Abstract
Fibrosis is a pathological manifestation of wound healing that replaces dead/damaged tissue with collagen-rich scar tissue to maintain homeostasis, and complications from fibrosis contribute to nearly half of all deaths in the industrialized world. Ageing is closely associated with a progressive decline in organ function, and the prevalence of tissue fibrosis dramatically increases with age. Despite the heavy clinical and economic burden of organ fibrosis as the population ages, to date, there is a paucity of therapeutic strategies that are specifically designed to slow fibrosis. Aryl hydrocarbon receptor (AhR) is an environment-sensing transcription factor that exacerbates aging phenotypes in different tissues that has been brought back into the spotlight again with economic development since AhR could interact with persistent organic pollutants derived from incomplete waste combustion. In addition, gut microbiota dysbiosis plays a pivotal role in the pathogenesis of numerous diseases, and microbiota-associated tryptophan metabolites are dedicated contributors to fibrogenesis by acting as AhR ligands. Therefore, a better understanding of the effects of tryptophan metabolites on fibrosis modulation through AhR may facilitate the exploitation of new therapeutic avenues for patients with organ fibrosis. In this review, we primarily focus on how tryptophan-derived metabolites are involved in renal fibrosis, idiopathic pulmonary fibrosis, hepatic fibrosis and cardiac fibrosis. Moreover, a series of ongoing clinical trials are highlighted.
Keywords: fibrosis, tryptophan, metabolites, aryl hydrocarbon receptor, aging, gut microbiota
1.Introduction
Fibrosis is a pathological extension of the wound healing process with high mortality that is majorly characterized by the formation and deposition of excess fibrous connective tissue resulting in progressive architectural remodeling in nearly all tissues and organs, eventual organ deformation and functional failure [1, 2]. It is difficult to diagnose at early stage and the pathogenesis is quite complex, so the efficacy and prognosis are not satisfactory, which is one of the major problems in clinical medical research. Aging is a main risk factor for numerous pathologies, and the risk of tissue fibrosis, including renal fibrosis, idiopathic pulmonary fibrosis (IPF), hepatic fibrosis and cardiac fibrosis, is significantly elevated with age [3]. In addition, progressive fibrosis is recognized as a sign of aging in many organs. Indeed, nearly half of deaths are attributed to the complications of organ fibrosis with the progression of industrialization, highlighting the importance of fibrosis inhibition in maintaining human health [4]. Fibrosis is often observed in many organs, causing architecture disruption and ultimately leading to irreversible damage to organ function, which includes but is not limited to the lung, kidney, liver and heart [5]. Although organ fibrosis was previously recognized as irreversible, recent advances indicate that certain circumstances permit fibrosis regression when the preventable causes are eradicated, bringing renewed hope that fibrosis could be curable instead of a death sentence [6].
Aryl hydrocarbon receptor (AhR) is a highly conserved environment-sensing transcription factor that integrates dietary, microbial, environmental, and metabolic cues to regulate complex transcriptional programs in a ligand-specific and cell-type specific manner [7-9]. It is composed of an amino (N-) terminal bHLH domain required for DNA binding, followed by two PER-ARNT-SIM (PAS) domains (A and B) and a carboxy (C-) terminal transactivation domain (TAD) [10]. In the cytoplasm, AhR forms a stable complex with HSP90 molecules, the AhR-interacting protein (HBV X associated protein 2), the co-chaperone p23 and the protein kinase SRC. Once activated by its ligand, the binding of a ligand at the PAS domain of the AhR leads to a conformational change in the exposing of the exposing of the nuclear localization signal. AhR dissociates from the complex and enters the nucleus. In the nucleus, AhR forms a dimer with the AhR nuclear translocator (ARNT) that regulates downstream gene transcription, and phosphorylation modification of AhR enhances its biological activity. Both AhR and ARNT can recruit a co-activator and then initiate the transcription of the AhR target genes, which including CYP1A1 and CYP1B1, the most topical genes and encode CYP1 family enzymes. Other AhR target genes include AhRR, INF-α [11] and Nrf2 [12]. AhR signaling also has crosstalk with other pathways such as Wnt/β-catenin, transforming growth factor-β/Smad (TGF-β/Smad) [13], nuclear factor-κB (NF-κB) [14], the renin-angiotensin system (RAS) [15] and matrix metalloproteinase (MMP) [16] due to the molecular interaction between activated AhR and other proteins, thereby regulating the cell functions (Fig. 2).
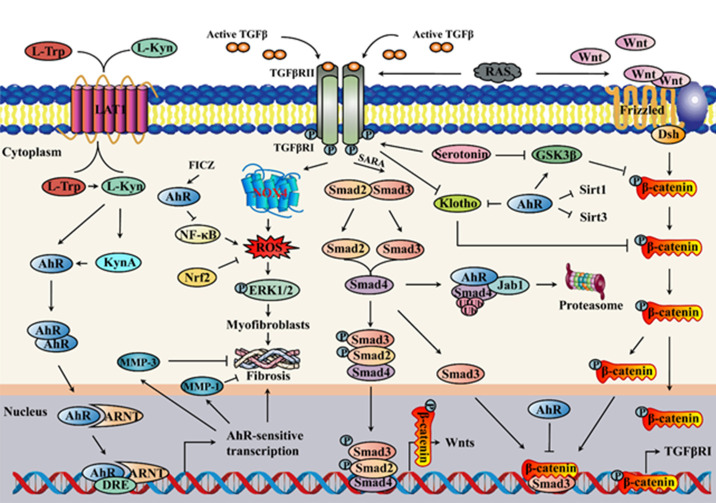
Figure 2.
The crosstalk among AhR and other signalling pathways in tissue fibrosis. Both L-Kyn and KynA can activate AhR, and AhR is translocated to the nucleus upon interaction with a ligand, leading to AhR-sensitive transcription and fibrosis development. In addition to canonical AhR signalling, AhR also interacts with other pathways. TGF-β/Smad and Wnt/β-catenin signalling are other core pathways of fibrosis progression that play pivotal roles in renal fibrosis, idiopathic pulmonary fibrosis, hepatic fibrosis and cardiac fibrosis. AhR not only attenuates lung fibrosis by inducing Smad4 degradation in the proteasome but also alleviates hepatic fibrosis by disrupting the interaction of Smad3 and β-catenin. GSK3β is an inhibitor of β-catenin, and AhR may also inhibit lung fibrosis by maintaining GSK3β in an active form. Serotonin is a dedicated contributor to cardiac fibrosis and pulmonary fibrosis by activating the TGF-β signalling pathway. Moreover, it also enhances β-catenin signalling by inhibiting GSK3β. Inflammation also contributes greatly to fibrogenesis, and AhR activated by FICZ improves acute kidney injury by inhibiting NF-κB. Furthermore, AhR has been proven to suppress fibrosis by activating MMP-1/MMP-3 in fibroblasts, but whether it has a similar effect on ageing-related fibrosis remains to be determined. ARNT: aryl hydrocarbon receptor nuclear translocator; DRE: dioxin response element; ERK: extracellular signal-related kinase; Jab: Jun-activation domain binding protein; KynA: kynurenic acid; Kyn: kynurenine; LAT1: L-type amino acid transporter 1; L-Kyn: L-kynurenine; L-Trp: L-tryptophan; NOX: nicotinamide adenine dinucleotide phosphate oxidase; SARA: Smad anchor for receptor activation.
AhR ligands are considerably selective in their activity, and the pharmacokinetic processes of ligands may be very different [17]. Well-known high-affinity AhR ligands include various toxic and hydrophobic chemicals, such as polychlorinated biphenyls, polycyclic aromatic hydrocarbons, and halogenated compounds [18]. A key to understanding the biological function of AhR is to identify endogenous ligands with high affinity, especially tryptophan derivatives. However, compared to TCDD, the affinities of most tryptophan metabolites, including kynurenine [19], tryptamine, indole acetic acid [20], indole, 3-methylindole, indole-3-aldehyde, indole-3-lactic acid, indole-3-propionic acid, and indole-3-pyruvate are quite low [21]. The discovery of 6-formylindolo[3,2-b] carbazole (FICZ) as an endogenous and high affinity agonist of AhR fills this gap. FICZ has been proven to be more potent than TCDD and can be efficiently metabolized by cytochrome CYP1A1, CYP1A2 and CYP1B1, thus keeping its concentration at a low level [22]. Nevertheless, its level is elevated by other AhR antagonists due to the inhibition of CYP1A1, CYP1A2 and CYP1B1, and AhR is subsequently activated [22]. In addition to mediating the toxic effects of various environmental pollutants, such as carcinogenic, teratogenic, and inflammatory effects, the functional role of AhR also plays an important role in various physiopathological processes, such as immune regulation [23], fibrosis, growth and development [24], and maintenance of physiological homeostasis.
AhR is implicated in various aging-related diseases and tissue fibrosis, as it modulates numerous genes that are involved in many molecular pathways and cellular processes [25, 26]. For example, klotho is a well-known aging suppressor [27], the expression of which is significantly decreased, likely through an AhR-associated mechanism after treatment with TCDD [28]. Sirtuins (Sirt) are other prominent regulators of replicative lifespan and AhR can accelerate senescence by suppressing the activity of Sirtl [29] and Sirt3 [30]. Moreover, from the outcome of recent physiological and epidemiological studies, it appears that a considerable portion of the environmental influence on human disease is mediated by microbial communities, highlighting the importance of gut microbiota intervention in therapeutic strategy development [31]. Although the complex interaction between the host and resident gut microbiota remains elusive, tryptophan metabolism is recognized to play a crucial role in microbiota-host crosstalk through the AhR signaling pathway [32-34]. Furthermore, coronavirus disease 2019 (COVID-19) is a severe respiratory disease caused by respiratory syndrome coronavirus 2 (SARS-CoV-2), which severely harms human health. A large amount of epidemiological, viral immunological and current clinical evidence supported the possibility of pulmonary fibrosis as one of the major complications in patients with COVID-19. In severe cases of COVID-19 infection, pulmonary fibrosis is rapidly progress at patient’s imaging [35]. At present there are no reports on the mechanism of COVID-19 induced pulmonary fibrosis. However, with the existing theoretical basis we find that in addition to pulmonary fibrosis caused by the virus itself, other factors also play an extremely important role such as normal immune mechanism and abnormal immune mechanism, possibly as a consequence of a cytokine storm [36]. George et al. found that COVID-19 shared major risk factors with IPF, and anti-fibrotic therapies for IPF may also have value in preventing or alleviating severe COVID-19, fueling considerable enthusiasm for exploiting new drugs simultaneously targeting COVID-19 and fibrosis [37]. Considering that coronaviruses could activate the AhR signaling pathway after entry into cells [38], a promising treatment for both COVID-19 and tissue fibrosis may be achieved by targeting the tryptophan-AhR pathway.
2. Tryptophan metabolism: An overview
Tryptophan is an essential amino acid for humans that is acquired exclusively from dietary intake. Tryptophan and its metabolites have great effects on diverse physiological processes in which they serve as signaling molecules and building blocks of proteins to coordinate body responses to dietary and environmental cues [39-41]. Generally, tryptophan is metabolized via three metabolic pathways: the kynurenine pathway, serotonin pathway and indole pathway. Approximately 95% of tryptophan is converted to kynurenine, kynurenic acid, anthranilic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid through the kynurenine pathway; 1-2% of tryptophan is degraded to 5-hydroxytryptophan, serotonin, 5-hydroxyindole-3-acetic acid and melatonin via the serotonin pathway; and 4-6% of tryptophan is metabolized to tryptamine, indole, indoxyl sulfate, indole-3-pyruvate, indole-3-lactic acid, indole propionic acid, indole acetic acid, 3-methylindole and indole-3-aldehyde through the indole pathway in the gut. Although AhR was first recognized as a receptor for exogenous aromatic hydrocarbons and hazardous environmental toxins, endogenous AhR ligands from tryptophan metabolism, including FICZ [42], kynurenine [43], kynurenic acid [44], xanthurenic acid [45], cinnabarinic acid [46], serotonin [47], 5-hydroxyindole-3-acetic acid [33], tryptamine, indole, indole propionic acid [48, 49], indole acetic acid, indoxyl sulfate [50], indole-3-aldehyde [9], indole-3-pyruvate [51], indole-3-lactic acid [52] and 3-methylindole [53], have also been identified (Fig. 1). In fact, the traditional AhR signaling pathway cannot fully explain all the biological processes attributed to AhR.
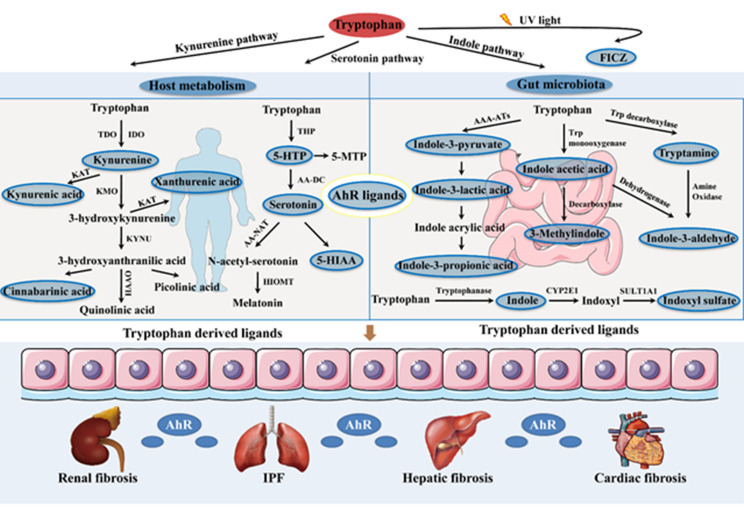
Figure 1.
Schematic representation of the effect of tryptophan metabolism in age-related tissue fibrosis through AhR. Tryptophan is mainly metabolized through three pathways in the host and gut microbiota: the kynurenine pathway, serotonin pathway and indole pathway. The endogenous ligands for AhR that are surrounded in blue, including FICZ, kynurenine, kynurenic acid, xanthurenic acid, cinnabarinic acid, 5-HTP, serotonin, 5-HIAA, indole-3-pyruvate, indole-3-lactic acid, indole-3-propionic acid, indole acetic acid, 3-methylindole, tryptamine, indole-3-aldehyde, indole and indoxyl sulfate, may modulate fibrosis progression after binding with AhR. AAA-ATs: aromatic amino acid aminotransferases; AA-DC: aromatic amino acid decarboxylase; AA-NAT: arylalkylamine N-acetyltransferase; CYP2E1: cytochrome P450 2E1; FICZ: 6-formylindolo[3,2-b]carbazole; HAAO: 3-hydroxyanthranilic acid oxygenase; 5-HIAA: 5-hydroxy- indole-3-acetic acid; HIOMT: hydroxyindole O-methyltransferase; 5-HTP: 5-hydroxy-tryptophan; 5-MTP: 5-methoxy-tryptophan; IDO: indoleamine 2,3-dioxygenase; KAT: kynurenine aminotransferase; KMO: kynurenine 3-monooxygenase; KYNU: kynureninase; SULT1A1: sulfotransferase 1A1; TDO: tryptophan 2,3-dioxygenase; THP: tryptophan hydroxylase; Try: tryptophan.
3. Role of tryptophan metabolism in age-related tissue fibrosis through AhR
3.1 Renal fibrosis
Chronic kidney disease (CKD) affects 8-16% of the population worldwide and is recognized as the 16th leading cause of death [54]. Aging leads to a significant decline in organ function, among which the kidney is particularly susceptible to age-associated damage [55]. Compared to young kidneys, aged kidneys have significantly increased fibrosis with impaired renal function, and it is highly recommended that kidney aging be explored along with the related mechanisms for the prevention of age-associated fibrosis [56]. The AhR signaling pathway has been reported to exacerbate aging phenotypes in different tissues, and here, we mainly focus on how tryptophan metabolism accelerates renal fibrosis via AhR [57, 58]. Epidemiological and experimental studies have indicated that the dysfunction of various metabolites is implicated in renal fibrosis, especially tryptophan-derived metabolites. Mesangial cells play important roles in the synthesis and degradation of type IV collagen, and serotonin can accelerate fibrosis by enhancing type IV collagen production in human mesangial cells, which is mediated by the activation of TGF-β/Smad signaling [59]. Moreover, AhR was activated in patients with CKD, and serum levels of kynurenic acid, kynurenine and quinolinic acid were increased with CKD severity [60]. Indoxyl sulfate is a representative protein-bound uraemic toxin, the excessive accumulation of which promotes CKD progression by activating both the canonical AhR and non-canonical AhR signaling pathways [61, 62], whereas indole-3-propionic acid could attenuate renal fibrosis by suppressing indoxyl sulfate-induced AhR expression [63]. Indole-3 acetic acid, another uremic toxin from the gut microbiota, was increased in parallel with CKD progression, while its level was substantially decreased and even normalized after kidney transplantation [64]. Furthermore, indole-3 acetic acid also contributed to the renal toxicity observed in CKD patients undergoing dialysis after binding to AhR, underscoring the importance of indole-3 acetic acid clearance in renoprotection [65].
Apart from the abovementioned factors, tryptophan metabolism is also highly associated with the development of complications in CKD, and a vicious cycle exists since CKD complications could exacerbate renal fibrosis. Cardiovascular disease is a global health threat, the risk of which is evidently increased in CKD [66]. Mounting evidence indicates that the progression of cardiovascular disease in CKD cannot be fully explained by traditional risk factors such as dyslipidemia, hypertension, and diabetic mellitus, and indoxyl sulfate-induced vascular inflammation may contribute to atherogenesis in CKD [67]. AhR-mediated tissue factor production [50] and immune dysfunction [68] also play pivotal roles in the pathogenesis of cardiovascular disease by accelerating atherosclerosis and provoking vascular endothelial damage, respectively. Similar results were observed for indole-3 acetic acid [50, 69]. Renal anemia is very common in CKD, and indoxyl sulfate could exacerbate anemia by inhibiting hypoxia-inducible factor-dependent erythropoietin production through the activation of AhR [70]. Additionally, iron deficiency was the major cause of anemia, and indoxyl sulfate also affected iron metabolism in CKD by participating in hepcidin regulation through AhR-dependent pathways, suggesting that indoxyl sulfate-induced AhR may be exploited as a therapeutic target for treating renal anemia [71]. Bone disorder is another CKD complication that appears at the early stage of renal failure, while the treatment for bone disorder remains a clinical challenge [72]. Kynurenine [73] and indoxyl sulfate [74] could exacerbate osteoporosis by decreasing bone strength and affecting osteoclastogenesis through AhR-associated mechanisms, respectively, the inhibition of which might provide new opportunities for the treatment of osteoporosis in CKD. Moreover, CKD patients with high indoxyl sulfate and kynurenine were predisposed to arteriovenous thrombosis after vascular injury through the uremic solute-AhR-tissue factor axis [75], hinting that AhR antagonists may also be novel targets for anti-thrombotic therapy.
3.2 Idiopathic pulmonary fibrosis
IPF is a fatal age-associated disease characterized by progressive and irreversible lung scarring, affecting approximately 130,000 people in the US and 3 million people worldwide, the prevalence and incidence of which dramatically increase with age [76]. In addition, the survival rate of IPF patients markedly decreases with age [77], and the median survival rate is less than 3 years [78, 79]. Although some risk factors have been identified, the etiology of IPF is still unknown, while age stands out as a major contributor to the pathogenesis since IPF is widely recognized as a disease of aging [80]. Therefore, it is critical to understand the age-associated mechanisms that play pivotal roles in disease pathogenesis with a growing population of elderly individuals. Kynurenine is increased with aging [81], and kynurenine elevation could signal through AhR to accelerate lung fibrosis by promoting epithelial-to-mesenchymal transition [82]. Reactive oxygen species (ROS) production and oxidative damage increase with age as well, which promotes the progression of age-related diseases, including IPF [83]. Zhang et al. discovered that AhR-deficient cells were susceptible to hypertoxic injury and that AhR activation could protect lung endothelial cells against ROS-induced damage [84]. However, Chen et al. uncovered that AhR downregulation ameliorated AhR-dependent oxidative damage caused by the environment and cigarette smoking exposure in lung epithelial cells [85], suggesting that lung endothelial cells respond differently to ROS than lung epithelial cells.
Inflammatory aging also contributes to the development of pulmonary disease in elderly individuals [86]. It is well known that regulatory T cells (Tregs) are critical for inflammation suppression [87], and AhR activation by FICZ attenuates IPF by increasing Tregs but inhibiting inflammatory T cell subsets in a bleomycin-induced lung fibrosis model [88]. Interestingly, pulmonary epithelial cells alleviated inflammation at least partially by suppressing their own IL-6 production through the kynurenine-AhR axis. Therefore, pulmonary epithelial cells may have a self-regulating system for inflammation [89]. Mesenchymal stem cells have been reported to protect against various diseases, and kynurenic acid pre-treatment enhances their therapeutic effect on lipopolysaccharide-induced acute lung damage through an AhR-associated mechanism [90]. 3-Methylindole is a pneumotoxicant derived from tryptophan metabolism in the human large intestine that causes acute pulmonary disease with highly selective toxicity to epithelial cells [91]. In addition to endogenous metabolism of tryptophan, cigarette smoke inhalation provides a considerable source of 3-methylindole exposure in humans, which directly brings the pneumotoxicant to its target organ. Weems et al. demonstrated that 3-methylindole led to DNA damage and induced cytochrome P450 enzyme expression in an AhR-dependent mechanism, providing mechanistic insights into 3-methylindole-mediated lung damage [92]. Of note, although AhR contributes to cigarette smoke- and environmental pollutant-induced lung damage, recent studies have indicated that AhR negatively regulates the progression of lung cancer. Lee et al. discovered that AhR overexpression attenuated lung cancer invasion by inducing Smad4 ubiquitination and proteasome degradation after binding to Smad4 under non-ligand conditions in lung cancer cells, providing new insight into the role of AhR in cancer formation and a novel target for therapeutic intervention [93] (Fig. 2). Individuals who suffering from SARS-CoV-2 infections are also at high risk of organ injure processing including the lung, heart, kidney. One case from clinical experience reported that idiopathic pulmonary fibrosis post-COVID-19 infection was the exacerbating factor causing progression of disease. Even she was treated with reversible inflammation, unfortunately, there was no improvement clinically [35]. It's because of CoV persistently activate AhRs, this may lead to up-regulation of multiple sets of downstream effectors resulting in pulmonary fibrosis. During late phase of the IPF, cytokine elevation related to persistent activation of AhRs by CoV, can also induce tissue injury in diverse organs, and contribute to worsening of clinical manifestations and disease outcomes [38].
3.3 Hepatic fibrosis
Hepatic disease is the fifth leading cause of premature life lost in the Western world, with irreversible damage caused by liver fibrosis, and ultimately cirrhosis, a primary contributor to mortality [94]. Aging is intimately associated with the physiological decline of the liver, which involves changes in hepatic structure and hepatic function, thus leading to the development of age-related hepatic diseases [95]. AhR is highly expressed in the liver, while the role of AhR in hepatic fibrosis is controversial since both loss and gain of AhR expression could lead to hepatic fibrosis. On the one hand, AhR has a great influence on liver development, and mice lacking AhR showed spontaneous hepatic fibrosis with significant liver architecture alteration, suggesting that the basal AhR activity may suppress fibrotic phenotypes in vivo [96]. On the other hand, AhR activation sensitized mice to hepatic fibrosis by regulating profibrotic pathways [97, 98]. Considering that there are multiple cell types in the liver, including hepatocytes, hepatic stellate cells and Kupffer cells, it is reasonable to postulate that AhR may have a cell type-specific role in hepatic fibrosis. Activated hepatic stellate cells are dominant contributors to hepatic fibrosis since they are major cells responsible for the production of extracellular matrix proteins and profibrogenic cytokines [99]. Yan et al. discovered that AhR knockout in hepatic stellate cells was sufficient to cause spontaneous hepatic fibrosis, whereas treatment of mice with non-toxic AhR ligand ameliorated hepatic fibrosis by preventing the interaction of Smad3 and β-catenin [100] (Fig. 2). Notably, similar results were not observed in hepatocytes/Kupffer cells [100], and the function of AhR can be differentially altered by binding to different ligands, which may explain the contradictory role of AhR in hepatic fibrosis.
Kynurenic acid exerted a hepatoprotective effect on thioacetamide-induced liver failure partly by mediating AhR, and the use of a diet containing high kynurenic acid can be beneficial to the recovery of liver injury, as kynurenic acid is a constituent of foods and is found in considerable amounts in selected products [101]. Indole-3-acetate may ameliorate hepatic fibrosis by inhibiting the release of pro-inflammatory cytokines through an AhR-associated mechanism [102]. Chronic alcohol consumption is an important contributor to liver-related deaths [103], while alcohol-induced hepatic fibrosis was reversed by AhR agonists generated by tryptophan metabolism in the intestine [32]. Given that there is no treatment that reverses alcohol-induced liver lesions except for liver transplantation, the modulation of AhR signaling by supplementation with AhR ligand-producing bacteria may hold promise in inspiring new therapeutic therapies for alcoholic liver disease. Additionally, emerging studies demonstrated that stanniocalcin 2 was a novel AhR target gene specifically responsive to cinnabarinic acid, and AhR-dependent stanniocalcin 2 activations induced by cinnabarinic acid protected against chemical insult or oxidative stress-induced liver damage [104, 105]. 3-Methylindole is a pulmonary toxin that activates AhR, while it acts as an antagonist of TCDD-induced AhR activation, suggesting that 3-methylindole is a partial AhR agonist and potentially influences liver function alone or in combination with other AhR ligands [106]. Approximately 90% of kynurenine is generated by indoleamine 2,3-dioxygenase (IDO), and the level of kynurenine is increased in the liver after CCl4 treatment accompanied by the activation of AhR signaling, whereas IDO2 inhibition attenuates hepatic fibrosis, hinting that kynurenine produced by IDO2 exacerbates liver injury by activating AhR [107]. Astonishingly, kynurenine could restrain fibrosis by upregulating MMP-1/MMP-3 and further promoting excessive fibrotic collagen degradation through an AhR-dependent mechanism, highlighting the potentially significant therapeutic value of kynurenine intervention for fibrotic disorders [16, 108, 109] (Fig. 2). However, it is unknown whether kynurenine has a similar effect in the liver, and future studies are urgently needed.
3.4 Cardiac fibrosis
Cardiac fibrosis is a hallmark of myocardial remodeling and is deeply implicated in the development of heart failure [110]. Aging induces cardiac structural changes linked to increased extracellular matrix protein deposition, leading to progressive interstitial fibrosis [111]. Gut microbiota dysfunction induced by excessive cardiac pressure contributes to adverse myocardial remodeling, while tryptophan metabolites ameliorate cardiac fibrosis in heart failure by activating AhR signalling [112]. In addition, AhR was cardioprotective against doxorubicin-induced cardiotoxicity [113], while AhR ablation promoted angiotensin II-induced cardiac fibrosis [114]. Kynurenine is a uremic toxin that plays a vital role in cardiac hypertrophy by mediating AhR, and the production of cardiac hypertrophy markers is reversed by AhR knockdown [115], suggesting that kynurenine and AhR are important mediators of cardiac injury that may be pharmacologically manipulated in vivo. In addition to the canonical AhR signaling pathway, kynurenine also accelerates cardiac fibrosis through the non-canonical AhR signaling pathway. Lekawanvijit et al. discovered for the first time that indoxyl sulfate had pro-fibrotic, pro-inflammatory and pro-hypertrophic effects, indicating that indoxyl sulfate might contribute to adverse cardiac remodeling by modulating cardiac fibroblasts and NF-κB signaling [116].
3.5 The crosstalk among AhR and other signaling pathways in aging-related fibrosis
TGF-β/Smad signaling is widely recognized as the core pathway of fibrosis, and AhR may attenuate lung fibrosis by inducing Smad4 degradation in the proteasome under non-ligand conditions [93]. Wnt/β-catenin signaling is another prominent signaling pathway that affects tissue fibrosis with aging, and Wnt/β-catenin dysfunction also plays a paramount role in aging-related tissue fibrosis [3]. Yan et al. discovered that Smad3 could promote fibrogenesis by binding to β-catenin, while AhR alleviated hepatic fibrosis by disrupting the interaction of Smad3 and β-catenin [100]. GSK3β is an inhibitor of β-catenin that induces β-catenin degradation by forming a complex together with axin and adenomatosis polyposis coli [117]. AhR could maintain GSK3β in an active form, thus suppressing epithelial-mesenchymal transition in lung cancer cells, which may be used to alleviate lung fibrosis [118]. Serotonin not only promoted cardiac fibrosis and pulmonary fibrosis by activating the TGF-β signaling pathway [119, 120] but also enhanced β-catenin signaling by inhibiting GSK3β [121]. In addition, klotho is an aging suppressor that represses Wnt/β-catenin signaling by binding to Wnt ligands, whereas klotho expression is markedly inhibited by AhR and TGF-β/Smad signaling [122]. Sirtl and Sirt3 are other aging suppressors, the loss of which contributes much to fibrosis progression [123, 124], and AhR could also signal with Sirtl [29] and Sirt3 [30]. Moreover, inflammation also plays an important role in fibrosis progression, and AhR signaling activated by FICZ can improve acute kidney injury by inhibiting the NF-κB pathway [125]. Furthermore, AhR has been reported to promote fibrillar collagen degradation by activating MMP1/MMP3 in fibroblasts, but whether it has a similar effect on renal fibrosis, idiopathic pulmonary fibrosis, hepatic fibrosis and cardiac fibrosis remains to be determined. In brief, AhR signaling is a dedicated modulator of aging-related diseases, and it also interacts with other signaling pathways, the intervention of which may yield promising candidates for anti-fibrotic therapies (Fig. 2).
4. Ongoing clinical trials for age-related tissue fibrosis
4.1 The trials for renal fibrosis
Worldwide estimates indicate that approximately 700 million individuals suffer from CKD, which contributes greatly to diminished quality of life and shorter life expectancy [126]. Despite the widespread availability of laboratory tests to identify patients with abnormal kidney function, very few clinical trials have been conducted to explore kidney diseases [127]. Until recently, angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers were the only classes of medicine that have been effective in slowing kidney function decline [128-131]. Empagliflozin [132], canagliflozin [133] and sotagliflozin [134] have been reported to reduce the risk of adverse events in patients with CKD and diabetes by inhibiting sodium-glucose cotransporter 2 (SGLT2), supporting the potential use of sodium-glucose cotransporter 2 inhibitors combined with other drugs in the treatment of CKD and diabetes. Similar results were observed in atrasentan [135] and finerenone [136]. It seems that the benefits of SGLT2 inhibitors are independent of the glucose-lowering effects, and their renal protective effects may persist in CKD patients caused by factors other than type 2 diabetes [137, 138]. Heerspink et al. investigated the effect of dapagliflozin (10 mg once a day) in CKD patients with or without type 2 diabetes and demonstrated that dapagliflozin significantly improved the clinical outcomes of CKD patients by inhibiting SGLT2 regardless of the absence or presence of diabetes [139].
In addition, higher plasma marine n-3 fatty acids were inversely associated with renal fibrosis development and independently associated with better survival in 1990 Norwegian renal transplant recipients, suggesting that marine n-3 fatty acid supplementation may assist renal function recovery [140]. However, meta-analyses of randomized controlled trials found that there was no statistically significant effect of marine n-3 fatty acids on renal function [141, 142]. Considering that the efficacy of marine n-3 fatty acids may be hampered by low sample sizes, Eide et al. conducted the largest randomized clinical trial to investigate the effect of marine n-3 fatty acids (2.6 g daily for 44 weeks) on renal function during the first year after kidney transplantation [143]. Astonishingly, marine n-3 fatty acid supplementation was safe, while it did not improve renal function compared to the controls. Collectively, the currently available randomized clinical trials cannot provide sufficient evidence to recommend marine n-3 fatty acid therapy to treat kidney disease. Given that the safety of marine n-3 fatty acids is well documented and its beneficial effect on cardiac fibrosis [144], more studies with longer durations are required to determine whether marine n-3 fatty acids could preserve renal function. Moreover, contrast-induced nephropathy is a serious health problem with increased morbidity and mortality, especially in patients with renal insufficiency. Sarpogrelate is a selective serotonin receptor subtype 2A antagonist that can block serotonin-induced platelet aggregation and has an anti-inflammatory effect, which has been shown to reduce renal fibrosis in animal models [145, 146], while the clinical outcome of sarpogrelate in contrast induced nephropathy is disappointing [147]. Of most patients who experienced an adverse event during the above trials, fracture and gastrointestinal discomfort were reported by more patients in the treat group. The severe adverse events and conflications are presented in Table1.
Table 1.
The ongoing clinical trials for age-related tissue fibrosis
| Drug | Target | Phase | Trial | Result | Ref. | Time | ADR | Complication |
|---|---|---|---|---|---|---|---|---|
| Renal fibrosis | ||||||||
| Empagliflozin | SGLT2 | - | NCT01131676 | Positive | [132] | 2.6y△ | Genital infection, volume depletion, bone fracture | - |
| Canagliflozin | SGLT2 | - | NCT02065791 | Positive | [133] | 2.5y | Amputation, fracture, diabetic ketoacidosis | - |
| Sotagliflozin | SGLT2 | - | NCT03315143 | Positive | [134] | 14.2m△ | Diarrhea, genital mycotic infections, volume depletion | - |
| Atrasentan | Endothelin A receptor | Phase 3 | NCT01858532 | Positive | [135] | 36m | Fluid retention, anaemia, cardiac toxicity | - |
| Finerenone | MR | Phase 3 | NCT02540993 | Positive | [136] | 36m | Hyperkalemia, modest reduction in blood pressure, change in bodyweight | Hyperkalemia, irrespective of history of CVD |
| Dapagliflozin | SGLT2 | - | NCT03036150 | Positive | [139] | 30m | Renal-related adverse event, volume depletion, fracture |
- |
| Marine n-3 FAs | - | - | NCT01744067 | NSB | [143] | 44w | Gastrointestinal discomfort, acute rejection episode, post transplantation diabetes mellitus |
- |
| Sarpogrelate* | 5-HT2A | - | NCT01165567 | NSB | [147] | 4w | - | - |
| Idiopathic pulmonary fibrosis | ||||||||
| Pirfenidone plus nintedanib | TKs | Phase 4 | NCT02598193 | Positive | [151] | 24w | Nausea, diarrhoea, vomiting | - |
| Pirfenidone plus nintedanib | TKs | - | NCT02579603 | Positive | [152] | 12w | Nausea, diarrhea, vomiting | - |
| PBI-4050 | GPR | Phase 2 | NCT02538536 | Positive | [154] | 12w | Diarrhoea, nausea, headache | - |
| GSK2126458 | PI3K/mTOR | - | NCT01725139 | Positive | [155] | 5d | ||
| GSK2126458 | PI3K/mTOR | - | 2012-001376-11 | Positive | [156] | 8d | Diarrhoea, nausea, hyperglycaemia | - |
| Thalidomide | - | - | NCT00600028 | Positive | [157] | 24w | Constipation, dizziness, malaise | Constipation, bradycardia |
| Omeprazole | Proton pump | - | NCT02085018 | Positive | [158] | 86d | Lower respiratory tract infection, abdominal pain, cough | - |
| Mesenchymal stromal cells | - | Phase 1b | NCT01385644 | Positive | [159] | 6m | Lightheadness,flushed in face, blurred vision | Worsing lung function, oxygenation |
| Pamrevlumab | CTGF | Phase 2 | NCT01890265 | Positive | [160] | 48w | Respiratory tract infection, cough, dyspnoea | Respiratory related |
| GLPG1690 | Autotaxin | Phase 2a | NCT02738801 | Positive | [161] | 12w | Lower respiratory tract infection, nasopharyngitis, cough | - |
| BMS-986020 | LPA1 | Phase 2 | NCT01766817 | Positive | [162] | 26w | Cough, headache, fatigue | IPF related |
| Pentraxin 2 | Monocyte | Phase 2 | NCT02550873 | Positive | [163] | 28w | Cough, fatigue, nasopharyngitis | Respiratory decline |
| FG-3019 | CTGF | Phase 2 | NCT01262001 | Positive | [164] | 45w | Cough, fatigue, dyspnoea | IPF, pneumonia |
| BIBF 1120 | TKs | Phase 2 | NCT00514683 | Positive | [165] | 52w | Diarrhea, cough, nausea | - |
| Acetylcysteine | - | - | 20130109-2 | Positive | [184] | 26w | Cough, drug-induced pneumonitis | - |
| Bosentan | - | - | NCT00391443 | NSB | [166] | 17.9w | Cough, dyspnea, bronchitis | Respiratory failure |
| Interferon γ-1b | - | - | NCT00075998 | NSB | [167] | 577d | Cough, headache, influenza-like illness | Respiratory-related, sepsis or multiorgan failure , cardiac |
| SAR156597 | Interleukin | Phase 2b | NCT02345070 | NSB | [168] | 52w | IPF, cough, diarrhoea | IPF, pneumonia, septic shock |
| Tralokinumab | Interleukin-13 | Phase 2 | NCT01629667 | NSB | [169] | 64w | Cough, IPF, upper respiratory tract infection | IPF, Pneumonia, cardiac arrest |
| Carbon monoxide | - | Phase 2a | NCT01214187 | NSB | [170] | 12w | Respiratory/thoracic/mediastinal, nervous system, gastrointestinal | - |
| Simtuzumab | LOXL2 | Phase 2 | NCT01769196 | NSB | [171] | 82w | IPF, fatigue, atrial fibrillation | IPF exacerbation or progression |
| Carlumab | CCL2 | Phase 2 | NCT00786201 | NSB | [172] | 48w | Respiratory, thoracic and mediastinal disorders, Infections and infestations, Cardiac disorders |
- |
| Macitentan | Endothelin receptor | Phase 2 | NCT00903331 | NSB | [173] | 12m | IPF, dyspnoea, cough | IPF worsening, respiratory failure |
| Imatinib | TKs | Phase 2 | NCT00131274 | NSB | [174] | 96w | Periorbital edema, diarrhea/cramping, nausea | Acute worsening of IPF |
| Thrombomodulin α | - | Phase 3 | NCT02739165 | NSB | [175] | 14d | Insomnia, delirium, hyperglycemia | The progression or recurrence of AE-IPF |
| Sildenafil | PDE5 | - | NCT00517933 | NSB | [176] | 24w | Worsening of idiopathic pulmo nary fibrosis, worsening of dyspnea, pneumonia |
- |
| Sildenafil plus nintedanib | PDE5; TKs | - | NCT02802345 | NSB | [177, 178] | 24w, 24w | Diarrhea, decreased appetite, nausea | -, cardiovascular event |
| Sildenafil plus pirfenidone | PDE5 | Phase 2b | NCT02951429 | NSB | [179] | 52w | Gastrointestinal disorder, clinically significant vascular event, hypotension event |
- |
| Lebrikizumab | Interleukin-13 | Phase 2 | NCT01872689 | NSB | [180] | 52w | Cough, IPF, dyspnoea | IPF, pulmonary embolism |
| Lebrikizumab plus pirfenidone | Interleukin-13 | Phase 2 | NCT01872689 | NSB | [180] | 52w | Nasopharyngitis, upper respiratory tract infection, cough | IPF, pneumonia, hot disease |
| Acetylcysteine | - | - | NCT00650091 | NSB | [183] | 60w | Respiratory, infectious, cardiac | Respiratory |
| Ambrisentan | Endothelin A receptor | Phase 3 | NCT00768300 | Negative | [181] | 34.7w | Edema peripheral, upper respiratory tract infection, headache | IPF, dyspnea, pneumonia |
| Warfarin | - | - | NCT00957242 | Negative | [182] | 48w | Bleeding | Cardiovascular, pulmonary hypertension, pneumonia |
| Acetylcysteine plus pirfenidone | TGF-β1 | Phase 3 | UMIN000015508 | Negative | [185] | 48w | Pneumonia, photosensitivity, decreased appetite |
- |
| Acetylcysteine plus pirfenidone | - | Phase 2 | 2012-000564-14 | Negative | [186] | 24w | Photosensitivity, cardiac, diarrhoea | worsening IPF |
| Hepatic fibrosis | ||||||||
| Elafibranor | PPAR | Phase 2 | NCT01694849 | Positive | [188] | 52w | Nausea, heahache, diarrhea | serum creatinine |
| GS-0976 | ACC | Phase 2 | NCT02856555 | Positive | [189] | 12w | Nausea, heahache, diarrhea | night sweats, hypertriglyceridemia |
| Resveratrol | - | - | NCT02030977 | Positive | [190] | 12w | - | - |
| GR-MD-02 | Galectin 3 | Phase 1 | - | Positive | [192] | 6/7w | Headache, dizziness, diarrhoea | - |
| Diacerein | Interleukin-1 | - | NCT02242149 | Positive | [196] | 24m | - | - |
| Dapagliflozin | SGLT2 | - | UMIN000022155 | Positive | [197] | 24w | - | - |
| Candesartan | Angiotensin | - | NCT00990639 | Positive | [198] | 6m | - | - |
| Capsule oxymatrine | - | - | - | Positive | [199] | 52w | Nausea, rash, chest discomfort | - |
| Interferon-γ | TGF-β1 | - | - | Positive | [200] | 6m | Limb pain, nausea, muscle pain | - |
| Silibinin | - | Phase 2 | NCT01518933 | Positive | [204] | 14d | Feeiling hot, diarrhea, headache | - |
| Telbivudine | - | - | NCT00877149 | Positive | [206] | 261w | - | - |
| Synbiotic agents | - | Phase 2 | NCT01680640 | NSB | [191] | 10-14m | Weight loss | - |
| GR-MD-02 | Galectin 3 | Phase 2 | NCT02462967 | NSB | [193] | 52w | Nausea, diarrhea, nasopharyngitis | Spasmodic cough# |
| GR-MD-02 | Galectin 3 | Phase 2 | NCT02421094 | NSB | [194] | 16w | - | - |
| Peg-interferon α-2a | - | - | NCT00122616 | NSB | [201] | 96w | Liver failure with ascites and edema, neutropenia, anemia | - |
| Oltipraz | TGF-β1 | Phase 2 | NCT00956098 | NSB | [202] | 24w | - | - |
| Simtuzumab | LOXL2 | Phase 2 | NCT01707472 | NSB | [203] | 22w | Fever, headache, asymptomatic elevations in amylase and lipase | - |
| Cardiac fibrosis | ||||||||
| Empagliflozin | SGLT2 | - | NCT03057977 | Positive | [208] | 124w | Uncomplicated genital tract infection, hypoglycemia, lower limb amputation |
- |
| Dapagliflozin | SGLT2 | - | NCT03036124 | Positive | [209] | 8w | Volume depletion, renal AE, fracture | - |
| Omega-3 PUFAs | - | - | - | Positive | [210] | 8w | - | - |
| Omega-3 acid ethyl ester | - | - | NCT00729430 | Positive | [144] | 6m | Nausea, fishy taste, bleeding | - |
| Lisinopril | ACE | - | 1262GR/0006 | Positive | [214] | 24w | - | - |
| Torasemide | Type I collagen | - | NCT00409942 | NSB | [216] | 32w | Diabetes mellitus, hyperuricemia, hypokalemia | - |
Drugs that interfere with the tryptophan-AhR pathway,
Time that the median duration if treatment,
Complication probably related to study drug ACC: acetyl-coenzyme A carboxylase; ACE: angiotensin-converting enzyme; CCL2: CC-chemokine ligand 2; CTGF: connective tissue growth factor; GPR: G-protein coupled receptor; 5-HT2A: serotonin receptor subtype 2A; LOXL2: lysyl oxidase-like 2; LPA1: lysophosphatidic acid receptor 1; MR: mineralocorticoid receptor; mTOR: mammalian target of rapamycin; NSB: no significant benefit; PDE: phosphodiesterase; PI3K: pan-PI3 kinase; PPAR: peroxisome proliferator-activated receptor; PUFAs: polyunsaturated fatty acids; ROS: reactive oxygen species; TGF-β: transforming growth factor-β; TKs: tyrosine kinases
4.2 The trials for idiopathic pulmonary fibrosis
The development of pirfenidone and nintedanib has been an exciting landmark in the treatment of IPF, and both of them are recommended for clinical use in the current clinical practice guidelines [148]. Unfortunately, although pirfenidone and nintedanib slow IPF progression, the disease continues to progress, indicating that additional therapies are needed to cure this deadly disease. Current efforts are primarily focused on developing new drugs and the combination of new agents with available therapies. Pirfenidone and nintedanib are thought to mediate different aspects of fibrotic cascades, the combination of which might provide additive effects on IPF outcomes compared with either monotherapy [149, 150]. Flaherty et al. assessed the safety of treatment with pirfenidone and nintedanib in 89 patients and discovered that treatment with pirfenidone (1602-2403 mg/day) and nintedanib (200-300 mg/day) for 24 weeks was well tolerated by the majority of IPF patients, supporting further research into combination regimens for patients with IPF [151]. Similar results were observed in another open-label, randomized trial [152]. PBI-4050 is a novel small-molecule compound that demonstrates anti-fibrotic effects in several fibrosis models, including lung fibrosis [153]. Khalil et al. conducted the first clinical study of PBI-4050 in patients with IPF and found that no safety concerns arose among patients receiving PBI-4050 alone or in conjunction with pirfenidone/nintedanib [154]. However, there was a drug-drug interaction between pirfenidone and PBI-4050, while the pharmacokinetic profiles were similar among patients with PBI-4050 alone or in conjunction with nintedanib, encouraging further study of PBI-4050 alone or PBI-4050 plus nintedanib in patients with IPF [154]. Phosphatidylinositol 3-kinase signaling, and mammalian target of rapamycin also play vital roles in IPF pathogenesis, the inhibition of which has been shown to attenuate fibrosis progression in randomized and placebo-controlled trials [155, 156]. Persistent, non-productive and often disabling cough that has no effective treatment is one of the most prominent symptoms of IPF, affecting up to 80% of IPF patients [157]. Thalidomide [157] and omeprazole [158] were reported to alleviate cough and improve the respiratory quality of patients with IPF, while large-scale prospective clinical trials are still warranted to further evaluate their efficacy and safety.
Moreover, mesenchymal stromal cells [159], pamrevlumab [160], GLPG1690 [161], BMS-986020 [162], pentraxin 2 [163], FG-3019 [164] and BIBF 1120 [165] also showed potential clinical benefits for patients with IPF in phase 1/2 trials. However, although a series of anti-fibrotic agents exhibited a promising future in clinical use, many ongoing clinical trials failed to demonstrate benefit in treating IPF patients compared to placebo, such as bosentan [166], interferon γ-1b [167], SAR156597 [168], tralokinumab [169], inhaled carbon monoxide [170], simtuzumab [171], carlumab [172], macitentan [173], imatinib [174] and thrombomodulin α [175]. Additionally, both sildenafil monotherapy [176] and in combination with nintedanib [177, 178] or pirfenidone [179] did not provide a significant benefit in patients with IPF. Similar results were observed for lebrikizumab [180]. Of note, endothelin-1 and the coagulation cascade were believed to be implicated in the pathogenesis of IPF, while treatments with ambrisentan [181] and warfarin [182] were associated with even worse outcomes in IPF patients and should not be used for clinical use. Furthermore, up to one-third of IPF patients in Europe are reported to treat IPF by using pirfenidone and acetylcysteine, while the efficacy of acetylcysteine on IPF remains controversial [183, 184], and the combination of acetylcysteine with pirfenidone may be harmful to patients with IPF [185, 186]. Therefore, more large-scale clinical trials are urgently needed to further evaluate its safety and efficacy. The most common reported ADRs were nausea, diarrhea, and cough ff most patients who experienced an adverse event during the above trials. The severe adverse events and conflictions are presented in Table1.
4.3 The trials for hepatic fibrosis
Non-alcoholic fatty liver disease is the most common liver disease with complex pathophysiology that is characterized by hepatic steatosis and can potentially progress to advanced fibrosis or end-stage liver disease, affecting approximately 24% of the population worldwide [187]. Elafibranor [188], GS-0976 [189] and resveratrol [190] were proven to be effective in hepatic fibrosis regression for patients with non-alcoholic fatty liver disease, while patients receiving a synbiotic combination (prebiotic and probiotic) failed to slow hepatic fibrosis progression [191]. Galectin 3 contributed greatly to the pathogenesis of hepatic fibrosis, including inflammation and fibrosis caused by non-alcoholic fatty liver disease, whereas GR-MD-02 alleviated liver fibrosis by inhibiting galectin 3 and was well tolerated in phase I studies [192]. Unfortunately, the results of two-phase II studies evaluating GR-MD-02 in hepatic fibrosis were disappointing, as it did not exhibit robust efficacy in fibrosis improvement, which needs to be validated in future studies [193, 194]. In addition, type 2 diabetes and non-alcoholic fatty liver disease, both of which severely threaten human health, share many physiopathological pathways [195]. Diacerein [196] and dapagliflozin [197] have been reported to reduce hepatic fibrosis in diabetic patients with non-alcoholic fatty liver disease in randomized controlled trials, fueling considerable enthusiasm for the conduction of further clinical trials. Moreover, alcohol is a primary cause of hepatic fibrosis, and chronic alcohol consumption contributes much to the development of end-stage liver disease. The administration of candesartan plus ursodeoxycholic acid significantly improved hepatic fibrosis in patients with alcoholic liver disease without serious side effects, suggesting that candesartan can be beneficial to alcohol-induced hepatic fibrosis [198]. Hepatic fibrosis due to chronic viral infection also has an enormous socioeconomic impact. In addition to strategies targeting virus elimination, inhibition, or prevention of fibrogenesis is amenable. Oxymatrine capsule [199] and interferon-γ [200] were effective in the treatment of hepatic fibrosis caused by chronic viral hepatitis, while no significant changes were observed in hepatic fibrosis in patients receiving peg-interferon α-2a [201], oltipraz [202] and simtuzumab [203]. Rendina et al. discovered that silibinin monotherapy had a significant anti-viral effect in patients with established hepatitis C virus recurrence, and there was no interaction with other drugs for the first time, encouraging the evaluation of silibinin in combined therapy with other anti-viral drugs [204]. Furthermore, although anti-viral therapy is important for ameliorating liver fibrosis in patients with chronic viral infection, the complete regression of hepatic cirrhosis remains an intractable problem, and many patients with hepatitis B infection will progress to cirrhosis within 5 years [205]. Hou et al. demonstrated that prolonged telbivudine treatment led to durable virologic inhibition and significant improvement in hepatic fibrosis, thus achieving the long-term goals of anti-viral treatment in patients with chronic hepatitis B infection, which may help resolve the problem of cirrhosis progression in hepatitis B-infected persons [206]. During the above clinical trials, side effects mostly occurred with nausea, diarrhea, headache and so on during the treatment (Table 1).
4.4 The trials for cardiac fibrosis
Heart failure is a major public health problem with substantial morbidity and mortality, the burden of which has increased to 23 million people worldwide [207]. Empagliflozin [208] and dapagliflozin [209] have been reported to reduce the risk of cardiovascular death and hospitalization for heart failure by inhibiting SGLT2 in randomized, double-blind, controlled trials. Omega-3 polyunsaturated fatty acids have multiple cardioprotective effects, and the beneficial role of omega-3 poly-unsaturated fatty acids in cardiac fibrosis in ischemic heart failure patients has recently been demonstrated in a double-blind, placebo-controlled trial [210]. Additionally, Bobak et al. investigated the effect of omega-3 acid ethyl esters on patients with acute myocardial infarction and found that treatment with high-dose omega-3 acid ethyl esters could ameliorate myocardial fibrosis in addition to the current guideline-based standard of care [144]. Moreover, a prolonged state of ventricular pressure overload, caused by hypertension or aortic valve diseases, promotes myocardial remodeling that progresses to cardiac fibrosis and heart failure [211]. Therefore, suppressing hypertensive ventricular remodeling may assist cardiac fibrosis recovery. Previous studies uncovered that lisinopril could restrain cardiac fibrosis by halting angiotensin-converting enzyme in spontaneously hypertensive rats, but whether cardiac fibrosis can also be alleviated in patients with hypertensive heart disease was unknown [212, 213]. Brilla et al. first evaluated the effect of lisinopril on patients with hypertensive heart disease and demonstrated its efficacy in cardiac fibrosis regression, providing evidence for large-scale clinical trials [214]. However, to date, no further trials have been conducted. Furthermore, torasemide was reported to alleviate cardiac fibrosis in biopsy specimens of hypertensive patients with symptomatic heart failure [215], while the results of a randomized, open-label trial evaluating prolonged-release torasemide for cardiac fibrosis of hypertensive patients with chronic heart failure were disappointing [216]. Considering the differential effect of dietary and other treatments on the efficacy variables of open-label trials, more clinical trials are warranted to further evaluate torasemide’s efficacy. The adverse drug reactions mainly included hypoglycemia, bleeding, nausea etc in above trials. The main events ae listed in Table1.
5. Concluding remarks
Fibrosis is a common sequela of organ injury that results in organ dysfunction and potential death. Although it accounts for a considerable burden of diseases globally, there are no effective therapeutic strategies that successfully mitigate fibrosis. Aging-related tissue fibrosis, including renal fibrosis, IPF, hepatic fibrosis and cardiac fibrosis, has become more acute with the aging of populations. Moreover, COVID-19 is spreading rapidly across the world with substantial morbidity and mortality, which has given rise to a global pandemic of unprecedented proportions in the modern era since it is highly contagious and has an enormous impact on human health [217-219]. As the spread of SARS-CoV-2 is increasingly becoming out of control, the epidemic is now in the most formidable phase. COVID-19 is characterized by multiorgan failure, and patients with renal fibrosis, IPF, hepatic fibrosis and cardiac fibrosis are susceptible to COVID-19 and to severe outcomes from the disease [220-224]. Therefore, it is essential to develop mechanism-based therapies that effectively halt fibrosis progression, especially in the background of the COVID-19 outbreak.
Accumulating evidence indicates that the gut microbiota is an important contributor to the metabolic health of humans, the dysregulation of which has a pathogenic effect on multiple diseases, including tissue fibrosis [225-228]. AhR signaling also plays a crucial role in modulating fibrosis progression [25, 26]. Many metabolites are involved in the crosstalk between the host and gut microbiota, among which tryptophan metabolites are dedicated players in fibrogenesis by acting as endogenous AhR ligands. However, to date, only a few studies have shed light on the impact of tryptophan metabolites on fibrosis modulation through AhR, especially for renal fibrosis and cardiac fibrosis. In addition, natural products are fertile ground for drug discovery, while only a few studies have evaluated the effect of natural products on fibrosis regression by AhR-associated mechanisms over the past few decades [229-231]. Randomized trials that provide evidence-based guidelines for clinical decisions are prerequisites before reaching clinical application. Unfortunately, there are fewer clinical trials for renal fibrosis, hepatic fibrosis, and cardiac fibrosis than for IPF, and more than half of the trials for IPF have failed to demonstrate benefit in IPF patients. Given the enormous influence of AhR signaling in fibrosis development and the limited number/efficacy of ongoing clinical trials for aging-related tissue fibrosis, it is highly recommended to exploit novel therapies targeting the AhR signaling pathway.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant numbers 81873263).
Footnotes
Declarations of interest
The authors declare that there is no conflict of interest.
References
- [1].Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY (2020). Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev, 60:101063. [DOI] [PubMed] [Google Scholar]
- [2].Weiskirchen R, Weiskirchen S, Tacke F (2019). Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol Aspects Med, 65:2-15. [DOI] [PubMed] [Google Scholar]
- [3].Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY (2020). Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev, 60::101063. [DOI] [PubMed] [Google Scholar]
- [4].Wynn TA (2007). Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest, 117:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF (2020). Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov, 19:57-75. [DOI] [PubMed] [Google Scholar]
- [6].Jun JI, Lau LF (2018). Resolution of organ fibrosis. J Clin Invest, 128:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim JB, Zhao Q, Nguyen T, Pjanic M, Cheng P, Wirka R, et al. (2020). Environment-sensing aryl hydrocarbon receptor inhibits the chondrogenic fate of modulated smooth muscle cells in atherosclerotic lesions. Circulation, 142:575-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Suzuki T, Hidaka T, Kumagai Y, Yamamoto M (2020). Environmental pollutants and the immune response. Nat Immunol, 21:1486-1495. [DOI] [PubMed] [Google Scholar]
- [9].Rothhammer V, Quintana FJ (2019). The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol, 19:184-197. [DOI] [PubMed] [Google Scholar]
- [10].Schulte KW, Green E, Wilz A, Platten M, Daumke O (2017). Structural Basis for Aryl Hydrocarbon Receptor-Mediated Gene Activation. Structure, 25:1025-1033.e1023. [DOI] [PubMed] [Google Scholar]
- [11].Shi Y, Zeng Z, Yu J, Tang B, Tang R, Xiao R (2020). The aryl hydrocarbon receptor: An environmental effector in the pathogenesis of fibrosis. Pharmacol Res, 160:105180. [DOI] [PubMed] [Google Scholar]
- [12].Furue M, Hashimoto-Hachiya A, Tsuji G (2019). Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan J, Tung HC, Li S, Niu Y, Garbacz WG, Lu P, et al. (2019). Aryl Hydrocarbon Receptor Signaling Prevents Activation of Hepatic Stellate Cells and Liver Fibrogenesis in Mice. Gastroenterology, 157:793-806.e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. (2019). Control of tumor-associated macrophages and T cells in glioblastoma via AhR and CD39. Nat Neurosci, 22:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu CN, Lin IC, Yu HR, Huang LT, Tiao MM, Tain YL (2020). Maternal tryptophan supplementation protects adult rat offspring against hypertension programmed by maternal chronic kidney disease: Implication of tryptophan-metabolizing microbiome and aryl hydrocarbon receptor. Int J Mol Sci, 21:4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Poormasjedi-Meibod MS, Salimi Elizei S, Leung V, Baradar Jalili R, Ko F, Ghahary A (2016). Kynurenine modulates MMP-1 and type-I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts. J Cell Physiol, 231:2749-2760. [DOI] [PubMed] [Google Scholar]
- [17].Safe S, Jin UH, Park H, Chapkin RS, Jayaraman A (2020). Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs). Int J Mol Sci, 21:6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stejskalova L, Dvorak Z, Pavek P (2011). Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab, 12:198-212. [DOI] [PubMed] [Google Scholar]
- [19].Hubbard TD, Murray IA, Perdew GH (2015). Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos, 43:1522-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, et al. (1998). Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry, 37:11508-11515. [DOI] [PubMed] [Google Scholar]
- [21].Vyhlidalova B, Krasulova K, Pecinkova P, Marcalikova A, Vrzal R, Zemankova L, et al. (2020). Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: A detailed characterization. Int J Mol Sci, 21:2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wincent E, Bengtsson J, Mohammadi Bardbori A, Alsberg T, Luecke S, Rannug U, et al. (2012). Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A, 109:4479-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stockinger B, Di Meglio P, Gialitakis M, Duarte JH (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol, 32:403-432. [DOI] [PubMed] [Google Scholar]
- [24].Jackson DP, Joshi AD, Elferink CJ (2015). Ah Receptor Pathway Intricacies; Signaling Through Diverse Protein Partners and DNA-Motifs. Toxicol Res (Camb), 4:1143-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, et al. (2016). Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology, 151:1206-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yao L, Wang C, Zhang X, Peng L, Liu W, Zhang X, et al. (2016). Hyperhomocysteinemia activates the aryl hydrocarbon receptor/CD36 pathway to promote hepatic steatosis in mice. Hepatology, 64:92-105. [DOI] [PubMed] [Google Scholar]
- [27].Chen GZ, Liu Y, Goetz R, Fu LL, Jayaraman S, Hu MC, et al. (2018). α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature, 553:461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jin MH, Lou J, Yu HH, Miao M, Wang GC, Ai H, et al. (2018). Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin promotes inflammation in mouse testes: The critical role of klotho in Sertoli cells. Toxicol Lett, 295::134-143. [DOI] [PubMed] [Google Scholar]
- [29].Koizumi M, Tatebe J, Watanabe I, Yamazaki J, Ikeda T, Morita T (2014). Aryl hydrocarbon receptor mediates indoxyl sulfate-induced cellular senescence in human umbilical vein endothelial cells. J Atheroscler Thromb, 21:904-916. [DOI] [PubMed] [Google Scholar]
- [30].He JH, Hu BF, Shi XJ, Weidert ER, Lu PP, Xu MS, et al. (2013). Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol, 33:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fan Y, Pedersen O (2021). Gut microbiota in human metabolic health and disease. Nat Rev Microbiol, 19:55-71. [DOI] [PubMed] [Google Scholar]
- [32].Wrzosek L, Ciocan D, Hugot C, Spatz M, Dupeux M, Houron C, et al. (2020). Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. [DOI] [PubMed] [Google Scholar]
- [33].Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. (2020). Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab, 31:837-851 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, et al. (2018). Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab, 28:737-749 e734. [DOI] [PubMed] [Google Scholar]
- [35].Earl N, Schoeneberg D, Davidson PD (2021). Severe progression of idiopathic pulmonary fibrosis post-COVID-19 infection. BMJ Case Rep, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang J, Wang BJ, Yang JC, Wang MY, Chen C, Luo GX, et al. (2020). [Research advances in the mechanism of pulmonary fibrosis induced by coronavirus disease 2019 and the corresponding therapeutic measures]. Zhonghua Shao Shang Za Zhi, 36:691-697. [DOI] [PubMed] [Google Scholar]
- [37].George PM, Wells AU, Jenkins RG (2020). Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med, 8:807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L (2020). AhR and IDO1 in pathogenesis of Covid-19 and the "Systemic AhR Activation Syndrome:" a translational review and therapeutic perspectives. Restor Neurol Neurosci, 38:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cervenka I, Agudelo LZ, Ruas JL (2017). Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science, 357. [DOI] [PubMed] [Google Scholar]
- [40].Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov, 18:379-401. [DOI] [PubMed] [Google Scholar]
- [41].Modoux M, Rolhion N, Mani S, Sokol H (2021). Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci, 42:60-73. [DOI] [PubMed] [Google Scholar]
- [42].Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, et al. (2013). Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A, 110:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478:197-203. [DOI] [PubMed] [Google Scholar]
- [44].DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, et al. (2010). Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci, 115:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, et al. (2016). An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Mol Pharmacol, 90:674-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, et al. (2014). Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One, 9:e87877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nieto C, Rayo I, de Las Casas-Engel M, Izquierdo E, Alonso B, Bechade C, et al. (2020). Serotonin (5-HT) shapes the macrophage gene profile through the 5-HT2B-dependent activation of the aryl hydrocarbon receptor. J Immunol, 204:2808-2817. [DOI] [PubMed] [Google Scholar]
- [48].Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature, 557:724-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. (2016). Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med, 22:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gondouin B, Cerini C, Dou L, Sallee M, Duval-Sabatier A, Pletinck A, et al. (2013). Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int, 84:733-744. [DOI] [PubMed] [Google Scholar]
- [51].Scott SA, Fu J, Chang PV (2020). Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A, 117:19376-19387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lamas B, Hernandez-Galan L, Galipeau HJ, Constante M, Clarizio A, Jury J, et al. (2020). Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci Transl Med, 12:eaba0624. [DOI] [PubMed] [Google Scholar]
- [53].Kurata K, Kawahara H, Nishimura K, Jisaka M, Yokota K, Shimizu H (2019). Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem Biophys Res Commun, 510:649-655. [DOI] [PubMed] [Google Scholar]
- [54].Chen TK, Knicely DH, Grams ME (2019). Chronic kidney disease diagnosis and management: A review. JAMA, 322:1294-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].O'Sullivan ED, Hughes J, Ferenbach DA (2017). Renal aging: Causes and consequences. J Am Soc Nephrol, 28:407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chen YY, Yu XY, Chen L, Vaziri ND, Ma SC, Zhao YY (2019). Redox signaling in aging kidney and opportunity for therapeutic intervention through natural products. Free Radic Biol Med, 141:141-149. [DOI] [PubMed] [Google Scholar]
- [57].Kondrikov D, Elmansi A, Bragg RT, Mobley T, Barrett T, Eisa N, et al. (2020). Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol, 130::110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bravo-Ferrer I, Cuartero MI, Medina V, Ahedo-Quero D, Pena-Martinez C, Perez-Ruiz A, et al. (2019). Lack of the aryl hydrocarbon receptor accelerates aging in mice. FASEB J, 33:12644-12654. [DOI] [PubMed] [Google Scholar]
- [59].Kasho M, Sakai M, Sasahara T, Anami Y, Matsumura T, Takemura T, et al. (1998). Serotonin enhances the production of type IV collagen by human mesangial cells. Kidney Int, 54:1083-1092. [DOI] [PubMed] [Google Scholar]
- [60].Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, et al. (2009). Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant, 24:1901-1908. [DOI] [PubMed] [Google Scholar]
- [61].Walker JA, Richards S, Belghasem ME, Arinze N, Yoo SB, Tashjian JY, et al. (2020). Temporal and tissue-specific activation of aryl hydrocarbon receptor in discrete mouse models of kidney disease. Kidney Int, 97:538-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yu Y, Guan X, Nie L, Liu Y, He T, Xiong J, et al. (2017). DNA hypermethylation of sFRP5 contributes to indoxyl sulfate-induced renal fibrosis. J Mol Med (Berl), 95:601-613. [DOI] [PubMed] [Google Scholar]
- [63].Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T (2017). Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci, 79:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liabeuf S, Laville SM, Glorieux G, Cheddani L, Brazier F, Titeca Beauport D, et al. (2020). Difference in profiles of the gut-derived tryptophan metabolite indole acetic acid between transplanted and non-transplanted patients with chronic kidney disease. Int J Mol Sci, 21:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Brito JS, Borges NA, Anjos JSD, Nakao LS, Stockler-Pinto MB, Paiva BR, et al. (2019). Aryl hydrocarbon receptor and uremic toxins from the gut microbiota in chronic kidney disease patients: Is there a relationship between them? Biochemistry, 58:2054-2060. [DOI] [PubMed] [Google Scholar]
- [66].Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, et al. (2020). Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: A scientific statement from the American heart association. Circulation, 142:e265-e286. [DOI] [PubMed] [Google Scholar]
- [67].Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, et al. (2019). Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-Notch signaling. Circulation, 139:78-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, et al. (2017). Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci Rep, 7:3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dou L, Sallee M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, et al. (2015). The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol, 26:876-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Asai H, Hirata J, Hirano A, Hirai K, Seki S, Watanabe-Akanuma M (2016). Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulfate. Am J Physiol Cell Physiol, 310:C142-150. [DOI] [PubMed] [Google Scholar]
- [71].Hamano H, Ikeda Y, Watanabe H, Horinouchi Y, Izawa-Ishizawa Y, Imanishi M, et al. (2018). The uremic toxin indoxyl sulfate interferes with iron metabolism by regulating hepcidin in chronic kidney disease. Nephrol Dial Transplant, 33:586-597. [DOI] [PubMed] [Google Scholar]
- [72].Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. (2018). Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: Synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann Intern Med, 168:422-430. [DOI] [PubMed] [Google Scholar]
- [73].Kalaska B, Pawlak K, Domaniewski T, Oksztulska-Kolanek E, Znorko B, Roszczenko A, et al. (2017). Elevated levels of peripheral kynurenine decrease bone strength in rats with chronic kidney disease. Front Physiol, 8:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu WC, Shyu JF, Lim PS, Fang TC, Lu CL, Zheng CM, et al. (2020). Concentration and duration of indoxyl sulfate exposure affects osteoclastogenesis by regulating NFATc1 via aryl hydrocarbon receptor. Int J Mol Sci, 21:3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kolachalama VB, Shashar M, Alousi F, Shivanna S, Rijal K, Belghasem ME, et al. (2018). Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol, 29:1063-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Richeldi L, Collard HR, Jones MG (2017). Idiopathic pulmonary fibrosis. Lancet, 389:1941-1952. [DOI] [PubMed] [Google Scholar]
- [77].Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. (2010). Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 181:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006). Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 174:810-816. [DOI] [PubMed] [Google Scholar]
- [79].Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med, 183:788-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mora AL, Rojas M, Pardo A, Selman M (2017). Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov, 16:755-772. [DOI] [PubMed] [Google Scholar]
- [81].Kaiser H, Parker E, Hamrick MW (2020). Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp Gerontol, 130::110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Duan Z, Li Y, Li L (2018). Promoting epithelial-to-mesenchymal transition by D-kynurenine via activating aryl hydrocarbon receptor. Mol Cell Biochem, 448:165-173. [DOI] [PubMed] [Google Scholar]
- [83].Scialo F, Sriram A, Fernandez-Ayala D, Gubina N, Lohmus M, Nelson G, et al. (2016). Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab, 23:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, et al. (2015). Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB. Toxicol Appl Pharmacol, 286:92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen J, Yakkundi P, Chan WK (2019). Down-regulation of p23 in normal lung epithelial cells reduces toxicities from exposure to benzo[a]pyrene and cigarette smoke condensate via an aryl hydrocarbon receptor-dependent mechanism. Toxicol Sci, 167:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Murray MA, Chotirmall SH (2015). The impact of immunosenescence on pulmonary disease. Mediators Inflamm, 2015:692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Miyara M, Ito Y, Sakaguchi S (2014). TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol, 10:543-551. [DOI] [PubMed] [Google Scholar]
- [88].Takei H, Yasuoka H, Yoshimoto K, Takeuchi T (2020). Aryl hydrocarbon receptor signals attenuate lung fibrosis in the bleomycin-induced mouse model for pulmonary fibrosis through increase of regulatory T cells. Arthritis Res Ther, 22:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lee SM, Park HY, Suh YS, Yoon EH, Kim J, Jang WH, et al. (2017). Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc Natl Acad Sci U S A, 114:E5881-E5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, et al. (2018). Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ, 25:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Carlson JR, Yokoyama MT, Dickinson EO (1972). Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science, 176:298-299. [DOI] [PubMed] [Google Scholar]
- [92].Weems JM, Yost GS (2010). 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol, 23:696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lee CC, Yang WH, Li CH, Cheng YW, Tsai CH, Kang JJ (2016). Ligand independent aryl hydrocarbon receptor inhibits lung cancer cell invasion by degradation of Smad4. Cancer Lett, 376:211-217. [DOI] [PubMed] [Google Scholar]
- [94].Peveler WJ, Landis RF, Yazdani M, Day JW, Modi R, Carmalt CJ, et al. (2018). A rapid and robust diagnostic for liver fibrosis using a multichannel polymer sensor array. Adv Mater, 30:e1800634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Maeso-Diaz R, Ortega-Ribera M, Lafoz E, Lozano JJ, Baiges A, Frances R, et al. (2019). Aging influences hepatic microvascular biology and liver fibrosis in advanced chronic liver disease. Aging Dis, 10:684-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. (1995). Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science, 268:722-726. [DOI] [PubMed] [Google Scholar]
- [97].Pierre S, Chevallier A, Teixeira-Clerc F, Ambolet-Camoit A, Bui LC, Bats AS, et al. (2014). Aryl hydrocarbon receptor-dependent induction of liver fibrosis by dioxin. Toxicol Sci, 137:114-124. [DOI] [PubMed] [Google Scholar]
- [98].Duval C, Teixeira-Clerc F, Leblanc AF, Touch S, Emond C, Guerre-Millo M, et al. (2017). Chronic exposure to low doses of dioxin promotes liver fibrosis development in the C57BL/6J diet-induced obesity mouse model. Environ Health Perspect, 125:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun, 4:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yan J, Tung HC, Li S, Niu Y, Garbacz WG, Lu P, et al. (2019). Aryl hydrocarbon receptor signaling prevents activation of hepatic stellate cells and liver fibrogenesis in mice. Gastroenterology, 157:793-806 e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Marciniak S, Wnorowski A, Smolinska K, Walczyna B, Turski W, Kocki T, et al. (2018). Kynurenic acid protects against thioacetamide-induced liver injury in rats. Anal Cell Pathol (Amst), 2018:1270483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. (2018). Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep, 23:1099-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Collaborators GBDA (2018). Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 392:1015-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Joshi AD, Hossain E, Elferink CJ (2017). Epigenetic regulation by agonist-specific aryl hydrocarbon receptor recruitment of metastasis-associated protein 2 selectively induces stanniocalcin 2 expression. Mol Pharmacol, 92:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Joshi AD, Carter DE, Harper TA Jr, Elferink CJ (2015). Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. J Pharmacol Exp Ther, 353:201-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rasmussen MK, Balaguer P, Ekstrand B, Daujat-Chavanieu M, Gerbal-Chaloin S (2016). Skatole (3-methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS One, 11:e0154629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoshi M, Osawa Y, Nakamoto K, Morita N, Yamamoto Y, Ando T, et al. (2020). Kynurenine produced by indoleamine 2,3-dioxygenase 2 exacerbates acute liver injury by carbon tetrachloride in mice. Toxicology, 438::152458. [DOI] [PubMed] [Google Scholar]
- [108].Li Y, Kilani RT, Rahmani-Neishaboor E, Jalili RB, Ghahary A (2014). Kynurenine increases matrix metalloproteinase-1 and -3 expression in cultured dermal fibroblasts and improves scarring in vivo. J Invest Dermatol, 134:643-650. [DOI] [PubMed] [Google Scholar]
- [109].Dolivo DM, Larson SA, Dominko T (2018). Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci, 75:3663-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schimmel K, Jung M, Foinquinos A, Jose GS, Beaumont J, Bock K, et al. (2020). Natural compound library screening identifies new molecules for the treatment of cardiac fibrosis and diastolic dysfunction. Circulation, 141:751-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, et al. (2018). Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation, 138:809-822. [DOI] [PubMed] [Google Scholar]
- [112].Carrillo-Salinas FJ, Anastasiou M, Ngwenyama N, Kaur K, Tai A, Smolgovsky SA, et al. (2020). Gut dysbiosis induced by cardiac pressure overload enhances adverse cardiac remodeling in a T cell-dependent manner. Gut Microbes, 12:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Volkova M, Palmeri M, Russell KS, Russell RR (2011). Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc Res, 90:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ichihara S, Li P, Mise N, Suzuki Y, Izuoka K, Nakajima T, et al. (2019). Ablation of aryl hydrocarbon receptor promotes angiotensin II-induced cardiac fibrosis through enhanced c-Jun/HIF-1α signaling. Arch Toxicol, 93:1543-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang Y, Wang S, Huang Y, Yang K, Liu Y, Bi X, et al. (2020). Inhibition of CYP1B1 ameliorates cardiac hypertrophy induced by uremic toxin. Mol Med Rep, 21:393-404. [DOI] [PubMed] [Google Scholar]
- [116].Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H (2010). Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J, 31:1771-1779. [DOI] [PubMed] [Google Scholar]
- [117].Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, et al. (2013). Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science, 340:867-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li CH, Liu CW, Tsai CH, Peng YJ, Yang YH, Liao PL, et al. (2017). Cytoplasmic aryl hydrocarbon receptor regulates glycogen synthase kinase 3β, accelerates vimentin degradation, and suppresses epithelial-mesenchymal transition in non-small cell lung cancer cells. Arch Toxicol, 91:2165-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N (2009). Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol, 46:518-525. [DOI] [PubMed] [Google Scholar]
- [120].Chen C, Han X, Fan F, Liu Y, Wang T, Wang J, et al. (2014). Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-β1/Smad3-mediated fibrotic responses through 5-HT(2A) receptors. Mol Cell Biochem, 397:267-276. [DOI] [PubMed] [Google Scholar]
- [121].Polter AM, Li X (2011). Glycogen synthase kinase-3 is an intermediate modulator of serotonin neurotransmission. Front Mol Neurosci, 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013). Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol, 24:771-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shaikh SB, Prabhu A, Bhandary YP (2018). Targeting anti-aging protein sirtuin (Sirt) in the diagnosis of idiopathic pulmonary fibrosis. [J] Cell Biochem. [DOI] [PubMed] [Google Scholar]
- [124].Ramirez T, Li YM, Yin S, Xu MJ, Feng D, Zhou Z, et al. (2017). Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol, 66:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tao S, Guo F, Ren Q, Liu J, Wei T, Li L, et al. (2021). Activation of aryl hydrocarbon receptor by 6-formylindolo[3,2-b]carbazole alleviated acute kidney injury by repressing inflammation and apoptosis. J Cell Mol Med, 25:1035-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Webster AC, Nagler EV, Morton RL, Masson P (2017). Chronic Kidney Disease. Lancet, 389:1238-1252. [DOI] [PubMed] [Google Scholar]
- [127].Chatzimanouil MKT, Wilkens L, Anders HJ (2019). Quantity and reporting quality of kidney research. J Am Soc Nephrol, 30:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. (1999). Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet, 354:359-364. [DOI] [PubMed] [Google Scholar]
- [129].Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, et al. (2006). Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med, 354:131-140. [DOI] [PubMed] [Google Scholar]
- [130].Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. (2001). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med, 345:861-869. [DOI] [PubMed] [Google Scholar]
- [131].Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. (2001). Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med, 345:851-860. [DOI] [PubMed] [Google Scholar]
- [132].Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. (2016). Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med, 375:323-334. [DOI] [PubMed] [Google Scholar]
- [133].Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med, 380:2295-2306. [DOI] [PubMed] [Google Scholar]
- [134].Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. (2021). Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med, 384:129-139. [DOI] [PubMed] [Google Scholar]
- [135].Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, et al. (2019). Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet, 393:1937-1947. [DOI] [PubMed] [Google Scholar]
- [136].Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. (2021). Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation, 143:540-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, et al. (2020). The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int, 97:202-212. [DOI] [PubMed] [Google Scholar]
- [138].Packer M (2020). SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: A paradigm shift in understanding their mechanism of action. Diabetes Care, 43:508-511. [DOI] [PubMed] [Google Scholar]
- [139].Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. (2020). Dapagliflozin in patients with chronic kidney disease. N Engl J Med, 383:1436-1446. [DOI] [PubMed] [Google Scholar]
- [140].Eide IA, Jenssen T, Hartmann A, Diep LM, Dahle DO, Reisaeter AV, et al. (2015). The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin J Am Soc Nephrol, 10:1246-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lim AK, Manley KJ, Roberts MA, Fraenkel MB (2016). Fish oil for kidney transplant recipients. Cochrane Database Syst Rev:CD005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tatsioni A, Chung M, Sun Y, Kupelnick B, Lichtenstein AH, Perrone R, et al. (2005). Effects of fish oil supplementation on kidney transplantation: a systematic review and meta-analysis of randomized, controlled trials. J Am Soc Nephrol, 16:2462-2470. [DOI] [PubMed] [Google Scholar]
- [143].Eide IA, Reinholt FP, Jenssen T, Hartmann A, Schmidt EB, Asberg A, et al. (2019). Effects of marine n-3 fatty acid supplementation in renal transplantation: A randomized controlled trial. Am J Transplant, 19:790-800. [DOI] [PubMed] [Google Scholar]
- [144].Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, et al. (2016). Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: The OMEGA-REMODEL randomized clinical trial. Circulation, 134:378-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hamasaki Y, Doi K, Maeda-Mamiya R, Ogasawara E, Katagiri D, Tanaka T, et al. (2013). A 5-hydroxytryptamine receptor antagonist, sarpogrelate, reduces renal tubulointerstitial fibrosis by suppressing PAI-1. Am J Physiol Renal Physiol, 305:F1796-F1803. [DOI] [PubMed] [Google Scholar]
- [146].Xu J, Pei Z, Yu M, Li X, Wang L, Lin Y, et al. (2020). Combinational use of antiplatelet medication sarpogrelate with therapeutic drug rosuvastatin in treating high-cholesterol diet-induced chronic kidney disease in ApoE-deficient mice. Biomed Res Int, 2020::1809326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ki YJ, Kwon SA, Kim HL, Seo JB, Chung WY (2019). The prevention of contrast induced nephropathy by sarpogrelate: a prospective randomized controlled clinical trial. J Korean Med Sci, 34:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. (2015). An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med, 192:e3-19. [DOI] [PubMed] [Google Scholar]
- [149].Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. (2015). Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J, 45:1434-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C (2014). Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci, 58:13-19. [DOI] [PubMed] [Google Scholar]
- [151].Flaherty KR, Fell CD, Huggins JT, Nunes H, Sussman R, Valenzuela C, et al. (2018). Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J, 52:1800230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. (2018). Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY trial. Am J Respir Crit Care Med, 197:356-363. [DOI] [PubMed] [Google Scholar]
- [153].Gagnon L, Leduc M, Thibodeau JF, Zhang MZ, Grouix B, Sarra-Bournet F, et al. (2018). A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am J Pathol, 188:1132-1148. [DOI] [PubMed] [Google Scholar]
- [154].Khalil N, Manganas H, Ryerson CJ, Shapera S, Cantin AM, Hernandez P, et al. (2019). Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur Respir J, 53:1800663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Mercer PF, Woodcock HV, Eley JD, Plate M, Sulikowski MG, Durrenberger PF, et al. (2016). Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax, 71:701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, et al. (2019). A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J, 53:1801992. [DOI] [PubMed] [Google Scholar]
- [157].Horton MR, Santopietro V, Mathew L, Horton KM, Polito AJ, Liu MC, et al. (2012). Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med, 157:398-406. [DOI] [PubMed] [Google Scholar]
- [158].Dutta P, Funston W, Mossop H, Ryan V, Jones R, Forbes R, et al. (2019). Randomised, double-blind, placebo-controlled pilot trial of omeprazole in idiopathic pulmonary fibrosis. Thorax, 74:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, et al. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology, 19:1013-1018. [DOI] [PubMed] [Google Scholar]
- [160].Richeldi L, Fernandez Perez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, et al. (2020). Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med, 8:25-33. [DOI] [PubMed] [Google Scholar]
- [161].Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, Dupont S, et al. (2018). Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med, 6:627-635. [DOI] [PubMed] [Google Scholar]
- [162].Palmer SM, Snyder L, Todd JL, Soule B, Christian R, Anstrom K, et al. (2018). Randomized, double-blind, placebo-controlled, phase 2 trial of BMS-986020, a lysophosphatidic acid receptor antagonist for the treatment of idiopathic pulmonary fibrosis. Chest, 154:1061-1069. [DOI] [PubMed] [Google Scholar]
- [163].Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, et al. (2018). Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: A randomized clinical trial. JAMA, 319:2299-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Raghu G, Scholand MB, de Andrade J, Lancaster L, Mageto Y, Goldin J, et al. (2016). FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J, 47:1481-1491. [DOI] [PubMed] [Google Scholar]
- [165].Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. (2011). Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med, 365:1079-1087. [DOI] [PubMed] [Google Scholar]
- [166].King TE Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. (2011). BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 184:92-99. [DOI] [PubMed] [Google Scholar]
- [167].King TE Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. (2009). Effect of interferon γ-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): A multicentre, randomised, placebo-controlled trial. Lancet, 374:222-228. [DOI] [PubMed] [Google Scholar]
- [168].Raghu G, Richeldi L, Crestani B, Wung P, Bejuit R, Esperet C, et al. (2018). SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772). Eur Respir J, 52:1801130. [DOI] [PubMed] [Google Scholar]
- [169].Parker JM, Glaspole IN, Lancaster LH, Haddad TJ, She D, Roseti SL, et al. (2018). A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 197:94-103. [DOI] [PubMed] [Google Scholar]
- [170].Rosas IO, Goldberg HJ, Collard HR, El-Chemaly S, Flaherty K, Hunninghake GM, et al. (2018). A phase II clinical trial of low-dose inhaled carbon monoxide in idiopathic pulmonary fibrosis. Chest, 153:94-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, et al. (2017). Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med, 5:22-32. [DOI] [PubMed] [Google Scholar]
- [172].Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, et al. (2015). CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J, 46:1740-1750. [DOI] [PubMed] [Google Scholar]
- [173].Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J, Group MS (2013). Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J, 42:1622-1632. [DOI] [PubMed] [Google Scholar]
- [174].Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR, et al. (2010). Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med, 181:604-610. [DOI] [PubMed] [Google Scholar]
- [175].Kondoh Y, Azuma A, Inoue Y, Ogura T, Sakamoto S, Tsushima K, et al. (2020). Thrombomodulin α for acute exacerbation of idiopathic pulmonary fibrosis. A randomized, double-blind placebo-controlled trial. Am J Respir Crit Care Med, 201:1110-1119. [DOI] [PubMed] [Google Scholar]
- [176].Idiopathic Pulmonary Fibrosis Clinical Research N, Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, et al. (2010). A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med, 363:620-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Behr J, Kolb M, Song JW, Luppi F, Schinzel B, Stowasser S, et al. (2019). Nintedanib and sildenafil in patients with idiopathic pulmonary fibrosis and right heart dysfunction. A prespecified subgroup analysis of a double-blind randomized clinical trial (INSTAGE). Am J Respir Crit Care Med, 200:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, et al. (2018). Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med, 379:1722-1731. [DOI] [PubMed] [Google Scholar]
- [179].Behr J, Nathan SD, Wuyts WA, Mogulkoc Bishop N, Bouros DE, Antoniou K, et al. (2021). Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med, 9:85-95. [DOI] [PubMed] [Google Scholar]
- [180].Maher TM, Costabel U, Glassberg MK, Kondoh Y, Ogura T, Scholand MB, et al. (2021). Phase 2 trial to assess lebrikizumab in patients with idiopathic pulmonary fibrosis. Eur Respir J, 57:1902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, et al. (2013). Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med, 158:641-649. [DOI] [PubMed] [Google Scholar]
- [182].Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, et al. (2012). A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 186:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Idiopathic Pulmonary Fibrosis Clinical Research N, Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G (2014). Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med, 370:2093-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Okuda R, Matsushima H, Oba T, Kawabe R, Matsubayashi M, Amano M, et al. (2016). Efficacy and safety of inhaled N-acetylcysteine in idiopathic pulmonary fibrosis: A prospective, single-arm study. Respir Investig, 54:156-161. [DOI] [PubMed] [Google Scholar]
- [185].Sakamoto S, Kataoka K, Kondo Y, Kato M, Okamoto M, Mukae H, et al. (2021). Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomised trial. Eur Respir J, 57:2000348. [DOI] [PubMed] [Google Scholar]
- [186].Behr J, Bendstrup E, Crestani B, Gunther A, Olschewski H, Skold CM, et al. (2016). Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med, 4:445-453. [DOI] [PubMed] [Google Scholar]
- [187].Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. (2018). Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol, 15:11-20. [DOI] [PubMed] [Google Scholar]
- [188].Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. (2016). Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology, 150:1147-1159 e1145. [DOI] [PubMed] [Google Scholar]
- [189].Loomba R, Kayali Z, Noureddin M, Ruane P, Lawitz EJ, Bennett M, et al. (2018). GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology, 155:1463-1473 e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A (2014). Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res, 34:837-843. [DOI] [PubMed] [Google Scholar]
- [191].Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. (2020). Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology, 158:1597-1610 e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, et al. (2016). Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther, 44:1183-1198. [DOI] [PubMed] [Google Scholar]
- [193].Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. (2020). Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology, 158:1334-1345 e1335. [DOI] [PubMed] [Google Scholar]
- [194].Harrison SA, Dennis A, Fiore MM, Kelly MD, Kelly CJ, Paredes AH, et al. (2018). Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One, 13:e0203054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Diehl AM, Day C (2017). Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med, 377:2063-2072. [DOI] [PubMed] [Google Scholar]
- [196].Leite NC, Viegas BB, Villela-Nogueira CA, Carlos FO, Cardoso CRL, Salles GF (2019). Efficacy of diacerein in reducing liver steatosis and fibrosis in patients with type 2 diabetes and non-alcoholic fatty liver disease: A randomized, placebo-controlled trial. Diabetes Obes Metab, 21:1266-1270. [DOI] [PubMed] [Google Scholar]
- [197].Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. (2019). Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab, 21:285-292. [DOI] [PubMed] [Google Scholar]
- [198].Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, et al. (2012). Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int, 32:977-987. [DOI] [PubMed] [Google Scholar]
- [199].Mao YM, Zeng MD, Lu LG, Wan MB, Li CZ, Chen CW, et al. (2004). Capsule oxymatrine in treatment of hepatic fibrosis due to chronic viral hepatitis: a randomized, double blind, placebo-controlled, multicenter clinical study. World J Gastroenterol, 10:3269-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Weng HL, Wang BE, Jia JD, Wu WF, Xian JZ, Mertens PR, et al. (2005). Effect of interferon-γ on hepatic fibrosis in chronic hepatitis B virus infection: A randomized controlled study. Clin Gastroenterol Hepatol, 3:819-828. [DOI] [PubMed] [Google Scholar]
- [201].Chapplain JM, Bellissant E, Guyader D, Molina JM, Poizot-Martin I, Perre P, et al. (2013). The effects of a maintenance therapy with peg-interferon α-2a on liver fibrosis in HIV/HCV co-infected patients: A randomized controlled trial. J Infect, 67:313-321. [DOI] [PubMed] [Google Scholar]
- [202].Kim SG, Kim YM, Choi JY, Han JY, Jang JW, Cho SH, et al. (2011). Oltipraz therapy in patients with liver fibrosis or cirrhosis: A randomized, double-blind, placebo-controlled phase II trial. J Pharm Pharmacol, 63:627-635. [DOI] [PubMed] [Google Scholar]
- [203].Meissner EG, McLaughlin M, Matthews L, Gharib AM, Wood BJ, Levy E, et al. (2016). Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial. Liver Int, 36:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Rendina M, D'Amato M, Castellaneta A, Castellaneta NM, Brambilla N, Giacovelli G, et al. (2014). Antiviral activity and safety profile of silibinin in HCV patients with advanced fibrosis after liver transplantation: A randomized clinical trial. Transpl Int, 27:696-704. [DOI] [PubMed] [Google Scholar]
- [205].Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. (1991). Natural history and prognostic factors for chronic hepatitis type B. Gut, 32:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hou JL, Xu D, Shi G, Wan M, Goodman Z, Tan D, et al. (2015). Long-term telbivudine treatment results in resolution of liver inflammation and fibrosis in patients with chronic hepatitis B. Adv Ther, 32:727-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Murphy SP, Ibrahim NE, Januzzi JL Jr, (2020). Heart failure with reduced ejection fraction: A review. JAMA, 324:488-504. [DOI] [PubMed] [Google Scholar]
- [208].Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. (2020). Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med, 383:1413-1424. [DOI] [PubMed] [Google Scholar]
- [209].Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, et al. (2020). Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: Insights from DAPA-HF. Circulation, 141:100-111. [DOI] [PubMed] [Google Scholar]
- [210].Oikonomou E, Vogiatzi G, Karlis D, Siasos G, Chrysohoou C, Zografos T, et al. (2019). Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin Nutr, 38:1188-1197. [DOI] [PubMed] [Google Scholar]
- [211].Oatmen KE, Cull E, Spinale FG (2020). Heart failure as interstitial cancer: emergence of a malignant fibroblast phenotype. Nat Rev Cardiol, 17:523-531. [DOI] [PubMed] [Google Scholar]
- [212].Brilla CG, Janicki JS, Weber KT (1991). Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation, 83:1771-1779. [DOI] [PubMed] [Google Scholar]
- [213].Brilla CG, Matsubara L, Weber KT (1996). Advanced hypertensive heart disease in spontaneously hypertensive rats. Lisinopril-mediated regression of myocardial fibrosis. Hypertension, 28:269-275. [DOI] [PubMed] [Google Scholar]
- [214].Brilla CG, Funck RC, Rupp H (2000). Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation, 102:1388-1393. [DOI] [PubMed] [Google Scholar]
- [215].Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J (2004). Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol, 43:2028-2035. [DOI] [PubMed] [Google Scholar]
- [216].Group TI (2011). Effects of prolonged-release torasemide versus furosemide on myocardial fibrosis in hypertensive patients with chronic heart failure: a randomized, blinded-end point, active-controlled study. Clin Ther, 33:1204-1213 e1203. [DOI] [PubMed] [Google Scholar]
- [217].Lenihan D, Carver J, Porter C, Liu JE, Dent S, Thavendiranathan P, et al. (2020). Cardio-oncology care in the era of the coronavirus disease 2019 (COVID-19) pandemic: An International Cardio-Oncology Society (ICOS) statement. CA Cancer J Clin, 70:480-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. (2021). SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med, 384:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Zheng J, Wong LR, Li K, Verma AK, Ortiz M, Wohlford-Lenane C, et al. (2021). COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature, 589:603-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Taz TA, Ahmed K, Paul BK, Kawsar M, Aktar N, Mahmud SMH, et al. (2021). Network-based identification genetic effect of SARS-CoV-2 infections to Idiopathic pulmonary fibrosis (IPF) patients. Brief Bioinform, 22:1254-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Hayek SS, Brenner SK, Azam TU, Shadid HR, Anderson E, Berlin H, et al. (2020). In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ, 371::m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. (2021). Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol, 74:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. (2020). Extrapulmonary manifestations of COVID-19. Nat Med, 26:1017-1032. [DOI] [PubMed] [Google Scholar]
- [224].Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. (2020). Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ, 369::m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, et al. (2020). A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab, 32:878- 888.e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Wang X, Yang S, Li S, Zhao L, Hao Y, Qin J, et al. (2020). Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut, 69:2131-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Tang WHW, Li DY, Hazen SL (2019). Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol, 16:137-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Dayama G, Priya S, Niccum DE, Khoruts A, Blekhman R (2020). Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med, 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wang M, Hu HH, Chen YY, Chen L, Wu XQ, Zhao YY (2020). Novel poricoic acids attenuate renal fibrosis through regulating redox signalling and aryl hydrocarbon receptor activation. Phytomedicine, 79::153323. [DOI] [PubMed] [Google Scholar]
- [230].Ye M, Xu M, Fan S, Zhang M, Zhou B, Yang S, et al. (2020). Protective effects of three propolis-abundant flavonoids against ethanol-induced injuries in HepG2 cells involving the inhibition of ERK1/2-AHR-CYP1A1 signaling pathways. J Funct Foods, 73. [Google Scholar]
- [231].Huang S, Chen P, Shui X, He Y, Wang H, Zheng J, et al. (2014). Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J Pharm Pharmacol, 66:1469-1477. [DOI] [PubMed] [Google Scholar]