Abstract
While studies have demonstrated concept formation in animals, only humans are known to label concepts to use them in mental simulations or predictions. To investigate whether other animals use labels comparably, we studied cross-modal, individual recognition in bottlenose dolphins (Tursiops truncatus) that use signature whistles as labels for conspecifics in their own communication. First, we tested whether dolphins could use gustatory stimuli and found that they could distinguish between water and urine samples, as well as between urine from familiar and unfamiliar individuals. Then, we paired playbacks of signature whistles of known animals with urine samples from either the same dolphin or a different, familiar animal. Dolphins investigated the presentation area longer when the acoustic and gustatory sample matched than when they mismatched. This demonstrates that dolphins recognize other individuals by gustation alone and can integrate information from acoustic and taste inputs indicating a modality independent, labeled concept for known conspecifics.
Dolphins can identify others by taste alone and link those inputs to vocal cues to form multimodal, labeled concepts.
INTRODUCTION
Cross-modal recognition is an adaptation that allows animals to identify a relevant entity in their environment from multiple sensory inputs, making detection faster and more effective. It has been reported for object recognition in insects (1), fish (2), birds (3), and mammals (4) and for the recognition of conspecifics or human caretakers in crows (5), African penguins (6), cats (7), dogs (8), horses (9), goats (10), African lions (11), ring-tailed lemurs (12), squirrel monkeys (13), gray-cheeked mangabeys (14), rhesus macaques (15), and chimpanzees (16). These studies provide evidence for the presence of concepts in an animal’s mind, because cross-modal recognition requires the integration of information received via different sensory pathways, possibly facilitated by a kind of mental model of the perceived entity, and not just a generalization along one physical stimulus parameter (17). We follow Murphy (18) here and define a concept as a representation of a class of entities in the world with “representation” referring to a physical state in the brain to store mental content (19). While quite complex concepts thus defined have been demonstrated widely in animals (20), it has been argued that concepts need labels to be functional in mental simulations or predictions and that it is unlikely that animals are able to generate or use labels in their thinking because they do not have language (21). A label in this context is a kind of shorthand for a concept, which can be used to refer to it either in thinking or in communication. While there clearly is meaning attached to animal signals (22), it has been argued that the emergence of an ability to develop labels and use them changes the quality of a representation (23), allowing a holistic concept to evolve (24). These views have been questioned given that infants can reason before they have words and that at least some animals use concepts in planning actions (25). Nevertheless, the use of labels in human language has a significant positive effect on cognitive flexibility and its development (26, 27), posing the question of whether similar effects can be found in animals.
Labeling in animals appears to be rare, and it is difficult to demonstrate that labels exist in an animal’s mind. For example, predator-specific alarm calls are effective signals to warn others of the presence of a predator, and it has been discussed whether these calls represent labels, especially in primates where these could be a precursor to words. Studies on Diana monkeys (28) have shown that different alarm calls indicate the presence of specific predator species rather than an imperative for a particular avoidance action, but it is unclear whether the underlying concept has a label in the receiver’s brain. One study using playbacks of heterospecific alarm calls and objects resembling a snake demonstrated that coal tits have cross-modal representation of snakes that includes the alarm call (29). Thus, cross-modal perception studies to demonstrate concepts in combination with an investigation of whether animals use labels for underlying concepts in their own communication system can help to understand the evolution of labeling and provide the foundation to ask whether benefits of labeling for cognitive development can be found in animals and humans. This is particularly interesting in animals that are capable of vocal learning, which are potentially able to create novel labels in their communication, similar to what has occurred in the evolution of human languages.
While active labeling has been investigated extensively in studies teaching animals artificial communication systems (3, 30), little is known about the occurrence of active labeling by vocal learners in natural animal communication systems. Parrots and dolphins are the only nonhuman animals that have been successfully trained to copy novel acoustic signals and then use them in vocal labeling (3, 31). Both groups also use labeling in their natural communication systems (32, 33). Bottlenose dolphins (Tursiops truncatus and Tursiops aduncus) use individually distinctive signature whistles (34, 35) that are developed by animals early in life apparently by copying and then changing whistles they hear (36, 37). This novel signature whistle is then used by not only the owner to broadcast its identity (38, 39) but also conspecifics to address the whistle owner (32, 40–42). Approximately 38 to 70% of bottlenose dolphin whistles in the wild are signature whistles (35). Unlike isolation or contact calls in most other animals, identity information is not encoded in general voice features, often called by-product distinctiveness (43), but by a novel frequency modulation pattern invented by the caller in early life. This has been confirmed by playback experiments with computer-generated whistles that removed all by-product distinctiveness (44). Dolphins reacted to these in the same way as if they were produced by the signature owner, a result that has led to the comparison of signature whistles with human names (45). With their remarkable vocal learning abilities, bottlenose dolphins are also able to copy signature whistles of others (42). This copying has been observed in zoos (39, 40, 42) and in the wild (40, 41) and appears to be primarily a contact behavior between closely affiliated animals such as mothers and their calves or males that formed alliances (40). Wild animals reply vocally specifically to hearing their own signature whistle (32, 46), and wild dolphins can sometimes be found to produce signature whistles of others in their absence (47), a behavior that can help to find others at sea. When different groups of bottlenose dolphins meet at sea, they exchange signature whistles before joining each other (48). Signature whistles also form the basis for social concept formation in multilevel alliances of bottlenose dolphins (49) where dolphins react more strongly to signature whistles of members of alliance levels that they have cooperated with. Long-term social memory of signature whistles has also been demonstrated with bottlenose dolphins reacting with approaches and vocalizations to signature whistles of close associates even if they have not heard or interacted with the whistle owner for over 20 years (50).
A crucial piece of information that is missing in all of these studies, however, is whether the animals have a concept for known conspecifics that includes their signature whistles. Alternatively, dolphins may represent signature whistles as independent stimuli that through experience have been associated with positive or negative outcomes rather than a label of identity (45). If a dolphin has an inclusive concept, then it should know who to expect when hearing a signature whistle, while this would not be the case if signature whistles were represented independently. If an inclusive concept exists, then we can conclude that dolphins copying signature whistles of others are labeling these animals in a way similar to how humans use names (45, 51). Here, we demonstrate that bottlenose dolphins perceive identity cross-modally and know whose identity a signature whistle refers to using signature whistle playbacks and urine samples in an expectation violation paradigm that is the first to provide evidence for gustatory-only social recognition in animals.
RESULTS
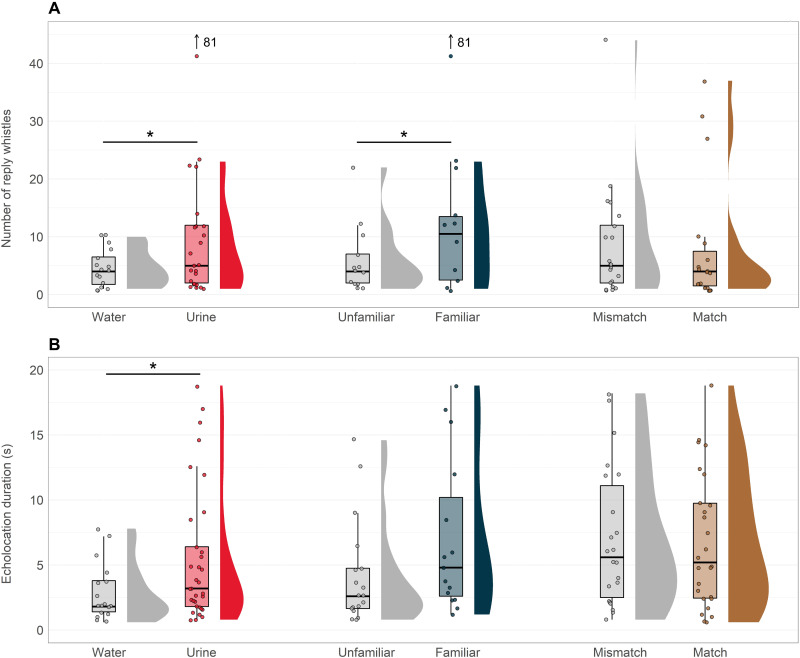
Because gustation in dolphins had only been studied in the context of food preferences (52), we tested the dolphins’ chemosensory abilities in three steps. First, we compared differences in investigation time when animals were presented with samples of water or samples of dolphin urine. Samples approximating 20 ml were poured into the enclosure in front of each dolphin, and the duration of the resulting open mouth sampling was measured (Fig. 1). In addition, we analyzed vocalizations of the animal as an indicator of the animal attending to and trying to interact with the source of the stimuli. Comparisons of open-mouth sampling durations showed that dolphins (n = 8) spent approximately twice as long sampling urine cues relative to water controls [generalized linear mixed effects model (GLMM), β = 0.764 ± 0.177 and P < 0.001; Fig. 2A and Table 1]. All animals explored urine samples longer than water samples (fig. S1). Though the likelihood of any type of whistling or echolocating did not vary significantly between water and urine presentations, vocalizing dolphins produced more whistles (GLMM, β = 0.826 ± 0.149 and P < 0.001) (Fig. 3A) and longer echolocation bouts in response to urine (GLMM, β = 0.541 ± 0.218 and P = 0.013) (Fig. 3B).
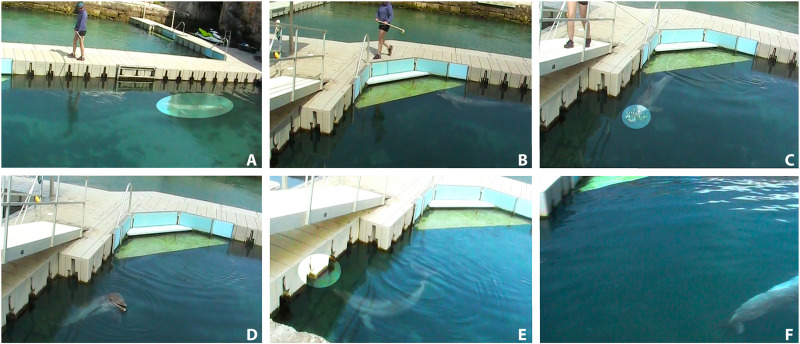
Fig. 1. Chronology of the cross-modal experiment.
(A and B) The test subject (highlighted) is led toward the trial area by the experimenter. (C and D) The subject is presented with a conspecific’s urine (highlighted) and is allowed to sample the stimulus. (E) A conspecific’s signature whistle is played, and the subject approaches the speaker apparatus (highlighted). (F) The subject exits the trial area. Photo credit: Jason Bruck, Stephen F. Austin State University.
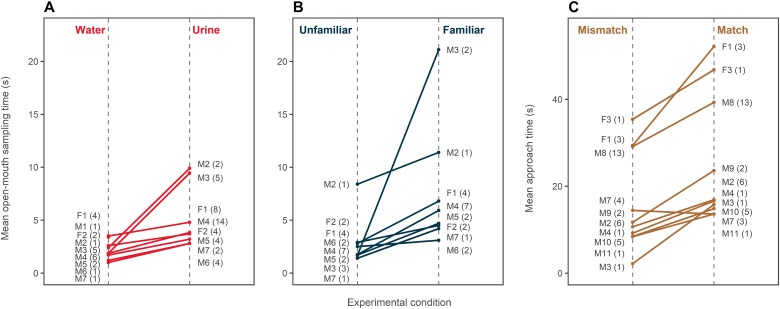
Fig. 2. Responses of individual dolphins in chemical familiarity and cross-modal experiments.
Each line connects the response data for one individual. (A) Mean duration of open-mouth sampling by individual, showing that dolphins sample urine longer than water. (B) Mean duration of open-mouth sampling by individual, showing that dolphins sample urine from familiar conspecifics longer than urine from unfamiliar individuals. (C) Mean approach duration by individual, showing that most individuals approach matched cross-modal presentations longer than mismatched presentations. Numbers in parentheses represent the number of observations for each individual and each condition. M, male; F, female. Full boxplots representing variation in individual response times are available in the Supplementary Materials (figs. S1 to S3). Note that the difference in each of the three panels reflects statistically significant effects in the multivariate models. Means and SEs are given in Table 1.
Table 1. Summary statistics for all stimulus comparisons.
| Mean (s) | SE (s) | |
| Open-mouth sampling duration: water-urine | ||
| Water | 2.23 | 0.31 |
| Urine | 4.73 | 0.72 |
| Open-mouth sampling duration: familiar-unfamiliar urine | ||
| Unfamiliar urine | 2.38 | 0.38 |
| Familiar urine | 7.19 | 1.21 |
| Speaker exploration duration: cross-modal match-mismatch | ||
| Matched urine and signature whistle |
28.44 | 3.39 |
| Mismatched urine and signature whistle |
19.17 | 2.01 |
Fig. 3. Vocal responses of dolphins in chemical familiarity and cross-modal experiments.
(A) Boxplots and density curves showing the number of whistles produced by subjects to different experimental stimuli. One outlying point (81 reply whistles produced in response to familiar urine) is referred to but not plotted directly for improved visualization of the central portion of the distribution. (B) Boxplots and density curves showing the duration of echolocation produced by dolphins in response to different experimental stimuli. The asterisks represent statistical significance. Dots represent raw data, while boxes show the interquartile range (IQR) centered on the median response duration, and whiskers show the smallest and largest values up to 1.5 times the IQR.
Next, we presented dolphins with urine from familiar and unfamiliar dolphins. Dolphins (n = 8) spent approximately three times as long sampling urine cues from familiar individuals compared to urine from animals they had never associated with (GLMM, β = 1.119 ± 0.179 and P < 0.001; Fig. 2B and Table 1). Subject explained about 11% of the overall variance in this test indicating a lack of consistent individual variation in the response measure. Included in the same model, neither the sex of the urine donor (GLMM, β = −0.319 ± 0.276 and P = 0.249) nor the age of the urine sample (GLMM, β = 0.007 ± 0.013 and P = 0.598) was a significant factor in responses. Urine samples in this test varied from 0 to 37 days in age. All animals explored urine from familiar animals for longer than that from unfamiliar ones (fig. S2). During trials in which whistling was detected, dolphins produced more whistles in response to urine from a familiar donor (GLMM, β = 0.932 ± 0.141 and P < 0.001) (Fig. 3A), although a large amount of variation was attributable to subject-specific differences (see table S3). Note also that the response to unfamiliar urine was similar to the response to water, demonstrating that it was not an aversive stimulus.
In the final experiment, we paired urine presentations with the acoustic playback of signature whistles in an expectation violation paradigm. After pouring a urine sample in front of a dolphin close to an underwater speaker, we played either the signature whistle of the urine donor (matched presentation) or a signature whistle of another known individual (mismatched presentation). We then measured the time the animal spent in the vicinity of the playback speaker (exploration time). Duration of attention is a common parameter to use in exposures to novel stimuli and in expectation violation paradigms. Dolphins remained close to the playback speaker longer during matched chemical and signature whistle presentations than they did during mismatched ones (GLMM, β = 0.42 ± 0.10 and P < 0.001; Fig. 2C and Table 1). As before, the sex of the urine donor and the sample’s age did not significantly affect dolphins’ behavioral responses (donor sex: GLMM, β = −0.286 ± 0.303 and P = 0.345; sample age: GLMM, β = 0.000 ± 0.004 and P = 0.982). Urine samples varied from 0 to 46 days of age. Subject explained approximately 30% of the overall variance in this test, suggesting that dolphins differ in their tendency to approach the speaker. However, all but one animal explored matching stimuli for longer than nonmatching ones (fig. S3).
Presentation type (matching or mismatching presentation) did not significantly alter the presence of vocal responses (whistles: GLMM, β = −0.284 ± 0.547 and P = 0.604; echolocation: GLMM, β = 1.034 ± 0.638 and P = 0.105) or the respective number or duration of these responses (number of whistles: GLMM, β = −0.036 ± 0.113 and P = 0.750; Fig. 3A; duration of echolocation: GLMM, β = 0.061 ± 0.182 and P = 0.738; Fig. 3B).
DISCUSSION
Our study presents the first case of identity perception by taste alone in animals. Crustaceans have been found to recognize conspecifics by general chemoreception (53), but there is no distinction between gustatory and olfactory systems in their perception. In mammals, olfactory and gustatory inputs use different cranial nerves to transmit information to the brain and project to different cortical areas (54). Dolphins do not have an olfactory bulb, and the corresponding cranial nerve is underdeveloped. These structures are necessary for the transmission of olfactory information to the brain. Furthermore, their nasal tract is isolated from the mouth, pharynx, and esophagus (55). Thus, it is clear that the performance of our animals was based on taste and not on olfaction.
Dolphins are unlikely to recognize an individually different composition of components in the urine of conspecifics using basic mammalian taste sensors, because they experienced a loss of taste receptor genes responsible for the perception of four of the five basic tastes in mammals (56). Instead, major urinary proteins (MUPs) as used in olfactory recognition by mice (57) might be a good candidate for the transmission of individual information by taste in dolphins. Another possible information carrier for dolphins are lipids, which are often transported by MUPs. Dolphins have positively selected, orthologous genes for CD36 proteins (58), offering the possibility of specialized lipid receptors as these same genes enable lipid taste perception in other mammals (59). Dolphins also have cells that resemble gustatory von Ebner’s glands that help with lipid hydrolysis on the tongue and modified taste receptors at the base of the tongue, both unusual for an animal that mostly swallows fish whole rather than process it in its mouth (55). Furthermore, the neural pathways for chemoreception through taste in dolphins are preserved and even expanded relative to humans, as is the case with the olfactory tubercle, epithalamus, and the mediodorsal nucleus (55). This is likely to allow the perception of taste signals through the facial or trigeminal nerves despite the loss of the olfactory and vomeronasal cranial nerves.
The use of taste is highly beneficial in the open ocean because urine plumes will persist for a while after an animal has left. By recognizing who caused a plume, dolphins would be alerted to the recent presence of that individual even if it had not signaled its presence vocally. Genital inspection in which there is rostrum to genital contact is relatively common in dolphin social interactions (60) and provides a good opportunity to learn the taste of a conspecific’s urine. Given the recognition skills revealed in our study, we think that it is likely that dolphins can also extract other information from urine, such as reproductive state, or use pheromones to influence each other’s behavior.
While cross-modal representation of identity has been shown in a variety of animals (5, 7–13, 16), none of these previous studies used species that invent their own recognition signals and use those of other individuals when addressing them. It is possible that labels commonly occur in animal minds. However, labeling in communication by vocal learners currently serves as the only evidence for the presence of labels in nonhuman systems. The large-billed crow (Corvus macrorhynchos) is the only studied animal capable of cross-modal identity perception that belongs to a taxon of vocal production learners (the song birds) (5), but little is known about the development of this species’ contact calls and how they use them. Bottlenose dolphins not only use learned, individually distinctive signature whistles to address others in their own communication system (32, 40) but can also be trained to use artificial labels to report on the presence or absence of objects in a pool (31, 61). This could suggest that they have labels for other objects in their own communication system. Alternatively, this could be an adaptation specific to group cohesion contexts and not be used elsewhere.
Dolphins spent longer investigating matched presentations of urine and whistles, compared to mismatched ones, an effect in the opposite direction typical of expectation violation paradigms where subjects often respond longer to mismatches than matches. This could reflect the more common occurrence of mismatches in their natural interactions because urine plumes linger in the water while new whistles are produced all the time and can be heard over long distances (62). We also found a general preference for familiar over unfamiliar urine, and the same has been reported for reactions to familiar and unfamiliar signature whistles alone (50). This is likely caused by the unstable nature of groups in individualized fission-fusion societies as found in bottlenose dolphins. In the open ocean, it is difficult to find others, and hearing or tasting a familiar individual is an important indicator of where the signaler is. In species in which group cohesion or stability is higher and encounters between preferred associates are more predictable, there is often a preference for unfamiliar stimuli over familiar ones, most likely because familiar stimuli are needed less to locate and maintain relationships with familiar individuals. In those cases, animals usually show a stronger response to unfamiliar stimuli [e.g., birds defending territories against intruders (63), odor-driven novelty preference in mice exploration (64), or female guppy mate preference for novel phenotypes (65)].
It is important to consider that our study was restricted to animals that were available in the facilities we worked with, which leaves some open questions for future studies. For example, we mostly tested males with only two females available to us. More data on possible sex differences are needed, but it is interesting that the two females in the sample showed the same behavior as the males. Other factors that could have influenced responses were animal age and the sex of urine donors. While our sample size is too small to test this comprehensively, all animals spent longer exploring urine over water and urine from familiar over that from unfamiliar individuals. All but one animal also explored matching stimuli for longer than nonmatching ones. This suggests that age or urine donor sex had little influence on our main results. Another factor to consider is rearing environment. While captive animals are likely to have an overall longer exposure to the conspecifics they live with and interact with fewer of them than wild ones, a previous study on cross-modal object recognition in bottlenose dolphins demonstrated that concept formation does not need many exposures (4). This suggests that rearing environment has little effect on recognition abilities.
Because bottlenose dolphins use signature whistles selectively when addressing specific individuals (40) and can remember these for over 20 years (50), signature whistles are effective vocal labels for the representation of conspecifics. In combination with these previous studies, our results show that dolphins form persistent modality-independent representations that have learned labels just as in human concept formation. The resulting concepts of conspecifics may be used in mental operations such as planning, mental time travel, or the simulation of social scenarios.
MATERIALS AND METHODS
Animal models/study population
Bottlenose dolphins were studied in three facilities operated by the Dolphin Quest organization between 2016 and 2017. Complete details of animals involved the study are shown in table S1. Dolphins in our study were of known sex and age and mostly of known ancestry (78%). Fourteen bottlenose dolphins 7 to 48 years old (5 females and 9 males) were urine donors. Eight naïve healthy bottlenose dolphins (formerly housed at the same facilities as the donors or at a sister facility), ages 6 to 31 years (two females, mean age of 8.5 years; six males, mean age of 18.17 years) participated as full test subjects in the tests comparing urine to water and looking at urine from familiar and unfamiliar individuals. One additional individual (male, age 17) participated only in the control condition of the urine presentations of familiar and unfamiliar individuals. Accordingly, we did not include this individual in the overall test sample size; however, data from their control trial were included in subsequent modeling.
In the familiarity chemical-only presentations, each dolphin in this study was exposed to familiar individuals that they were housed with for at least 5 years before relocation (if the animals were not cohabitators). To keep urine ages as constant as possible between stimulus sets, urine from familiar and unfamiliar individuals usually came from a sister facility with the target dolphin only having familiarity with one of the two urine donors. There was an exception as one individual was relocated to a sister facility during the study and could serve as a fresh unfamiliar urine donor (unfamiliar cohabitator in a separate lagoon) to test against fresh familiar urine from a cohabitator. In cases where urine was used from two different facilities, urine ages were matched as closely as possible.
For the chemical and acoustic cross-modal study, 19 Atlantic bottlenose dolphins ages 3 to 48 years (12 females and 7 males) were urine donors. Ten naïve healthy Atlantic bottlenose dolphins (mostly housed at the same facilities as the donors), ages 3 to 31 years (2 females, average age of 6.5 years; 8 males, average age of 16 years) participated as test subjects in this study. All but one of these test subjects were born under human care. In all but two trials in the match and mismatch paradigm, dolphins were presented with urine and whistles for individuals they cohabitated with at the time of the study (table S1). For most tests, animals were separated from their pool mates in a netted area of the enclosure for 30 to 60 min. A few tests took place without separations when one animal was by itself at the test end of the pool. All animals were used to separations, which are common for husbandry procedures. Both experiments were conducted under the approval of the University of St. Andrews Animal Welfare Committee and Dolphin Quest’s Institutional Animal Care and Use Committee.
Urine collection procedures
Dolphins were trained to voluntarily provide urine, which was collected in 60-ml syringes. Dolphins would regularly provide 20- to 60-ml samples during this process. After collection, dolphins were rewarded with food reinforcement for the behavior. All dolphins were fed a mix of capelin, herring, and squid. After collection, urine was labeled, cataloged, and placed in 20-m Nalgene Brand cryo-tubes and then stored in a portable −86°C freezer (Stirling Ultracold Shuttle ULT-25 Ultra Low Temperature Freezer; Athens, OH USA).
Prestudy procedures
Most dolphins under human care are conditioned to follow and place their rostrums on small round buoys at the end of up to 5-m-long polyvinyl chloride pipes. This is a type of target training that allows marine mammal care personnel to train more complex behaviors, including leaps and precise positioning of the animals. Modified versions of these training poles (or “target” poles) were used for this experiment, where the buoy was replaced with a 20-ml cup with a hole in the lid screwed to the pole. The exterior of the cup was taped to inhibit the use of visual cues by subjects as this is from where chemical stimuli were delivered. Dolphins transferred their training to this modified target readily, allowing experimenters to lead the dolphins to a test zone at the end of the pool using the pole.
Initially, ice and fresh water were placed in the cup and dropped by an experimenter in the test zone after leading the dolphin for 6- to 9- m along the docks. Dolphins would move in-sync with the experimenter along the docks. If the dolphin got ahead or fell too far behind the experimenter, the experimenter would reposition 6 to 9 m from the test zone and wait for the subject to circle back. If the subject followed the experimenter to the test zone successfully, the test liquid would be dropped 0.5 to 1 m in front of the subject’s rostrum.
In this prestudy phase, dolphins readily consumed the ice and fresh water as these substances are often given to the animals as part of enrichment protocols. After the dolphin successfully completed this process with ice water three times, they were considered primed and ready for the experiment.
Testing procedures
Water versus urine and chemical familiarity experiments
Presentations of water with ice preceded all test sessions. This allowed us to gauge the animals’ willingness to participate as animals unwilling to sample water with ice were unlikely to sample our experimental presentations. If the dolphins tracked the experimenter and sampled the water with ice correctly, the session could begin. First, dolphins were given water only to serve as the control. Dolphins were then given two urine samples per session. One sample was from a familiar individual, while another sample was from an unfamiliar one (presentation order was randomized by coin toss). Within each trial, urine presentations were matched by sex and age of the donor and age of the sample as closely as possible. In the water versus urine and chemical familiarity study, urine samples varied from 0 to 37 days of age with a mean of 18.56 days. The procedures for urine delivery were the same for ice water delivery as outlined above.
During data collection, animals were videotaped from above water with underwater audio from hydrophones recorded on the soundtrack on a Canon (Ohta-ku, Tokyo) Model FS200 camera. Dolphin vocalizations were recorded using two SENSOR Technology Ltd. (Collingwood, Ontario) model SS03 Sea Phone hydrophones (frequency response range, 0.02 to 50 kHz) and a PreSonus (Baton Rouge, Louisiana) two-channel AudioVox USB A/D device (sampling frequency, 48 kHz). Audio was also recorded in Praat v 5.4.08 running on a 2012 Apple MacBook Pro with an Intel i7 processor. Acoustic behavior was attributed to the subject by comparing the time of arrival of received sounds between two hydrophones, one close to the test zone (within 0.5 m) and one about 10 m from the subject.
Chemical/acoustic cross-modal experiments
As before, presentations of ice water preceded all test sessions. Three seconds after delivery of the water, a simulated tonal sound or “test whistle” of less than 1-s duration was played from an Apple iPod (Cupertino, California; model sixth generation). All playbacks were projected from a Lubell Labs (Columbus, Ohio; model LL916) underwater speaker (range, 0.6 to 21 kHz ± 8 dB) connected to a hertz amplifier (Electromedia-Potenza Picena, Italy, model HCP 2) powered by a 12-V battery (Fig. 1). Spaced in 5-min increments, repeated presentations of these control playbacks were made to ensure that dolphin responses were to the social nature of the experimental stimuli and not to the novelty of the procedure, and when the animals stopped approaching the speaker after the presentation of the test whistle, they were considered habituated. These control procedures also allowed for comparison of signature whistle/urine playbacks to the first water/test whistle playbacks.
After habituation, dolphins were given two urine samples, each paired with a signature whistle (fundamental frequencies, 800 Hz to 28.5 kHz). Urine samples varied from 0 to 46 days of age with a mean of 10.42 days. One urine sample was presented right before the signature whistle of the urine donor, while the other sample was followed by a signature whistle from an animal other than the urine donor (presentation order was randomized by coin toss). To avoid condition independence issues, donor dolphin stimuli were not repeated within these single sessions (i.e., urine or whistles from the same dolphin was not used in both the match and the mismatch conditions). However, urine presentations were matched by sex, age of the donor, and age of the sample as closely as possible, whereas whistle presentations were matched by age and sex. Ninety-seven percent of the urine and whistles presented to the animals were from individuals that they were currently housed with. The procedures for urine/signature whistle delivery were the same for water delivery outlined above, and recording methodology was the same as in the chemical familiarity tests.
Extracting behavioral data
Videos were scored blindly by randomizing the presentation order to the scorer. In the chemical familiarity study, open-mouth sampling duration was the key index of dolphins’ behavioral response. This was measured from the first opening after the start of the drop until the animal closed its mouth without opening it again within 2 s (to account for multiple samplings). In the cross-modal study, the key dependent measure was duration of proximity to the speaker after cross-modal presentation. Speaker proximity response (defined as the subject’s head being within 1 m of speaker) was measured from the start of the drop until the animal swam away and maintained distance from the response area for 10 s. Subject responses were scored using Solomon Coder beta 15.11.19 running on a Windows 7–based HP Mini 110-4100. For both experiments, the presence and magnitude of acoustic responses (whistling and echolocation) were recorded for 1 min after chemical presentation. We expected movement-based and acoustic behavior to be distinct indicators of interest: While sampling duration was expected to capture the dolphin’s general interest in the stimulus, vocal replies would be indicative of a communicative response.
Statistical analysis
For the chemical familiarity study, we first used a GLMM with gamma family and log link function to test whether dolphins spent more time sampling urine compared to water, allowing individuals to vary in sampling time (model 1). We secondarily tested for differences in vocal behavior across experimental treatment. Both the presence and number of reply whistles (for trials where whistles were present) were compared across presentations of water and urine using GLMMs with binomial family (logistic link; model 2) and zero-truncated Poisson family (log link; model 3), respectively. An additional binomial GLMM with logistic link was used to test for differences in the probability of an echolocation response across treatment (model 4). Last, the duration of echolocation responses (when echolocation was detected) was compared across treatment with a gamma GLMM with log link (model 5). Complete results of these urine-water discrimination models are presented in table S2.
Having determined that dolphins exhibit behavioral responses to conspecific urine, we then sought to test whether dolphins discriminate the urine of their social associates from those of unknown animals. We fit a GLMM with gamma family and log link function comparing open-mouth sampling duration of urine extracted from unfamiliar versus familiar individuals (model 6). For this focal test, we also included the age of the urine sample and the sex of the urine donor as covariates, allowing us to estimate their possible influences on the sampling duration. Then, applying the same model structures as in the water versus urine comparison, we used GLMMs to test for differences in the occurrence and magnitude of acoustic responses across treatment (models 7 to 10). Complete results of these familiar versus unfamiliar discrimination models are presented in table S3.
For the cross-modal study, we used a GLMM to model approach duration as a function of experimental treatment (mismatched versus matched identity cues), also including the age of the urine sample and the sex of the urine donor as covariates (model 11). This allowed us to test whether dolphins recognize individual identity from both acoustic and chemical cues and expect cues from the same individual to occur in tandem. Then, as for the other experimental comparisons, we also tested for differences in vocal response (models 12 to 15). Complete results of these cross-modal recognition models are presented in table S4.
Each of these models was fit with test subject as a random effect, allowing for interindividual variation in behavioral response. We also considered fitting more complex random structures (i.e., random slopes). Limitations in the number of observations per individual meant that these would be estimated with limited confidence, however. The restricted maximum likelihood estimation used by R and the lmer4 package for mixed models are resistant to violations associated with uneven testing of subjects and were therefore seen as the most appropriate tools for analysis. All models were assessed for adequacy of fit before consideration of parameter estimates. This was accomplished by diagnostic plots of residuals versus fitted values, a Q-Q plot of residuals, and a Q-Q plot of random effects. Explanatory power was assessed using a pseudo-R2 metric optimized for GLMMs. Pseudo-R2 was calculated using the piecewiseSEM package in R (66) for models with binomial families and manually for the gamma models. In the absence of methods for estimating explained variance of zero-truncated Poisson GLMMs, we provide pseudo-R2 values based on standard Poisson distributions, calculated with piecewiseSEM. Accordingly, we advise caution in interpreting pseudo-R2 values for models 3, 8, and 13.
To test whether carryover effects could have influenced our results, we also ran the two models reporting our main results (models 6 and 11) with some trials and sessions removed, creating a dataset that fulfilled the requirements of a fully counterbalanced design. Model diagnostics for model 6 suggested a better fit without the random effect when using the smaller, counterbalanced dataset, so was fit as a standard GLM (model 6-counterbalanced) (see model descriptions below). These models provided the same results as those run with the full dataset (see tables S3 and S4). All analyses were conducted in R 4.0.2, with use of the lme4 (67) and glmmTMB packages for model fitting (68).
Formal model descriptions: Dolphin responses to water versus urine
Model 1
Here, S represents the duration of open-mouth sampling in seconds, indexed by measure i (n = 66) and individual j (n = 9). αwater represents the intercept of the sampling duration for water, making βurine the difference for trials using urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 2
Here, W represents the presence of whistles, indexed by trial i (n = 66) and individual dolphin j (n = 9). αwater represents the probability of whistles being produced during presentations of water, making βurine the difference for trials with urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 3
Here, W represents the count of whistles produced (when whistles were detected), indexed by trial i (nwater = 16 and nurine = 24) and individual dolphin j (n = 9). αwater represents the intercept of the number of whistles produced during presentations of water, making βurine the difference for trials with urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 4
Here, E represents the presence of echolocation clicks, indexed by trial i (n = 66) and individual dolphin j (n = 9). αwater represents the probability of echolocation being produced during presentations of water, making βurine the difference for trials with urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 5
Here, E represents duration of echolocation in seconds, indexed by trial i (n = 8) and individual dolphin j (nwater = 17 and nurine = 33). αwater represents the echolocation duration during presentations of water, making βurine the difference for trials with urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Formal model descriptions: Dolphin responses to familiar versus unfamiliar urine
Model 6
Here, S represents the duration of open-mouth sampling in seconds, indexed by measure i (n = 43) and individual j (n = 8). αunfam represents the intercept of the sampling duration for unfamiliar, female urine, making βfam the difference for trials with familiar urine and βmaleUrine the difference for trials using male urine. βage represents the effect of urine sample age, o, in days. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 6-counterbalanced
Here, S represents the duration of open-mouth sampling in seconds, indexed by measure i (n = 24). αunfam represents the intercept of the sampling duration for unfamiliar, female urine, making βfam the difference for trials with familiar urine and βmaleUrine the difference for trials using male urine. βage represents the effect of urine sample age, o, in days.
Model 7
Here, W represents the presence of whistles, indexed by trial i (n = 43) and individual dolphin j (n = 8). αunfamrepresents the probability of whistles being produced during presentations of unfamiliar urine, making βfam the difference for trials with familiar urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 8
Here, W represents the count of whistles produced, indexed by trial i (nunfam = 13 and nfam = 11) and individual dolphin j (n = 6). αunfam represents the intercept of the number of whistles produced during presentations of unfamiliar urine, making βfam the difference for trials with familiar urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 9
Here, E represents the presence of echolocation clicks, indexed by trial i (n = 43) and individual dolphin j (n = 8). αunfam represents the probability of echolocation being produced during presentations of unfamiliar urine, making βfam the difference for trials with familiar urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 10
Here, E represents duration of echolocation in seconds, indexed by trial i (nunfam = 18 and nfam = 15) and individual dolphin j (n = 7). αunfam represents the intercept of the echolocation duration during presentations of unfamiliar urine, making βfam the difference for trials with familiar urine. μj represents the dolphin-specific random effect, which has estimated variance d2.
Formal model descriptions: Dolphin responses to cross-modal identity cues
Model 11
Here, S represents approach duration in seconds, indexed by measure i (n = 73) and individual j (n = 10). αmismatch represents the intercept of the sampling duration for mismatched trials using female urine, making βmatch the difference for matched trials and βmaleUrine the difference for trials using male urine. βage represents the effect of urine sample age, o, in days. μj represents the dolphin-specific random effect, which has estimated variance d2.
M11-counterbalanced
Here, S represents approach duration in seconds, indexed by measure i (n = 44) and individual j (n = 8). αmismatch represents the intercept of the sampling duration for mismatched trials using female urine, making βmatch the difference for matched trials and βmaleUrine the difference for trials using male urine. βage represents the effect of urine sample age, o, in days. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 12
Here, W represents the presence of whistles, indexed by trial i (n = 73) and individual dolphin j (n = 10). αmismatchrepresents the probability of whistles being produced during mismatched trials, making βmatch the difference for matched trials. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 13
Here, W represents the count of whistles produced, indexed by trial i (nmismatch = 21 and nmatch = 19) and individual dolphin j (n = 8). αmismatch represents the intercept of the number of whistles produced during mismatched trials, making βmatch the difference for matched trials. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 14
Here, E represents the presence of echolocation clicks, indexed by trial i (n = 73) and individual dolphin j (n = 10). αmismatch represents the probability of echolocation being produced during mismatched trials, making βmatch the difference for matched trials. μj represents the dolphin-specific random effect, which has estimated variance d2.
Model 15
Here, E represents duration of echolocation in seconds, indexed by trial i (nmismatch = 22 and nmatch = 27) and individual dolphin j (n = 9). αmismatch represents the intercept of the echolocation duration during mismatched trials, making βmatch the difference for matched trials. μj represents the dolphin-specific random effect, which has estimated variance d2.
Acknowledgments
We thank the Dolphin Quest animal care staff personnel in Bermuda, Hawaii, and Oahu as well as R. Stone, J. Sweeney, M. Campbell, D. Hill, A. Hribar, and S. Sheppard for support during this study.
Funding: The study was supported by a Marie Skłodowska-Curie fellowship of the European Commission, grant number 661214 (J.N.B. and V.M.J.).
Author contributions: Conceptualization: J.N.B. and V.M.J. Methodology: J.N.B., S.F.W., and V.M.J. Investigation: J.N.B. and V.M.J. Visualization: S.F.W. and J.N.B. Supervision: V.M.J. Writing—original draft: V.M.J. and J.N.B. Writing—review and editing: J.N.B., S.F.W., and V.M.J.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S3
Tables S1 to S4
REFERENCES AND NOTES
- 1.Solvi C., Al-Khudhairy S. G., Chittka L., Bumble bees display cross-modal object recognition between visual and tactile senses. Science 367, 910–912 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Schumacher S., de Perera T. B., Thenert J., von der Emde G., Cross-modal object recognition and dynamic weighting of sensory inputs in a fish. Proc. Natl. Acad. Sci. U.S.A. 113, 7638–7643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepperberg I. M., Cognition in an African gray parrot (Psittacus erithacus): Further evidence for comprehension of categories and labels. J. Comp. Psychol. 104, 41–52 (1990). [Google Scholar]
- 4.Harley H. E., Putman E. A., Roitblat H. L., Bottlenose dolphins perceive object features through echolocation. Nature 424, 667–669 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Kondo N., Izawa E. I., Watanabe S., Crows cross-modally recognize group members but not non-group members. Proc. R. Soc. B 279, 1937–1942 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baciadonna L., Solvi C., la Cava S., Pilenga C., Gamba M., Favaro L., Cross-modal individual recognition in the African penguin and the effect of partnership. Proc. R. Soc. B 288, 20211463 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi S., Arahori M., Chijiiwa H., Saito A., Kuroshima H., Fujita K., Cats match voice and face: Cross-modal representation of humans in cats (Felis catus). Anim. Cogn. 22, 901–906 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Adachi I., Kuwahata H., Fiujita K., Dogs recall their owner’s face upon hearing the owner’s voice. Anim. Cogn. 10, 17–21 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Proops L., McComb K., Reby D., Cross-modal individual recognition in domestic horses (Equus caballus). Proc. Natl. Acad. Sci. U.S.A. 106, 947–951 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitcher B. J., Briefer E. F., Baciadonna L., McElligott A. G., Cross-modal recognition of familiar conspecifics in goats. R. Soc. Open Sci. 4, 160346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilfillan G., Vitale J., McNutt J. W., McComb K., Cross-modal individual recognition in wild African lions. Biol. Lett. 12, 20160323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulahci I. G., Drea C. M., Rubenstein D. I., Ghazanfar A. A., Individual recognition through olfactory – auditory matching in lemurs. Proc. R. Soc. B 281, 20140071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi I., Fujita K., Cross-modal representation of human caretakers in squirrel monkeys. Behav. Processes 74, 27–32 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Bovet D., Deputte B. L., Matching vocalizations to faces of familiar conspecifics in grey-cheeked mangabeys (Lophocebus albigena). Fol. Primatol. 80, 220–232 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Habbershon H. M., Ahmed S. Z., Cohen Y. E., Rhesus macaques recognize unique multimodal face-voice relations of familiar individuals and not of unfamiliar ones. Brain Behav. Evol. 81, 219–225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izumi A., Kojima S., Matching vocalizations to vocalizing faces in a chimpanzee (Pan troglodytes). Anim. Cogn. 7, 179–184 (2004). [DOI] [PubMed] [Google Scholar]
- 17.R. K. R. Thompson, Natural and relational concepts in animals, in Comparative Approaches to Cognitive Science, H. L. Roitblat, J.-A. Meyer, Eds. (MIT Press, 1995), pp. 175–224. [Google Scholar]
- 18.G. L. Murphy, The Big Book of Concepts (MIT Press, 2002). [Google Scholar]
- 19.Pearson J., Kosslyn S. M., The heterogeneity of mental representation: Ending the imagery debate. Proc. Natl. Acad. Sci. U.S.A. 112, 10089–10092 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zentall T. R., Wasserman E. A., Urcuioli P. J., Associative concept learning in animals. J. Exp. Anal. Behav. 101, 130–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chater N., Heyes C. M., Animal concepts: Content and discontent. Mind Lang. 9, 209–246 (1994). [Google Scholar]
- 22.J. R. Hurford, The Origins of Meaning: Language in the Light of Evolution (Oxford Univ. Press, 2007). [Google Scholar]
- 23.C. M. Heyes, Cognitive Gadgets (MIT Press, 2018). [Google Scholar]
- 24.Bickerton D., Language first, then shared intentionality, then a beneficent spiral. Behav. Brain Sci. 28, 691–692 (2005). [Google Scholar]
- 25.Fitch W. T., Animal cognition and the evolution of human language: Why we cannot focus solely on communication. Phil. Trans. R. Soc. B 375, 20190046 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buss A. T., Nikam B., Not all labels develop equally: The role of labels in guiding attention to dimensions. Cogn. Dev. 53, 100843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S. Jacques, P. D. Zelazo, Language and the development of cognitive flexibility: Implications for theory of mind, in Why Language Matters for Theory of Mind, J. W. Astington, J. A. Baird, Eds. (Oxford Univ. Press, 2005), pp. 144–162. [Google Scholar]
- 28.Zuberbühler K., Interspecies semantic communication in two forest primates. Proc. R. Soc. B 267, 713–718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T. N., Other species’ alarm calls evoke a predator-specific search image in birds. Curr. Biol. 30, 2616–2620.e2 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Krause M. A., Beran M. J., Words matter: Reflections on language projects with chimpanzees and their implications. Am. J. Primatol. 82, e23187 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Richards D. G., Wolz J. P., Herman L. M., Vocal mimicry of computer-generated sounds and vocal labeling of objects by a bottlenosed dolphin, Tursiops truncatus. J. Comp. Psychol. 98, 10–28 (1984). [PubMed] [Google Scholar]
- 32.King S. L., Janik V. M., Bottlenose dolphins can use learned vocal labels to address each other. Proc. Natl. Acad. Sci. U.S.A. 110, 13216–13221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanker R., Sugama Y., Prinage S., Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Anim. Behav. 70, 111–118 (2005). [Google Scholar]
- 34.Gridley T. G., Cockcroft V. G., Hawkins E. R., Blewitt M. L., Morisaka T., Janik V. M., Signature whistles in free-ranging populations of Indo-Pacific bottlenose dolphins, Tursiops aduncus. Mar. Mammal Sci. 30, 512–527 (2014). [Google Scholar]
- 35.Janik V. M., Sayigh L. S., Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A 199, 479–489 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Fripp D., Owen C., Quintana-Rizzo E., Shapiro A., Buckstaff K., Jankowski K., Wells R., Tyack P., Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim. Cogn. 8, 17–26 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Tyack P. L., Development and social functions of signature whistles in bottlenose dolphins Tursiops truncatus. Bioacoustics 8, 21–46 (1997). [Google Scholar]
- 38.M. C. Caldwell, D. K. Caldwell, P. L. Tyack, Review of the signature-whistle-hypothesis for the Atlantic bottlenose dolphin, in The Bottlenose Dolphin, S. Leatherwood, R. R. Reeves, Eds. (Academic Press, 1990), pp. 199–234. [Google Scholar]
- 39.Janik V. M., Slater P. J. B., Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim. Behav. 56, 829–838 (1998). [DOI] [PubMed] [Google Scholar]
- 40.King S. L., Sayigh L. S., Wells R. S., Fellner W., Janik V. M., Vocal copying of individually distinctive signature whistles in bottlenose dolphins. Proc. R. Soc. B 280, 20130053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janik V. M., Whistle matching in wild bottlenose dolphins (Tursiops truncatus). Science 289, 1355–1357 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Tyack P., Whistle repertoires of two bottlenosed dolphins, Tursiops truncatus: Mimicry of signature whistles? Behav. Ecol. Sociobiol. 18, 251–257 (1986). [Google Scholar]
- 43.J. W. Boughman, C. F. Moss, Social sounds: Vocal learning and development of mammal and bird calls, in Acoustic Communication, A. M. Simmons, A. N. Popper, R. R. Fay, Eds. (Springer, 2003), pp. 138–224. [Google Scholar]
- 44.Janik V. M., Sayigh L. S., Wells R. S., Signature whistle shape conveys identity information to bottlenose dolphins. Proc. Natl. Acad. Sci. U.S.A. 103, 8293–8297 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton R. A., Animal communication: Do dolphins have names? Curr. Biol. 16, R598–R599 (2006). [DOI] [PubMed] [Google Scholar]
- 46.King S. L., Harley H., Janik V. M., The role of signature whistle matching in bottlenose dolphins, Tursiops truncatus. Anim. Behav. 96, 79–86 (2014). [Google Scholar]
- 47.Watwood S. L., Owen E. C. G., Tyack P. L., Wells R. S., Signature whistle use by temporarily restrained and free-swimming bottlenose dolphins, Tursiops truncatus. Anim. Behav. 69, 1373–1386 (2005). [Google Scholar]
- 48.Quick N. J., Janik V. M., Bottlenose dolphins exchange signature whistles when meeting at sea. Proc. R. Soc. B 279, 2539–2545 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King S. L., Connor R. C., Krützen M., Allen S. J., Cooperation-based concept formation in male bottlenose dolphins. Nat. Comm. 12, 2373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruck J. N., Decades-long social memory in bottlenose dolphins. Proc. R. Soc. B 280, 20131726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janik V. M., Cognitive skills in bottlenose dolphin communication. Trends Cogn. Sci. 17, 157–159 (2013). [DOI] [PubMed] [Google Scholar]
- 52.P. E. Nachtigall, Vision, audition, and chemoreception in dolphins and other marine mammals, in Dolphin Cognition and Behavior: A Comparative Approach, R. J. Schusterman, J. A. Thomas, F. G. Wood, Eds. (Lawrence Erlbaum, 1986), pp. 79–113. [Google Scholar]
- 53.Gherardi F., Aquiloni L., Tricarico E., Revisiting social recognition systems in invertebrates. Anim. Cogn. 15, 745–762 (2012). [DOI] [PubMed] [Google Scholar]
- 54.C. U. M. Smith, Biology of Sensory Systems (Wiley, ed. 2, 2008). [Google Scholar]
- 55.B. Cozzi, S. Huggenberger, H. Oelschlaeger, Anatomy of Dolphins: Insights Into Body Structure and Function (Academic Press, 2017). [Google Scholar]
- 56.Feng P., Zheng J., Rossiter S. J., Wang D., Zhao H., Massive losses of taste receptor genes in toothed and baleen whales. Genome Biol. Evol. 6, 1254–1265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurst J. L., Payne C. E., Nevison C. M., Marie A. D., Humphries R. E., Robertson D. H. L., Cavaggioni A., Beynon R. J., Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Wang Z., Chen Z., Xu S., Ren W., Zhou K., Yang G., ‘Obesity’ is healthy for cetaceans? Evidence from pervasive positive selection in genes related to triacylglycerol metabolism. Sci. Rep. 5, 14187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P., CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115, 3177–3184 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.M. Nakamura, M. Sakai, Social touch in apes and dolphins, in Primates and Cetaceans, J. Yamagiwa, L. Karczmarski, Eds. (Springer, 2014), pp. 355–383. [Google Scholar]
- 61.Herman L. M., Forestell P. H., Reporting presence or absence of named objects by a language-trained dolphin. Neurosci. Biobehav. Rev. 9, 667–681 (1985). [DOI] [PubMed] [Google Scholar]
- 62.Janik V. M., Source levels and the estimated active space of bottlenose dolphin (Tursiops truncatus) whistles in the Moray Firth, Scotland. J. Comp. Physiol. A 186, 673–680 (2000). [DOI] [PubMed] [Google Scholar]
- 63.C. K. Catchpole, P. J. B. Slater, Bird Song: Biological Themes and Variations (Cambridge Univ. Press, ed. 2, 2008). [Google Scholar]
- 64.Smith D. R., Burruss D. R., Johnson A. W., An assessment of olfaction and responses to novelty in three strains of mice. Behav. Brain Res. 201, 22–28 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Daniel M. J., Koffinas L., Hughes K. A., Mating preference for novel phenotypes can be explained by general neophilia in female guppies. Am. Nat. 196, 414–428 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Lefcheck J., PiecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 571–579 (2016). [Google Scholar]
- 67.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2014). [Google Scholar]
- 68.Brooks M. E., Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., Skaug H. J., Mächler M., Bolker B. M., glmmTMB balances speed and flexibility among packages for zero-inflated generalized modelling. R Journal. 9, 378–400 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S3
Tables S1 to S4