Abstract
Background Helping parents quit smoking is a public health priority. However, parents are rarely, if ever, offered tobacco use treatment through pediatric settings. Clinical decision support (CDS) systems developed for the workflows of pediatric primary care may support consistent screening, treatment, and referral.
Objectives This study aimed to develop a CDS system by using human-centered design (HCD) that identifies parents who smoke, provides motivational messages to quit smoking (informed by behavioral science), and supports delivery of evidence-based tobacco treatment.
Methods Our multidisciplinary team applied a rigorous HCD process involving analysis of the work environment, user involvement in formative design, iterative improvements, and evaluation of the system's use in context with the following three cohorts: (1) parents who smoke, (2) pediatric clinicians, and (3) clinic staff. Participants from each cohort were presented with scenario-based, high-fidelity mockups of system components and then provided input related to their role in using the CDS system.
Results We engaged 70 representative participants including 30 parents, 30 clinicians, and 10 clinic staff. A key theme of the design review sessions across all cohorts was the need to automate functions of the system. Parents emphasized a system that presented information in a simple way, highlighted benefits of quitting smoking, and allowed direct connection to treatment. Pediatric clinicians emphasized automating tobacco treatment. Clinical staff emphasized screening for parent smoking via several modalities prior to the patient's visit. Once the system was developed, most parents (80%) reported that it was easy to use, and the majority of pediatricians reported that they would use the system (97%) and were satisfied with it (97%).
Conclusion A CDS system to support parental tobacco cessation in pediatric primary care, developed through an HCD process, proved easy to use and acceptable to parents, clinicians, and office staff. This preliminary work justifies evaluating the impact of the system on helping parents quit smoking.
Keywords: tobacco use treatment, clinical decision support, human-centered design
Background and Significance
By helping parents quit smoking, pediatric clinicians protect children and families from significant health harms. More than 40% of the U.S. pediatric population is regularly exposed to secondhand smoke (SHS), most often by a parent. 1 2 SHS exposure among children increases the risk of sudden infant death syndrome, chronic respiratory diseases, such as asthma, and lung cancer in adulthood. 3 Parents who quit smoking eliminate the majority of their children's SHS exposure, 1 decrease the risk of their children becoming smokers when they grow up, 4 and can increase parents' own life expectancy. 5 Pediatric clinicians are well-positioned to protect children from SHS exposure by promoting tobacco use treatment for parents who smoke, 6 7 but appropriate treatments to parent smokers are not routinely delivered in pediatric settings. 8 Major barriers to wider adoption include a lack of methods to support effective pediatrician–parent communication regarding tobacco use treatment and mechanisms to systematize, consistently deliver, and scale effective interventions. 8 9 10
Communication regarding smoking cessation likely influences parental decisions to engage in treatment. In pediatric settings, tobacco use treatment messages that emphasize the impact of smoking to parents on their child may increase acceptance of cessation treatment. 11 12 Effective messages and interventions for parental tobacco use treatment can be systematized through electronic health records' (EHRs') clinical decision support (CDS) systems. These systems are effective in improving health care process measures across a wide variety of care settings. 13 14 CDS systems may also improve engagement with clinical interventions for tobacco use. 15 In adult settings, CDS systems that connect patients to Quitlines (telephone-based counseling programs effective in helping smokers quit 16 ) led to a 13-fold increase in the proportion of smokers who enroll in treatment 17 and can be successfully implemented with minimal promotion. 18 In pediatric settings, CDS systems, including those developed by members of this study team, have shown potential in helping pediatricians screen for SHS exposure, provide treatment, and similarly increase Quitline enrollment. 19 20 21 22 23 24 25 Workflow limitations, however, have prevented their full incorporation into routine clinical practice. For CDS systems to be effective, the interface needs to be designed with the needs of parents and clinicians taken into consideration. In addition, it is critical to consider how other aspects of the CDS system are integrated into clinical workflows such that clinical staff can continue their work processes. These types of workflows are often not considered leading to failures during CDS system implementation.
Developing CDS systems using human-centered design (HCD) approaches may help move beyond simple prompts and achieve a more effective and integrated workflow for tobacco use treatment in pediatric settings. HCD, guided by usability experts, involves an analysis of the work environment, active user involvement in the development process, iterative systems development, and testing systems in real-word settings. 26 27 28 29 30 31 HCD approaches have been shown to help ensure that technology is easy-to-use and, thus, more consistently and effectively used. 27 28 30 32 While HCD approaches provide a methodological foundation for developing effective CDS and have been used to develop smoking cessation CDS system in adult care settings, 33 34 they have not been rigorously applied to systems focused on tobacco use treatment in pediatric settings. This critical gap in the application of HCD to smoking cessation efforts in pediatric settings has limited the effectiveness of efforts to reduce parent smoking.
Objective
This study aimed to develop a CDS system by using HCD processes that identifies parents who smoke, provides motivational messages to quit smoking (informed by behavioral science), and supports delivery of evidence-based tobacco treatment.
Methods
We applied a rigorous HCD process that engaged participants from three cohorts in workflow analysis and a series of iterative formative scenario-based usability testing sessions that captured a range of objective and subjective metrics on CDS system usability, utility, and intent to use. This work was performed by a multidisciplinary team with expertise in pediatric primary care, smoking cessation, CDS, HCD methods, and software engineering.
System Design
The CDS system was designed to support three cohorts of system users as follows: (1) parents/caregivers who smoke (hereafter referred to as parents), (2) primary care pediatric clinicians, and (3) clinical staff. The initial design of the CDS system was based on clinical expertise and research on tobacco use treatment in pediatric settings, 8 23 35 prior work developing and evaluating CDS systems for parent tobacco use, 20 22 expertise on EHR programming and CDS, and the application of HCD methods such as usability heuristics, scenario development, and cognitive walkthrough. 26 27 28 29 30 31 The final system design was developed through a more detailed HCD process, 29 30 31 as described below.
Study Setting, Participants, and Recruitment
This study took place with the Children's Hospital of Philadelphia (CHOP) Care Network, a highly productive primary care practice-based research network. 36 The study was conducted in practices identified as having higher rates of parent smoking. Inclusion criteria were as follows: parents were eligible if they were a self-identified smoker, aged ≥18 years, present at their child's health care visit, and able to communicate in English; pediatric clinicians were eligible if they were an attending pediatrician or nurse practitioner; and clinical staff were eligible if they were either the office manager or lead patient scheduler. Parent participants were identified through EHR patient chart reviews, and study procedures were performed immediately after the clinical encounter and audio recorded. Pediatric clinicians were recruited via e-mail, and sessions were performed remotely using a video-sharing platform and audio recorded as well. Pediatric clinicians were recruited via purposeful sampling to ensure a range of confidence and perceived roles in providing tobacco use treatment ( Table 1 ). 37 Clinical staff participants were recruited via e-mail, and sessions were performed remotely using a video-sharing platform. All sessions were audio recorded. The study was approved by the CHOP Institutional Review Board.
Table 1. System subjective and objective evaluation metrics.
| Parent CDS system development | |
|---|---|
| Qualitative | Quantitative |
| Outcome measure Think-aloud protocol: Parent review of prototype with interviewer Parent thinks aloud, communicating understanding of interface, task, and choice selection; Interviewer probes while not guiding Questionnaire: TAM: validated measure for usability and utility of CDS tools Semi-structured interview: Questions regarding system design |
Outcome measure Error rate: percentage of errors committed by participant, per scenario |
| Analysis Think-aloud protocol: Sessions recorded, reviewed, and debriefed by study team members Most serious usability problems identified and discussed Questionnaire: Parent rates prototype's usability, functionality, and general satisfaction Semi-structured interview: Identify missing components or concerns Content reviewed to inform the next iteration of prototype |
Analysis Error rate: measure where participant deviates from expected actions in performing task, recorded as either critical error or noncritical error |
| Pediatric clinician CDS system development | |
| Qualitative | |
| Outcome measure Think-aloud protocol: Clinician review of prototype with interviewer; prototypes addressed multiple scenarios of parent smoker status, including only one parent smokes, parent and other family member smokes, only other family members smoke, and scenarios where parent may change their mind about accepting treatment after a discussion with the clinician Clinicians think aloud, communicating understanding of interface, task, and choice selection; Interviewer probes while not guiding Questionnaire: TAM: validated measure for usability and utility of CDS tools Semi-structured interview: Questions regarding system design | |
| Analysis Think-aloud protocol: Sessions recorded, reviewed, and debriefed by study team members Most serious usability problems identified and discussed Questionnaire: Clinician rates prototype's usability, functionality, and general satisfaction Semi-structured interview: Identify missing components or concerns Content reviewed to inform the next iteration of prototype | |
| Clinical staff workflow analysis | |
| Qualitative | |
| Outcome measure Semistructured interview: Questions describe pros and cons of each workflow at their practice Questionnaire: Formal evaluated mock-ups of proposed workflow | |
| Analysis Semistructured interview: Staff asked to describe pros and cons of each workflow at their practice. Questionnaire: Staff rate suitability for their clinical site and whether the workflow was efficient and easy to manage | |
Abbreviations: CDS, clinical decision support; TAM, Technology acceptance model.
Usability Testing: Parent System Component
Functional interactive prototypes of the system's component intended for use by parents were developed using REDCap electronic survey building and data capture tools. 38 39 This component was a screening questionnaire with questions about the parent's and/or other family member's tobacco use, motivational smoking cessation messages, and options to connect with three evidence-based tobacco use treatments: nicotine replacement therapy (NRT) patch and/or gum, referral to the Quitline (counseling services via phone 16 ), and/or referral to SmokefreeTXT (counseling via text messaging 40 ). Parents interacted directly with system prototypes on a laptop, allowing the recording of objective measures including task completion and error rates. In these sessions, participants were encouraged to talk aloud while performing tasks, 41 following a think-aloud protocol, 42 communicating their understanding of the user interface and their selections in performing the tasks. The facilitator encouraged the parent to say whatever comes into their mind as they complete the task. This approach (1) gathered responses to open ended questions on the usability and functionality of the system and (2) encouraged subjects to add opinions, ideas, and suggestions. After the usability testing, participants completed a system usability, utility, and trust questionnaire based on the Technology Acceptance Model (TAM), 43 44 followed by a brief semistructured interview. Interview questions focused on desired features of the system, general process of screening for tobacco use, content of motivational smoking cessation messages, and tobacco treatment.
Usability Testing: Pediatric Clinician System Component
High fidelity, semi-interactive, and scenario-based mockups of the system components used by clinicians were created in a rapid prototyping application (Axure, San Diego, California, United States). Usability testing was conducted remotely to accommodate participants' availability. While the mockups were high fidelity, they did not fully support direct interaction. As a result, we applied a pluralistic walkthrough format 45 where test facilitators presented each scenario to participants and prompted them to describe the scenario-based action for each screen. If the participant selected the intended action of the design, the facilitator would proceed to the next screen. If a participant selected an unintended action, the facilitator would describe the result of that action and allow the participant to select another action. If the participant was unable to identify the correct action, the facilitator would explain the intended action and proceed. After the prototypes' review, participants responded to a questionnaire based on the TAM to assess perceived ease of use, perceived usefulness, and intent to use. 43 The questionnaire was followed by a brief semistructured interview with questions about most- and least-liked features, general satisfaction, suggested changes to the prototypes, and overall fit of the proposed system within the current visit workflow.
Workflow Analysis: Clinic Staff System Component
While clinical staff are not direct end users of the CDS system, they have both an important role in supporting parents using the system and valuable experience in how the system could best fit within the clinic workflow. Participants reviewed graphic representations of three distinct workflow options for delivering the parent system including completing the screening questionnaire prior to the appointment (via the EHR patient portal), in the office on a dedicated tablet, or in the office on the parent's own smartphone device. After workflow reviews, participants completed a TAM questionnaire rating the system on perceived ease of use, perceived usefulness, and intent to use. 43 Participants then responded to semistructured questions on the positives and negatives of each workflow and which one would fit best within their practice workflow.
Outcome Measures
Our outcome measures addressed the following two goals: (1) determine that the CDS system meets the functional objective of identifying parents who smoke, providing motivational messages, and connecting parents to treatments; and (2) ensure the system is usable, useful, and likely to be adopted. For the parent and pediatric clinician user testing, we captured a mix of objective and subjective data ( Table 1 ). Objective data included critical errors (defined as tasks that participants were unable to complete) and noncritical errors (defined as tasks where participants encountered difficulty but were able to recover without assistance to complete the task). Subjective data included participant response to a pretest demographic questionnaire, postscenario Likert's scale questions based on the TAM, comments from the think-aloud protocol, and semistructured interview questions administered at the end of the usability test sessions. For clinical staff, outcome measures focused on ensuring the system was usable, efficient, and fit within the workflow of the clinical staff. The evaluation approach leveraged the Hierarchical Task Analysis methods 46 47 in which staff reviewed, commented on, and evaluated mockups of proposed workflows.
Analysis
Demographic survey responses for each participant cohort were characterized using descriptive statistics. For parent and pediatric clinician design review sessions, subjective analysis involved assessment of think-aloud comments, questionnaires assessing usability, functionality, general satisfaction, and responses to open-ended questions that were coded to identify key themes ( Table 1 ). Recordings were analyzed using NVivo 11 (QSR International) analysis software to code and identify themes. Objective analysis assessed participant error rate while completing specific CDS prototype activities. The research team responded to the occurrence of errors and/or negative subjective results by modifying the system and continuing to test with new participants. For clinical staff sessions, analysis involved assessment of responses to open-ended questions and questionnaires assessing usability, functionality, and general satisfaction. In all, the optimization process ensured that the system design met users' needs ,a level of usability, functionality, and usefulness that supports screening parents for smoking, providing motivational messages, and connecting to tobacco use treatment.
Sample Size
For the parents and pediatric clinicians, we anticipated iteratively testing and refining the CDS system prototype with a minimum of 30 participants. At least 97% of all problems with software systems can be identified with 30 participants. 48 49 For the clinical staff, we performed workflow review with a sample of staff until we reached thematic saturation.
Results
Participants and Recruitment
The HCD process engaged 70 representative participants including 30 parents, 30 pediatric clinicians, and 10 clinic staff. Parents were recruited from 2 different practices, clinicians from 10 practices, and staff from 5 practices ( Table 2 ). The clinicians expressed a range of confidence in providing tobacco use treatment, including prescribing NRT.
Table 2. Participant demographics.
| Characteristic |
Parent caregivers (
n
= 30)
% ( n ) |
Pediatric clinicians (
n
= 30)
% ( n ) |
Clinical staff (
n
= 10)
% ( n ) |
|---|---|---|---|
| Age (y) | |||
| ≤ 24 | 7 (2) | – | – |
| 25–34 | 43 (13) | 17 (5) | 20 (2) |
| 35–44 | 43 (13) | 43 (13) | 40 (4) |
| 45–54 | 7 (2) | 17 (5) | 20 (2) |
| 55–64 | – | 20 (6) | 20 (2) |
| 65 or older | – | 3 (1) | – |
| Sex | |||
| Female | 77 (23) | 80 (24) | 100 (10) |
| Male | 23 (7) | 20 (6) | – |
| Race | |||
| White | 13 (3) | 83 (25) | – |
| Black/African American | 77 (23) | 7 (2) | – |
| Asian | 3 (1) | 10 (3) | – |
| American Indian or Alaska Native | 3 (1) | – | – |
| Other | 10 (3) | – | – |
| Multiracial | 7 (2) | – | – |
| Ethnicity | |||
| Hispanic | 17 (5) | – | – |
| Non-Hispanic | 83 (25) | 100 (30) | – |
| Relationship to patient | |||
| Parent | 93 (28) | – | – |
| Grandparent | 3 (1) | – | – |
| Other guardian | 3 (1) | – | – |
| Clinical title/role | |||
| Attending physician | – | 87 (26) | – |
| Nurse practitioner | – | 13 (4) | – |
| Office manager/director | – | – | 50 (5) |
| Patient services representative | – | – | 50 (5) |
| Experience in clinical practice (y) | |||
| 0–10 | – | 53 (16) | 30 (3) |
| 11–20 | – | 17 (5) | 40 (4) |
| 21–30 | – | 17 (5) | 20 (2) |
| > 30 | – | 13 (4) | 10 (1) |
| How comfortable do you feel providing smoking cessation counseling to parents or other caregivers who smoke? | |||
| Very uncomfortable | – | 0 (0) | – |
| Somewhat uncomfortable | – | 10 (3) | – |
| Neutral | – | 20 (6) | – |
| Somewhat comfortable | – | 50 (15) | – |
| Very comfortable | – | 20 (6) | – |
| How comfortable do you feel treating parents or other caregivers who smoke with nicotine replacement therapy? | |||
| Very uncomfortable | – | 37 (11) | – |
| Somewhat uncomfortable | – | 17 (5) | – |
| Neutral | – | 3 (1) | – |
| Somewhat comfortable | – | 20 (6) | – |
| Very comfortable | – | 23 (7) | – |
Parent System Component
Formative testing of the parent system (the screening questionnaire) was performed over four iterative versions of system prototypes. Modifications were primarily in the editing of system language but also included adding functionality. The initial version was tested with five participants and had a critical error rate of 20% and a noncritical error rate of 60%. All participants reviewing the initial version rated the system as easy to understand and easy to use. Usability testing continued for three additional versions. The final version was tested with 10 participants and had no critical or noncritical errors. Ninety percent of participants rated the questionnaire as easy to understand and 80% as easy to use ( Table 3 ).
Table 3. Parent system summary of prototype iterations.
| Version Information | Statistics | Design changes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prototype version | Participants ( n ) | % Critical error | % Noncritical error | Content clear and easy to understand (% strongly agree/agree) | Content easy to use (% strongly agree/agree) | Intro screening questions | Intro summary | Three treatment options | End info screen |
| 1 | 5 | 20 | 60 | 100 | 100 | NA | NA | NA | NA |
| 2 | 8 | 0 | 38 | 100 | 100 | None | None | Major edits | None |
| 3 | 7 | 0 | 29 | 86 | 100 | None | None | Minor wording edits | Minor wording edits |
| 4 | 10 | 0 | 0 | 90 | 80 | Minor edits | Minor edits | Minor grammar edits | Minor wording and display edits |
Abbreviation: NA, not available.
Several important design requirements were identified, including minimizing the likelihood that the questionnaire would elicit feelings from parents of being judged for their smoking, keeping the time spent completing the questionnaire as short as possible, and presenting information relevant to their child's health as a motivator for behavior change. Most parents did not feel “judged” regarding smoking behaviors by the content of the questionnaire (93%), and most parents agreed they would be comfortable filling out the questionnaire (90%) and having their child's pediatrician know about smoking in their family (77%; Fig. 1 ). The nonjudgmental aspect of the language and the screener was also reflected during the design screener review, “You are not judgmental, so yeah. I appreciate it.”
Fig. 1.
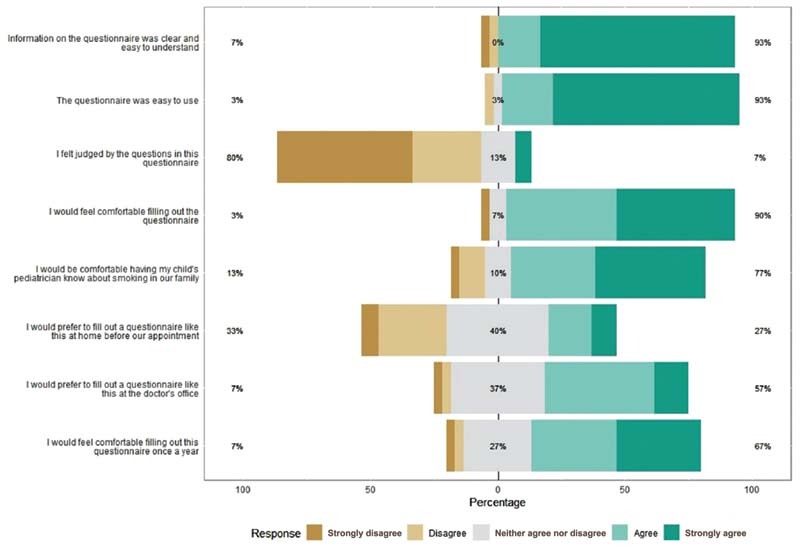
Parent technology acceptance model (TAM) questionnaire ( n = 30).
Parent participants shared that the parent CDS system was easy to complete, and that the information presented was helpful and informative. One illustrative comment included: “It was short, sweet and simple because it was getting straight to the point ….” Many mentioned their child's health as their motivation to quit. We received overwhelmingly positive feedback on the motivational messages that lists the benefits of quitting smoking. Despite its length and level of detail, respondents found it self-explanatory, helpful and the appropriate length: “I think that it's all good components to helping to quit smoking. Making it brighter in your brain to think about your kids ….” Participants found the variety of treatment options helpful, and none of the subjects expressed concerns about the default opt-in to the treatments.
Clinician System Component
Formative testing of the clinician system was performed over six iterative versions of system prototypes. Modifications included redesign of interaction components, workflow, content, and adding functionality. The initial version was tested with only two participants but identified severe issues requiring a reorganization of user interface elements and workflow. The subsequent five iterations identified more minor issues that required modest changes but also resulted in discovering requirements for added functionality and information. During most sessions, questions would arise from clinician participants about features, functionality, and workflows. We used data from the usability sessions to add key features, reorganize and change layouts, optimize workflows, and provide information about treatment resources ( Fig. 2 ).
Fig. 2.

Pediatric clinician system summary of prototype iterations. Version, participants, design changes; version: 1; participants: 2; scenarios in testing: caregiver smokes and accepts treatment; caregiver smokes and declines treatment; design changes: changed notification about parent options and treatment option presentation. Version: 2; participants: 3; scenarios in testing: same as version 1; design changes: reorganized main decision support screen and moderate edits to documentation feature. Version: 3; participants: 3; scenarios in testing: same as version 1 plus other family member smokes, no smokers in family; design changes: added main discussion prompts to main decision support screen and information to submission feature, minor edits to documentation. Version: 4; participants: 11; scenarios in testing: same as above; design changes: minor edits to main decision support screen layout and tips for talking with caregiver screen, simplified documentation. Version: 5; participants: 7; scenarios in testing: same as above; design changes: minor edits to main decision support screen, treatment options screen, and submission feature. Version: 6; participants: 4; scenarios in testing: same as above; design changes: minor edits to main decision support screen to clarify parent/caregiver respondent information, tips for talking with parents, treatment options, and submission feature; added billing support, hover tips for quick information, and contact information for treatment options.
The responses across all iterations to the pediatric clinician TAM questionnaire were positive. Overall, across all system versions, more than 95% of participants strongly agreed/agreed that the system would help them provide treatment to parent smokers and would improve outcomes in parent smoking cessation ( Fig. 3 ). Further, more than 95% of participants strongly agreed/agreed that they would use the system and that they were satisfied with the system.
Fig. 3.
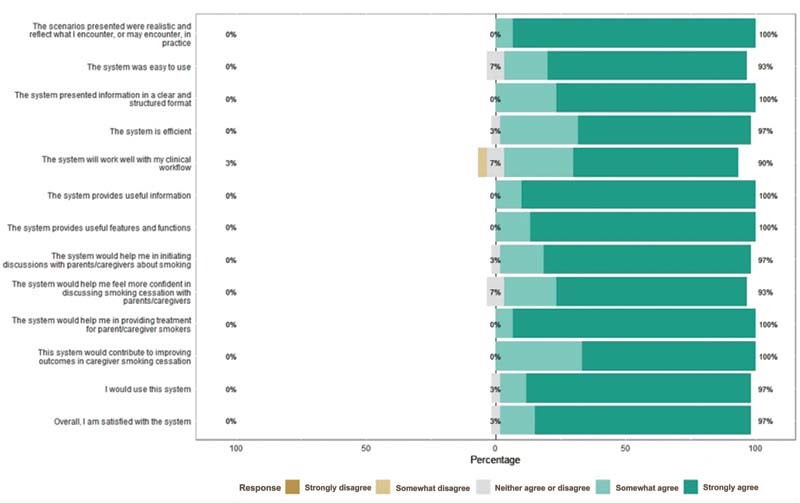
Pediatric clinician technology acceptance model (TAM) questionnaire ( n = 30).
Pediatric clinician participants were supportive of the system and its role in helping parents quit smoking. One illustrative comment included, “I like that it identifies and uses what I knew was evidence-based tactics for smoking cessation and it is aligned with my philosophy as far as what I'm offering.” Some clinician participants expressed the need for some time to get used to the system before feeling completely confident but still confirmed that the system was easy to use and well presented. One participant explained: “It's easy to use. It provides the information needed. It sort of walks you through it step by step. Resources are right there at your fingertips … I would think for many like me we're not so aware of the options for nicotine replacement and, again, just for smoking cessation programs.” Clinicians emphasized the value of automating tobacco treatments and documentation as much as possible to reduce the burden on them during visits with competing priorities. They were comfortable with treatment being provided, regardless of a discussion with the parent.
Workflow Analysis: Clinic Staff System Component
Review and qualitative assessment of three different workflows revealed that staff perceived the EHR patient portal reminder workflow as the most preferred for staff, parents, and clinicians at their practice (90% agreed/strongly agreed). They also rated it as the workflow that would be the most efficient and easy to manage (100% agreed/strongly agreed). However, during the postreview, semistructured interview participants unanimously preferred having all three workflows available as part of the questionnaire completion, as opposed to relying solely on EHR patient portal workflow. Participants reported that their parent population prefers to complete questionnaires through a range of modalities. A multifaceted approach with a combination of questionnaire completion at home via the EHR patient portal, in the office via tablet, or on a parent's own smartphone device is widely utilized for other questionnaires in these practices, and therefore would be a preferred strategy for this system.
Final System Development
An overarching theme of the design review sessions across all cohorts was automating key functions as much as possible. We iteratively developed the final CDS system based on the subjective and objective data from the design review sessions and workflow analysis. This system is now live at five primary care practices in our electronic health record (EHR). The system supports screening for parent smoking via several modalities (personal computer or smartphone device) prior to the patient's visit to decrease the burden on in-office completion or in the office on a tablet device. If the parent indicates that they are a smoker, the parent portion of the system displays information designed to motivate treatment engagement, followed by presentation of three treatment options for them to accept (1) NRT, (2) Quitline, and/or (3) SmokefreeTXT. The system automatically connects the parent with the treatment options they selected, and the NRT is directly delivered to the parent, if interested. The pediatric clinician is notified of the parent's selections within several key sections in the EHR, including the best practice alert section, questionnaire review section, and main note, all to account for different clinician workflows. The system includes information to help the clinician in what is often a difficult conversation about smoking, with simple guidance on key conversation prompts that motivate treatment. The system supports automated documentation content including the office visit progress note, after visit summary, and billing. Finally, the system will periodically remind the clinician to follow-up on the parent's effort to quit. See Fig. 4 for system workflow.
Fig. 4.
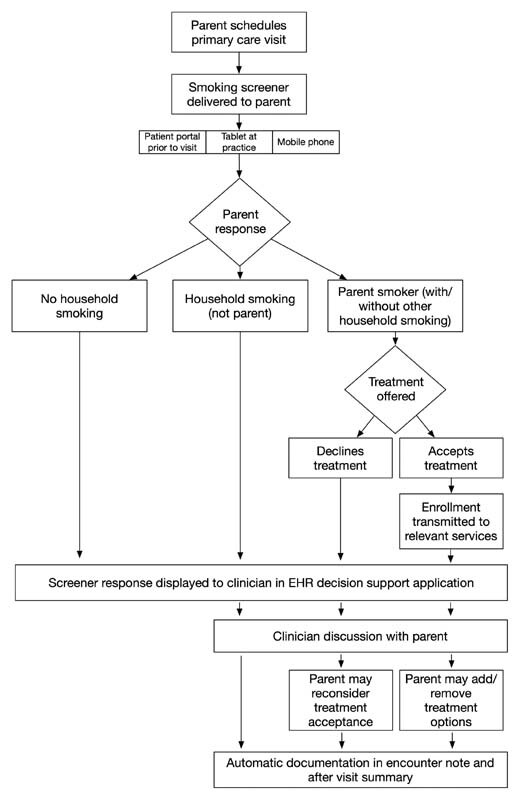
Automated process for family tobacco control screening and treatment delivery. EHR, electronic health record.
Discussion
We applied HCD processes to develop a CDS system that supports parent tobacco use treatment through pediatric settings. We identified key system functions and features through initial prototyping. These features were prioritized and refined though iterative end-user feedback. Previous work had shown that a CDS system targeted at clinicians could improve process measures related to parent tobacco use, 23 24 but further work was needed to better incorporate these systems into office workflows. 20 22 In this study, parents, pediatric clinicians, and clinical staff all identified directly screening parents for tobacco use and then directly offering treatment through an electronic questionnaire as critical system features. Parents valued this approach, as it allowed them to disclose tobacco use in a nonjudgmental way. Pediatric clinicians and staff felt this approach could most efficiently identify parents and then systematize treatment. Participant interactions with iterative system prototypes helped identify how to make these key functions acceptable and usable. Parents valued motivational messages, informed by behavioral science, that focused on the benefits of smoking cessation to their child's health 11 12 and simple, easy-to-understand explanations of treatment options. Pediatric clinicians valued automation of parent screening, treatment, and documentation with simple guidance on key conversation prompts that support treatment. Similarly, clinical staff valued methods for parents to complete key system features ahead of the visit to decrease in-office workflow burdens.
Developing CDS systems using HCD approaches is a growing area of research interest, as it helps clinical application developers move beyond simple prompts and alerts to systems that more effectively support clinical workflows. 50 51 52 Clinicians spend a significant portion of their professional time using the EHR, with a large percentage of clinicians spending more time interacting with the EHR than interacting with patients. 53 54 EHR-related stress is prevalent, and there may be a particularly strong relationship between poor EHR usability and physician burnout. 55 Developing CDS systems with the end user of the system at the center of the processes is critical, considering the well-known time burdens and disproportionate stress on clinicians through the current processes. 53 55 56 Further, our HCD approach provides a more holistic approach to system development, incorporating usability assessment of the CDS from both the interface level (parents and pediatric clinicians) and the office workflow level (clinical staff).
Despite our success in achieving our objectives, the EHR presented many barriers to usability and functionality, primarily due to the inability to account for family relationships. EHR vendors have focused on communication and data privacy for adult health care settings, with technological services developed for the physician–adult patient dyad. These systems fail to account for the family aspect of child health care, including the inability to readily link family members and address health at the family or household-level. 57 58
Limitations
Our study has several limitations. First, we do not have data on the CDS system's impact on parent smoking cessation rates. Nonetheless, by leveraging the team's previous experience developing effective CDS systems for tobacco use treatment 20 22 and grounding this work in rigorous HCD methods, our design process maximizes the likelihood of CDS effectiveness. Second, pediatric clinician usability testing was conducted remotely, using web conferencing software, which did not fully support direct interaction with the prototypes. Thus, we did not have recorded error metrics for participant interactions with the system. We felt the improved accessibility to pediatric clinicians using this approach outweighed the effort of in-person testing during the pandemic. Third, we did not perform a formal power analysis. We performed formative iterative testing where the objective was to apply an HCD approach to system design using high-fidelity mockups prior to development. Literature on number of participants for summative testing lacks consensus and is less clear on formative testing. 59 60 Fourth, the facilitator-led usability testing and workflow analysis sessions may be prone social to desirability bias. Fifth, while we sought a representative sample for usability testing and workflow analysis, participant feedback may not be representative of all parent, pediatric clinician, or staff perspectives. Nonetheless, for pediatric clinicians, we did not identify meaningful differences in TAM questionnaire responses by demographic or professional characteristics, including, for example, experience in clinical practice. Finally, the CDS system to support parent tobacco use treatment was limited to a single institution as part of a research effort.
Conclusion
A CDS system to support parental tobacco cessation in pediatric primary care, developed through a HCD process, proved easy to use and acceptable to parents, clinicians, and office staff, justifying future efforts to test its impact on parental smoking cessation outcomes in the clinical setting.
Clinical Relevance Statement
In this study, parents, pediatric clinicians, and clinical staff, all identified directly screening parents for tobacco use and then directly offering treatment through an electronic questionnaire as critical system features. Parents valued this approach, as it allowed them to disclose tobacco use in a nonjudgmental way. Pediatric clinicians and staff felt this approach could identify parents most efficiently and then systematize treatment.
Multiple Choice Questions
-
One of the key features of human-centered design (HCD) approaches is:
A stochastic analysis describing a sequence of possible events.
Active use involvement in the development process.
Information present at the right time in the workflow.
A formatting system for displaying material within the electronic health record.
Correct Answer: The correct answer is option b. HCD approaches, guided by usability experts, involve an analysis of the work environment, active user involvement in the development process, iterative systems development, and testing systems in real-word settings.
-
A think-aloud protocol is a data-gathering method in which:
Individuals are observed performing their typical clinical workflows.
Electronic health record data are queried to answer a clinical question.
Participants talk aloud while performing key prototype tasks.
Participants give feedback as a group, describing key desired features for a new system.
Correct Answer: The correct answer is option c. The think-aloud protocol involves participants talking aloud while performing tasks, communicating their understanding of the user interface, and their selections in performing the tasks.
Acknowledgments
We thank the parents, pediatric clinicians, and clinical staff for their contribution to this study.
Conflict of Interest None declared.
Protection of Human and Animal Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and was reviewed by the Children's Hospital of Philadelphia Institutional Review Board.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. . Atlanta, GA: Centers for Disease Control and Prevention (US); 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 2.Tsai J, Homa D M, Gentzke A S. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. Morb Mortal Wkly Rep. 2018;67(48):1342–1346. doi: 10.15585/mmwr.mm6748a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office on Smoking and Health (US) . Atlanta, GA: Centers for Disease Control and Prevention (US); 2006. Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 4.den Exter Blokland E AW, Engels R CME, Hale W W, III, Meeus W, Willemsen M C. Lifetime parental smoking history and cessation and early adolescent smoking behavior. Prev Med. 2004;38(03):359–368. doi: 10.1016/j.ypmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(04):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 6.Rosen L J, Noach M B, Winickoff J P, Hovell M F. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(01):141–152. doi: 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 7.Section on Tobacco Control . Farber H J, Walley S C, Groner J A, Nelson K E. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136(05):1008–1017. doi: 10.1542/peds.2015-3108. [DOI] [PubMed] [Google Scholar]
- 8.Winickoff J P, Nabi-Burza E, Chang Y. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics. 2013;132(01):109–117. doi: 10.1542/peds.2012-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly J B, Mackenzie L J, Freund M, Wolfenden L, Roseby R, Wiggers J H. Interventions by health care professionals who provide routine child health care to reduce tobacco smoke exposure in children: a review and meta-analysis. JAMA Pediatr. 2016;170(02):138–147. doi: 10.1001/jamapediatrics.2015.3342. [DOI] [PubMed] [Google Scholar]
- 10.Behbod B, Sharma M, Baxi R, Roseby R, Webster P. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2018;1:CD001746. doi: 10.1002/14651858.CD001746.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenssen B P, Kelly M K, Faerber J. Parent preferences for pediatric clinician messaging to promote smoking cessation treatment. Pediatrics. 2020;146(01):e20193901. doi: 10.1542/peds.2019-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenssen B P, Kelly M K, Faerber J. Pediatrician delivered smoking cessation messages for parents: a latent class approach to behavioral phenotyping. Acad Pediatr. 2021;21(01):129–138. doi: 10.1016/j.acap.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bright T J, Wong A, Dhurjati R. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(01):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 14.Kwan J L, Lo L, Ferguson J. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ. 2020;370:m3216. doi: 10.1136/bmj.m3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2014;12(12):CD008743. doi: 10.1002/14651858.CD008743.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matkin W, Ordóñez-Mena J M, Hartmann-Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019;5:CD002850. doi: 10.1002/14651858.CD002850.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidrine J I, Shete S, Li Y. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(06):737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood-Medland E A, Stewart S L, Nguyen H. Health system implementation of a tobacco quitline eReferral. Appl Clin Inform. 2019;10(04):735–742. doi: 10.1055/s-0039-1697593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharifi M, Adams W G, Winickoff J P, Guo J, Reid M, Boynton-Jarrett R. Enhancing the electronic health record to increase counseling and quit-line referral for parents who smoke. Acad Pediatr. 2014;14(05):478–484. doi: 10.1016/j.acap.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Jenssen B P, Bryant-Stephens T, Leone F T, Grundmeier R W, Fiks A G. Clinical decision support tool for parental tobacco treatment in primary care. Pediatrics. 2016;137(05):e20154185. doi: 10.1542/peds.2015-4185. [DOI] [PubMed] [Google Scholar]
- 21.Jenssen B P, Shelov E D, Bonafide C P, Bernstein S L, Fiks A G, Bryant-Stephens T. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(02):399–411. doi: 10.4338/aci-2015-12-ra-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenssen B P, Muthu N, Kelly M K. Parent eReferral to tobacco quitline: a pragmatic randomized trial in pediatric primary care. Am J Prev Med. 2019;57(01):32–40. doi: 10.1016/j.amepre.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabi-Burza E, Drehmer J E, Hipple Walters B. Treating parents for tobacco use in the pediatric setting: the clinical effort against secondhand smoke exposure cluster randomized clinical trial. JAMA Pediatr. 2019;173(10):931–939. doi: 10.1001/jamapediatrics.2019.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabi-Burza E, Winickoff J P, Drehmer J E. Innovations in parental smoking cessation assistance delivered in the child healthcare setting. Transl Behav Med. 2020;10(04):1039–1052. doi: 10.1093/tbm/ibz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffers-van Schayck T, Hipple Walters B, Otten R, Kleinjan M. Implementation of a proactive referral tool for child healthcare professionals to encourage and facilitate parental smoking cessation in the Netherlands: a mixed-methods study. BMC Health Serv Res. 2021;21(01):973. doi: 10.1186/s12913-021-06969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulliksen J, Göransson B, Boivie I, Blomkvist S, Persson J, Cajander Å. Key principles for user-centred systems design. Behav Inf Technol. 2003;22(06):397–409. [Google Scholar]
- 27.Belden J L, Wegier P, Patel J. Designing a medication timeline for patients and physicians. J Am Med Inform Assoc. 2019;26(02):95–105. doi: 10.1093/jamia/ocy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran R L, Kukhareva P V, Taft T. Integrated displays to improve chronic disease management in ambulatory care: A SMART on FHIR application informed by mixed-methods user testing. J Am Med Inform Assoc. 2020;27(08):1225–1234. doi: 10.1093/jamia/ocaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ISO 9241–210:2019 Ergonomics of human-system interaction—Part 210: Human-centred design for interactive systemsAccessed September 29, 2021 at:https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/75/77520.html
- 30.Nielsen J. Boston, MA: Academic Press; 1993. Usability Engineering. [Google Scholar]
- 31.Kushniruk A W, Patel V L. Cognitive and usability engineering methods for the evaluation of clinical information systems. J Biomed Inform. 2004;37(01):56–76. doi: 10.1016/j.jbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Thayer J G, Ferro D F, Miller J M. Human-centered development of an electronic health record-embedded, interactive information visualization in the emergency department using fast healthcare interoperability resources. J Am Med Inform Assoc. 2021;28(07):1401–1410. doi: 10.1093/jamia/ocab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcy T W, Kaplan B, Connolly S W, Michel G, Shiffman R N, Flynn B S. Developing a decision support system for tobacco use counselling using primary care physicians. Inform Prim Care. 2008;16(02):101–109. doi: 10.14236/jhi.v16i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rush W A, Schleyer T KL, Kirshner M. Integrating tobacco dependence counseling into electronic dental records: a multi-method approach. J Dent Educ. 2014;78(01):31–39. [PMC free article] [PubMed] [Google Scholar]
- 35.Winickoff J P, Buckley V J, Palfrey J S, Perrin J M, Rigotti N A. Intervention with parental smokers in an outpatient pediatric clinic using counseling and nicotine replacement. Pediatrics. 2003;112(05):1127–1133. doi: 10.1542/peds.112.5.1127. [DOI] [PubMed] [Google Scholar]
- 36.Fiks A G, Grundmeier R W, Margolis B. Comparative effectiveness research using the electronic medical record: an emerging area of investigation in pediatric primary care. J Pediatr. 2012;160(05):719–724. doi: 10.1016/j.jpeds.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong E K, Strouse R, Hall J, Kovac M, Schroeder S A. National survey of U.S. health professionals' smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12(07):724–733. doi: 10.1093/ntr/ntq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris P A, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(02):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.REDCap Consortium . Harris P A, Taylor R, Minor B L. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10(10):CD006611. doi: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Britto M T, Jimison H B, Munafo J K, Wissman J, Rogers M L, Hersh W. Usability testing finds problems for novice users of pediatric portals. J Am Med Inform Assoc. 2009;16(05):660–669. doi: 10.1197/jamia.M3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright P C, Monk A F. The use of think-aloud evaluation methods in design. SIGCHI Bull. 1991;23(01):55–57. [Google Scholar]
- 43.Davis F D. User acceptance of information technology: system characteristics, user perceptions and behavioral impacts. Int J Man Mach Stud. 1993;38(03):475–487. [Google Scholar]
- 44.Ortega Egea J M, Román González M V. Explaining physicians' acceptance of EHCR systems: an extension of TAM with trust and risk factors. Comput Human Behav. 2011;27(01):319–332. [Google Scholar]
- 45.Nielson J, Mack R L. New York, NY: John Wiley and Sons; 1994. Usability Inspection Methods. [Google Scholar]
- 46.Salmon P, Jenkins D, Stanton N, Walker G. Hierarchical task analysis vs. cognitive work analysis: comparison of theory, methodology and contribution to system design. Theor Issues Ergon Sci. 2010;11(06):504–531. [Google Scholar]
- 47.Stanton N A. Hierarchical task analysis: developments, applications, and extensions. Appl Ergon. 2006;37(01):55–79. doi: 10.1016/j.apergo.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003;35(03):379–383. doi: 10.3758/bf03195514. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administration Applying human factors and usability engineering to medical devices: guidance for industry and food and drug administration staffAvailable at: Applying Human Factors and Usability Engineering to Medical Devices | FDA. Accessed March 29, 2022
- 50.Wang X, Kim T C, Hegde S. Design and evaluation of an integrated, patient-focused electronic health record display for emergency medicine. Appl Clin Inform. 2019;10(04):693–706. doi: 10.1055/s-0039-1695800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGeorge N, Hegde S, Berg R L. Assessment of innovative emergency department information displays in a clinical simulation center. J Cogn Eng Decis Mak. 2015;9(04):329–346. doi: 10.1177/1555343415613723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark L N, Benda N C, Hegde S. Usability evaluation of an emergency department information system prototype designed using cognitive systems engineering techniques. Appl Ergon. 2017;60:356–365. doi: 10.1016/j.apergo.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overhage J M, Johnson K B. Pediatrician electronic health record time use for outpatient encounters. Pediatrics. 2020;146(06):e20194017. doi: 10.1542/peds.2019-4017. [DOI] [PubMed] [Google Scholar]
- 54.Fiks A G, Alessandrini E A, Forrest C B, Khan S, Localio A R, Gerber A. Electronic medical record use in pediatric primary care. J Am Med Inform Assoc. 2011;18(01):38–44. doi: 10.1136/jamia.2010.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner R L, Cooper E, Haskell J. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(02):106–114. doi: 10.1093/jamia/ocy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arndt B G, Beasley J W, Watkinson M D. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(05):419–426. doi: 10.1370/afm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Committee on Adolescence ; Council on Clinical and Information Technology . Blythe M J, Del Beccaro M A. Standards for health information technology to ensure adolescent privacy. Pediatrics. 2012;130(05):987–990. doi: 10.1542/peds.2012-2580. [DOI] [PubMed] [Google Scholar]
- 58.Council on Clinical Information Technology ; Committee on Medical Liability and Risk Management ; Section on Telehealth Care . Webber E C, Brick D, Scibilia J P, Dehnel P. Electronic communication of the health record and information with pediatric patients and their guardians. Pediatrics. 2019;144(01):e20191359. doi: 10.1542/peds.2019-1359. [DOI] [PubMed] [Google Scholar]
- 59.Furniss D.Beyond problem identification: valuing methods in a ‘system usability practice’Available at: Beyond Problem Identification: Valuing methods in a ‘system of usability practice’ (ucl.ac.uk). Accessed March 28, 2022
- 60.Yusop N, Grundy J, Vasa R. Reporting usability defects: a systematic literature review. IEEE Trans Softw Eng. 2019;43(09):848–867. [Google Scholar]


