interv Imaging 2015;96(1):37–43. [DOI] [PubMed] [Google Scholar]
- 105.Santamato A, Micello MF, Valeno G, et al. Ultrasound-Guided Injection of Botulinum Toxin Type A for Piriformis Muscle Syndrome: A Case Report and Review of the Literature. Toxins (Basel). 2015;7(8):3045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lang AM. Botulinum toxin type B in piriformis syndrome. Am J Phys Med Rehabil 2004;83(3):198–202. [DOI] [PubMed] [Google Scholar]
- 107.Fishman LM, Konnoth C, Rozner B. Botulinum neurotoxin type B and physical therapy in the treatment of piriformis syndrome: a dose-finding study. Am J Phys Med Rehabil 2004;83(1):42–50, quiz 51–3. [DOI] [PubMed] [Google Scholar]
- 108.Yoon SJ, Ho J, Kang HY, et al. Low-dose botulinum toxin type A for the treatment of refractory piriformis syndrome. Pharmacotherapy 2007;27(5):657–65. [DOI] [PubMed] [Google Scholar]
- 109.Childers MK, Wilson DJ, Gnatz SM, et al. Botulinum toxin type A use in piriformis muscle syndrome: a pilot study. Am J Phys Med Rehabil 2002;81(10):751–9. [DOI] [PubMed] [Google Scholar]
- 110.Fishman LM, Anderson C, Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil 2002;81(12):936–42. [DOI] [PubMed] [Google Scholar]
- 111.Fishman LM, Wilkins AN, Rosner B. Electrophysiologically identified piriformis syndrome is successfully treated with incobotulinum toxin a and physical therapy. Muscle Nerve 2017;56(2):258–63. [DOI] [PubMed] [Google Scholar]
- 112.Rodríguez-Piñero M, Vidal Vargas V, Jiménez Sarmiento AS. Long-Term Efficacy of Ultrasound-Guided Injection of IncobotulinumtoxinA in Piriformis Syndrome. Pain Med 2018;19(2):408–11. [DOI] [PubMed] [Google Scholar]
- 113.Yan K, Xi Y, Hlis R, et al. Piriformis syndrome: pain response outcomes following CT-guided injection and incremental value of botulinum toxin injection. Diagn Interv Radiol 2021;27(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


