Abstract
Background & Aims
Gastrointestinal cancer risk is influenced by the presence of metabolic syndrome (MetS). However, previous epidemiologic studies lacked full serological biomarker data for the classification of MetS, and the interaction of MetS with germline cancer risk variants is unknown.
Methods
We investigated the associations between MetS and gastrointestinal cancer risk (overall, colorectal, pancreatic, esophageal adenocarcinoma, esophageal squamous cell carcinoma, stomach cardia, stomach non-cardia, hepatocellular carcinoma, and intrahepatic bile duct cancer) in 366,016 United Kingdom Biobank participants with comprehensive serum biomarker and genotype data. MetS status was determined by 3 different definitions at baseline, and, in 15,152 participants, at a repeat assessment after a median of 4.3 years of follow-up. Multivariable hazard ratios and 95% confidence intervals for cancer outcomes were estimated using Cox proportional hazards models. Analyses stratified by polygenic risk score were conducted for colorectal and pancreatic cancers.
Results
During a median follow-up of 7.1 years, 4238 incident cases of a gastrointestinal cancer occurred. MetS at baseline was associated with higher risk of overall gastrointestinal cancer by any definition (hazard ratio, 1.21; 95% confidence interval, 1.13–1.29, harmonized definition). MetS was associated with increased risks of colorectal cancer, colon cancer, rectal cancer, hepatocellular carcinoma, pancreatic cancer in women, and esophageal adenocarcinoma in men. Associations for colorectal cancer and pancreatic cancer did not differ by polygenic risk score strata (P-heterogeneity 0.70 and 0.69, respectively), and 80% of participants with MetS at baseline retained this status at the repeat assessment.
Conclusions
These findings underscore the importance of maintaining good metabolic health in reducing the burden of gastrointestinal cancers, irrespective of genetic predisposition.
Keywords: Cancer Genetic Risk, Cancer Prevention, Gastrointestinal Neoplasms, Molecular Epidemiology
Abbreviations used in this article: AGM, abnormal glucose metabolism; BP, blood pressure; CI, confidence interval; HbA1c, glycated hemoglobin; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; HR, hazard ratio; IBDC, intrahepatic bile duct cancer; IDF 2005, International Diabetes Federation, 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III; PRS, polygenic risk score; UK, United Kingdom
Graphical abstract
What You Need to Know.
Background
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities that is reported to be a risk factor for some gastrointestinal cancers. However, the use of inconsistent methods or proxies for recognized MetS definitions have limited previous cancer studies.
Findings
Prevalent MetS, as defined by standard molecular criteria, was associated with increased risks of colorectal cancer, colon cancer, rectal cancer, hepatocellular carcinoma, pancreatic cancer, and esophageal adenocarcinoma. For colorectal cancer and pancreatic cancer, associations did not vary across strata of polygenic risk score.
Implications for patient care
Given that long-term MetS status is unlikely to change in the absence of intervention, these findings highlight the importance of maintaining good metabolic health in reducing the burden of gastrointestinal cancers and developing preventative strategies.
Metabolic syndrome (MetS) refers to the simultaneous presence of several metabolic abnormalities, including abdominal obesity, abnormal glucose metabolism, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, and hypertension.1 The presence of these abnormalities promotes insulin resistance and therefore increases risk of clinical diabetes.2, 3, 4 MetS is a proposed risk factor for developing specific gastrointestinal cancers, including colon cancer, hepatocellular carcinoma (HCC),3,5, 6, 7, 8, 9, 10, 11 and pancreatic cancer in women.3,12 The relationships between MetS and other less common gastrointestinal cancers, such as stomach cancer and intrahepatic bile duct cancer (IBDC), are less clear as relatively few studies have been conducted to date.
Methods used to define MetS in previous cancer studies have varied, with inconsistent associations reported depending on whether recognized criteria or proxy indicators were used to replace original data (eg, using prevalent diabetes status in place of circulating glucose markers).3,13 Therefore, additional large-scale studies with high-quality prediagnostic epidemiologic, biomarker, and clinical data are needed to comprehensively examine the MetS and gastrointestinal cancer association. In addition, the availability of extensive genotyping data in large cohorts allows polygenic risk scores (PRS) to be derived, which may improve prediction of cancer risk at the population level,14 particularly where there is evidence suggesting an interaction between PRS and lifestyle or environmental risk factors. To our knowledge, the interaction between PRS and MetS and its association with cancer risk has not previously been examined.
In this study, we leverage the wealth of molecular measurements available in the United Kingdom (UK) Biobank prospective cohort to investigate the associations between MetS and risk of gastrointestinal cancers (overall, colorectal, pancreatic, esophageal adenocarcinoma and squamous cell carcinoma, stomach cardia and non-cardia, HCC, and IBDC). The availability of data on circulating concentrations of triglycerides, HDL cholesterol, and glycated hemoglobin (HbA1c) for the majority of participants at recruitment allowed us to fully adhere to the standard criteria and cut points used for MetS definitions and not rely on proxy indicators as previous studies have done. In addition, we constructed PRS for colorectal cancer and pancreatic cancer and examined the associations between MetS and these malignancies according to genetic risk strata.
Methods
Study Population
The UK Biobank is a large cohort of 502,656 adults initiated in 2006 that aims to investigate the genetic, lifestyle, and environmental causes of a range of diseases.15,16 Ethical approval was obtained from the North West Multicentre Research Ethics Committee, the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. All participants provided written informed consent. The present study was undertaken under application number 25897. At baseline, participants completed questionnaire on socio-demographics (including age, sex, education, and Townsend deprivation score), health and medical history, lifestyle exposures (including smoking habits, dietary intakes, and alcohol consumption), early life exposures, and medication use. Physical measurements, including weight, height, and waist circumference, were taken. Systolic and diastolic blood pressure (BP) was measured from 2 separate automated readings and an average taken. Around 20,000 participants attended a repeat assessment visit between 2012 and 2013. Exclusions were made for prevalent cancer at recruitment (n = 30,296), missing MetS component data (n = 106,344), and voluntary withdrawal from the study (n = 44), leaving a final sample of 366,016 participants.
Laboratory Methods
Blood samples (non-fasting) were collected from all participants at each assessment. Serum concentrations of triglycerides and HDL cholesterol were determined by a chemiluminescent immunoassay on a Beckman Coulter DXI 800 analyzer (Beckman Coulter, High Wycombe, UK). Coefficients of variation (CVs) of measurement ranged from 1.7% to 2.3%. HbA1c levels were determined in erythrocytes using a Variant II Turbo 2.0 high-performance liquid chromatography analyzer (Bio-Rad, Watford, UK), with CVs of 1.5% to 2.1%. Methods and quality control have previous been described.17 Genotyping was performed on the UKB Affymetrix Axiom array or the UK BiLEVE array18 with imputation using the Haplotype Reference Consortium as the main reference panel, supplemented with the UK10K and 1000 Genomes phase 3 reference panels.
Assessment of Cancer Outcome
Incident cancer cases and cancer cases recorded first in death certificates within the UK Biobank cohort were identified through linkage to national cancer and death registries. Complete follow-up was available through March 31, 2016, for England and Wales and October 31, 2015, for Scotland. Cancer incidence data were coded using the Tenth Revision of the International Classification of Diseases. Gastrointestinal cancers included colon cancers (C18), rectal cancers (C19-20), esophageal adenocarcinomas and squamous cell carcinomas (C15), gastric cardia (C16.0) and non-cardia (C16.1-16.6) cancers, pancreatic cancers (C25), hepatocellular carcinomas (C22.0), and intrahepatic bile duct cancers (C22.1).
Components and Definition of Metabolic Syndrome
The components of MetS are abdominal obesity, elevated circulating triglycerides, reduced circulating HDL cholesterol, abnormal glucose metabolism (AGM), and elevated BP (Supplementary Table 1). MetS status were computed based on 3 definitions: (1) the latest harmonized definition (any 3 of the components, abdominal obesity defined as per harmonized criteria),19 the original National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATPIII) definition which used stricter cut points for abdominal obesity,1 and the International Diabetes Federation (IDF) 2005 definition (abdominal obesity required, plus any 2 of the other components).20 Abdominal obesity was defined as waist circumference ≥102 cm or ≥88 cm (NCEP-ATPIII) or ≥94 cm and ≥80 cm (IDF 2005) in men and women, respectively. Triglycerides were considered elevated if measured at ≥1.7 mmol/L. Reduced HDL was defined as ≤1.03 mmol/L in men and ≤1.29 mmol/L in women, or regular use of cholesterol-lowering medication. AGM was defined if HbA1c ≥5.7%, regardless of diabetes status. Elevated BP was defined as ≥130 mm/Hg for systolic BP and ≥85 mm/Hg for diastolic BP, previously diagnosed high BP, or regular use of BP-lowering medication.
Calculation of Polygenic Risk Scores for Colorectal and Pancreatic Cancer
We calculated PRS for colorectal cancer and pancreatic cancer for 363,294 (99.3%) of the eligible participants. These accounted for the majority of gastrointestinal cancers diagnosed in UK Biobank and the assembly of PRS that are strongly associated with cancer risk (hazard ratio [HR] per standard deviation increase in PRS = 1.4–1.5) has recently been described.21 PRS used single nucleotide polymorphisms (SNPs) that have previously been associated with colorectal cancer (n = 99) and pancreatic cancer (n = 26) at the genome-wide significance level (P <5 × 10-8).22,23 These were selected for independence (linkage disequilibrium r2 < 0.3), high imputation score, absence of allele mismatches or minor allele frequency differences >0.10 relative to the 1000 Genomes reference population, and palindromic SNPs with MAF ≥0.45. Genotypes for risk SNPs were extracted for each chromosome from imputed UK Biobank genotyping data using plink2 software, converted to dosages, and inverse variance weights applied for risk alleles. PRS for individuals were calculated as the sum of these weighted dosages. Participants were stratified into low, medium, and high PRS groups using 20th and 80th percentile cut points.
Statistical Analysis
HRs and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models. Time at entry was age at recruitment. Exit time was age at first diagnosis of incident cancer, loss to follow-up or death, or the last date at which follow-up was considered complete.
Multivariable models were adjusted for total physical activity (<10, 10–20, 20–40, 40–60, >60 MET hours/week), height (cm, continuous), alcohol consumption frequency (never, special occasions only, 1–3 times/month, 1–2 times per week, 3–4 times/week, daily or almost daily, unknown/prefer not to answer), smoking intensity (never, previous, current <15 per day, current >15 per day, current unknown intensity, unknown/prefer not to answer), frequency of red and processed meat consumption (<2 per week, 2–2.99 times/week, 3–3.99 times/week, >4/week, unknown), highest educational level (CSE/GCSE/O-level, NVQ/HND/A-level/AS-level, other professional qualification, college/university degree, missing/prefer not to answer), regular aspirin or ibuprofen use (yes/no), ever use of hormone replacement therapy (yes/no) and, where necessary, fasting time (hours, continuous). These adjustments were made for all gastrointestinal cancers, and colorectal cancer models were additionally adjusted for family history of colorectal cancer in first degree relatives (yes/no). Stratification variables were age at recruitment in 5-year categories, Townsend deprivation index quintiles, and region of the recruitment assessment center. Subgroup analyses by sex were conducted where cases >100. PRS models were additionally adjusted for genotyping array and the first 15 genetic ancestry principal components to account for population stratification. Heterogeneity across subgroups was evaluated by performing likelihood ratio tests comparing models with and without appropriate interaction terms.
Because repeat measurements of all MetS components were available for 15,152 participants, we assessed the long-term stability of MetS classifications, and additionally fit 2-way mixed models to obtain intra-class correlation coefficients as an assessment of consistency for each MetS component. Sensitivity analyses were also performed for gastrointestinal cancers overall by smoking status (never, previous, current) and excluding those participants diagnosed with a cancer within 2 years of baseline. Finally, as an additional control for bias due to different MetS durations at baseline, the analysis was performed as a nested case-control study with each case of gastrointestinal cancer matched to 5 controls on age, sex, and recruitment region, and the same adjustments as the main models.
Statistical analyses were performed using Stata 16.1 (StataCorp Inc) and R (3.6.2) statistical software. Forest plots were generated using the R package metaphor.24
Results
Baseline Characteristics of Participants
After a median follow-up of 7.1 years, 4238 incident cases of overall gastrointestinal cancers were recorded (2525 colorectal cancers, 478 pancreatic cancers, 290 esophageal adenocarcinomas, 100 esophageal squamous cell carcinomas, 111 stomach cardia cancers, 74 stomach non-cardia cancers, 112 HCC, and 108 IDBC). The prevalence of harmonized MetS among study participants was 31.9% (n = 116,624), and this group was predominantly male, of higher body mass index, lower physical activity, and higher tobacco use than participants without MetS (Table 1).
Table 1.
Characteristics of the Study Population (n = 366,016)
| No metabolic syndrome at baseline (n = 249,392) | Prevalent metabolic syndrome (harmonized definition; n = 116,624)a | |
|---|---|---|
| Gastrointestinal cancer diagnosed | ||
| No | 247,022 (99.0) | 114,756 (98.4) |
| Yes | 2370 (1.0) | 1868 (1.6) |
| Age when attended assessment center, years | 55.48 ± 8.15 | 58.40 ± 7.61 |
| Follow-up time at cancer diagnosis, years | 3.80 ± 2.12 | 3.88 ± 2.09 |
| Participants with second assessment (of which unchanged metabolic syndrome status) | ||
| Yes | 11,036 (8635) | 4116 (3275) |
| Sex | ||
| Female | 152,691 (61.2) | 42,083 (36.1) |
| Male | 96,701 (38.8) | 74,541 (63.9) |
| BMI, kg/m2 | 25.9 ± 3.8 | 30.8 ± 4.9 |
| Waist circumference, cm | 85.3 ± 11.1 | 101.6 ± 11.3 |
| Standing height, cm | 167.8 ± 9.1 | 170.4 ± 9.4 |
| Total physical activity level, MET hours/week | 36.8 ± 49.5 | 31.6 ± 48.1 |
| Smoking status | ||
| Never | 144,013 (57.7) | 55,189 (47.3) |
| Previous | 79,776 (32.0) | 46,530 (39.9) |
| Current | 24,548 (9.8) | 14,170 (12.2) |
| Unknown | 1055 (0.4) | 735 (0.6) |
| Alcohol intake | ||
| Never | 9560 (3.8) | 6338 (5.4) |
| Former | 7520 (3.0) | 5381 (4.6) |
| Current | 231,765 (92.9) | 104,568 (89.7) |
| Unknown | 547 (0.2) | 337 (0.3) |
| Socioeconomic status (Townsend deprivation index) | ||
| Quartile 1 | 65,046 (26.1) | 26,386 (22.7) |
| Quartile 4 | 57,996 (23.3) | 33,391 (28.7) |
| Family history of colorectal cancer | ||
| No | 218,148 (87.5) | 100,376 (86.1) |
| Yes | 26,358 (10.6) | 13,167 (11.3) |
| Unknown | 4886 (2.0) | 3081 (2.6) |
| Regular use of aspirin or ibuprofen | ||
| No | 190,483 (76.4) | 71,242 (61.1) |
| Yes | 56,054 (22.5) | 43,521 (37.3) |
| Unknown | 2855 (1.1) | 1861 (1.6) |
| Red or processed meat intake, times/week | 4.5 ± 10.3 | 5.6 ± 12.5 |
| Blood pressure, mmHg | ||
| Systolic | 136.4 ± 19.2 | 146.9 ± 18.4 |
| Diastolic | 80.3 ± 10.2 | 86.6 ± 10.5 |
| Glycated hemoglobin, mmol/mol | 34.45 ± 4.03 | 39.71 ± 9.56 |
| HDL cholesterol, mmol/L | 1.56 ± 0.37 | 1.20 ± 0.28 |
| Triglycerides, mmol/L | 1.42 ± 0.74 | 2.45 ± 1.19 |
| Polygenic risk score, colorectal cancer (99 SNPs) | 653.3 ± 45.4 | 653.0 ± 45.4 |
| Polygenic risk score, pancreatic cancer (26 SNPs) | 146.0 ± 21.4 | 146.1 ± 21.6 |
Note: Data are presented as mean ± SD or number (%).
BMI, Body mass index; HDL, high-density lipoprotein; MET, metabolic equivalent of task; SD, standard deviation; SNP, single nucleotide polymorphism.
Rate of metabolic syndrome in 353 participants excluded from the study due to prevalent gastrointestinal cancers was 44.8%, compared with 31.9% for cancer-free participants.
Metabolic Syndrome and Risk of Gastrointestinal Cancers
Overall Gastrointestinal Cancer
MetS, as classified by the harmonized definition, was associated with higher risk of developing gastrointestinal cancers (HR, 1.21 for presence versus absence of MetS; 95% CI, 1.13-1.29) (Figure 1), with similar associations for women (HR, 1.12; 95% CI, 1.00-1.25) and men (HR, 1.26; 95% CI, 1.16-1.37; P heterogeneity = .09). Associations were not appreciably different for the NCEP-ATPIII and IDF 2005 MetS classifications. The presence of all individual MetS components were associated with increased gastrointestinal cancer risk.
Figure 1.
Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for overall gastrointestinal cancer risk and prevalent metabolic syndrome, defined by the presence of 3 or more components. AGM, Abnormal glucose metabolism; HDL, high density lipoprotein; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Colorectal Cancer
MetS was associated with higher colorectal cancer risk (harmonized definition: HR, 1.17; 95% CI, 1.08–1.28), with a similar association observed for men (HR, 1.27; 95% CI, 1.14–1.42), but not women (HR, 1.01; 95% CI, 0.88–1.17; P-heterogeneity = .04). Of the individual MetS components, the presence of obesity by either definition was most strongly associated with colorectal cancer (Figure 2). Associations of similar strength were found for colon and rectal cancer (P-heterogeneity = .88, harmonized definition).
Figure 2.
Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for colorectal cancer risk and prevalent metabolic syndrome, defined by the presence of 3 or more components. AGM, Abnormal glucose metabolism; HDL, high density lipoprotein; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Esophageal Cancer and Stomach Cancer
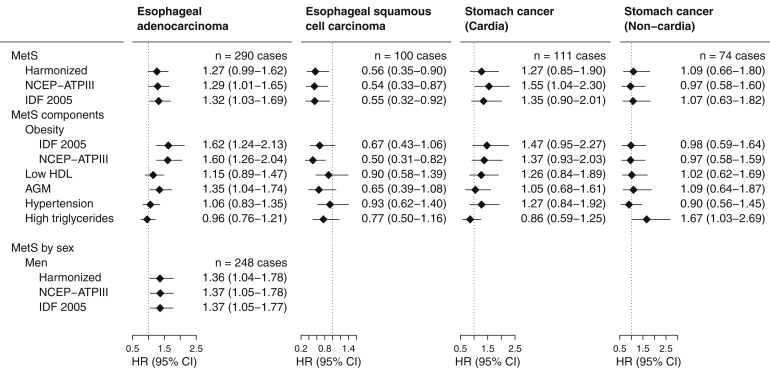
MetS was associated with an increased risk of esophageal adenocarcinoma by all definitions in men (85.5% of all cases; harmonized MetS: HR, 1.36; 95% CI, 1.04–1.78) (Figure 3). Of the assessed components of MetS, the presence of obesity, by either definition, was most notably associated with risk. In contrast, there was evidence for an inverse association between MetS and esophageal squamous cell carcinoma risk (HR, 0.56; 95% CI, 0.35–0.90), driven similarly by the obesity component. A positive association between MetS and cancer of the stomach cardia was found for NCEP-ATPIII MetS (HR, 1.55; 95% CI, 1.04–2.30).
Figure 3.
Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for stomach and esophageal cancer risk and prevalent metabolic syndrome, defined by the presence of 3 or more components. Sex-stratified results are only given where cases >100. AGM, Abnormal glucose metabolism; HDL, high density lipoprotein; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Pancreatic Cancer, Hepatocellular Carcinoma, and Intrahepatic Bile Duct Cancers
MetS was associated with increased risk of pancreatic cancer (HR, 1.39; 95% CI, 1.14–1.69), with positive associations found particularly for obesity and AGM (Figure 4). In contrast to other cancers, associations were stronger in women than men, with a suggestion of heterogeneity for the MetS association (P-heterogeneity = .06, harmonized MetS) (see Supplementary Table 2 for MetS components by sex). Harmonized MetS was also associated with HCC risk (HR, 1.61; 95% CI, 1.07–2.43) but not IBDC risk (HR, 1.16; 95% CI, 0.77–1.76).
Figure 4.
Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for hepatocellular carcinoma, pancreatic cancer, and bile duct cancer risk and prevalent metabolic syndrome, defined by the presence of 3 or more components. Sex-stratified results are only given where cases >100. P Heterogeneity by sex for pancreatic cancer was 0.06, 0.09, and 0.05 for harmonized, NCEP-ATPIII, and IDF 2005 MetS definitions, respectively. AGM, Abnormal glucose metabolism; HDL, high density lipoprotein; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Associations According to PRS Strata
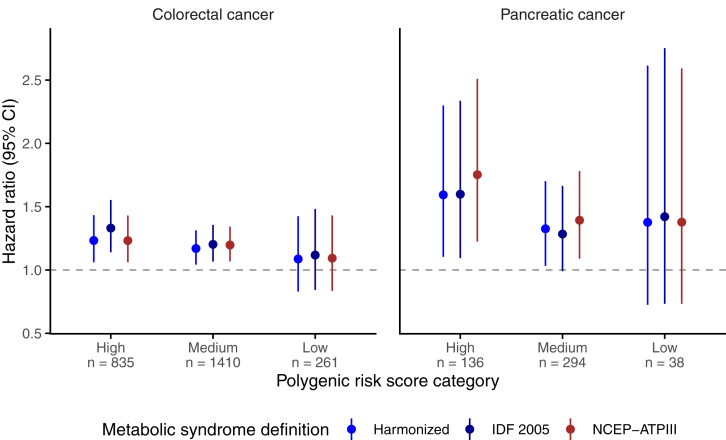
Positive associations between harmonized MetS and colorectal cancer or pancreatic cancer were generally maintained within PRS strata (eg, HR, 1.17; 95% CI, 1.04–1.31 and HR, 1.33; 95% CI, 1.03–1.70 for medium PRS categories in the 2 cancers respectively) (Figure 5) and no evidence of interaction was detected for either cancer (P = .70 and .69, respectively).
Figure 5.
Multivariable-adjusted hazard ratios and 95% confidence intervals (CIs) for associations of prevalent metabolic syndrome with colorectal cancer and pancreatic cancer by polygenic risk score category. IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III. P Heterogeneity across PRS strata was 0.70 and 0.69 for colorectal and pancreatic cancers, respectively.
MetS Stability
Of those participants with prevalent MetS were reassessed by the UK Biobank after a median of 4.3 years, 80.0%, 78.1%, and 77% retained this status by harmonized, NCEP-ATPIII, and IDF 2005 definitions, respectively (Supplementary Table 3).
Sensitivity Analysis
Associations for gastrointestinal cancers were weakened in current smokers, although no heterogeneity was detected (P = .18) (Supplementary Table 4). Associations were systematically unchanged or became stronger when cases diagnosed within the first 2 years of follow-up were excluded. Intra-class correlation coefficients for 2 timepoints over a median of 4.3 years were highest for waist circumference (0.86; 95% CI, 0.85–0.86) and lowest for diastolic BP (0.62; 95% CI, 0.61–0.63) (Supplementary Table 5, Supplementary Table 6). Associations for gastrointestinal cancers were unchanged when expressed as odds ratios via an equivalent nested case-control study design (Supplementary Table 7).
Discussion
We comprehensively examined the association between MetS and gastrointestinal cancer risk. MetS, independently of prevalent diabetes, was positively associated with overall gastrointestinal cancer risk for both men and women, as were its individual components. MetS was strongly associated with increased risks of colorectal cancer, HCC, pancreatic cancer, and esophageal adenocarcinoma in men. MetS associations with risks of colorectal and pancreatic cancers remained consistent across PRS groups, and most participants with prevalent MetS at baseline retained this status after more than 4 years of follow-up.
Chronic obesity-associated inflammation, hyperglycemia, and hyperinsulinemia are the mechanisms associated with MetS that are considered to influence gastrointestinal neoplasia.2,25,26 Visceral adipose tissue, for example, produces adipokines that inhibit apoptosis while promoting cell proliferation.25 Also, the exposure of cancer cells to high insulin levels stimulates mitogenesis.2 This was reflected in the strength of associations for individual obesity and AGM components. However, the large sample size and rigorous adherence to standard MetS definitions confirmed the presence of associations for the dyslipidemia and BP components. Elevated triglycerides remained associated with the risk of colorectal and colon cancer. Obesity is linked to elevated triglycerides; in visceral adiposity, energy is stored in this form.25
Overall, associations for colon cancer appeared to be driven by male cancers, with higher magnitude HRs observed, although there was weak evidence suggesting heterogeneity by sex. Rectal cancer followed a similar pattern, with a significant association detected for harmonized and NCEP-ATPIII definitions, unlike in the EPIC study.11 In contrast, MetS-pancreatic cancer associations were driven by female cancers, as reported by a Korean prospective study12 and a large meta-analysis on MetS and cancer.3 Metabolic dysregulation promotes insulin resistance, and the pancreas is exposed to high levels of endogenous insulin, which has mitogenic and anti-apoptotic effects.2 Although both obesity and AGM were strongly associated with pancreatic cancer risk overall, obesity was the main driver of the stronger MetS associations in women. This finding is consistent with an National Institues of Health-AARP study that reported a positive association between waist circumference and pancreatic cancer for women but not men.27 Additional studies are required to examine which specific aspects of central obesity-related metabolic dysregulation may be driving pancreatic cancer development and how this process may differ by sex.
Stratification of study participants by PRS has usually been implemented with the aim of optimizing colorectal cancer screening strategies. We assessed whether genetic risk category was a modifier of the association between MetS and colorectal or pancreatic cancer, and found no evidence in support. This suggests that all the population, regardless of their genetic profile, may be susceptible to the adverse tumorigenic effects of poor metabolic health, and priority for intervention in metabolic health should therefore not be based on genetic risk. To our knowledge, this is the first study to examine genetic risk of cancer in conjunction with overall metabolic dysregulation. However, a similar conclusion was reached by a German study that found no heterogeneity of the association between colorectal cancer risk and non-steroidal anti-inflammatory drug use across strata of PRS.28
HCC and IBDC are less prevalent than pancreatic cancer in Western populations, although the global incidence of the latter is rising.29 In support of a recent meta-analysis,6 we found MetS to be strongly associated with HCC, whereas the presence of AGM, but not MetS overall, was associated with IBDC risk. Few studies have examined MetS in relation to minor hepatobiliary cancers; a composite MetS score was found to be associated with gallbladder cancer in women in the Me-Can study,30 but no other data were available.
Esophageal adenocarcinoma and squamous cell carcinoma differ markedly in their patterns of incidence and etiologic factors. Adenocarcinoma risk was positively associated with MetS in men and squamous cell cancer risk inversely associated with MetS overall. Obesity exerted a disproportionate influence in these associations, consistent with findings from the large Me-Can study.31 As well as influencing adenocarcinoma risk through chronic inflammation, obesity is thought to increase risk separately through gastroesophageal reflux and its known progression to Barrett’s esophagus.32 Inverse associations of squamous cell carcinoma risk with obesity have previously been reported.33 Residual confounding by smoking, which is often more common in leaner study participants, has been proposed as an explanation for this finding.
The study manifests notable strengths. Firstly, high-quality baseline measurements of serum biomarkers allowed AGM and dyslipidemia to be objectively assessed, independently of diabetes. Comprehensive genotype data allowed the incorporation of PRS into the analysis. Furthermore, owing to the cohort’s repeat assessment, we have shown for the first time that MetS status is unlikely to change over a 4-year period in the absence of any intervention. Some limitations should be considered. Full MetS data were not available for around one-quarter of the cohort, and the duration of MetS at baseline was unknown. Participants did not fast prior to blood draw, potentially leading to inconsistencies in biomarker measurements. Furthermore, the relatively short follow-up limited the examination of rarer gastrointestinal cancers. Finally, generalization to other populations should be made with caution, given the “healthy participant” bias within the UK Biobank.34
In summary, predominantly long-term MetS was robustly associated with risk of developing overall gastrointestinal cancer, colorectal cancer, pancreatic cancer, and HCC, regardless of baseline genetic risk. Although obesity and AGM were most influential in these associations, all other components were associated with overall gastrointestinal cancer risk. Given that MetS status is unlikely to change long-term, these findings highlight the importance of maintaining good metabolic health in reducing the burden of gastrointestinal cancers and should assist in the development of preventative strategies.
Acknowledgments
This work was conducted using the UK Biobank Resource (Application Number 25897) and the authors gratefully acknowledge the participants and those involved in building the resource. All code for data analysis is stored in an online centralized repository and is available from the authors upon reasonable request.
CRediT Authorship Contributions
Joseph Rothwell, PhD (Formal analysis: Lead; Writing – original draft: Lead)
Mazda Jenab (Writing – review & editing: Equal)
Mojgan Karimi (Writing – review & editing: Equal)
Thérèse Truong (Writing – review & editing: Equal)
Yahya Mahamat-Saleh (Writing – review & editing: Equal)
Pietro Ferrari (Writing – review & editing: Equal)
S. Ghazaleh Dashti (Writing – review & editing: Equal)
Tilman Kühn (Writing – review & editing: Equal)
Amanda J. Cross (Writing – review & editing: Equal)
Gianluca Severi (Supervision: Equal; Writing – review & editing: Equal)
Marc J. Gunter (Writing – review & editing: Equal)
Neil Murphy (Conceptualization: Lead; Supervision: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the French National Cancer Institute (INCa SHSESP17, grant no. 2017-127).
Disclaimer Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Data transparency statement The UK Biobank is an open access resource. Bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/.
Note: To access the supplementary material accompanying this article, please click here.
Supplementary Material
Supplementary Table 1.
Definitions of Metabolic Syndrome as used in the Study
| Metabolic syndrome definition | Harmonized (2009) |
National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATPIII) (2005) |
International Diabetes Federation 2005 (IDF 2005) |
|---|---|---|---|
| Three or more of the following: | Three or more of the following: | Required: | |
| Abdominal obesity | ≥94 cm in men, ≥80 cm in women | ≥102 cm in men, ≥88 cm in women | ≥94 cm in men, ≥80 cm in women |
| Plus any two of the following: | |||
| Elevated triglycerides | ≥150 mg/dL (1.7 mmol/L) | ≥150 mg/dL (1.7 mmol/L) | ≥150 mg/dL (1.7 mmol/L) |
| Reduced HDL cholesterol | ≤40 mg/dL (1.03 mmol/L) in men, ≤50 mg/dL (1.29 mmol/L) in women, or use of cholesterol-lowering medications | ≤40 mg/dL (1.03 mmol/L) in men, ≤50 mg/dL (1.29 mmol/L) in women, or use of cholesterol-lowering medications | ≤40 mg/dL (1.03 mmol/L) in men, ≤50 mg/dL (1.29 mmol/L) in women, or use of cholesterol-lowering medications |
| Abnormal glucose metabolism | Glycated hemoglobin ≥5.7% of total | Glycated hemoglobin ≥5.7% of total | Glycated hemoglobin ≥5.7% of total |
| Elevated blood pressure | Systolic ≥130, diastolic ≥85 mmHg, previously diagnosed hypertension, or use of anti-hypertensive medications | Systolic ≥130, diastolic ≥85 mmHg, previously diagnosed hypertension, or use of anti-hypertensive medications | Systolic ≥130, diastolic ≥85 mmHg, previously diagnosed hypertension, or use of anti-hypertensive medications |
HDL, high density lipoprotein.
Supplementary Table 2.
HRs and 95% CIs for Associations Between Metabolic Syndrome Components and Pancreatic Cancer Risk by Sex
| Metabolic syndrome component | HR (95% CI)a,b |
|
|---|---|---|
| Women | Men | |
| Obesity (IDF 2005 definition) | 1.63 (1.21–2.19) | 1.21 (0.92–1.58) |
| Obesity (NCEP-ATPIII definition) | 1.38 (1.04–1.83) | 1.30 (1.01–1.69) |
| HDL cholesterol | 1.45 (1.09–1.93) | 1.08 (0.83–1.41) |
| Abnormal glucose metabolism | 1.82 (1.35–2.44) | 1.61 (1.23–2.09) |
| Hypertension | 1.44 (1.09–1.91) | 0.99 (0.76–1.28) |
| High triglycerides | 1.13 (0.85–1.50) | 1.00 (0.78–1.28) |
NOTE: Boldface indicates statistical significance.
CI, Confidence interval; HR, hazard ratio; IDF 2005, International Diabetes Federation 2005; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Multivariable models were adjusted for total physical activity (<10, 10–20, 20–40, 40–60, >60 MET hour/week), height (cm, continuous), alcohol consumption frequency (never, special occasions only, 1–3 times/month, 1–2 times per week, 3–4 times/week, daily or almost daily, unknown/prefer not to answer), smoking intensity (never, previous, current <15 per day, current ≥15 per day, current unknown intensity, unknown/prefer not to answer), frequency of red and processed meat consumption (<2 per week, 2–2.99 times/week, 3–3.99 times/week, ≥4/week, unknown), educational level (CSE/GCSE/O-level, NVQ/HND/A-level/AS-level, other professional qualification, college/university degree, missing/prefer not to answer), regular aspirin or ibuprofen use (yes/no), ever use of hormone replacement therapy (yes/no) and, where appropriate, fasting time (hours, continuous).
HRs are given for the presence compared with the absence of each component.
Supplementary Table 3.
Assessment of Metabolic Syndrome at Baseline and Repeat Assessment in a Subset of Participants
| Metabolic syndrome status | N at baseline | Of which unchanged at repeat assessmenta n (%) |
|---|---|---|
| Harmonized | ||
| Absent | 11,036 | 8635 (78.2) |
| Prevalent | 4116 | 3275 (80.0) |
| Total | 15,152 | 11,910 (78.6) |
| NCEP-ATPIII | ||
| Absent | 11,450 | 9031 (78.9) |
| Prevalent | 3702 | 2892 (78.1) |
| Total | 15,152 | 11,923 (78.7) |
| IDF 2005 | ||
| Absent | 11,831 | 9999 (84.5) |
| Prevalent | 3321 | 2555 (77.0) |
| Total | 15,152 | 12,554 (82.9) |
IDF 2005, International Diabetes Federation 2005; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
After a median duration of 4.3 years.
Supplementary Table 4.
HRs and 95% CIs for Associations Between Metabolic Syndrome, Its Individual Components, and Gastrointestinal Cancer Risk, by Smoking Status
| Metabolic syndrome definition or component | Never-smokers | HR (95% CI)a,b |
Current smokers |
|---|---|---|---|
| Former smokers | |||
| N for cases | 1813 | 1862 | 536 |
| MetS (Harmonized)c | 1.17 (1.06–1.30) | 1.27 (1.15–1.41) | 1.11 (0.93–1.34) |
| MetS (NCEP-ATPIII) | 1.18 (1.07–1.31) | 1.30 (1.18–1.43) | 1.24 (1.03–1.48) |
| MetS (IDF 2005) | 1.24 (1.12–1.38) | 1.32 (1.20–1.46) | 1.16 (0.97–1.40) |
| Obesity (IDF 2005 definition) | 1.23 (1.11–1.36) | 1.28 (1.16–1.41) | 1.11 (0.93–1.34) |
| Obesity (NCEP-ATPIII definition) | 1.30 (1.18–1.44) | 1.35 (1.22–1.51) | 1.07 (0.88–1.28) |
| HDL cholesterol | 1.05 (0.95–1.16) | 1.22 (1.11–1.35) | 1.15 (0.96–1.39) |
| Abnormal glucose metabolism | 1.10 (0.98–1.23) | 1.39 (1.26–1.54) | 1.20 (1.00–1.45) |
| Hypertension | 1.11 (1.01–1.22) | 1.03 (0.93–1.13) | 1.24 (1.04–1.49) |
| High triglycerides | 1.10 (1.00–1.21) | 1.09 (1.00–1.20) | 0.94 (0.79–1.12) |
Note: Boldface indicates statistical significance.
CI, Confidence interval; HDL, high-density lipoprotein; HR, hazard ratio; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Multivariable models were adjusted for total physical activity (<10, 10–20, 20–40, 40–60, >60 MET hour/week), height (cm, continuous), alcohol consumption frequency (never, special occasions only, 1–3 times/month, 1–2 times per week, 3–4 times/week, daily or almost daily, unknown/prefer not to answer), frequency of red and processed meat consumption (<2 per week, 2–2.99 times/week, 3–3.99 times/week, ≥4/week, unknown), educational level (CSE/GCSE/O-level, NVQ/HND/A-level/AS-level, other professional qualification, college/university degree, missing/prefer not to answer), regular aspirin or ibuprofen use (yes/no), ever use of hormone replacement therapy (yes/no) and, where appropriate, fasting time (hours, continuous).
HRs are given for classified positive compared with negative for each component.
P value for heterogeneity across strata of smoking status = .18.
Supplementary Table 5.
HRs and 95% CIs for Associations Between MetS and Gastrointestinal Cancers Overall and Excluding Cases Diagnosed Within 2 Years of Baseline
| N for cases | HR and 95% CI for prevalent MetSa,b |
|||
|---|---|---|---|---|
| Harmonized | NCEP-ATPIII | IDF 2005 | ||
| All gastrointestinal cancers | ||||
| All | 4238 | 1.21 (1.13–1.29) | 1.24 (1.16–1.33) | 1.27 (1.18–1.36) |
| <2-year cases excluded | 3224 | 1.25 (1.16–1.35) | 1.27 (1.18–1.37) | 1.33 (1.23–1.44) |
| Colorectal cancer | ||||
| All participants | 2,525 | 1.17 (1.08–1.28) | 1.19 (1.10–1.30) | 1.23 (1.13–1.35) |
| <2-year cases excluded | 1,893 | 1.20 (1.09–1.32) | 1.20 (1.09–1.33) | 1.28 (1.16–1.42) |
| Colon cancer | ||||
| All | 1,670 | 1.18 (1.06–1.31) | 1.21 (1.09–1.34) | 1.30 (1.16–1.45) |
| <2-year cases excluded | 1,253 | 1.19 (1.06–1.35) | 1.20 (1.06–1.36) | 1.32 (1.16–1.49) |
| Rectal cancer | ||||
| All | 855 | 1.16 (1.00–1.35) | 1.17 (1.01–1.35) | 1.13 (0.97–1.31) |
| <2-year cases excluded | 640 | 1.21 (1.02–1.43) | 1.20 (1.01–1.42) | 1.22 (1.03–1.46) |
| Esophageal adenocarcinoma | ||||
| All | 248 | 1.27 (0.99–1.62) | 1.29 (1.01–1.65) | 1.32 (1.03–1.69) |
| <2-year cases excluded | 184 | 1.38 (1.04–1.83) | 1.35 (1.02–1.79) | 1.44 (1.08–1.91) |
| Esophageal squamous cell carcinoma | ||||
| All | 100 | 0.56 (0.35–0.90) | 0.54 (0.33–0.87) | 0.55 (0.32–0.92) |
| <2-year cases excluded | 77 | 0.54 (0.31–0.93) | 0.54 (0.31–0.94) | 0.54 (0.30–0.98) |
| Pancreatic cancer | ||||
| All | 478 | 1.39 (1.14–1.69) | 1.48 (1.22–1.79) | 1.37 (1.12–1.68) |
| <2-year cases excluded | 379 | 1.35 (1.08–1.67) | 1.49 (1.20–1.84) | 1.29 (1.03–1.62) |
| Hepatocellular carcinoma | ||||
| All | 112 | 1.61 (1.07–2.43) | 1.48 (0.99–2.19) | 2.01 (1.34–3.03) |
| <2-year cases excluded | 100 | 1.90 (1.22–2.97) | 1.63 (1.07–2.48) | 2.34 (1.51–3.64) |
| Stomach cancer, cardia | ||||
| All | 111 | 1.27 (0.85–1.90) | 1.55 (1.04–2.30) | 1.35 (0.90–2.01) |
| <2-year cases excluded | 81 | 1.20 (0.75–1.91) | 1.46 (0.92–2.32) | 1.42 (0.89–2.26) |
| Stomach cancer, non-cardia | ||||
| All | 74 | 1.09 (0.66–1.80) | 0.97 (0.58–1.60) | 1.07 (0.63–1.82) |
| <2-year cases excluded | 59 | 1.09 (0.62–1.91) | 0.97 (0.55–1.71) | 1.15 (0.64–2.07) |
| Intrahepatic bile duct cancer | ||||
| All | 108 | 1.16 (0.77–1.76) | 1.41 (0.94–2.11) | 1.34 (0.87–2.05) |
| <2-year cases excluded | 77 | 1.19 (0.73–1.94) | 1.49 (0.93–2.40) | 1.43 (0.86–2.35) |
Note: Boldface indicates statistical significance.
AGM, Abnormal glucose metabolism; CI, confidence interval; HDL, high density lipoprotein; HR, hazard ratio; IDF 2005, International Diabetes Federation 2005; MetS, metabolic syndrome; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III.
Multivariable models were adjusted for total physical activity (<10, 10–20, 20–40, 40–60, >60 MET hour/week), height (cm, continuous), alcohol consumption frequency (never, special occasions only, 1–3 times/month, 1–2 times per week, 3–4 times/week, daily or almost daily, unknown/prefer not to answer), smoking intensity (never, previous, current <15 per day, current ≥15 per day, current unknown intensity, unknown/prefer not to answer), frequency of red and processed meat consumption (<2 per week, 2–2.99 times/week, 3–3.99 times/week, ≥4/week, unknown), educational level (CSE/GCSE/O-level, NVQ/HND/A-level/AS-level, other professional qualification, college/university degree, missing/prefer not to answer), regular aspirin or ibuprofen use (yes/no), ever use of hormone replacement therapy (yes/no), family history of colorectal cancer in first degree relatives (yes/no) and, where appropriate, fasting time (hours, continuous).
HRs are given for MetS detected versus not detected.
Supplementary Table 6.
Intra-class Correlation Coefficients for Metabolic Syndrome Components
| Number of time points | N for subset | Intra-class correlation coefficient (95% CI)a | |
|---|---|---|---|
| Waist circumference | |||
| All | 3 | 7061 | 0.86 (0.85–0.86) |
| Male | 3569 | 0.81 (0.80–0.82) | |
| Female | 3492 | 0.81 (0.08–0.82) | |
| Triglycerides | |||
| All | 2 | 15775 | 0.64 (0.63–0.65) |
| Male | 8011 | 0.61 (0.59–0.62) | |
| Female | 7764 | 0.66 (0.65–0.68) | |
| HDL cholesterol | |||
| All | 2 | 12790 | 0.85 (0.84–0.85) |
| Male | 6627 | 0.81 (0.81–0.82) | |
| Female | 6163 | 0.81 (0.80–0.82) | |
| Glycated hemoglobin | |||
| All | 2 | 12240 | 0.78 (0.77–0.79) |
| Male | 6058 | 0.80 (0.79–0.81) | |
| Female | 6182 | 0.76 (0.75–0.77) | |
| Blood pressure, diastolic | |||
| All | 2 | 18387 | 0.62 (0.61–0.63) |
| Male | 9090 | 0.57 (0.56–0.59) | |
| Female | 9297 | 0.64 (0.63–0.65) | |
| Blood pressure, systolic | |||
| All | 2 | 18383 | 0.66 (0.65–0.67) |
| Male | 9088 | 0.62 (0.61–0.63) | |
| Female | 9295 | 0.69 (0.68–0.70) |
CI, confidence interval; HDL, high density lipoprotein.
Calculated from 2-way linear mixed effects models in a subset of around 20,000 participants who were reassessed after a median of 4.3 years after baseline.
Supplementary Table 7.
ORs and 95% CIs for Associations Between Metabolic Syndrome, Its Individual Components, and Gastrointestinal Cancer Risk, by Smoking Status
| Metabolic syndrome definition or component | OR (95% CI)a,b | HR (95% CI)a,b |
|---|---|---|
| N for study | 4238 cases + 21,190 controls | 366,016 |
| All participants | ||
| MetS (Harmonized) | 1.23 (1.15–1.32) | 1.21 (1.13–1.29) |
| MetS (NCEP-ATPIII) | 1.26 (1.17–1.35) | 1.24 (1.16–1.33) |
| MetS (IDF 2005) | 1.29 (1.20–1.39) | 1.27 (1.18–1.36) |
| Women | ||
| MetS (Harmonized) | 1.12 (0.99–1.27) | 1.12 (1.00–1.25) |
| MetS (NCEP-ATPIII) | 1.15 (1.02–1.29) | 1.14 (1.03–1.27) |
| MetS (IDF 2005) | 1.20 (1.05–1.38) | 1.21 (1.07–1.37) |
| Men | ||
| MetS (Harmonized) | 1.30 (1.19–1.42) | 1.26 (1.16–1.37) |
| MetS (NCEP-ATPIII) | 1.34 (1.22–1.46) | 1.31 (1.20–1.42) |
| MetS (IDF 2005) | 1.33 (1.22–1.46) | 1.29 (1.19–1.40) |
| Individual components | ||
| Obesity (IDF 2005 definition) | 1.26 (1.17–1.35) | 1.24 (1.16–1.32) |
| Obesity (NCEP-ATPIII definition) | 1.32 (1.23–1.42) | 1.29 (1.20–1.38) |
| HDL cholesterol | 1.14 (1.07–1.23) | 1.13 (1.06–1.21) |
| Abnormal glucose metabolism | 1.29 (1.19–1.39) | 1.25 (1.17–1.34) |
| Hypertension | 1.08 (1.01–1.16) | 1.09 (1.03–1.16) |
| High triglycerides | 1.08 (1.01–1.16) | 1.08 (1.01–1.15) |
Note: Boldface indicates statistical significance.
CI, Confidence interval; HR, hazard ratio; IDF 2005, International Diabetes Federation 2005; NCEP-ATPIII, National Cholesterol Education Program – Adult Treatment Panel III; OR, odds ratio.
Multivariable models were adjusted for total physical activity (<10, 10–20, 20–40, 40–60, >60 MET hour/week), height (cm, continuous), alcohol consumption frequency (never, special occasions only, 1–3 times/month, 1–2 times per week, 3–4 times/week, daily or almost daily, unknown/prefer not to answer), smoking intensity (never, previous, current <15 per day, current ≥15 per day, current unknown intensity, unknown/prefer not to answer), frequency of red and processed meat consumption (<2 per week, 2–2.99 times/week, 3–3.99 times/week, ≥4/week, unknown), educational level (CSE/GCSE/O-level, NVQ/HND/A-level/AS-level, other professional qualification, college/university degree, missing/prefer not to answer), regular aspirin or ibuprofen use (yes/no), ever use of hormone replacement therapy (yes/no) and, where appropriate, fasting time (hours, continuous).
ORs and HRs and are given for classified positive compared with negative for each component.
References
- 1.Alberti K., Zimmet P., Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E., Harlan D.M., Archer M.C., et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito K., Chiodini P., Colao A., et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsilidis K.K., Kasimis J.C., Lopez D.S., et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. Brit Med J. 2015;350 doi: 10.1136/bmj.g7607. g7607. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:836S–842S. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Li X., Wu S., et al. Metabolic syndrome and the incidence of hepatocellular carcinoma: a meta-analysis of cohort studies. OncoTarg Ther. 2018;11:6277–6285. doi: 10.2147/OTT.S154848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borena W., Strohmaier S., Lukanova A., et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 8.Kasmari A.J., Welch A., Liu G., et al. Independent of cirrhosis, hepatocellular carcinoma risk is increased with diabetes and metabolic syndrome. Am J Med. 2017;130:746.e1–746.e7. doi: 10.1016/j.amjmed.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Ren H., Wang J., Gao Y., et al. Metabolic syndrome and liver-related events: a systematic review and meta-analysis. BMC Endocr Disord. 2019;19:40. doi: 10.1186/s12902-019-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed R.L., Schmitz K.H., Anderson K.E., et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 11.Aleksandrova K., Boeing H., Jenab M., et al. Metabolic syndrome and risks of colon and rectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Cancer Prev Res. 2011;4:1873–1883. doi: 10.1158/1940-6207.CAPR-11-0218. [DOI] [PubMed] [Google Scholar]
- 12.Park S.K., Oh C.M., Kim M.H., et al. Metabolic syndrome, metabolic components, and their relation to the risk of pancreatic cancer. Cancer. 2020;126:1979–1986. doi: 10.1002/cncr.32737. [DOI] [PubMed] [Google Scholar]
- 13.Cao Z., Zheng X.M., Yang H.X., et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Brit J Cancer. 2020;123:1336–1344. doi: 10.1038/s41416-020-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert S.A., Abraham G., Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28:R133–R142. doi: 10.1093/hmg/ddz187. [DOI] [PubMed] [Google Scholar]
- 15.Allen N., Sudlow C., Downey P., et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1:123–126. [Google Scholar]
- 16.Bycroft C., Freeman C., Petkova D., et al. 2018. The UK Biobank cohort [dataset]https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access Available at: [Google Scholar]
- 17.Fry D., Almond R., Moffat S., et al. 2019. UK Biobank Biomarker Project: companion document to accompany serum biomarker data.https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf Available at: [Google Scholar]
- 18.Bycroft C., Freeman C., Petkova D., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti K.G.M.M., Eckel R.H., Grundy S.M., et al. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.Alberti K.G.M.M., Zimmet P., Shaw J. Metabolic syndrome - a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Kachuri L., Graff R.E., Smith-Byrne K., et al. Pan-cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat Commun. 2020;11:6084. doi: 10.1038/s41467-020-19600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huyghe J.R., Bien S.A., Harrison T.A., et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51:76–87. doi: 10.1038/s41588-018-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein A.P., Wolpin B.M., Risch H.A., et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun. 2018;9:556. doi: 10.1038/s41467-018-02942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 25.Micucci C., Valli D., Matacchione G., et al. Current perspectives between metabolic syndrome and cancer. Oncotarget. 2016;7:38959–38972. doi: 10.18632/oncotarget.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J Clin Investig. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolzenberg-Solomon R.Z., Adams K., Leitzmann M., et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 28.Chen X.C., Guo F., Hoffmeister M., et al. Non-steroidal anti-inflammatory drugs, polygenic risk score and colorectal cancer risk. Aliment Pharmacol Ther. 2021;54:167–175. doi: 10.1111/apt.16438. [DOI] [PubMed] [Google Scholar]
- 29.Khan S.A., Thomas H.C., Davidson B.R., et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 30.Borena W., Edlinger M., Bjorge T., et al. A prospective study on metabolic risk factors and gallbladder cancer in the Metabolic Syndrome and Cancer (Me-Can) collaborative study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089368. e89368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindkvist B., Johansen D., Stocks T., et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer. 2014;14:103. doi: 10.1186/1471-2407-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandar A.K., Iyer P.G. Role of obesity in the pathogenesis and progression of Barrett’s esophagus. Gastroenterol Clin North Am. 2015;44:249–264. doi: 10.1016/j.gtc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Smith M., Zhou M., Whitlock G., et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer. 2008;122:1604–1610. doi: 10.1002/ijc.23198. [DOI] [PubMed] [Google Scholar]
- 34.Fry A., Littlejohns T.J., Sudlow C., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]