Abstract
IMPORTANCE:
Myeloperoxidase (MPO)-DNA complexes, biomarkers of neutrophil extracellular traps (NETs), have been associated with arterial and venous thrombosis. Their role in aneurysmal subarachnoid hemorrhage (aSAH) is unknown.
OBJECTIVES:
To assess whether serum MPO-DNA complexes are present in patients with aSAH and whether they are associated with delayed cerebral ischemia (DCI).
DESIGN, SETTING, AND PARTICIPANTS:
Post-hoc analysis of a prospective, observational single-center study, with de novo serum biomarker measurements in consecutive patients with aSAH between July 2018 and September 2020, admitted to a tertiary care neuroscience ICU.
MAIN OUTCOMES AND MEASURES:
We analyzed serum obtained at admission and hospital day 4 for concentrations of MPO-DNA complexes. The primary outcome was DCI, defined as new infarction on brain CT. The secondary outcome was clinical vasospasm, a composite of clinical and transcranial Doppler parameters. We used Wilcoxon signed-rank-test to assess for differences between paired measures.
RESULTS:
Among 100 patients with spontaneous subarachnoid hemorrhage, mean age 59 years (sd ± 13 yr), 55% women, 78 had confirmed aSAH. Among these, 29 (37%) developed DCI. MPO-DNA complexes were detected in all samples. The median MPO-DNA level was 33 ng/mL (interquartile range [IQR], 18–43 ng/mL) at admission, and 22 ng/mL (IQR, 11–31 ng/mL) on day 4 (unpaired test; p = 0.015). We found a significant reduction in MPO-DNA levels from admission to day 4 in patients with DCI (paired test; p = 0.036) but not in those without DCI (p = 0.17). There was a similar reduction in MPO-DNA levels between admission and day 4 in patients with (p = 0.006) but not in those without clinical vasospasm (p = 0.47).
CONCLUSIONS AND RELEVANCE:
This is the first study to detect the NET biomarkers MPO-DNA complexes in peripheral serum of patients with aSAH and to associate them with DCI. A pronounced reduction in MPO-DNA levels might serve as an early marker of DCI. This diagnostic potential of MPO-DNA complexes and their role as potential therapeutic targets in aSAH should be explored further.
Keywords: extracellular traps, intracranial aneurysm, ischemic stroke, neutrophils, subarachnoid hemorrhage
Aneurysmal subarachnoid hemorrhage (aSAH) is a severe form of hemorrhagic stroke, associated with 18% inhospital mortality, and about 30% poor long-term outcome (1–3). In about one third of cases, the hospitalization is complicated by delayed cerebral ischemia (DCI), ischemic infarcts, identified on brain imaging (4). Most DCI occurs 4 to 14 days after the initial hemorrhage and is strongly associated with poor outcome (5, 6). However, effective prediction and prevention measures of DCI are lacking (6). While DCI development might be preceded by clinical deterioration, angiographic vasospasm, increase in transcranial Doppler (TCD) flow velocities, or electroencephalographic changes, DCI often occurs silently and is mostly identified in retrospect (7–10). Finding markers of impending DCI could lead to a paradigm shift in the diagnosis and possibly in the prevention and treatment of this severe complication of subarachnoid hemorrhage (SAH) (11).
Neutrophil extracellular traps (NETs) have been described as a central mechanism of arterial and venous thrombus formation (12). Since their definition in 2004 as a neutrophil innate immune mechanism (13), they have also been detrimentally associated with ischemic stroke, deep vein thrombosis, as well as immunothrombosis driven by COVID-19 (14–16). NETs are the product of neutrophil hyperactivation leading to the release of extracellular “net-like” structures consisting of chromatin lined with neutrophil proteins. When released into the blood, they facilitate thrombus formation by promoting coagulation and binding platelets and other blood components. NETs have been visualized using electron microscopy (13), and components of NETs can be detected in plasma of patients with acute thrombotic processes (13). Furthermore, NETs can be therapeutically targeted by degradation with DNAses, as demonstrated in animal studies (17–19) and ex vivo thrombolysis experiments (14, 20). While an inflammatory surge and an increased neutrophil-lymphocyte ratio have been linked to vasospasm progression, DCI, and outcome after aSAH, only limited data is available on the detection of NETs in patients with SAH (21–24).
In this exploratory study, we focused on one major NET biomarker, myeloperoxidase (MPO)-DNA complexes. We leveraged prospective data from a cohort of patients with SAH undergoing prospective neurologic and TCD examinations, as well as standardized DCI assessment. We aimed to test two hypotheses: 1) MPO-DNA complexes are present in peripheral serum of patients with aSAH and 2) changes in MPO-DNA complex levels between admission and hospital day 4 are associated with the occurrence of DCI.
MATERIALS AND METHODS
Study Design and Population
We conducted a post hoc analysis of the high mobility group box 1 in aneurysmal subarachnoid hemorrhage (HIMOBASH) study, a prospective, blinded, single tertiary care center biomarker observational study (25). For the HIMOBASH study, consecutive patients with spontaneous SAH, 18 years old or older, admitted to the neurosurgical ICU at one tertiary care center, were evaluated for study eligibility. The diagnosis of SAH was established through an admission brain CT. An aneurysmal etiology of SAH was confirmed through CT angiography or digital subtraction angiography (DSA). If the DSA was negative for an aneurysmal bleeding source, patients underwent additional brain MRI and repeat-DSA after 7–10 days to rule out other pathologies and establish the diagnosis of nonaneurysmal SAH.
Clinical Management
Patients were treated according to current guidelines including monitoring in a dedicated neurointensive care unit, aneurysm treatment via clipping or coiling no later than 24 hours from admission, treatment of hydrocephalus via insertion of an external ventricular drain, and oral nimodipine administration. Bedside TCD studies were performed bid and additionally as required to corroborate suspected vasospasm (25). If a delayed neurologic deficit (DND) was identified (as defined in the Outcomes paragraph), hyperdynamic therapy was initiated, avoiding hypovolemia and increasing the mean arterial blood pressure levels using vasopressors. If vasospasm was suspected, decisions on additional imaging or neurointerventional rescue therapies were made on a case-by-case basis after reaching interdisciplinary consensus.
Outcomes
Delayed Cerebral Ischemia.
DCI was defined as a new ischemic infarct on brain imaging that was not present on the routine post-clipping/-coiling CT brain 1 day after aneurysm treatment (4). Patients who died before another follow-up CT scan was obtained, were deemed ineligible for DCI assessment, and were thus excluded from this study.
Clinical Vasospasm.
Clinical vasospasm was defined as a composite outcome consisting of at least one of the two following: 1) the diagnosis of DND and 2) abnormal TCD findings.
Delayed Neurologic Deficit.
The definition of DND followed the consensus statement of the Neurocritical Care Society (26). Glasgow Coma Scale (GCS) scores were assessed hourly for each patient. DND was diagnosed if a reduction of greater than or equal to 2 on the GCS occurred or if a new focal neurologic deficit was identified while other potential causes were eliminated.
Transcranial Doppler Studies.
Bedside TCD were conducted at least bid to diagnose suspected vasospasm. The following TCD criteria were considered indicative of potential vasospasm: a middle cerebral artery (MCA) peak systolic velocity (PSV) of greater than or equal to 120 cm/s that was not attributable to generalized hyperperfusion, a PSV increase of greater than or equal to 50 cm/s compared with the admission TCD examination, or a Lindegaard ratio (mean flow velocity in the MCA divided by mean flow velocity in the ipsilateral extracranial internal carotid artery) of greater than or equal to 3.
Isolated angiographic vasospasm was a priori excluded as an endpoint for the purpose of this study for its poor correlation with DCI (25).
Serum Collection and Laboratory Measurements
For laboratory analysis of MPO-DNA complex concentrations, we used serum samples collected at admission (day 0) and on day 4, as previously described, which were frozen and thawed immediately before batch laboratory analysis (25, 27). Anti-MPO polyclonal antibody (Invitrogen PA5-16672, RRID AB_11006367, 1:1,000 dilution) was coated overnight on a 96-well MediSORP immunoassay plate (ThermoFisher, Waltham, MA) in buffer consisting of 0.05 M sodium carbonate/sodium bicarbonate buffer (pH 9.6). After four washes with 0.05% phosphate buffered saline containing Tween-20, wells were blocked with 2% low-endotoxin bovine serum albumin (Carl Roth, Karlsruhe, Germany) for 2 hours and subsequently washed another four times. Samples were diluted 1:2 in incubation buffer from the Cell Death Detection Enzyme-Linked Immunosorbent Assay (ELISA) kit (11544675001; Roche, Basel, Switzerland), added to the plate described above, incubated for 90 minutes at room temperature, then washed four times before addition of anti-double-stranded DNA (ds-DNA)-peroxidase antibody from the Cell Death Detection ELISA diluted 1:40 in incubation buffer from the same kit (Roche). Wells were washed another four times before development with ready-to-use tetramethylbenzidine substrate (Life Technologies, Carlsbad, CA). The reaction was stopped with 1N hydrochloric acid, and the absorbance measured at 450 nM with 630 nM background subtraction using a Gen5 microplate reader (BioTek, Santa Clara, CA). A standard concentration curve was prepared from a known amount of MPO standard from the BioLegend Human Myeloperoxidase LEGEND MAX ELISA kit (BioLegend, San Diego, CA) preincubated with an excess of lambda DNA Invitrogen (ThermoFisher) and human native nucleosomes (Merck Millipore, Merck KGaA, Darmstadt, Germany), run over a range from 10 ng/mL down to 0.15 ng/mL. All samples measured fell within the limit of quantification based on this standard curve.
Statistical Analysis
Data were reported as counts (percentage) or median (interquartile range [IQR]). Intergroup differences of baseline characteristics were assessed using the Mann-Whitney U test for all continuous variables except for age, which given its normal distribution was assessed using the Student t test. Intergroup differences between categorical variables were assessed using the chi-square test. Differences in laboratory marker serum concentrations between admission and hospital day 4, pooling results of the entire cohort (unpaired analysis), were assessed using the Mann-Whitney U test, and for paired laboratory markers for each individual participant (paired analysis) using the Wilcoxon signed-rank test. Cohen’s criteria were used to calculate effect sizes. We performed statistical analyses using SPSS, version 28 (IBM, Armonk, NY). The threshold for statistical significance was set at less than 0.05.
Standard Protocol Approvals, Registrations, and Patients Consents
The study was approved by the Saarland Medical Association ethics committee (Number 118/17). Written informed consent was obtained from all participants or their legal representatives.
RESULTS
Among 100 enrolled patients, 83 had a confirmed aneurysmal SAH, five of which died before follow-up CT scan. In the analytical cohort (n = 78), there were 43 (55%) women, and the mean age was 59 years (± 13.0 yr) (Table 1). The median World Federation of Neurosurgeons Scale (WFNS) at admission was 2 (IQR, 1–4), and the median modified Fisher scale (mFS) score at admission was 4 (IQR 3–4). Twenty-nine patients (37%) developed DCI. Those who later developed DCI compared with those who did not tended to have a higher mFS score (median 4 vs 3; p = 0.03) and a numerically greater disease severity, as illustrated by higher scores on the WFNS (median 3 vs 2; p = 0.09) and the Hunt & Hess scale (median 3 vs 2; p = 0.06).
TABLE 1.
Baseline Characteristics of Patients With Aneurysmal Subarachnoid Hemorrhage, Stratified by Presence/Absence of Delayed Cerebral Ischemia
| Demographics and Medical History | All Patients (n = 78) | DCI (n = 29) | No DCI (n = 49) | p |
|---|---|---|---|---|
| Mean age (sd), yr | 59.0 (13.0) | 59.7 (12.6) | 58.6 (13.3) | 0.73 |
| Female | 43 (55.1) | 16 (55.2) | 25 (51.0) | 1.0 |
| Hypertension | 41 (52.6) | 16 (55.2) | 25 (51.0) | 0.72 |
| Smoking | 38 (48.7) | 15 (51.7) | 23 (46.9) | 0.68 |
| Coronary artery disease | 9 (11.5) | 4 (13.8) | 5 (10.2) | 0.72 |
| Clinical data | ||||
| WFNS score on admissiona | 2 (1–4.25) | 3 (1–5) | 2 (1–4) | 0.09 |
| WFNS III–V | 29 (37.2) | 15 (51.7) | 19 (38.8) | 0.27 |
| Hunt & Hess score on admissiona | 3 (2–4) | 3 (2–5) | 2 (2–4) | 0.06 |
| Hunt & Hess IV–V | 25 (37.2) | 12 (41.4) | 13 (26.5) | 0.17 |
| Modified Fisher score on admissiona | 4 (3–4) | 4 (3–4) | 3 (3–4) | 0.03 |
| Modified Fisher III–V | 65 (83.3) | 27 (93.1) | 38 (77.6) | 0.12 |
| Intraventricular hemorrhage at admission CT | 50 (64.1) | 22 (75.9) | 28 (57.1) | 0.10 |
| Intracerebral hemorrhage at admission CT | 15 (19.2) | 6 (20.7) | 9 (18.4) | 0.80 |
| Hydrocephalus at admission CT | 54 (69.2) | 23 (79.3) | 31 (63.3) | 0.14 |
| Generalized cerebral edema at admission CT | 5 (6.4) | 3 (10.3) | 2 (4.1) | 0.36 |
| Clinical vasospasm | 29 (37.2) | 17 (58.6) | 12 (24.5) | 0.003 |
| Nosocomial infection | 53 (67.9) | 19 (65.5) | 34 (69.4) | 0.72 |
| Admission WBC (×103/μL)a | 12.4 (9.6–15.9) | 12.8 (10.7–16.3) | 11.6 (9.4–15.8) | 0.36 |
DCI = delayed cerebral ischemia, WFNS = World Federation of Neurosurgeons Scale.
aValues presented as median (interquartile range).
p < 0.05 was considered significant.
Detection of Serum MPO-DNA Complexes
MPO-DNA complexes were detected in all admission and day-4 serum samples. In the pooled unpaired analysis, there was a significant decrease in MPO-DNA levels between admission (median, 33 ng/mL; IQR, 18–43 ng/mL) and day 4 (median, 22 ng/mL; IQR, 11–31 ng/mL) (Mann-Whitney U test, p = 0.02). The reduction in MPO-DNA complex levels coincided with a reduction in WBC from admission day to day 4 (median 12.4 vs 9.7 × 103/μL, Mann-Whitney U test, p < 0.001) and an increase in C-reactive protein concentrations (median 2.4, IQR, 1.3–8.3 vs median 174, IQR, 87–255, Mann-Whitney U test, p < 0.001).
Primary Analysis: MPO-DNA Complexes in Patients With and Without DCI
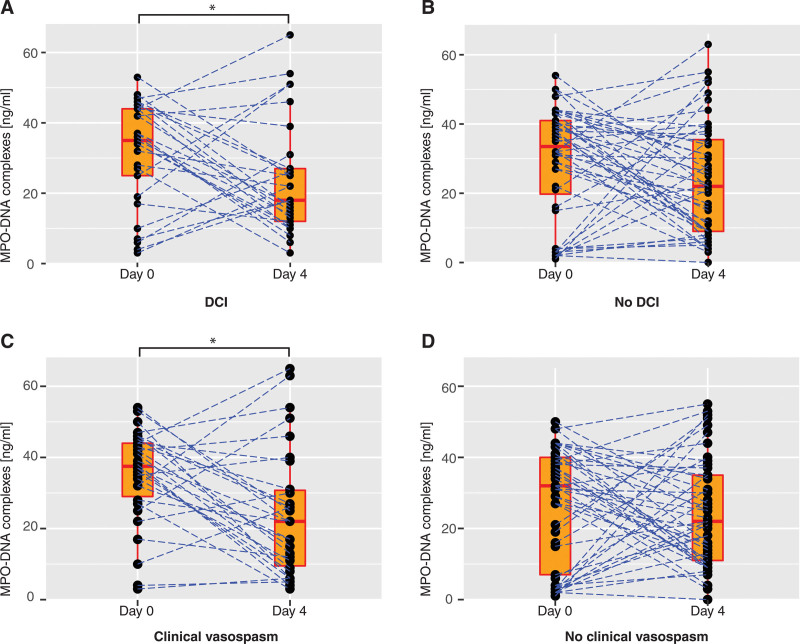
Admission MPO-DNA levels were similar between those with DCI and those without DCI (median, 35 ng/mL, IQR, 22–44 ng/mL vs median 34 ng/mL, IQR, 17–41 ng/mL, p = 0.6) and those with clinical vasospasm and those without clinical vasospasm (median 38 ng/mL, IQR, 28–44 ng/mL vs median 32 ng/mL, IQR, 7–40 ng/mL, p = 0.5). In a paired data analysis, there was a significant reduction in MPO-DNA levels between admission and day 4 in patients with DCI (z = –2.095, Wilcoxon signed-rank test: p = 0.04, r = 0.3, medium effect size) but not in those without DCI (Wilcoxon signed-rank test: p = 0.17) (Fig. 1). Like in the unpaired analysis, there was a significant reduction in WBC in both groups in the paired data analysis (DCI group p = 0.007, no DCI group p < 0.001) as well as a significant increase in C-reactive protein (DCI group p < 0.001, no DCI group p < 0.001) between admission and day 4, respectively.
Figure 1.
Change in serum myeloperoxidase-(MPO) DNA complex levels between admission and hospital day 4. Paired data points in the cohort stratified by delayed cerebral ischemia (DCI) (present in n = 29, absent in n = 49, A and B) and stratified by clinical vasospasm (present in n = 29, absent in n = 52, C and D). Admission is defined as day 0. Boxes represent median, interquartile range, and ranges. Dotted blue lines connect paired data points for each participant. Statistical significance (Wilcoxon signed-rank test) is indicated by an asterisk.
Secondary Analysis: MPO-DNA Complexes in Patients With and Without Clinical Vasospasm
When stratifying the cohort by presence or absence of clinical vasospasm, we found a significant reduction in MPO-DNA levels between admission and day 4 in patients with clinical vasospasm (paired analysis, z = –2.757, p = 0.006, r = 0.4, medium effect size), but not in those without clinical vasospasm (p = 0.5) (Fig. 1).
DISCUSSION
NETs, the product of hyperactivated neutrophils, promote immune-mediated thrombus formation. In this exploratory study in patients with aSAH, we found high levels of the NET marker MPO-DNA complexes in all peripheral serum samples and a significant reduction in MPO-DNA complex levels in patients who developed DCI. Our study suggests that NETs might be of relevance in aSAH, possibly contributing to DCI, a severe ischemic brain complication, strongly associated with poor outcome.
A prior study aimed to detect NET markers in mice and in patients with SAH (23). This study used ds-DNA as a surrogate for the presence of NETs. However, ds-DNA has been shown to be an unspecific remnant of cell death (28). MPO-DNA complexes, in contrast, are established measures of NET release (18). Three further studies—in humans, rats, and rabbits, respectively—assessed MPO (not MPO-DNA complex) serum levels in SAH (29–31). Secreted MPO levels are indicative of neutrophil activation, including by degranulation, in addition to NET release, which limits the utility of MPO levels in quantifying NETs. A recent study reported that blocking NET-mediated inflammation does not affect neutrophil recruitment suggesting that NETs are not merely a byproduct of enhanced neutrophilic inflammation (32). Our study adds a novel element to the existing literature, demonstrating the detection of a specific NET biomarker in a prospective cohort of patients with aSAH, as well as its potential implication in the development of DCI.
Among pathophysiologic correlates of DCI, prior studies have investigated vascular dysfunction (leading to vasoconstriction-induced hypoperfusion) and spreading depolarization (33–35). In addition, a systemic inflammatory response after SAH, including an increase in the circulating neutrophil-lymphocyte ratio, has been consistently demonstrated across many studies and is associated with DCI and poor outcome (21, 22, 36, 37). Disruption of the blood-brain barrier with influx of cell-free heme into the subarachnoid space may stimulate local macrophages and the heme scavenger system, leading to further recruitment of other immune cells and excessive inflammation (6, 38). Direct activation of neutrophils through free heme is conceivable as well, with free heme being a driving factor for NET release in sickle cell disease (39, 40). There is also data to suggest that free heme itself may lead to endothelial injury, which in turn may promote recruitment of macrophages and neutrophils (41). As a downstream mechanism, the concept of NET-induced thrombus formation is intriguing, as it provides a framework that links inflammation, thrombus formation, and DCI (6, 40). NETs have been therapeutically targeted in animal models and human pulmonary disease such as COVID-19, which might inform future studies assessing new treatments in patients with aSAH (42–44).
We found high levels of MPO-DNA complexes in all patients with aSAH, not only in those with DCI which contradicts a previous Chinese animal model study that found higher NET levels in those with greater SAH severity (45). While lower levels of MPO-DNA complexes have been measured in the general population (46), the high levels in our study may reflect an overall inflammatory environment after aSAH, in line with high levels of C-reactive protein in all our patients.
A speculative explanation for the decline in MPO-DNA complex levels, seen predominantly in patients with DCI, may be NET-driven thrombus formation that binds MPO-DNA complexes to thrombus material and removes it from the serum where it can be measured. It is worth noting that using the clinical consensus definition of DCI (which requires diagnosis of an infarct on brain imaging) might be too crude of a parameter to accurately distinguish between patients with and without NET-driven thrombosis, as microthrombosis might not always lead to macroscopic infarcts on CT. This might explain why patients with declining MPO-DNA levels were seen in both the DCI and the no DCI groups. Similarly, in both groups, there were patients who had increasing NET levels between admission and day 4. Further investigations into factors that influence serum MPO-DNA levels are needed before considering them as biomarkers potentially indicating DCI. Furthermore, in future studies, it may be worth acquiring MRI to detect DCI and other ischemic lesions for its superior spatial resolution and sensitivity compared with CT. Differences in serum NET levels might be more pronounced when comparing patients with any ischemic MRI lesions with those without any ischemic MRI lesions (4, 47). Demonstrating internal consistency, our results were similar when we stratified the cohort by presence or absence of clinical vasospasm, a more inclusive composite endpoint. This likely reflects previously described associations between DND and DCI on the one hand and vasospasm and DCI on the other. Our study might serve as a first step toward an early detection assay to identify patients who develop DCI. Toward this end, future studies should consider using an endpoint of an MRI-based definition of delayed brain ischemia and pairing NET measurements from serum with those from plasma and from cerebrospinal fluid. Apart from a potential diagnostic value in DCI detection, NETs might serve as therapeutic targets. DNase or janus kinase inhibitors in humans and neonatal NET inhibitory factor in mice have shown promise to reduce NET formation and NET-mediated ischemic injury (32, 48).
Our study has limitations. First, we measured MPO-DNA complex levels in serum. In contrast to plasma, serum preparations may result in the release of NETs during the coagulation phase and may thus artificially increase NET levels, a confounding factor that, however, should not systematically vary between serum samples and patients since serum collection, storage, and analysis were highly standardized in our study. Second, due to the exploratory character of this study, we refrained from analyzing NET markers other than MPO-DNA complexes. Third, we did not analyze samples beyond hospital day 4. Since DCI typically occurs between post-SAH days 4 to 14, it is possible that in those patients with DCI, further reductions in MPO-DNA complex levels might have been appreciated at later time points. Fourth, we did not compare baseline MPO-DNA levels in our patient cohort with healthy controls, which would help to quantify NET-mediated inflammation in patients with SAH. Last, because of the exploratory nature of our study, we were unable to systematically adjust for other variables associated with DCI. Future studies should ideally use paired NET measurements of plasma and serum samples with matching neutrophil counts and include measurements of further NET biomarkers with additional mechanistic insight, for example, citrullinated histones and peptidylarginine deiminase 4.
CONCLUSIONS
MPO-DNA complexes, a component of NETs, are detectable in the peripheral serum of patients with aSAH. MPO-DNA complex levels undergo a marked reduction in those with DCI over the first days of hospitalization, suggesting a potential role of NETs in the pathophysiology of DCI.
Footnotes
Drs. Witsch and Hendrix shared the corresponding authorship.
Intramural research grants from the Homburger Forschungsförderung (to Drs. Hendrix and Hemmer) and the Dr. Theiss Research Award (to Dr. Hendrix).
Dr. Martinod has received consulting fees from PEEL Therapeutics. She is an inventor on U.S. patent application 62/594,266 for the method of inhibition of JAK-STAT signaling to prevent neutrophil extracellular trap formation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Muehlschlegel S: Subarachnoid hemorrhage. Continuum (Minneap Minn) 2018; 24:1623–1657 [DOI] [PubMed] [Google Scholar]
- 2.Witsch J, Frey HP, Patel S, et al. : Prognostication of long-term outcomes after subarachnoid hemorrhage: The FRESH score. Ann Neurol 2016; 80:46–58 [DOI] [PubMed] [Google Scholar]
- 3.Witsch J, Kuohn L, Hebert R, et al. : Early prognostication of 1-year outcome after subarachnoid hemorrhage: The FRESH score validation. J Stroke Cerebrovasc Dis 2019; 28:104280. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwen MD, Vermeulen M, van Gijn J, et al. : Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010; 41:2391–2395 [DOI] [PubMed] [Google Scholar]
- 5.Al-Mufti F, Roh D, Lahiri S, et al. : Ultra-early angiographic vasospasm associated with delayed cerebral ischemia and infarction following aneurysmal subarachnoid hemorrhage. J Neurosurg 2017; 126:1545–1551 [DOI] [PubMed] [Google Scholar]
- 6.Dodd WS, Laurent D, Dumont AS, et al. : Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: A review. J Am Heart Assoc 2021; 10:e021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarrafzadeh AS, Vajkoczy P, Bijlenga P, et al. : Monitoring in neurointensive care - the challenge to detect delayed cerebral ischemia in high-grade aneurysmal SAH. Front Neurol 2014; 5:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megjhani M, Terilli K, Weiss M, et al. : Dynamic detection of delayed cerebral ischemia: A study in 3 centers. Stroke 2021; 52:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HY, Elmer J, Zafar SF, et al. : Combining transcranial Doppler and EEG data to predict delayed cerebral ischemia after subarachnoid hemorrhage. Neurology 2022; 98:e459–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lissak IA, Locascio JJ, Zafar SF, et al. : Electroencephalography, hospital complications, and longitudinal outcomes after subarachnoid hemorrhage. Neurocrit Care 2021; 35:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabbarli R, Pierscianek D, Darkwah Oppong M, et al. : Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: A systematic review. Neurosurg Rev 2020; 43:825–833 [DOI] [PubMed] [Google Scholar]
- 12.Martinod K, Wagner DD: Thrombosis: Tangled up in NETs. Blood 2014; 123:2768–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V, Reichard U, Goosmann C, et al. : Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 14.Laridan E, Denorme F, Desender L, et al. : Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 2017; 82:223–232 [DOI] [PubMed] [Google Scholar]
- 15.Martinod K, Witsch T, Farley K, et al. : Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost 2016; 14:551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasco A, Coronado MJ, Hernandez-Terciado F, et al. : Assessment of neutrophil extracellular traps in coronary thrombus of a case series of patients with COVID-19 and myocardial infarction. JAMA Cardiol 2020; 6:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulos V: Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18:134–147 [DOI] [PubMed] [Google Scholar]
- 18.Sorvillo N, Cherpokova D, Martinod K, et al. : Extracellular DNA NET-works with dire consequences for health. Circ Res 2019; 125:470–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peña-Martínez C, Durán-Laforet V, García-Culebras A, et al. : Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke 2019; 50:3228–3237 [DOI] [PubMed] [Google Scholar]
- 20.Ducroux C, Di Meglio L, Loyau S, et al. : Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018; 49:754–757 [DOI] [PubMed] [Google Scholar]
- 21.Claassen J, Albers D, Schmidt JM, et al. : Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol 2014; 75:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Mufti F, Amuluru K, Damodara N, et al. : Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurointerv Surg 2019; 11:1135–1140 [DOI] [PubMed] [Google Scholar]
- 23.Früh A, Tielking K, Schoknecht F, et al. : RNase A inhibits formation of neutrophil extracellular traps in subarachnoid hemorrhage. Front Physiol 2021; 12:724611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucke-Wold B, Hosaka K, Dodd W, et al. : Interleukin-6: Important mediator of vasospasm following subarachnoid hemorrhage. Curr Neurovasc Res 2021; 18:364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmer S, Senger S, Griessenauer CJ, et al. : Admission serum high mobility group box 1 (HMGB1) protein predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurg Rev 2022; 45:807–817 [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira Manoel AL, van der Jagt M, Amin-Hanjani S, et al. ; Unruptured Aneurysms and SAH − CDE Project Investigators: Common data elements for unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage: Recommendations from the Working Group on Hospital Course and Acute Therapies-Proposal of a Multidisciplinary Research Group. Neurocrit Care 2019; 30:36–45 [DOI] [PubMed] [Google Scholar]
- 27.Vanderbeke L, Van Mol P, Van Herck Y, et al. : Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun 2021; 12:4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langseth MS, Helseth R, Ritschel V, et al. : Double-stranded DNA and NETs components in relation to clinical outcome after ST-elevation myocardial infarction. Sci Rep 2020; 10:5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim M, Bower RS, Wang Y, et al. : The predictive value of serum myeloperoxidase for vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Rev 2012; 35:413–419; discussion 419 [DOI] [PubMed] [Google Scholar]
- 30.Erşahin M, Toklu HZ, Erzik C, et al. : The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma 2010; 27:1143–1155 [DOI] [PubMed] [Google Scholar]
- 31.Zhou ML, Shi JX, Hang CH, et al. : Potential contribution of nuclear factor-kappaB to cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. J Cereb Blood Flow Metab 2007; 27:1583–1592 [DOI] [PubMed] [Google Scholar]
- 32.Denorme F, Portier I, Rustad JL, et al. : Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest 2022. Mar 31. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saber H, Desai A, Palla M, et al. : Efficacy of cilostazol in prevention of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: A meta-analysis. J Stroke Cerebrovasc Dis 2018; 27:2979–2985 [DOI] [PubMed] [Google Scholar]
- 34.Macdonald RL, Higashida RT, Keller E, et al. : Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10:618–625 [DOI] [PubMed] [Google Scholar]
- 35.Dreier JP, Major S, Manning A, et al. ; COSBID study group: Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009; 132:1866–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tam AK, Ilodigwe D, Mocco J, et al. : Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: Exploratory analysis of CONSCIOUS-1 database. Neurocrit Care 2010; 13:182–189 [DOI] [PubMed] [Google Scholar]
- 37.Badjatia N, Monahan A, Carpenter A, et al. : Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 2015; 84:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hugelshofer M, Buzzi RM, Schaer CA, et al. : Haptoglobin administration into the subarachnoid space prevents hemoglobin-induced cerebral vasospasm. J Clin Invest 2019; 129:5219–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, Zhang D, Fuchs TA, et al. : Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 2014; 123:3818–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goursaud S, Martinez de Lizarrondo S, Grolleau F, et al. : Delayed cerebral ischemia after subarachnoid hemorrhage: Is there a relevant experimental model? A systematic review of preclinical literature. Front Cardiovasc Med 2021; 8:752769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodd WS, Patel D, Lucke-Wold B, et al. : Adropin decreases endothelial monolayer permeability after cell-free hemoglobin exposure and reduces MCP-1-induced macrophage transmigration. Biochem Biophys Res Commun 2021; 582:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaventura A, Vecchié A, Dagna L, et al. : Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021; 21:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. : Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 2020; 217:e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyers S, Crescente M, Verhamme P, et al. : Staphylococcus aureus and neutrophil extracellular traps: The master manipulator meets its match in immunothrombosis. Arterioscler Thromb Vasc Biol 2022; 42:261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, Fu X, Cai J, et al. : Neutrophil extracellular traps may be a potential target for treating early brain injury in subarachnoid hemorrhage. Transl Stroke Res 2022; 13:112–131 [DOI] [PubMed] [Google Scholar]
- 46.Donkel SJ, Wolters FJ, Ikram MA, et al. : Circulating Myeloperoxidase (MPO)-DNA complexes as marker for Neutrophil Extracellular Traps (NETs) levels and the association with cardiovascular risk factors in the general population. PLoS One 2021; 16:e0253698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dreier JP, Sakowitz OW, Harder A, et al. : Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol 2002; 52:825–829 [DOI] [PubMed] [Google Scholar]
- 48.Wolach O, Martinod K: Casting a NET on cancer: The multiple roles for neutrophil extracellular traps in cancer. Curr Opin Hematol 2022; 29:53–62 [DOI] [PubMed] [Google Scholar]