Abstract
Background
COVID-19 may negatively impact the prognosis of patients with chronic HFrEF and vice versa.
Methods
This study included 2 parallel analyses of patients in the United States who were in the TriNetX health database and who underwent polymerase chain reaction testing for SARS-CoV-2 as an inpatient or outpatient between January and September of 2020. Analysis A included patients with positive tests for COVID-19 and compared patients with histories of worsening heart failure with reduced ejection fraction (HFrEF) (hospitalization due to heart failure (HF) or IV diuretic use during the prior 12 months), HFrEF without worsening, and no prior HF. Analysis B included patients with histories of HFrEF and compared patients with positive vs negative COVID-19 tests. Outcomes included mortality and worsening HF. In both analyses, prespecified subgroup analyses were stratified by inpatient vs outpatient settings of the COVID-19 tests.
Results
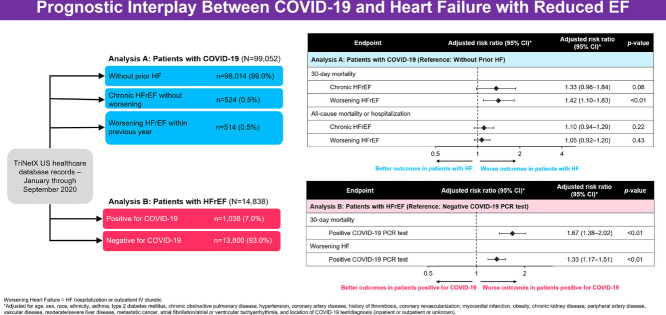
In Analysis A, of 99,052 patients with positive COVID-19 tests, 514 (0.5%) and 524 (0.5%) patients had histories of worsening HFrEF and HFrEF without worsening, respectively. After adjustment, compared to patients without HF, worsening HFrEF (risk ratio [RR] 1.42, 95% CI 1.10–1.83; P< 0.001) and HFrEF without worsening (RR 1.33, 95% CI 0.96–1.84; P= 0.06) were associated with higher 30-day mortality rates. Excess risk of mortality tended to be pronounced in patients initially diagnosed with COVID-19 as outpatients (P for interaction, 0.12 and 0.006, respectively). In Analysis B, of 14,838 patients with HFrEF tested for COVID-19, 1038 (7.0%) had positive tests. After adjustment, testing positive was associated with excess 30-day mortality risk (RR 1.67, 95% CI 1.38–2.02; P< 0.001) and worsening HF (RR 1.33, 95% CI 1.17–1.51; P< 0.001). Mortality risk was nominally more pronounced among patients presenting as outpatients (P for interaction 0.07).
Conclusion
In this large cohort of patients tested for COVID-19, among patients testing positive, a history of HFrEF with or without worsening was associated with excess mortality rates, particularly among patients diagnosed with COVID-19 as outpatients. Among patients with established HFrEF, compared with testing negative, testing positive for COVID-19 was independently associated with higher risk of death and worsening HF.
Key Words: COVID-19, heart failure, outcomes, mortality
Graphical Abstract
Although Coronavirus Disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is most commonly recognized as a pathogen targeting the lungs and respiratory tract, cardiac manifestations and complications are common and contribute substantially to death and adverse outcomes.1 , 2 In particular, patients with pre-existing heart failure (HF) have shown significant vulnerability to death and respiratory failure in the days to weeks following COVID-19 infection.3 , 4 As such, history of HF has been proposed as being potentially useful for informing patient risk-stratification and for guiding decisions regarding intensity of surveillance and therapy for patients.3
Nonetheless, many facets of the relationship between COVID-19 and HF remain unclear.5 , 6 For example, it is unknown whether poor clinical outcomes in patients with COVID-19 and with HF are driven by a subset of higher risk HF patients, including those with recent hospitalization due to HF or outpatient clinical worsening. Likewise, it is unknown whether excess risk of death among patients with HF and with COVID-19 is concentrated in patients who present with severe COVID-19 symptoms, prompting inpatient diagnosis and hospitalization, or whether significant clinical risk extends to milder initial presentations of COVID-19 that are diagnosed in the outpatient setting. In addition, although multiple studies have assessed associations between COVID-19 and HF in terms of in-hospital and 30-day outcomes, longer-term clinical consequences and prognostic implications remain poorly characterized, including associations with downstream HF-specific endpoints, such as hospitalizations for worsening HF.3 , 4 , 7 In this context, the objectives of the current study were to leverage a large, longitudinal, real-world dataset of patients tested for SARS-CoV-2 to characterize: (1) associations between history of HF with reduced ejection fraction (HFrEF) with vs without a recent worsening heart failure event and clinical outcomes in patients diagnosed with COVID-19; (2) the prognostic implications of testing positive vs negative for COVID-19 in patients with HFrEF; and (3) the interaction between HFrEF, clinical outcomes and the locations of initial COVID-19 presentations in the inpatient vs outpatient setting.
Methods
Data Source
This study used a de-identified patient dataset from the TriNetX Dataworks USA Network (Cambridge, MA). TriNetX is a global health-research database that contains de-identified data that are sourced and continuously updated from electronic medical records (EMRs). Clinical organizations, such as hospitals and integrated delivery networks, own all rights, consents and approvals of transferring data to TriNetX. The current study used data from the United States (US), including data from > 37 health care organizations responsible for the care of > 58 million patients in the US. Patients with a variety of health insurance coverage were included (ie, private insurance, Medicare, Medicaid, and no insurance). Data include longitudinal outpatient and in-hospital data and encompass patient demographics, medical diagnoses, laboratory test results, outpatient visits, hospitalizations, and mortality.
TriNetX, LLC is compliant with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law that protects the privacy and security of health care data, and any additional data privacy regulations applicable to the contributing health care organizations. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System to ensure the protection of the health care data it has access to and to meet the requirements of the HIPAA Security Rule. Any data displayed on the TriNetX Platform in aggregate form, and any patient-level data provided in a data set generated by the TriNetX Platform, contain only de-identified data as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. Because this study used only de-identified patient records and did not involve the collection, use or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
Study Population and Design
This retrospective observational study included adult patients ≥ 18 years of age who had received ≥ 1 polymerase chain reaction (PCR) test for SARS-CoV-2 between January 1, 2020, and September 30, 2020; had EMR data for the 12 months prior to the index COVID-19 PCR test; and had both inpatient and outpatient EMR records that were accessible. Patients were included if they had either a diagnosis of HFrEF or no prior HF diagnosis, as determined by International Classification of Diseases-10th Revision (ICD-10) codes during the 12 months before COVID-19 testing. To be defined as having a history of HFrEF, a patient was required to have 1 inpatient diagnosis for HFrEF, 2 outpatient diagnoses for HFrEF on 2 different dates or 1 outpatient diagnosis for HFrEF plus 1 outpatient diagnosis for any HF on 2 different dates (Supplementary Table 1). Given established differences in patients’ profiles, pathophysiologies and outcomes in patients with HFrEF vs HF with preserved ejection fraction (HFpEF), patients with any diagnosis of HFpEF (ICD-10: I50.3X) were prespecified for exclusion so as to focus specifically on the HFrEF phenotype.
For all analyses, the date of the PCR test for SARS-CoV-2 was considered the index date and study baseline. For patients with multiple positive PCR tests for COVID-19, the first positive test was used as the index test. For purposes of analysis, patients with both positive and negative tests were considered to be positive for COVID-19; the date of the positive test was considered the index date.
From this overall study population, 2 sets of analyses involving 2 distinct cohorts were prespecified. Analysis A was designed to assess the clinical implications of prior history of HFrEF with vs without a worsening heart failure event among patients newly diagnosed with COVID-19. Only patients with positive PCR tests for SARS-CoV-2 were included. Patients were then categorized into 1 of 3 mutually exclusive groups as defined by their histories of HF during the 12 months prior to the positive COVID-19 test: (1) worsening HFrEF, defined as a hospitalization for HF or receipt of intravenous (IV) diuretics (either inpatient or outpatient) in the prior 12 months; (2) HFrEF, without worsening; and (3) no history of HF. Analysis B was designed to assess the clinical implications of COVID-19 among the subset of patients with a history of HFrEF. Patients with HFrEF who tested positive for COVID-19 were compared with patients who tested negative. In both Analysis A and Analysis B, patients were included regardless of whether their COVID-19 test occurred in the setting of a hospitalization or in an outpatient setting.
Study Endpoints
For Analysis A among patients with COVID-19, prespecified study endpoints included (1) 30-day all-cause mortality; (2) 90-day all-cause mortality; and (3) composite of all-cause mortality or hospitalization. For Analysis B in patients with HFrEF, endpoints included: (1) 30-day all-cause mortality; (2) 90-day all-cause mortality; and (3) worsening HF, defined as a hospitalization for HF or receipt of IV diuretics (either inpatient or outpatient). Hospitalization for HF was defined as an inpatient claim with primary diagnosis of HF, and IV diuretic use was defined by relevant procedure codes (Supplementary Table 2).
Statistical Analysis
For both analyses (A and B), baseline characteristics were described for each patient group. To assess associations between patient group and each outcome, 3 levels of statistical modeling were employed in all analyses to account for potential confounders: unadjusted, base adjustment (accounting for demographics and comorbidities) and comprehensive adjustment (base adjustment plus adjustment for inpatient vs outpatient status at time of index test for COVID-19). Prespecified covariates in the base-adjusted model included age, gender, race, ethnicity, and medical history (asthma, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, coronary artery disease, history of thrombosis, coronary revascularization, myocardial infarction, obesity, chronic kidney disease, peripheral arterial disease, vascular disease, moderate/severe liver disease, metastatic solid tumor, and atrial or ventricular tachyarrhythmia) (Supplementary Table 3). For all endpoints (30-day mortality, 90-day mortality, composite all-cause mortality or all-cause hospitalization, worsening HF), unadjusted and adjusted log linear models (Poisson distribution, with a log link and a robust error variance) were used to estimate relative risk.
Recognizing that the location of COVID-19 testing (in-hospital vs outpatient) may suggest significant differences in health status at time of testing and that the prognostic interplay between HFrEF and COVID-19 may differ based on the clinical severity of the initial suspected or confirmed COVID-19 presentation, associations with study endpoints were stratified by inpatient vs outpatient location of COVID-19 testing. Thus, in Analysis A, associations between prior HF history and study endpoints were assessed separately in patients testing positive for COVID-19 during inpatient vs outpatient settings. Likewise, in Analysis B, associations between testing positive vs negative for COVID-19 were separately assessed in patients being tested for COVID-19 as inpatients vs outpatients. Inpatient location was defined as an associated inpatient encounter at the time of testing. To capture patients being tested in the emergency department or ambulatory setting and directly admitted to the hospital, inpatient location was also defined as a test in the emergency department or ambulatory setting with an associated inpatient encounter within 3 days. Outpatient location was defined as testing performed in the emergency department or ambulatory setting without an inpatient encounter within 3 days. In both Analyses A and B, interaction p values were calculated to assess for a differential association between patient group and clinical outcome by the location of COVID-19 testing. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), and a 2-tailed p value of 0.05 was considered statistically significant.
Results
Patient Cohort
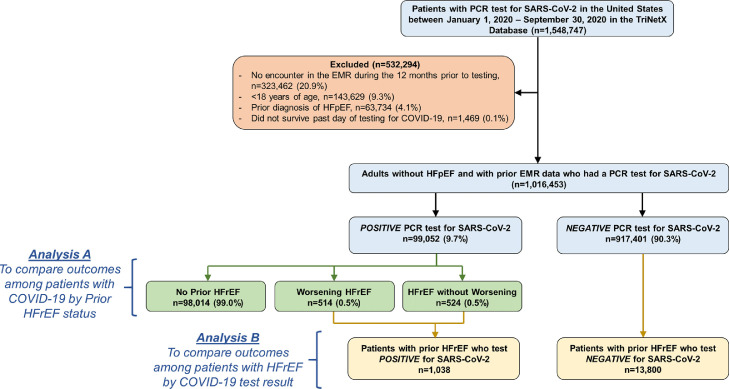
Between January 1, 2020, and September 30, 2020, the study identified 1,548,747 unique patients in the US who received ≥ 1 PCR test for SARS-CoV-2. After excluding patients who did not have at least 1 encounter in EMRs during the 12 months prior to testing, were < 18 years old, had known diagnoses of HFpEF, and died on the date of COVID-19 testing, the final study population included 1,016,453 (65.6%) adults who underwent PCR testing for SARS-CoV-2. Of this sample, 99,052 (9.7%) of patients had positive PCR tests, and 14,838 (1.5%) patients had histories of HFrEF (Fig. 1 ).
Fig. 1.
Selection of the study cohorts. EMR, electronic medical record; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PCR, polymerase chain reaction.
Clinical Outcomes of Patients with COVID-19 by Heart Failure Status (Analysis A)
Patients’ Characteristics According to Prior Heart Failure Status
Among 99,052 patients with positive PCR tests for SARS-CoV-2, 514 (0.5%) had histories of worsening HFrEF, 524 (0.5%) had histories of HFrEF without worsening, and 98,014 (99.0%) patients had no prior histories of HF (Table 1 ). The location of COVID-19 testing varied across groups. The proportion of inpatient diagnoses of COVID-19 was lowest among patients without prior HFrEF (8.9%), intermediate among patients with HFrEF without worsening (22.5%) and highest among patients with worsening HFrEF, in whom the majority of patients were hospitalized due to their index COVID-19 diagnoses (59.3%).
Table 1.
Baseline Characteristics of Patients with COVID-19 by Prior History of Heart Failure
| No HF(n = 98,014) | HFrEF without Worsening (n = 524) | Worsening HFrEF(n = 514) | P Value | |
|---|---|---|---|---|
| Age (years) | 43 (29–58) | 66 (55–76) | 66 (56–75) | <0.001 |
| Female | 56,739 (57.9) | 173 (33.0) | 164 (31.9) | <0.001 |
| Race/ethnicity | <0.001 | |||
| White | 37,337 (38.1) | 182 (34.7) | 235 (45.7) | |
| Black | 14,469 (14.8) | 115 (21.9) | 149 (29.0) | |
| Hispanic | 17,375 (17.7) | 67 (12.8) | 57 (11.1) | |
| Other/unknown | 28,833 (29.4) | 160 (30.5) | 73 (14.2) | |
| Location of SARS-CoV-2 testing | <0.001 | |||
| Inpatient | 8768 (8.9) | 118 (22.5) | 305 (59.3) | |
| Outpatient | 62,195 (63.5) | 219 (41.8) | 120 (23.3) | |
| Unknown | 27,051 (27.6) | 187 (35.7) | 89 (17.3) | |
| Medical history | ||||
| Hypertension | 19,470 (19.9) | 391 (74.6) | 388 (75.5) | <0.001 |
| Coronary artery disease | 2796 (2.9) | 224 (42.7) | 307 (59.7) | <0.001 |
| History of thromboembolism | 1213 (1.2) | 26 (5.0) | 61 (11.9) | <0.001 |
| History of PCI/CABG | 484 (0.5) | 45 (8.6) | 104 (20.2) | <0.001 |
| Myocardial infarction | 879 (0.9) | 90 (17.2) | 186 (36.2) | <0.001 |
| Peripheral artery disease | 1598 (1.6) | 154 (29.4) | 200 (38.9) | <0.001 |
| Vascular disease | 3219 (3.3) | 189 (36.1) | 262 (51.0) | <0.001 |
| Atrial or ventricular tachyarrhythmia/atrial fibrillation | 1619 (1.7) | 185 (35.3) | 223 (43.4) | <0.001 |
| Type 2 diabetes | 9744 (9.9) | 225 (42.9) | 237 (46.1) | <0.001 |
| Chronic kidney disease | 3040 (3.1) | 158 (30.2) | 219 (42.6) | <0.001 |
| Obesity | 11455 (11.7) | 114 (21.8) | 147 (28.6) | <0.001 |
| Asthma | 5287 (5.4) | 36 (6.9) | 40 (7.8) | 0.02 |
| COPD | 7770 (7.9) | 112 (21.4) | 155 (30.2) | <0.001 |
| Moderate/severe liver disease | 296 (0.3) | 2 (0.4) | 15 (2.9) | <0.001 |
| Metastatic solid tumor | 554 (0.6) | 4 (0.8) | 18 (3.5) | <0.001 |
| Laboratory Results | ||||
| White blood cell count (x 103/µL)* | 6.9 (5.4–8.8) | 7.0 (5.5–8.9) | 7.3 (5.7–9.1) | 0.911 |
| Lymphocytes (%)† | 27 (19–34) | 22 (15–29) | 18 (11–26) | <0.001 |
| Hemoglobin (g/dL)‡ | 13.3 (12.0–14.5) | 12.7 (11.3–14.1) | 11.6 (9.9–13.3) | <0.001 |
| Serum creatinine (mg/dL)§ | 0.8 (0.7–1.0) | 1.2 (0.9–1.7) | 1.2 (0.9–1.8) | <0.001 |
| Albumin (g/dL)|| | 4.1 (3.7–4.4) | 3.9 (3.5–4.2) | 3.5 (3.0–4.0) | 0.88 |
Data represent median (quartile 1–quartile 3) or n (%).
There were 37,769, 340 and 478 patients with available data in the No HF, HFrEF without worsening, and Worsening HFrEF groups, respectively.
There were 25,504, 290 and 288 patients with available data in the No HF, HFrEF without worsening, and Worsening HFrEF groups, respectively.
There were 40,264, 404 and 496 patients with available data in the No HF, HFrEF without worsening, and Worsening HFrEF groups, respectively.
There were 40,824, 419 and 476 patients with available data in the No HF, HFrEF without worsening, and Worsening HFrEF groups, respectively.
There were 24,933, 260 and 301 patients with available data in the No HF, HFrEF without worsening, and Worsening HFrEF groups, respectively.
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention.
Patients without HF tended to be younger (median age 43 years vs 66 years for worsening HFrEF and 66 years for HFrEF without worsening) and were more likely to be female (57.9% vs 33.0% and 31.9%). Across all 3 groups, patients without HF tended have lower rates of comorbidities, higher hemoglobin and lymphocyte values, higher serum albumin values, and lower serum creatinine levels.
Comparing patients with HFrEF with vs without histories of worsening, patients were generally similar in terms of demographics, but those with worsening HFrEF tended to have higher rates of cardiac and noncardiac comorbidities. Compared with HFrEF without worsening, patients with worsening HFrEF tended to have lower lymphocyte percentage, serum hemoglobin and serum albumin but generally had similar serum creatinine levels.
Clinical Outcomes of Patients with COVID-19 According to Prior Heart Failure Status
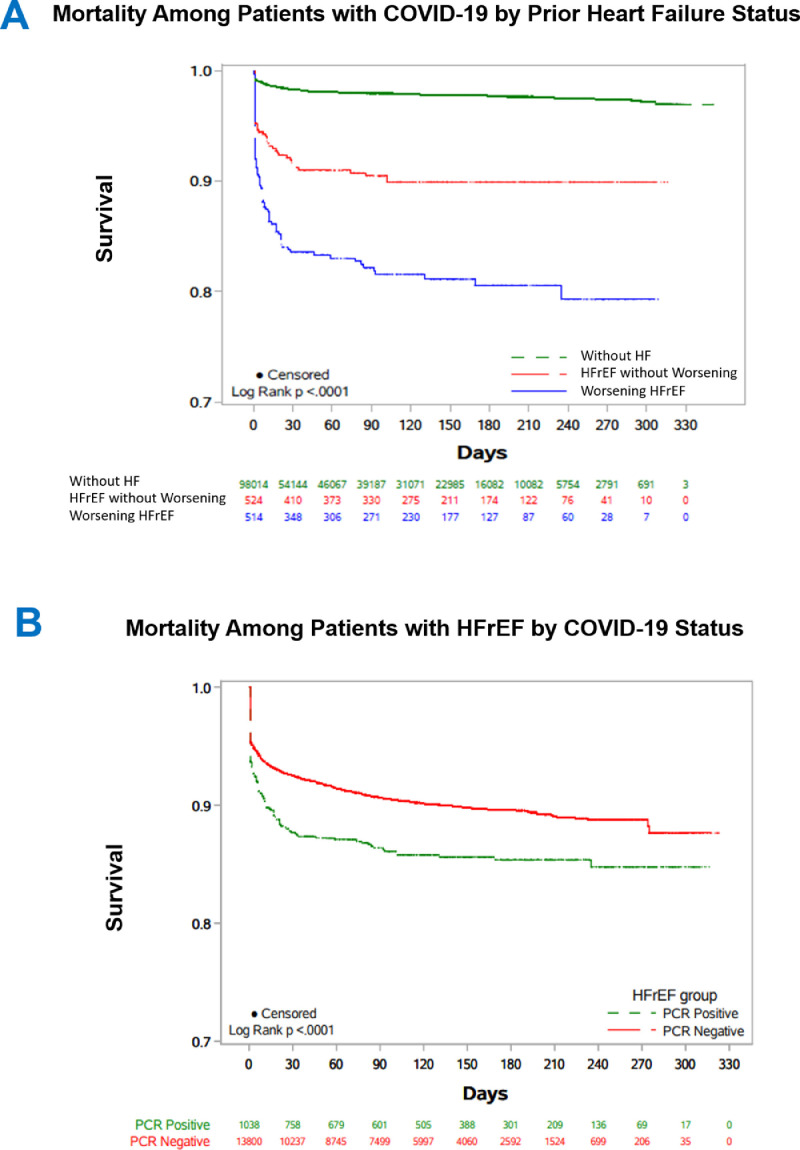
Rates of 30-day and 90-day mortality were lowest among patients without HF (1.4% and 1.6%), intermediate among patients with HFrEF without worsening (8.0% and 9.0%), and highest among patients with worsening HFrEF (15.6% and 16.5%) (Table 2 ) (Fig. 2 ). After base adjustment, relative to patients without HF, worsening HFrEF was associated with higher risks of 30-day (risk ratio [RR] 1.87, 95% confidence interval [CI] 1.45–2.43) and 90-day mortality (RR 1.69, 95% CI 1.32-2.18), whereas HFrEF was not. However, after further adjustment for inpatient/outpatient location of COVID-19 testing, associations with excess mortality rates were similar for worsening HFrEF (30-day mortality: RR 1.42, 95% CI 1.10–1.83; 90-day mortality: 1.28, 95% CI 1.00–1.63) and HFrEF without worsening (30-day mortality: RR 1.33, 95% CI 0.96–1.84; 90-day mortality: 1.29, 95% CI 0.96–1.75), and generally had marginal statistical significance (Table 2) (Supplementary Table 4).
Table 2.
Mortality and Hospitalization of Patients with COVID-19 According to Prior Heart Failure Status
| Risk Ratio (95% CI) |
||||
|---|---|---|---|---|
| Event Rate (%) | Unadjusted | Base Adjustment* | Base Adjustment + Inpatient/Outpatient Diagnosis† | |
| 30-Day mortality | ||||
| No prior HF | 1.4% | Reference | Reference | Reference |
| HFrEF without Worsening | 8.0% | 5.71 (4.20–7.76) P < 0.001 |
1.20 (0.87–1.66) P = 0.26 |
1.33 (0.96–1.84) P = 0.078 |
| Worsening HFrEF | 15.6% | 11.08 (8.84–13.88)P < 0.001 | 1.87 (1.45–2.43) P < 0.001 |
1.42 (1.10–1.83) P = 0.007 |
| 90-Day mortality | ||||
| No prior HF | 1.6% | Reference | Reference | Reference |
| HFrEF without worsening | 9.0% | 5.69 (4.26–7.61 P < 0.001 |
1.16 (0.86–1.57) P = 0.34 |
1.29 (0.96–1.75) P = 0.095 |
| Worsening HFrEF | 16.5% | 10.50 (8.44–13.06) P < 0.001 |
1.69 (1.32–2.18) P < 0.001 |
1.28 (1.00–1.63) P = 0.052 |
| All-cause Mortality or Hospitalization‡ | ||||
| No prior HF | 12.1% | Reference | Reference | Reference |
| HFrEF without worsening | 31.9% | 2.64 (2.27–3.08) P < 0.001 |
1.05 (0.90–1.23) P = 0.53 |
1.10 (0.94–1.29) P = 0.22 |
| Worsening HFrEF | 56.8% | 4.71 (4.19–5.29) P < 0.001 |
1.34 (1.18–1.53) P < 0.001 |
1.05 (0.92–1.20) P = 0.43 |
Adjusted for age, sex, race, ethnicity, asthma, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, coronary artery disease, history of thrombosis, coronary revascularization, myocardial infarction, obesity, chronic kidney disease, peripheral artery disease, vascular disease, moderate/severe liver disease, metastatic cancer, and atrial fibrillation/atrial or ventricular tachyarrhythmia.
Includes covariates in base-adjusted model, with addition of location of COVID-19 test/diagnosis (ie, inpatient or outpatient or unknown).
Median (IQR) follow-up was 48 (2–144) days.
CI, confidence interval; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range.
Fig. 2.
Associations between COVID-19 and HFrEF for all-cause mortality. Kaplan-Meier curves of all-cause mortality among patients diagnosed with COVID-19 by prior heart failure status (Panel A) and Kaplan-Meier curves of all-cause mortality among patients with HFrEF who tested positive vs negative for COVID-19 (Panel B). HFrEF, heart failure with reduced ejection fraction.
For the composite all-cause mortality or hospitalization endpoint, median (interquartile range [IQR]) follow-up was 48 (2–144) days. Rates of the composite endpoint increased in graded fashion across the groups of no HF (12.1%), HFrEF without worsening (31.9%) and worsening HFrEF (56.8%). After base adjustment, compared with patients without HF, worsening HFrEF was independently associated with higher risk of the composite endpoint (RR 1.34, 95% CI 1.18–1.53), and HFrEF without worsening was not (RR 1.05, 95% CI 0.90–1.23). After additionally accounting for inpatient/outpatient location of COVID-19 testing, neither HFrEF with or without worsening was independently associated with the composite endpoint (Table 2) (Supplementary Table 5).
Clinical Outcomes, Prior Heart Failure Status and Location of COVID-19 Presentation
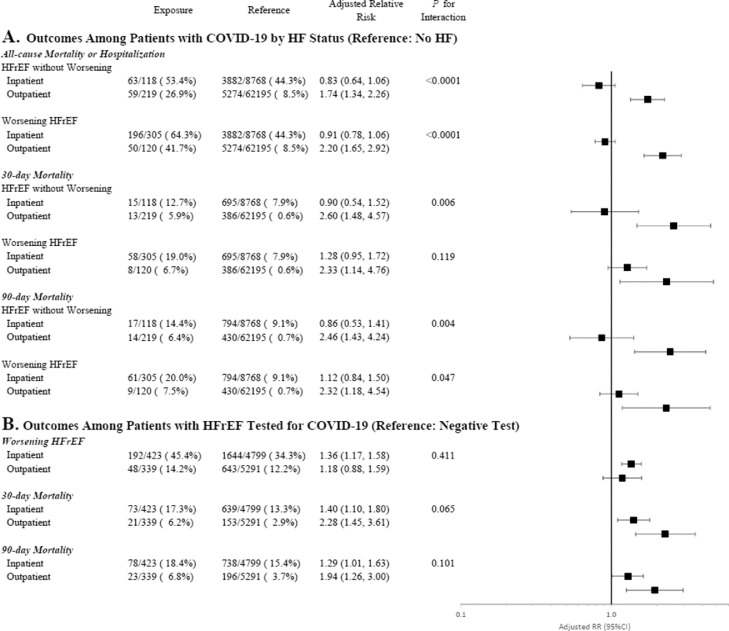
In stratified analyses accounting for inpatient vs outpatient testing for COVID-19, the association between prior HF status and each clinical outcome differed significantly by location of COVID-19 diagnosis (Fig. 3 A). For each endpoint, excess risk associated with HFrEF (with or without worsening) was driven by patients who were diagnosed with COVID-19 as outpatients. In the subset of patients diagnosed with COVID-19 during a hospitalization, although HFrEF with or without worsening were both associated with substantially higher unadjusted event rates, there was no independent association with mortality or composite outcomes after adjustment for potential confounders.
Fig. 3.
Interaction between prior HF status, COVID-19 status, and clinical outcomes by location of testing for COVID-19. Forest plots display risk of clinical outcomes by heart failure (HF) status among patients who tested positive for COVID-19 (Panel A) and clinical outcomes among patients with HFrEF who tested positive vs negative for COVID-19 (Panel B). Analyses excluded patients with unknown locations of COVID-19 testing.
Clinical Outcomes Among Patients with HFrEF by COVID-19 Status (Analysis B)
Patient Characteristics by COVID-19 Status
Among 14,838 patients with HFrEF who underwent testing for COVID-19, 1038 (7.0%) patients tested positive, and 13,800 (93.0%) tested negative (Table 3 ). Irrespective of test results, patients were generally similar with regard to age, gender, comorbidities, and laboratory findings. However, patients testing positive were more likely to be Black or of Hispanic ethnicity and less likely to be white.
Table 3.
Baseline Characteristics in Patients with HFrEF Tested for COVID-19
| Positive COVID-19 Test (n = 1,038) | Negative COVID-19 Test (n = 13,800) | P Value | |
|---|---|---|---|
| Age (years) | 66 (55–75) | 67 (57–76) | 0.17 |
| Female | 337 (32.5) | 4662 (33.8) | 0.18 |
| Race/ethnicity | <0.001 | ||
| White | 417 (40.2) | 8072 (58.5) | |
| Black | 264 (25.4) | 2508 (18.2) | |
| Hispanic | 124 (11.9) | 603 (4.4) | |
| Other/unknown | 233 (22.4) | 2617 (19.0) | |
| Location of SARS-CoV-2 testing | <0.001 | ||
| Inpatient | 423 (40.8) | 4799 (34.8) | |
| Outpatient | 339 (32.7) | 5291 (38.3) | |
| Unknown | 276 (26.6) | 3710 (26.9) | |
| Medical History | |||
| Hypertension | 779 (75.0) | 10890 (78.9) | 0.003 |
| Coronary artery disease | 531 (51.2) | 8188 (59.3) | <0.001 |
| History of thrombosis | 87 (8.4) | 1394 (10.1) | 0.07 |
| History of PCI/CABG | 140 (13.5) | 2361 (17.1) | 0.003 |
| Myocardial infarction | 276 (26.6) | 4380 (31.7) | <0.001 |
| Peripheral artery disease | 354 (34.1) | 5264 (38.1) | 0.001 |
| Vascular disease | 451 (43.4) | 6527 (47.3) | 0.02 |
| Atrial or ventricular tachyarrhythmia/atrial fibrillation | 408 (39.3) | 6483 (47.0) | <0.001 |
| Type 2 diabetes | 462 (44.5) | 5508 (39.9) | 0.004 |
| Chronic kidney disease | 377 (36.3) | 4790 (34.7) | 0.29 |
| Obesity | 261 (25.1) | 3502 (25.4) | 0.87 |
| Asthma | 76 (7.3) | 1075 (7.8) | 0.59 |
| COPD | 267 (25.7) | 4268 (30.9) | <0.001 |
| Moderate/severe liver disease | 17 (1.6) | 307 (2.2) | 0.21 |
| Metastatic solid tumor | 22 (2.1) | 511 (3.7) | 0.008 |
| Laboratory Results | |||
| White blood cell count (x 103/µL)* | 7.1 (5.6–9.0) | 7.5 (5.9–9.7) | 0.45 |
| Lymphocytes (%)† | 20.0 (13.0–28.0) | 19.1 (12.3–27.0) | 0.052 |
| Hemoglobin (g/dL)‡ | 12.2 (10.5–13.6) | 12.2 (10.3–13.8) | 0.84 |
| Serum creatinine (mg/dL)§ | 1.2 (0.9–1.7) | 1.1 (0.9–1.5) | <0.001 |
| Albumin (g/dL)|| | 3.7 (3.2–4.1) | 3.7 (3.2–4.1) | 0.75 |
Data represent median (quartile 1 – quartile 3) or n (%).
There were 818 and 11,834 patients with available data in the positive test and negative test groups, respectively.
There were 578 and 8377 patients with available data in the positive test and negative test groups, respectively.
There were 900 and 12,277 patients with available data in the positive test and negative test groups, respectively.
There were 895 and 11703 patients with available data in the positive test and negative test groups, respectively.
There were 561 and 7801 patients with available data in the positive test and negative test groups, respectively.
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention.
Clinical Outcomes of Patients with HFrEF Testing Positive vs Negative for COVID-19
Rates of 30-day and 90-day mortality were higher among patients with HFrEF who tested positive for COVID-19 (11.8% and 12.7%) than among HFrEF patients who tested negative (7.2% and 8.5%) (Table 4 ) (Fig. 2). After base statistical adjustment, testing positive for COVID-19 was independently associated with higher risk of mortality (30-day mortality: RR 1.79, 95% CI 1.48–2.17; 90-day mortality: RR 1.63, 95% CI 1.36–1.96). These findings persisted after further adjustment for inpatient vs outpatient location of COVID-19 testing (30-day mortality: 1.67, 95% CI 1.38–2.02; RR 1.52, 95% CI 1.27–1.83) (Table 4) (Supplementary Table 6).
Table 4.
Mortality and Worsening Heart Failure in Patients with HFrEF Tested for COVID-19
| Risk Ratio (95% CI) |
||||
|---|---|---|---|---|
| Event Rate (%) | Unadjusted | Base Adjustment* | Base Adjusted + Inpatient/Outpatient Diagnosis† | |
| 30-Day mortality | ||||
| Negative COVID-19 Test | 7.2% | Reference | Reference | Reference |
| Positive COVID-19 Test | 11.8% | 1.64 (1.35–1.97) P < 0.001 |
1.79 (1.48–2.17) P < 0.001 |
1.67 (1.38–2.02) P < 0.001 |
| 90-Day mortality | ||||
| Negative COVID-19 Test | 8.5% | Reference | Reference | Reference |
| Positive COVID-19 Test | 12.7% | 1.50 (1.25–1.79) P < 0.001 |
1.63 (1.36–1.96) P < 0.001 |
1.52 (1.27–1.83) P < 0.001 |
| Worsening Heart Failure‡ | ||||
| Negative COVID-19 Test | 19.0% | Reference | Reference | Reference |
| Positive COVID-19 Test | 26.0% | 1.37 (1.21–1.56) P < 0.001 |
1.44 (1.27–1.64) P < 0.001 |
1.33 (1.17–1.51) P < 0.001 |
Adjusted for age, sex, race, ethnicity, asthma, type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, coronary artery disease, history of thrombosis, coronary revascularization, myocardial infarction, obesity, chronic kidney disease, peripheral artery disease, vascular disease, moderate/severe liver disease, metastatic cancer, and atrial fibrillation/atrial or ventricular tachyarrhythmia.
Includes covariates in base-adjusted model, with addition of location of COVID-19 test/diagnosis (ie, inpatient or outpatient or unknown).
Median (interquartile range) follow-up was 104 (27–162) days.
CI, confidence interval; HFrEF, heart failure with reduced ejection fraction.
For the worsening HF endpoint, median (IQR) follow-up was 104 (27–162) days. Unadjusted rates of worsening HF were higher among patients positive for COVID-19 (26.0%) than among those negative for COVID-19 (19.0%). After statistical adjustment, including location of COVID-19 testing, testing positive for COVID-19 was independently associated with excess subsequent risk of worsening HF (RR 1.33, 95% CI 1.17–1.51) (Table 4) (Supplementary Table 7) (Supplementary Fig. 1).
Clinical Outcomes, COVID-19 Test Results and Location of Clinical Presentation
Although not reaching formal statistical significance, there were borderline statistical interactions whereby the relationship between testing positive for COVID-19 and 30-day and 90-day mortality differed for patients who presented to the inpatient vs the outpatient setting (Fig. 3B). Although a positive COVID-19 test was independently associated with excess risk of death across both locations, the magnitude of excess risk was higher among patients tested as outpatients. In contrast, for the worsening HF endpoint, the higher clinical risk with a positive COVID-19 test was consistent, irrespective of inpatient vs outpatient testing.
Discussion
In this large cohort of US adults tested for COVID-19 in ambulatory and hospitalized settings, there are several notable findings. First, among patients testing positive for COVID-19, patients with worsening HFrEF represented a particularly high-risk subset and were more likely to require hospitalization at the time of COVID-19 diagnosis. However, although unadjusted rates of mortality and hospitalization were markedly higher among patients with HFrEF, after comprehensive risk adjustment for potential confounders, neither a history of worsening HFrEF nor a history of HFrEF without worsening was consistently associated with worse clinical outcomes in the setting of concurrent COVID-19. Second, among patients with HFrEF, relative to patients who tested negative, a diagnosis of COVID-19 was independently associated with substantially heightened risk of mortality and subsequent worsening HF. Third, these data highlight the importance of the presenting clinical setting on the interplay between COVID-19 and HFrEF. Specifically, among patients with COVID-19, a history of stable or worsening HFrEF carried independent prognostic value predominantly when COVID-19 was diagnosed as an outpatient and less so when patients were hospitalized for COVID-19. Likewise, among patients with established HFrEF who received COVID-19 testing, the relative increase risk of death with a positive test was highest among patients diagnosed in the ambulatory setting.
To our knowledge, we present the first large-scale analysis evaluating the bidirectional prognostic interplay between COVID-19 and HFrEF. In this regard, several strengths and novel features of this analysis warrant consideration. First, we examined a unique population tested for COVID-19 in either the inpatient or the outpatient setting, allowing a unique comparison of the prognostic interplay of testing and diagnosis of COVID-19 among patients initially receiving in-hospital vs ambulatory care. Although prior studies have centered almost exclusively on patients hospitalized or presenting to the emergency department who are likely to be highly symptomatic, comparatively little is known regarding the implications of ambulatory COVID-19 testing for patients with HFrEF or the anticipated clinical courses for patients who are diagnosed with COVID-19 but initially have milder symptoms not requiring hospitalization.3 , 4 , 7, 8, 9, 10 Second, in a large population of > 1 million patients tested for SARS-CoV-2 in the US in 2020, we report both the overall test positivity rate (9.7%) and the test positivity rate among patients with established HFrEF (7.0%). Although potentially subject to confounding and requiring further validation, the lower test positivity rate among patients with HFrEF does not suggest that patients with HFrEF face heightened vulnerability to acquiring COVID-19. Third, although prior studies have examined clinical outcomes in patients with HF and COVID-19, comparator groups have generally been loosely defined as patients with HF without COVID-19.4 In contrast, by exclusively studying a population that underwent PCR testing, the present analysis offers a more secure control group of patients confirmed to be COVID-19-negative. Likewise, given that all patients receiving tests presumably had some degree of new or worsening symptoms or clinical suspicion compatible with active COVID-19 infection, the current study may better address potential confounding by indication stemming from the decision to test for COVID-19. Fourth, recognizing that clinical risk may vary within the broad HFrEF population, the current study subcategorized patients into those with vs without a recent worsening HF episode (ie, HF hospitalization or IV diuretic administration). In doing so, the results highlight that although a graded relationship in unadjusted clinical event rates was observed, the incremental and independent prognostic value of a worsening HF event seen in prior analyses may not necessarily extend to the setting of active COVID-19 infection.11 , 12 Last, to the best of our knowledge, this analysis is the first to evaluate the association between COVID-19 and longer-term HF-specific outcomes in clinical practice. Among patients with established HFrEF, compared with testing negative, a diagnosis of COVID-19 was independently associated with a 33% greater risk of a worsening HF event over a median follow-up of 3.5 months. These data highlight the potential impact of COVID-19 on HFrEF disease progression, even after recovery from acute infection, and are consistent with other data suggesting the potential for longer-term cardiac consequences of the disease.13 , 14
Prior analyses have documented high rates of in-hospital mortality among patients with histories of HF who have been hospitalized due to COVID-19. For example, an analysis from New York City studying an early and particularly severe phase of the pandemic through June 2020 reported exceptionally high event rates for patients hospitalized due to COVID-19, with in-hospital mortality rates of 40.0% and 24.9% for patients with and without histories of HF, respectively.3 High event rates were also reported in a more recent analysis by Bhatt et al. that extended through September 2020, with an in-hospital mortality rate of 24.2% among patients hospitalized for COVID-19 with histories of HF, compared with a mortality rates of 14.2% among patients without HF.4 In the current analysis, in the subset that with inpatient diagnoses of COVID-19, we observed lower, albeit still markedly elevated, rates of 30-day mortality. Specifically, 30-day mortality was 19.0% and 12.7% for hospitalized patients with histories of worsening HF and HF without worsening, respectively, as compared with 7.9% for patients without HF. Compared with the study by Bhatt et al. that examined a similar timeframe, reasons for lower mortality rates among hospitalized patients in the present study are unclear but could be related to (1) exclusion of patients with HFpEF, (2) differences in COVID-19 definition based on PCR results as opposed to diagnoses codes, or (3) differences in case-mix for primary clinical reasons for hospitalization (ie, hospitalized for primary or secondary diagnosis of COVID-19 in the prior study vs being diagnosed with COVID-19 during hospitalization in current study).
The low rate of mortality we observed among US patients diagnosed with COVID-19 in the ambulatory setting is notable. Indeed, we found risks of 90-day mortality among ambulatory patients without HF of < 1%. However, among patients with HFrEF, the adjusted relative risk of death compared with patients without HF was particularly high when patients with HFrEF were diagnosed with COVID-19 as outpatients. Among patients diagnosed with COVID-19 as inpatients, differences in adjusted mortality risk between patients with HFrEF and patients without HF were less pronounced. Although future analyses are needed to confirm the results of this subgroup analysis, these findings may speak to the ability of a history of HF to drive prognosis in patients presenting with mild or no COVID-19 symptoms. By comparison, it is possible that patients hospitalized with severe COVID-19 symptoms may have outcomes more strongly determined by the infection itself, and that HFrEF may have lesser prognostic importance after rigorously accounting for confounding risk factors and clinical markers of severe illness.
The results for patients with HFrEF who test positive vs negative for COVID-19 warrant mention. Although prior studies have supported adverse outcomes relative to HF patients without COVID-19, the current analysis exclusively examining patients receiving testing may more strongly support the independent prognostic consequences of COVID-19, as compared with other cardiorespiratory processes that may mimic COVID-19 symptoms. Likewise, similar to Analysis A, Analysis B found statistically significant interactions for mortality according to the location of COVID-19 testing. Despite lower absolute risks, the relative risk of mortality due to COVID-19 was particularly high in patients with HFrEF who were tested in the outpatient setting.
Limitations
Limitations of this analysis should be noted. First, this retrospective observational analysis cannot definitively prove cause-effect relationships, and residual or unmeasured confounding may remain. Likewise, data on vital signs, laboratory tests, examination findings, symptoms of HF and/or COVID-19, medications, and procedures were not available and, thus, could not be included as covariates in adjusted models. Moreover, this study cannot decipher the reasons patients presented for COVID-19 testing (eg, symptoms of COVID-19, close contact to confirmed case, requirement for inpatient hospitalization). Inpatient vs outpatient location of COVID-19 testing was added to models as a potential surrogate of clinical severity at presentation, but this may not necessarily capture the complete clinical risk reflected by vital signs and objective clinical characteristics. Second, data concerning location of COVID-19 testing were not available for all patients in this sample. We cannot exclude the potential of selection bias among patients with available data. Third, history of HFrEF was defined by administrative coding, and some degree of misclassification may have occurred. Fourth, despite sampling > 1 million patients tested for COVID-19, the proportions and absolute numbers of patients with HFrEF without worsening and with worsening HFrEF were modest. In the setting of some outcome results that have marginal Pvalues and wide confidence intervals, a larger sample of patients with HFrEF may have provided more statistical power and greater precision of risk estimates. Fifth, given established differences in pathophysiology and clinical profile, this analysis excluded patients with HFpEF and focused solely on those with HFrEF. The degree to which the current findings for HFrEF extend to patients with HFpEF requires future dedicated study in that population. Sixth, the current study prespecified exclusion of patients who died on the day of COVID-19 testing so as to ensure that all patients were alive at the beginning of the study and had at least some period of follow-up. Nonetheless, this may have introduced a bias, and the current findings should be interpreted in this context. Last, the study period included patients in the early pandemic period in 2020 prior to availability of vaccines. The degree to which these findings generalize to patients vaccinated with COVID-19 or to the more recent omicron variant of SARS-CoV-2 is unclear.
Conclusions
In this large cohort of US patients tested for COVID-19 in the inpatient or outpatient setting, among patients testing positive, a history of HFrEF (with or without recent clinical worsening) was not independently associated with excess risk of mortality and hospitalization. However, this association varied by location of COVID-19 testing, with HFrEF associated with substantial clinical risk among patients tested for COVID-19 as outpatients. Among patients with histories of HFrEF, compared with testing negative, a positive test for COVID-19 was independently associated with higher risk of death and worsening HF, with interaction testing identifying a nominal trend towards higher mortality risk being driven by patients tested for COVID-19 as outpatients. These findings support the strong prognostic interplay between COVID-19 and HFrEF, especially in patients who present for COVID-19 testing as outpatient and have potentially milder initial symptoms.
Declaration of Competing Interest
SJG has received research support from the Duke University Department of Medicine Chair's Research Award, American Heart Association, National Heart Lung and Blood Institute, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck & Co., Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim/Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; has received speaker fees from Boehringer Ingelheim; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, PharmaIN, Roche Diagnostics, Sanofi, Urovant Pharmaceuticals, and Vifor; DL, LY, XT, and JEB are employees of Merck Sharp & Dohme, a subsidiary of Merck & Co., Kenilworth, NJ, US.
Acknowledgments
Funding
This study was supported by Merck Sharp & Dohme, a subsidiary of Merck & Co., Kenilworth, NJ, US.
Acknowledgments
The authors thank Dr. Amy Puenpatom for statistical and study design consultation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2022.05.001.
Appendix. Supplementary materials
References
- 1.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation: searching for evidence from a new disease. Eur J Heart Fail. 2020;22:957–966. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumery K, Seo A, Jiang L, Choudhary G, Shah NR, Rudolph JL, et al. Outcomes of Coronavirus disease-2019 among veterans with pre-existing diagnosis of heart failure. ESC Heart Fail. 2021;8:2338–2344. doi: 10.1002/ehf2.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19: results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020;22:2238–2247. doi: 10.1002/ejhf.2052. [DOI] [PubMed] [Google Scholar]
- 9.Rey JR, Caro-Codon J, Rosillo SO, Iniesta AM, Castrejon-Castrejon S, Marco-Clement I, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Task force for the management of COVID-19 of the European Society of Cardiology: ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2: care pathways, treatment, and follow-up. Eur Heart J. 2022;43:1059–1103. doi: 10.1093/eurheartj/ehab697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon SD, Claggett B, Packer M, Desai A, Zile MR, Swedberg K, et al. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM-HF Trial. JACC Heart Fail. 2016;4:816–822. doi: 10.1016/j.jchf.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart: is heart failure the next chapter? JAMA Cardiol. 2020;5:1216–1217. doi: 10.1001/jamacardio.2020.3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.