Abstract
Background
Safe, effective, and easily implementable treatments that reduce the progression of respiratory failure in COVID-19 are urgently needed. Despite the increased adoption of prone positioning during the pandemic, the effectiveness of this technique on progression of respiratory failure among nonintubated patients is unclear.
Research Question
What is the effectiveness of smartphone-guided self-prone positioning recommendations and instructions compared with usual care in reducing progression of respiratory failure among nonintubated patients with COVID-19?
Study Design and Methods
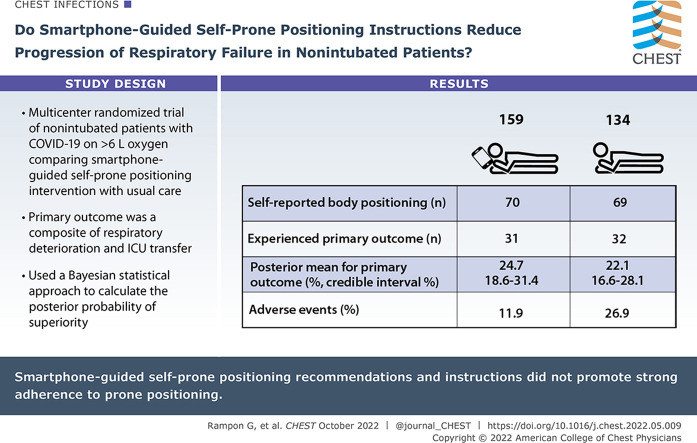
Awake Prone Position for Early Hypoxemia in COVID-19 (APPEX-19) is a multicenter randomized clinical trial that randomized nonintubated adults with COVID-19 on < 6 L/min of supplemental oxygen to receive a smartphone-guided self-prone positioning intervention or usual care. The primary outcome was the composite of respiratory deterioration (an increase in supplemental oxygen requirement) or ICU transfer. Using a Bayesian statistical approach, the posterior probability of superiority within each treatment arm (superiority threshold 95%) was calculated.
Results
The trial was stopped early for slow enrollment. A total of 293 participants were included in the modified intention-to-treat analysis (159 self-prone positioning intervention and 134 usual care). Among participants who self-reported body positioning (n = 139 [70 intervention, 69 usual care]), 71.4% in the intervention arm and 59.4% in the usual care arm attempted prone positioning. Thirty-one participants (posterior mean, 24.7%; 95% credible interval, 18.6-31.4) receiving usual care and 32 participants (posterior mean, 22.1%; 95% credible interval, 16.6-28.1) receiving the self-prone positioning intervention experienced the primary outcome; the posterior probability of superiority for the self-prone positioning intervention was 72.1%, less than the 95% threshold for superiority. Adverse events occurred in 26.9% of participants in the usual care arm and in 11.9% of participants in the intervention arm.
Interpretation
Among nonintubated patients with COVID-19, smartphone-guided self-prone positioning recommendations and instructions did not promote strong adherence to prone positioning.
Clinical Trial Registration
ClinicalTrials.gov; No.: NCT04344587; URL: www.clinicaltrials.gov.
Key Words: COVID-19, prone positioning, randomized clinical trial, respiratory failure, smartphone
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 731
Take-home Points.
Study Question: What is the effectiveness of smartphone-guided self-prone positioning recommendations and instructions compared with usual care to reduce progression of respiratory failure among nonintubated, non-ICU patients hospitalized with COVID-19?
Results: Among 293 participants, the posterior probability of superiority for the self-prone positioning intervention compared with usual care was 72.1%, below the predefined superiority margin; however, the study was stopped early owing to low enrollment.
Interpretation: Among nonintubated, non-ICU hospitalized patients with COVID-19, smartphone-guided self-prone positioning recommendations and instructions did not promote strong adherence to prone positioning.
COVID-19, the respiratory illness caused by SARS-CoV-2, is a global public health emergency. Nearly 25% of hospitalized patients with COVID-19 are cared for in the ICU,1 , 2 leading to potential shortages and rationing of scarce medical resources.3 Therefore, safe, effective, cost-efficient, and easily implementable treatments that reduce the progression of acute respiratory failure and the need for ICU transfer due to COVID-19 are urgently needed.
Prone positioning improves oxygenation and mortality in patients who are mechanically ventilated who have moderate to severe ARDS4 and potentially offers improvement for patients with COVID-19 on high-flow nasal cannula.5 However, despite increased adoption of prone positioning in nonintubated patients during the pandemic,6 and clinical guidelines7 that recommend prone positioning in patients on minimal respiratory support, the potential risks (eg, dislodgement of catheters, intubation delays) and benefits (eg, reduced progression of acute respiratory failure) in nonintubated, lower-acuity patients remain unclear. We present the results of an unblinded, multicenter, randomized controlled trial (Awake Prone Position for Early Hypoxemia in COVID-19 [APPEX-19]) comparing the effectiveness of smartphone-guided self-prone positioning recommendations and instructions with usual care among nonintubated, non-ICU patients with COVID-19.
Study Design and Methods
Objectives, Participants, and Oversight
The study protocol (e-Appendix 1) was registered on ClinicalTrials.gov 8 and prepublished.9 Patients were recruited from EDs and general medical wards at participating hospitals. Eligible patients were English-speaking and English-reading or Spanish-speaking and Spanish-reading adults aged ≥ 18 years with confirmed or suspected COVID-19 per the treating clinician, were within 48 h of admission to a medical ward, were not intubated, and had access to a functioning smartphone during their hospitalization. Patients receiving supplemental oxygen with flow rates ≥ 6 L/min were excluded because of concern from consulting physicians during the design phase and from the institutional review board that self-prone positioning in patients receiving higher oxygen flow rates was rapidly becoming standard care and that clinical equipoise may not exist. Patients were excluded if they had contraindications for prone positioning (eg, unstable fracture, indwelling chest tube, recent facial trauma or surgery4), were unable to safely self-pronate (eg, unable to operate the hospital bed or unable to turn from prone to supine without assistance), or had a diagnosis of dementia. Complete inclusion and exclusion criteria are provided in e-Table 1.
The trial protocol was approved by the institutional review board at each of the 12 study sites (e-Table 2) and was overseen by an independent data and safety monitoring board that reviewed unblinded effectiveness and safety data (e-Appendix 2). Data coordination was provided by the study team at Boston University School of Medicine. All participants provided written informed consent. The study began enrollment on April 25, 2020, and halted enrollment on March 25, 2021, after the study met stopping criteria for low enrollment (one or fewer participants enrolled per week for 3 consecutive weeks).
Randomization
Participants were allocated to the prone positioning intervention arm or the usual care arm using response adaptive randomization based on the posterior probability of the intervention being superior to usual care. The randomization was initially 1:1, and at each predetermined interim analysis, a beta-binomial conjugate model10 was used to update the randomization probabilities to preferentially assign more patients to the better-performing study arm. Three of six planned interim analyses were completed prior to halting the study. Study personnel enrolling patients were blinded to the randomization allocation sequence. Additional details concerning the adaptive randomization are available in the study protocol (e-Appendix 1).
Trial Procedures
Study personnel approached patients for enrollment via in-room hospital telephones or patient cellphones or as part of entering a hospital room for routine medical care. Participants who enrolled in APPEX-19 received a text message to their personal smartphone containing a link to a Qualtrics-based (Qualtrics XM) welcome message customized to their assigned study arm (prone positioning intervention or usual care). The welcome message for participants randomized to the self-prone position intervention arm contained the following: (1) an overview of the potential benefits of prone positioning in COVID-19; (2) a recommendation to lie in the prone position up to four times daily for 1 to 2 h each session and nightly for a total of 12 h; (3) pictorial instructions to safely turn to the self-prone position while in the hospital; and (4) instructions to keep track of time spent in different body positions while in bed. The welcome message for participants randomized to the usual care arm contained the following: (1) instructions to lie in bed in whichever position was comfortable and (2) instructions to keep track of time spent in different body positions while in bed. All participants were then sent separate monitoring survey links twice daily via text messages that contained the initial treatment arm instructions (including a reminder to prone position in the intervention arm) and prompts to report complications and estimates of time spent in different positions. Surveys were sent until any of the following occurred: the primary outcome was reached, the participant was discharged from the hospital, or 14 days had passed since enrollment. Intervention adherence was assessed by participant self-report on twice-daily surveys.
An amendment to the study protocol (e-Appendix 1) allowed remote contact of participants who had not responded to a survey in 24 h to troubleshoot technical issues and to remind participants to complete surveys. Screenshots of the treatment arm smartphone messages and surveys are included in e-Figure 1. There were no restrictions to prone positioning in the usual care group (neither participants nor providers were advised against prone positioning), and clinical staff were blinded to treatment assignment. Study investigators selected a smartphone-based approach to avoid the need for study staff to enter patient rooms, because of prior reported success of smartphone-based interventions11 , 12 to change behavior, and because smartphones have been widely adopted across demographic and socioeconomic groups,13 making them widely implementable. Participant study materials were developed iteratively by the study investigators, including experts in ecological momentary assessment (C. S. R.) and implementation (A. J. W.), and were translated into Spanish by certified medical translators.
Data Collection
Electronic medical record data, including outcome data, were collected at enrollment and daily thereafter by study personnel and were entered into centralized Research Electronic Data Capture and Qualtrics databases.14 , 15 Participants recorded prone position adherence information, adverse events, and their level of dyspnea twice daily on electronic surveys.
Outcomes
The primary outcome, specified to assess the effectiveness of self-prone positioning on reducing the progression of acute respiratory failure, was the composite of (1) respiratory deterioration or (2) transfer to the ICU. Respiratory deterioration was defined as an increase in the supplemental oxygen flow rate of ≥ 2 L/min compared with the flow rate at the time of the initial welcome text message and sustained for ≥ 12 h, or a switch to a higher level of oxygen support (eg, a transition from nasal cannula to nonrebreather mask, high-flow nasal cannula, noninvasive positive pressure ventilation, or mechanical ventilation). Secondary effectiveness outcomes included each individual component of the primary composite outcome, receipt of mechanical ventilation, hospital mortality, diagnosis of ARDS, and each participant’s median self-reported level of dyspnea as measured by using the modified Borg dyspnea scale.16 , 17 Secondary safety outcomes were the self-reported degree of discomfort with self-prone positioning, and loss of venous or urinary catheters, captured through survey prompts (e-Fig 1). Prone positioning adherence was recorded for time categories of no time, up to 6 h, 6 to 11 h, and ≥ 12 h since the last monitoring survey. Outcome data collection was conducted until the participant was discharged from the hospital or 14 days following enrollment, whichever came first.
Sample Size
Based on preliminary data at Boston Medical Center from spring 2020, we determined a maximum total of 560 participants would have 90% power to reject the null hypothesis, assuming a primary outcome rate of 24.5% in the self-prone positioning arm and 35.0% in the usual care arm.
Statistical Analysis
In the primary analysis, a modified intention-to-treat approach was used; this approach included all participants who were randomized to treatment and subsequently received the initial welcome text message instructions prior to discharge from the hospital, transfer to the ICU, or study withdrawal. Bayesian analyses were used to calculate the posterior probability: the probability of the primary outcome accounting for the probability prior to randomization and the data gathered from the study. A priori, we selected a prior probability (beta [12+ events, 28+ nonevents]), which assumed that the effects of the prone positioning intervention in the study cohort would be small. This prior probability has a mean of 30% (average of assumed rates) and was selected to express a priori skepticism of the alternative hypothesis being true; indeed, under this prior probability, the probability of the alternative hypothesis is 50%, whereas the probability of observing an improvement in the true rate of 10.5% or larger (the hypothesized improvement) is only 15%. Thus, only strong study data supporting the use of self-prone positioning would be taken as evidence that self-prone positioning was superior to usual care.
For each treatment arm, the posterior mean (the posterior probability distribution average for the primary outcome) and 95% credible interval are reported. We predefined that self-prone positioning would be considered superior to usual care if the data showed that there was a > 95% probability that the posterior probability in the intervention arm was less than that of the usual care arm. Unlike the P value, the posterior probability for superiority provides the probability of the alternative hypothesis being true (ie, that the intervention is superior to usual care) and thus may be informative even when below the predefined superiority threshold of 95%. Additional details of the Bayesian approach are provided in e-Appendix 1.
In addition to the intention-to-treat analysis, we performed an exploratory analysis that compared participants in the intervention arm who self-reported prone positioning for ≥ 6 h at least once vs participants in the usual care arm who did not self-report prone positioning on any survey. The single 6-h prone positioning duration for inclusion in the exploratory analysis was selected owing to the improvement in oxygenation using this duration in a prior prone positioning clinical trial18; there was also concern from study investigators that longer durations used for patients who were mechanically ventilated and had ARDS4 would not be feasible in nonintubated patients. We were unable to adjust for a priori selected covariates in the exploratory analysis given the low number of outcomes.
Prespecified subgroups tested for heterogeneity of treatment effect were as follows: (1) enrollment BMI < 30 kg/m2 vs ≥ 30 kg/m2; (2) enrollment age < 65 years vs ≥ 65 years; (3) no history of congestive heart failure vs history of congestive heart failure; (4) no receipt of oxygen at the time of enrollment vs receipt of oxygen at the time of enrollment; (5) no opacities or infiltrates on admission chest radiograph vs opacities or infiltrates on admission chest radiograph; and (6) negative or pending vs positive SARS-CoV-2 test result at the time of enrollment.
Dichotomous secondary effectiveness outcomes were compared by using risk differences with 95% CIs. Continuous secondary effectiveness outcomes were presented as medians and interquartile ranges (IQRs) for each treatment arm. No corrections for multiple testing were made for the exploratory analysis, subgroup, or secondary outcomes. Adverse events were reported as the number of participants and associated percentages that experienced at least one adverse event per treatment arm. The complete statistical analysis plan is included in the study protocol (e-Appendix 1). R version 4.0.5 (R Foundation for Statistical Computing) was used for analyses.
Results
Participants
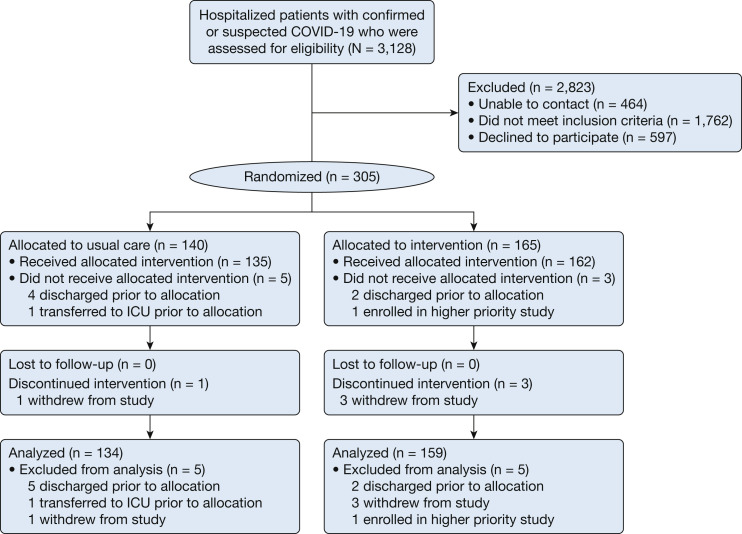
From April 25, 2020, to March 25, 2021, a total of 3,128 patients were screened, 305 participants underwent randomization, and 293 participants (134 in the usual care arm and 159 in the self-prone positioning intervention arm) were included in the modified intention-to-treat analysis (Fig 1 ). Participants were on average 53 (IQR, 41-63) years old and had COVID-19 symptoms for 7 (IQR, 4-10) days prior to hospital admission (Table 1 ),19 and 91.1% had a positive SARS-CoV2 test result at the time of enrollment. More than one-half of participants (n = 153 [52.2%]) were receiving supplemental oxygen at the time of enrollment, of whom 72 (47.1%) were receiving oxygen flow rates of ≥ 3 L/min.
Figure 1.
Enrollment and randomization of study participants for the awake prone position for early hypoxemia in COVID-19 trial.
Table 1.
Baseline Characteristics of Participants According to Treatment Arm
| Characteristic | Total (N = 293) | Usual Care (n = 134) | Self-Prone Positioning Intervention (n = 159) |
|---|---|---|---|
| Age, median (IQR), y | 53 (41-63) | 54 (43-63) | 52 (39-62) |
| Sex | |||
| Female | 117 (39.9) | 54 (40.3) | 63 (39.6) |
| Male | 176 (60.1) | 80 (59.7) | 96 (60.4) |
| Race | |||
| White | 180 (61.4) | 83 (61.9) | 97 (61.0) |
| Black | 52 (17.7) | 24 (17.9) | 28 (17.6) |
| Asian | 8 (2.7) | 2 (1.5) | 6 (3.8) |
| Other or not reported | 53 (18.1) | 25 (18.7) | 28 (17.6) |
| Ethnicity | |||
| Hispanic or Latino | 59 (20.1) | 24 (17.9) | 35 (22.0) |
| Not Hispanic or Latino | 220 (75.1) | 106 (79.1) | 114 (71.7) |
| Unknown/not reported | 14 (4.8) | 4 (3.0) | 10 (6.3) |
| Preferred language | |||
| English | 253 (86.3) | 109 (81.3) | 144 (90.6) |
| Spanish | 40 (13.7) | 25 (18.7) | 15 (9.4) |
| Randomized following release of dexamethasone RECOVERY19 on June 22, 2020 | 242 (82.6) | 108 (80.6) | 134 (84.3) |
| Medical history | |||
| Asthma | 48 (16.4) | 19 (14.2) | 29 (18.2) |
| Chronic pulmonary disease, not asthma | 30 (10.2) | 14 (10.4) | 16 (10.1) |
| Coronary artery disease | 28 (9.6) | 11 (8.2) | 17 (10.7) |
| Congestive heart failure | 18 (6.1) | 9 (6.7) | 6 (3.8) |
| Diabetes | 84 (28.7) | 39 (29.1) | 45 (28.3) |
| HIV/AIDS, or other immunocompromising condition | 19 (6.5) | 10 (7.5) | 9 (5.7) |
| Hypertension | 138 (47.1) | 62 (46.3) | 76 (47.8) |
| Malignancy | 15 (5.1) | 9 (6.7) | 6 (3.8) |
| Organ transplant | 20 (6.8) | 7 (5.2) | 13 (8.2) |
| Current smoker | 15 (5.1) | 8 (6.0) | 7 (4.4) |
| SARS-CoV-2 virus test result | |||
| Positive | 267 (91.1) | 118 (88.1) | 149 (93.7) |
| Negative | 19 (6.5) | 13 (9.7) | 6 (3.8) |
| Not performed or pending | 7 (2.4) | 3 (2.2) | 4 (2.5) |
| Days from earliest symptoms to admission, median (IQR) | 7 (4-10) | 7 (4-10) | 7 (4-10) |
| Chest radiograph findings | |||
| Multifocal distribution | 158 (53.9) | 70 (52.2) | 88 (55.3) |
| Opacities | 197 (67.2) | 88 (65.7) | 109 (68.6) |
| Interstitial pattern | 54 (18.4) | 30 (22.4) | 24 (15.1) |
| Pleural effusion | 12 (4.1) | 8 (6.0) | 4 (2.5) |
| No acute pathology | 48 (16.4) | 26 (19.4) | 22 (13.8) |
| Chest radiograph not performed | 16 (5.5) | 6 (4.5) | 10 (6.3) |
| Medications received | |||
| Anti-IL-6 or anti-IL-1 | 1 (0.3) | 0 | 1 (0.6) |
| Azithromycin or doxycycline | 63 (21.5) | 26 (19.4) | 37 (23.3) |
| Hydroxychloroquine | 5 (1.7) | 2 (1.5) | 3 (1.9) |
| Remdesivir | 77 (26.3) | 29 (21.6) | 48 (30.2) |
| Mean arterial pressure, median (IQR), mm Hg | 90 (82-100) | 89 (83-99) | 91 (82-101) |
| Respiratory rate, median (IQR), breaths/min | 18 (18-20) | 18 (18-20) | 18 (18-20) |
| Peripheral venous oxygen saturation, median (IQR), % | 95 (93-97) | 95 (94-97) | 95 (92-96) |
| Sequential organ failure assessment score, median (IQR) | 1 (0-2) | 1 (0-2) | 0 (0-2) |
| BMI ≥ 30 kg/m2a | 160 (55.0) | 67 (50.0) | 93 (59.2) |
| Spo2/Fio2 ratio at enrollment, median (IQR) | 396 (306-378) | 402 (311-457) | 396 (308-457) |
| Supplemental oxygen delivery device at the time of initial study text messageb | |||
| None | 140 (47.8) | 66 (49.3) | 74 (46.5) |
| Nasal cannula | 148 (50.5) | 65 (48.5) | 83 (52.2) |
| Mask | 2 (0.7) | 2 (1.5) | 0 (0.0) |
| High-flow nasal cannula | 3 (1.0) | 1 (0.7) | 2 (1.3) |
| Supplemental oxygen flow rate, L/minc | |||
| Room air | 140 (47.8) | 66 (49.3) | 74 (46.5) |
| 1 | 15 (5.1) | 5 (3.7) | 10 (6.3) |
| 2 | 66 (22.5) | 30 (22.4) | 36 (22.6) |
| 3 | 33 (11.3) | 14 (10.4) | 19 (11.9) |
| 4 | 19 (6.5) | 10 (7.5) | 9 (5.7) |
| 5 | 7 (2.4) | 4 (3.0) | 3 (1.9) |
| > 5 | 13 (4.4) | 5 (3.7) | 8 (5.0) |
Data are presented as No. (%) unless otherwise indicated. IL = interleukin; IQR = interquartile range; Spo2 = blood oxygen saturation; RECOVERY = Randomised Evaluation of COVID-19 Therapy.
Two participants were missing BMI values.
Four participants had an escalation in their supplemental oxygen delivery device to Venturi mask or high-flow nasal cannula following randomization but prior to the initial study intervention.
Thirteen participants had an escalation in their supplemental oxygen delivery rate above 5 L/min following randomization but prior to initial study intervention. These participants were included in the modified intention-to-treat analysis.
Adherence to Prone Positioning Recommendations
A total of 99 of 159 (62.3%) participants in the self-prone positioning intervention arm and 83 of 134 (61.9%) participants in the usual care arm opened the survey link on their smartphone and received their treatment assignment instructions. In total, 139 (47.4%) participants self-reported their body position at least once during the study period (70 in the intervention arm and 69 in the usual care arm). Among participants in the self-prone position intervention arm who self-reported their body position and received their initial treatment assignment, 50 (71.4%) reported lying in the prone position at least once and 25 (35.7%) reported lying in the prone position for ≥ 6 h at least once. In the usual care arm, 41 (59.4%) reported lying in the prone position at least once and nine (13.0%) reported lying in the prone position for ≥ 6 h at least once. In total, among those who received their initial treatment assignment, 25 participants in the self-prone positioning intervention arm documented prone positioning and 60 participants in the usual care arm documented not positioning themselves in the prone position and thus were included in the exploratory analysis. The self-reported time spent in the self-prone position according to treatment arm and survey is displayed in e-Table 3.
Primary Outcome
The primary outcome of respiratory deterioration of ICU transfer was experienced by 31 participants (posterior mean, 24.7%; 95% credible interval, 18.6 to 31.4) in the usual care arm and 32 participants (posterior mean, 22.1%; 95% credible interval, 16.6 to 28.1) in the self-prone positioning intervention arm (posterior mean difference, –2.6%; 95% credible interval, –11.2 to 6.0). The posterior probability of superiority for the self-prone positioning intervention was 72.1%, less than the 95% prespecified threshold for superiority (Table 2 ). The posterior probability of inferiority of the self-prone positioning intervention was 10.0%, also less than the 95% prespecified threshold for inferiority. Characteristics of patients (n = 85) included in the exploratory analysis are presented in e-Table 4. In the exploratory analysis comparing participants who reported self-prone positioning in the intervention arm vs participants who did not self-prone position from the usual care arm, the primary outcome occurred in one participant in the self-prone positioning intervention arm (posterior mean, 20.0%; 95% credible interval, 11.3 to 30.5) and in 17 participants in the usual care arm (posterior mean, 29.0%; 95% credible interval, 20.6 to 38.2) for a posterior mean difference of –9.0% (95% credible interval, –21.8 to 4.4). The posterior probability of superiority for the self-prone positioning intervention in the exploratory analysis was 90.9%. There was no evidence of heterogeneity of treatment effect in the subgroup analyses.
Table 2.
Main and Subgroup Analyses
| Analysis | Usual Care Primary Outcome Events (N) | Self-Prone Positioning Primary Outcome Events (N) | Usual Care: Posterior Mean (95% Credible Interval) | Self-Prone Positioning Arm: Posterior Mean (95% Credible Interval) | Posterior Mean Difference (95% Credible Interval) | Posterior Probability of Superiority of Self-Prone Positioning | Probability of Significant Interaction |
|---|---|---|---|---|---|---|---|
| MITT | 31 (134) | 32 (159) | 24.7% (18.6 to 31.4) | 22.1% (16.6 to 28.1) | –2.6% (–11.2 to 6.0) | 72.1% | |
| Exploratory | 17 (60) | 1 (25) | 29.0% (20.6 to 38.2) | 20.0% (11.3 to 30.5) | –9.0% (–21.8 to 4.4) | 90.9% | |
| Age | 0.77 | ||||||
| < 65 y | 23 (105) | 23 (125) | 24.1% (17.6 to 31.4) | 21.2% (15.3 to 27.8) | –2.9% (–12.3 to 6.3) | 73.1% | |
| ≥ 65 y | 8 (29) | 9 (34) | 29.0% (19.0 to 40.2) | 28.4% (18.8 to 39.1) | –0.6% (–15.4 to 14.1) | 53.2% | |
| BMI | 0.94 | ||||||
| < 30 kg/m2 | 14 (67) | 11 (64) | 24.3% (16.7 to 32.8) | 22.1% (14.7 to 30.5) | –2.2% (–13.5 to 9.2) | 64.8% | |
| ≥ 30 kg/m2 | 17 (67) | 20 (93) | 27.1% (19.2 to 35.9) | 24.1% (17.2 to 31.7) | –3.1% (–14.2 to 7.9) | 70.4% | |
| History of congestive heart failure | 0.97 | ||||||
| Yes | 2 (9) | 1 (9) | 28.6% (17.0 to 41.9) | 26.5% (15.3 to 39.6) | –2.0% (–19.5 to 15.5) | 59.1% | |
| No | 29 (125) | 31 (150) | 24.9% (18.6 to 31.7) | 22.6% (17.0 to 28.8) | –2.2% (–11.1 to 6.6) | 68.8% | |
| Receipt of supplemental oxygen at the time of enrollment | 0.46 | ||||||
| Yes | 17 (68) | 22 (85) | 26.9% (19.0 to 35.6) | 27.2% (19.8 to 35.3) | 0.4% (–11.1 to 11.7) | 47.5% | |
| No | 14 (66) | 10 (74) | 24.5% (16.9 to 33.1) | 19.3% (12.6 to 27.0) | –5.2% (–16.2 to 5.6) | 82.7% | |
| Opacities or infiltrates on admission chest radiograph | 0.57 | ||||||
| Yes | 26 (97) | 30 (117) | 27.7% (20.6 to 35.5) | 26.8% (20.1 to 33.9) | –1.0% (–11.2 to 9.2) | 57.4% | |
| No | 5 (37) | 2 (42) | 22.1% (13.6 to 31.9) | 17.1% (9.8 to 25.9) | –5.0% (–17.4 to 7.2) | 78.9% | |
| Positive SARS-CoV-2 test result at the time of enrollment | 0.49 | ||||||
| Yes | 30 (118) | 31 (149) | 26.6% (20.0 to 33.7) | 22.8% (17.1 to 29.0) | –3.8% (–13.0 to 5.2) | 79.5% | |
| No | 1 (16) | 1 (10) | 23.2% (13.2 to 35.0) | 26.0% (15.0 to 38.9) | 2.8% (–13.4 to 19.2) | 37.0% | |
MITT = modified intention-to-treat; NA = not applicable.
Secondary Outcomes
There were no differences between the self-prone positioning intervention arm and the usual care arm across all secondary effectiveness outcomes (Table 3 ). Participants’ respiratory parameters over time are shown in e-Figure 2.
Table 3.
Secondary Effectiveness Outcomes
| Outcome or Analysis | Usual Care (n = 134) | Self-Prone Positioning Intervention (n = 159) |
|---|---|---|
| Increase in the supplemental oxygen flow rate, No. (%) | 26 (19.4) | 30 (18.9) |
| Risk difference, % (95% CI) | ... | –0.5 (–9.6 to 8.5) |
| Switch to a higher level of oxygen support, No. (%) | 18 (13.4) | 16 (10.1) |
| Risk difference, % (95% CI) | ... | –3.4 (–10.8 to 4.1) |
| ICU transfer, No. (%) | 6 (4.5) | 9 (5.7) |
| Risk difference, % (95% CI) | ... | 1.2 (–3.8 to 6.2) |
| Invasive mechanical ventilation, No. (%) | 4 (3.0) | 2 (1.3) |
| Risk difference, % (95% CI) | ... | –1.7 (–5.1 to 1.6) |
| Diagnosis of ARDS, No. (%) | 1 (0.7) | 4 (2.5) |
| Risk difference, % (95% CI) | ... | 1.8 (–1.1 to 4.6) |
| Hospital mortality, No. (%) | 2 (1.5) | 2 (1.3) |
| Risk difference, % (95% CI) | ... | –0.2 (–2.9 to 2.5) |
| Hospital length of stay, median (IQR), d | 4 (1 to 6) | 3 (2 to 6) |
| Median Modified Borg Dyspnea Scale,15,16 median (IQR) | 1 (0.5 to 2) | 1 (0 to 3) |
IQR = interquartile range.
Adverse Events
Adverse events occurred in 19 (11.9%) participants in the self-prone positioning intervention arm and in 36 (26.9%) participants in the usual care arm. Fourteen (8.8%) participants in the self-prone positioning intervention arm and 28 (20.9%) in the usual care arm reported being very uncomfortable with self-prone positioning. Nine (5.7%) participants in the self-prone positioning intervention arm and 12 (9.0%) in the usual care arm reported loss of an IV catheter and one (0.6%) and zero (0.0%) reported loss of a urinary catheter in the intervention and usual care arms, respectively. There were no serious adverse events.
Discussion
In this multicenter, unblinded, randomized clinical trial, smartphone-guided self-prone positioning recommendations and instructions did not promote strong adherence to self-prone positioning. Notably, the study was terminated prematurely owing to low enrollment; fewer than two-thirds of participants accessed their treatment assignment on their smartphones; and the self-reported time spent in the prone position was short. Thus, the study was underpowered to make conclusions regarding the effectiveness of self-prone positioning recommendations and instructions or self-prone positioning itself in reducing clinical deterioration.
The current study highlights three important aspects of self-prone positioning for non-ICU, nonintubated, hospitalized patients during the COVID-19 pandemic. First, smartphone-guided delivery of trial instructions among hospitalized patients with COVID-19 had low rates of adherence. Only 60% of participants accessed their randomization group, and < 40% reported self-prone positioning for ≥ 6 h, suggesting participant difficulty with both accessing and adhering to the smartphone-guided interventions. Thus, time spent in the prone position in this study was substantially shorter in duration compared with a recent meta-trial5 among critically ill patients with COVID-19 on high-flow nasal cannula, which found that prone positioning reduced rates of treatment failure. Importantly, the meta-trial intervention used assisted prone positioning. In contrast, clinical trials20 (including ours) that involved recommendations for self-prone positioning but no direct assistance have had shorter durations and lower adherence to prone positioning. These results suggest that the efficacy of prone positioning in nonintubated patients may strongly depend on the degree to which staff assist with the maneuver. In contrast to our findings of low adherence, studies of smartphone-based interventions for chronic disease management11 , 12 have reported high rates of adherence. We speculate that the low adherence and low access rate in our study were due in part to the inclusion of hospitalized patients with acute illness who may be less able or motivated to navigate their smartphones compared with outpatients. It is also possible that the specific smartphone-guided recommendations and instructions in our study were ineffective, and the low rate of adherence may not be generalizable to other smartphone-guided interventions in the inpatient setting. Further research is required prior to more widespread use of smartphone-guided intervention delivery for hospitalized patients. Second, among patients who did self-prone position for ≥ 6 h, outcomes may be favorable. In the current study, participants who self-prone positioned in the intervention arm exhibited a 90% posterior probability for superiority in reducing the rate of respiratory deterioration and ICU transfer compared with participants in the usual care arm who did not self-prone. However, this exploratory analysis may be confounded by a strong association between postrandomization factors that predict adherence (eg, patient ability to access their smartphones and perform self-pronation) and outcome.21 Third, self-prone positioning among nonintubated, hospitalized patients who received instructions via smartphone was generally well tolerated and without serious side effects.
The current study has several limitations. First, the study was terminated for low enrollment. Thus, whether completion of the study to the planned sample size would have led to different results is unknown. Second, only about two-thirds of participants opened the smartphone-guided survey containing their treatment assignment and just less than one-half self-reported their time spent in the prone position. Thus, it is unclear if our primary findings of no difference were driven by lack of effectiveness of prone positioning in the study population, lack of effectiveness of the smartphone-guided delivery platform and instructions, not reaching the planned sample size, and/or the short duration spent in the prone position. Nineteen percent of approached patients declined to participate, and patients who were unable to self-prone position were excluded. Thus, the external validity of these results may be limited. A small percentage of participants (< 10%) did not have a positive SARS-CoV-2 test result at the time of enrollment. The effect of these patients with suspected COVID-19 on study outcomes and the ultimate status of subsequent test results are unknown. Lastly, the inclusion of patients receiving < 6 L/min of supplemental oxygen via nasal cannula was necessitated by concern that clinical equipoise may not exist for patients on higher flow rates of oxygen. However, prone positioning may be more beneficial in patients with severe respiratory disease.22 Thus, the lack of effectiveness of this study may be due to the lower severity of respiratory disease included in the study cohort.
Interpretation
Among nonintubated, non-ICU hospitalized patients with COVID-19, smartphone-guided self-prone positioning recommendations and instructions did not promote strong adherence to prone positioning. These results help to inform the use of self-guided prone positioning recommendations among nonintubated low-acuity patients with COVID-19, inpatient smartphone-based interventions, and self-prone positioning vs assisted prone positioning in nonintubated patients.
Acknowledgments
Author contributions: All authors assume responsibility for the overall content and integrity of the article. G. R., A. J. W., S. Q. S., G. D., C. S. R., and N. A. B. drafted the initial version of the manuscript, and all members of the writing committee edited and approved subsequent versions.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: K. M. is the Primary Investigator for the industry-funded trial NCT03808922 (STOP PIV-Phase III DAS181 Lower Tract PIV Infection in Immunocompromised Subjects). None declared (G. R., S. J., R. A., N. A., A. M.-Q., E. A. F., J. M., N. J., A. S., A. H. C., M. T., G. D., C. S. R., M. A. G., K. R. G., N. G. G., B. J., K. L. M., J. M. R., S. Q. S., A. J. W., N. A. B.).
Funding/support: The study was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through Boston University-Clinical and Translational Science Institute [Grant 1UL1TR001430].
Role ofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclaimer: The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
APPEX-19 Collaborators: A complete list of collaborators in the APPEX-19 trial is provided in e-Appendix 3.
Other contributions: The authors thank the participating patients in this trial; the allied health professionals, institutional review boards, research assistants, and research administrators at the 12 APPEX-19 sites in the United States and Spain (e-Appendix 3), especially Charlotte Alger, BS, and Mary-Tara Roth, RN, MSN, MPH; and the members of the independent data monitoring committee (Arthur C. Theodore, MD; Hasmeena Kathuria, MD; and Robert A. Lew, PhD).
Additional information: The e-Appendixes, e-Figures, and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 4.Guérin C., Reignier J., Richard J.-C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Ehrmann S., Li J., Ibarra-Estrada M., et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walkey A. COVID-19 Practice Variations Across the USA and Beyond [internet]. 2021 [cited April 13, 2021] https://www.eventscribe.net/2021/CON21/fsPopup.asp?efp=V1hHSVBHQ1AxMzc4NQ&PresentationID=802118&rnd=0.8714458&mode=presinfo
- 7.Bamford P, Bentley A, Dean J, Wilson-Baig N. ICS Guidance for Prone Positioning of the Conscious COVID Patient 2020 [internet]. [December 13, 2021]. https://emcrit.org/wp-content/uploads/2020/04/2020-04-12-Guidance-for-conscious-proning.pdf
- 8.Walkey A. Awake Prone Position for Early Hypoxemia in COVID-19 [internet]. clinicaltrials.gov; 2021 [cited October 25, 2021]. https://clinicaltrials.gov/ct2/show/NCT04344587
- 9.Garcia M.A., Rampon G.L., Doros G., et al. Rationale and design of the Awake Prone Position for Early Hypoxemia in COVID-19 (APPEX-19) study protocol. Ann Am Thorac Soc. 2021;18(9):1560–1566. doi: 10.1513/AnnalsATS.202009-1124SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunn D., Jackson C., Best N., Thomas A., Spiegelhalter D. Chapman and Hall/CRC; New York, NY: 2013. The BUGS Book: A Practical Introduction to Bayesian Analysis. [Google Scholar]
- 11.Xu H., Long H. The effect of smartphone app-based interventions for patients with hypertension: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2020;8(10):e21759. doi: 10.2196/21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenhersch E., Thrul J., Ungaretti J., Rosencovich N., Waitman C., Ceberio M.R. Virtual reality smartphone-based intervention for smoking cessation: pilot randomized controlled trial on initial clinical efficacy and adherence. J Medical Internet Res. 2020;22(7):e17571. doi: 10.2196/17571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pew Research Center Demographics of Mobile Device Ownership and Adoption in the United States [internet]. Pew Research Center: Internet, Science & Tech. [cited 2021 Jul 14] https://www.pewresearch.org/internet/fact-sheet/mobile/
- 14.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 17.Burdon J.G., Juniper E.F., Killian K.J., Hargreave F.E., Campbell E.J. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982;126(5):825–828. doi: 10.1164/arrd.1982.126.5.825. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor S.P., Bundy H., Smith W.M., Skavroneck S., Taylor B., Kowalkowski M.A. Awake-prone positioning strategy for non-intubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18(8):1360–1368. doi: 10.1513/AnnalsATS.202009-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray E.J., Hernán M.A. Adherence adjustment in the Coronary Drug Project: a call for better per-protocol effect estimates in randomized trials. Clin Trials. 2016;13(4):372–378. doi: 10.1177/1740774516634335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L., Carlesso E., Taccone P., Polli F., Guérin C., Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76(6):448–454. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.