Abstract
Background
A description of energy-based genitourinary non-surgical devices (energy-based devices) safety data is outlined given their rapid adoption.
Objectives
The authors sought to describe adverse events for energy-based devices in the Manufacturer and User Facility Device Experience database and to compare with similar devices and other subspecialty applications. We hypothesized that products with genitourinary applications had similar adverse events to dermatologic or general surgery applications.
Methods
The authors used Reed Tech Navigator to compile adverse events reports for all registered energy-based devices. Individual adverse events reports associated with (1) non-ablative, (2) fractionated, (3) unfractionated, (4) radiofrequency, and (5) hybrid laser technologies were categorized. Adverse event characteristics were compared among genitourinary applications (n = 39) and other subspecialty applications within the same devices (n = 79).
Results
Eighteen manufacturers were identified, which collectively manufacture 43 products with genitourinary applications. Thirty-nine genitourinary adverse events were reported and isolated to 6 manufacturers with 11 products, of which 82% (n = 32) were injuries, 15% (n = 6) were device malfunction, and 3% (n = 1) were related to improper maintenance. Local treatment reactions were the most commonly reported injury (62%, n = 21). Adverse events varied by device type, with CO2 lasers having more burns and radiofrequency devices having higher rates of sensation loss. Comparing similar technology types, genitourinary energy-based devices had the fewest adverse events reports per device in the Manufacturer and User Facility Device Experience database.
Conclusions
Adverse events were reported for one-quarter of the products currently available, and most were local treatment reactions. The reporting of adverse events is equal to that of other subspecialties, suggesting similar risk profiles. Improved reporting is needed to fully evaluate the safety of individual energy-based devices.
Level of Evidence: 4

See the Commentary on this article here.
Laser technology is an evolving tool in gynecology. Carbon dioxide (CO2) lasers were invented in the 1960s, and by 2000, low-energy minimally invasive lasers were utilized in dermatology. In 2013, fractionated CO2 lasers were cleared for utilization by the FDA for “incision, excision, ablation, vaporization, and coagulation of body soft tissues” in gynecology and other specialties.1 In the past few years, dozens of laser manufacturers have filed for 510(k) FDA clearance of energy-based devices. These devices frequently employ the same programable console unique to each manufacturer, with different site-specific removable handpieces for energy delivery across different applications. Newer devices employ fractionation, non-ablative, and radiofrequency techniques to allow for cutaneous or epithelial treatments with lower energy. Initial studies on fractionated CO2 lasers have demonstrated histologic and symptomatic improvement of vaginal atrophy by stimulating fibroblastic growth, collagen biosynthesis, and extracellular matrix restoration with organized collagen fibers of the vaginal epithelium.2,3 Event reporting of newer fractionated lasers has decreased in superficial scarring, discoloration, and pain compared with ablative techniques.4-6
In 2018, the FDA issued a warning that safety and effectiveness had not been established for vaginal “rejuvenation” devices or aesthetic genitourinary applications, cautioning physicians and manufacturers against such indications.7 They cited reports of adverse events, including burns, dyspareunia, and pain, and established a registry to improve reporting.
We first aimed to describe the reported adverse events for energy-based devices with genitourinary applications in a defined time frame from the Manufacturer and User Facility Device Experience (MAUDE) database (US FDA, Silver Spring, MD). Second, we aimed to compare the number of genitourinary adverse events with other subspecialty (non-genitourinary anatomic locations) applications the same devices and against other similar technology types. We hypothesized that products with genitourinary applications had similar adverse events to dermatologic or general surgery applications.
METHODS
The Reed Tech Navigator database (LexisNexis Company, Horsham, PA) was utilized to compile adverse events from the MAUDE database medical device reports for all energy-based devices with genitourinary applications registered with 510k FDA clearances from January 1999 to March 2019. Individual adverse events reports associated with lasers (non-ablative, fractionated, unfractionated, and hybrid types) and radiofrequency technologies were reviewed. To execute a comprehensive search, a list of all devices and their manufacturer currently available in the United States was compiled employing a combination of web-based search engines, society guidelines, literature review, and conference registrations.
Adverse event reports were searched within the Reed Tech Navigator database by first filtering using FDA product codes. These codes are typically utilized to organize comparable devices. For this study, these codes were employed to compare similarly classified products. The following product codes were individually reviewed for applicable products: HHR (laser, surgical, gynecologic), MUK (electrosurgical radiofrequency system, stress urinary incontinence, female, transvaginal or laparoscopic, pelvic tissue), ONG (powered laser surgical instrument with microbeam\fractional output), and ONF (powered light-based non-laser surgical instrument with thermal effect). Unfortunately, most products of interest were classified under the much broader codes GEX (laser powered surgical instruments) or GEI (electrosurgical, cutting, and coagulation). Due to the extensive number of reports under this product code (>2000), this category was refined utilizing the manufacturer name of specific devices with genitourinary applications. Duplicate reports were removed from analysis. Adverse events descriptions were individually reviewed and categorized from scanned narratives and medical records by date, subspecialty, manufacturer, technology type, reporter, injury type, injury description, and outcome for analysis by research team. As part of the characterization, adverse events for genitourinary applications were grouped together and compared with other subspecialty (dermatology and general surgery) applications for the same devices.
The Reed Tech Navigator (New York, NY) provides summary statistics by product code categories to allow for comparison of the number of adverse events reports for similar technologies. The number of adverse events for devices within the FDA product codes GEX (powered surgical instruments) and ONG (microbeam/fractionate output) were compared with energy-based devices.
Descriptive analysis techniques were employed to characterize adverse events into categories, expressed as frequencies. Values are reported as proportions and chi-square test for significance α < 0.05. Several adverse events reported device malfunction or misfire, and the intended application was unknown. These events were analyzed within the genitourinary-related group for comparison with the integumentary injury from aesthetic use group even though they were not necessarily related to the genitourinary tract for sensitivity. Data consist of tabular summaries from publically available MAUDE database and are available for data sharing with proper institutional IRB permissions.
RESULTS
An initial literature and web-search identified 24 manufacturers of energy-based devices. Two manufacturers with alternative technology types (electromagnetic and sonic vibration) were excluded. Four international manufacturers’ products were not found in the MAUDE database and were removed, leaving a total of 18 manufacturers owning 43 devices with genitourinary applications. No additional products were found in the review of product codes Reed Tech search for HHR, MUK, ONG, and ONF. A total 118 adverse events for these 43 devices were reported, 79 of which were integumentary injury from aesthetic utilization (non-genitourinary). There were 39 solely genitourinary-related adverse events for analysis (Table 1).
Table 1.
Comparison of Adverse Events in Energy-Based Devices With Genitourinary and Other Aesthetic Applications
| Adverse event characterization | Genitourinary adverse events, n (%) | Other aesthetic use adverse events, n (%) | Total, n |
|---|---|---|---|
| Adverse events, No. | 39 (33) | 79 (67) | 118 |
| Manufacturers with adverse events | 6 (33) | 12 (67) | 18 |
| Adverse event type | |||
| Injury | 32 (44) | 63 (66) | 95 |
| Malfunction | 6 (40) | 9 (60) | 15 |
| Other | 1 (15) | 6 (85) | 7 |
| Reporter type | |||
| Manufacturer | 16 (17) | 80 (83) | 96 |
| Distributor | 6 (100) | 0 | 6 |
| Voluntary | 17 (100) | 0 | 17 |
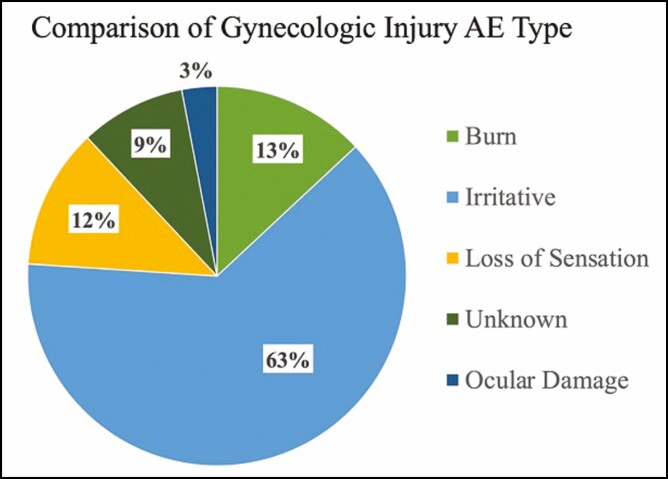
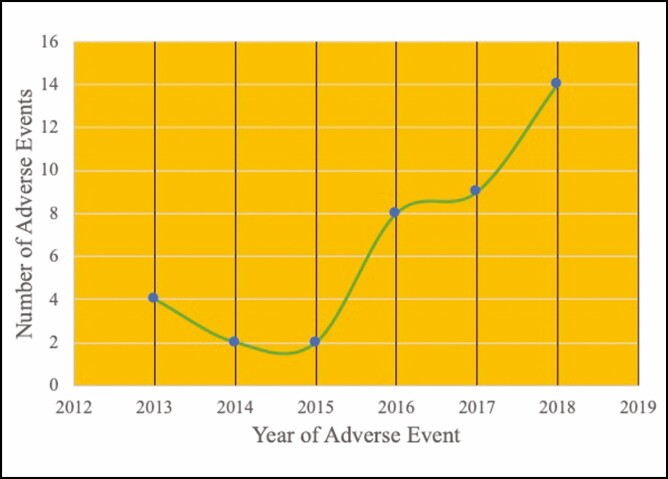
Genitourinary-related adverse events were isolated to 6 manufacturers and 11 products out of the 43 products. Of the genitourinary adverse events, 82% (n = 32) were injuries, 15% (n = 6) device malfunction, and 3% (n = 1) related to improper maintenance (Table 2). The primary anatomic location of injury was the vagina or vulva (67%, n = 22). The remaining injury locations included the urethra (6%, n = 2), other external body injury from misfire (9%, n = 3), eye without eye protection (3%, n = 1), and unknown (15%, n = 5) (Table 2). The need for medical treatment and device failure were the most common reasons for filing an adverse event report. Voluntary reports by patients and physicians outnumbered mandatory reporting by manufacturers, who are required to report any adverse event or device malfunction that could cause death or serious injury. Local treatment reactions to the vagina and vulva, including irritative symptoms of mild pain and discomfort, edema, and erythema, were the most commonly reported injury (61%, n = 21) (Figure 1). The number of adverse events reported each year for energy-based devices has increased yearly since 2015, with 36% (n = 14) of all complaints reported in 2018 (Figure 2).
Table 2.
Descriptive Features of Genitourinary Adverse Events
| Adverse event characterization | Genitourinary adverse events, n (%) |
|---|---|
| Injury type (n = 39) | |
| Injuries | 32 (82) |
| Device malfunction | 6 (15) |
| Device not maintained | 1 (3) |
| Injury location (n = 33) | |
| Vagina/vulva | 22 (67) |
| Urethra | 2 (6) |
| Eye | 1 (3) |
| Unknown | 5 (15) |
| Other extremity, misfire | 3 (9) |
| Report reason (n = 39) | |
| Medical treatment | 16 (41) |
| Device failure | 13 (33) |
| Improper technique | 3 (8) |
| Emotional stress | 2 (5) |
| Financial burden | 2 (5) |
| Unknown | 3 (8) |
| Reporter type (n = 39) | |
| Distributor | 6 (15) |
| Voluntary (patient or provider) | 17 (44) |
| Manufacturer | 16 (41) |
Figure 1.
Urogenital adverse event by injury type, n = 34. No injury was reported for 5 adverse events (AE). Irritative symptoms include discomfort, edema, and erythema. Loss of sensation was a patient-reported outcome described as numbness after intravaginal radiofrequency treatments. One patient reported sexual dysfunction secondary to sensation loss.
Figure 2.
Genitourinary adverse events by year. Cases prior to 2014 FDA clearance are related to misuse safety event for same devices.
The majority of adverse events for all genitourinary energy-based device products involved CO2 lasers (73%, n = 25), fewer in radiofrequency (35%, n = 12), and least in Er:YAG lasers (5%, n = 2). Irritative symptoms were the most commonly reported adverse event for all technology types, and burns and loss of sensation were reported with higher frequency in CO2 lasers and radiofrequency devices, respectively, during the observed timeframe.
When comparing genitourinary energy-based device products (n = 43) with similar technology within the FDA product code for laser-powered surgical instruments devices, the percentage of products with reported adverse events to total products registered (n = 11/43 vs 624/2064) was similar (25% vs 30%) and significantly lower than the microbeam/fractionate output products (n = 79/103 vs 11/43 [70%, P = .01]) used in topical dermatologic aesthetic applications.
DISCUSSION
Overall, at least 1 adverse event was reported for one-quarter of the products currently available for genitourinary applications. Most reports were submitted to document injuries, which corresponds to the most common types of reporters being patients and health care professionals. Most adverse events were known risks and side effects of this treatment type, with local reactions occurring at the treatment site. Most reports were intended to document increased need for postprocedure medical treatment. The increasing number of adverse events since 2015 is likely related to the expanding number of devices, utilization, and awareness from providers and registries. The medical community relies on reporting by fellow clinicians in their utilization of new technology.
In a review of uses of lasers and energy-based devices in urogynecology, Bhide et al reported on 25 studies that were without major adverse events. A few (n = 7/25) studies included side effects such as pain, edema, dysuria, minor bleeding, and urinary tract infection. Thus, they inferred that initial research experience with treatments and short-term follow-up in these technologies was safe without significant adverse events.8
Ahluwalia et al completed a similar review of the MAUDE database from October 2015 to January 2019 for events related to lasers and energy-based devices specifically for vaginal rejuvenation in which they reported a similar total number of events (n = 46) from 45 patients.9 The descriptions of the most common adverse events injury type were comparable, including pain (n = 19), burning (n = 11), scarring (n = 7), and dyspareunia (n = 6). They concluded that further research is needed to establish the safety profiles for specific gynecologic indications of this technology.9 Because the indications for treatment are often missing from the adverse event reports, we chose to more broadly include all adverse events for treatments on genital organs or with handpieces specific to genitourinary applications.
In a systematic review of current literature, the MAUDE database, and Bloomberg Law database, Guo et al also reported a similar 120 adverse events from the MAUDE database and 29 from current literature.10 Their analysis focused on genitourinary syndrome of menopause. Again, they noted that indications and preexisting symptoms are frequently missing from reports. Our more detailed review of adverse event reports allows for further characterization regarding those specifically with genitourinary application.
In a review of the 174 legal cases within the Westlaw Next database for cosmetic laser procedures between 1985 and 2012, injury reports were very similar to MAUDE reported adverse events.11 The most common adverse events were burns (47%, n = 86), followed by scars (38%, n = 71), pigmentation (24%, n = 43), disfigurement (16%, n = 29), emotional distress (12%, n = 21), and physical suffering (10%, n = 20). Hair removal was the most popular procedure (36%), followed by “rejuvenation” (24%).11 Likewise, Pierce et al reported on a review of 42 cases in the LexisNexis database of court records of legal trials from 1991 to 2015 related to medical malpractice of ablative lasers, not limited to urogenital applications, and again demonstrated that the most common injuries were scarring (75%), discoloration (14%), and infection (9.5%).12 Guo et al reported no cases in the Bloomberg Law database, established in 2010, for vaginal indications.10 Although these are bias samples of those seeking legal action, it further supports the severity and portion of injuries type for these technologies.
Aesthetic and dermatologic specialists are generally thought to have more experience and a broader knowledge base for the variety of applications of laser and energy-based technologies. In a survey of European dermatologists, 40% did not perform minimally invasive cutaneous procedures; however, they recommend them to 20% of their patients.13 The most common reason for not providing these treatments was lack of training 60%, and 17% were worried about complications and 11% cited legal concerns.13 These attitudes demonstrate the importance of provider education and training, particularly with these newly emerging techniques. We demonstrated that 8% of the adverse events for genitourinary applications were directly related to improper operation by the clinician.
Importantly, lack of informed consent is the most common cause of legal action in 53% to 55% of cases related to lasers and energy-based devices.11,12 Particularly given that the most common adverse events are known risks of the procedure, it is imperative to have a thorough discussion with the patient about potential adverse events prior to each procedure.11,14
The strengths of this study include a comprehensive search all of the available adverse events reported for energy-based devices with genitourinary applications. This research was not limited to single indication reports but further characterizes and compares with other aesthetic applications (general and plastic surgery) on the same devices for broader comparison. Our data can only include what was documented in the MAUDE database reports, and therefore a limitation is the self-reporting mechanism and a lack of verification or medical professional review. Another limitation of our study is that the total number of uses of energy-based devices is unknown; thus, without a denominator, we can only make conclusions based on comparisons between what is reported. Our comparisons of number of adverse events by device for genitourinary applications and other applications is meant to be a broad comparison of reporting trends, because the specific types of adverse events were not examined. Fortunately, there is no 1 dominating laser technology type with adverse events to consider an individual technology unsafe at this time.
All of the devices within the MAUDE database are FDA approved or cleared, and therefore all of the adverse events reviewed involved FDA-cleared devices. However, there are many examples of rapid technology adoption under FDA 510k clearances leading to unforeseen adverse and patient safety events. In 2018, the FDA announced some changes to the 510k clearance process for new devices, including stricter regulations with the use of predicate devices as the basis for clearance. Providers should be cautious when introducing new medical technology into practice, obtain proper training, and understand the research premise. Our review found that fractionated laser and radiofrequency technology does have similar risk profiles across different target organs and subspecialty applications. However, the reported adverse events indicate poor risk discussions with patients because they were primarily known side effects.
CONCLUSIONS
In conclusion, this is a comprehensive analysis of the reported adverse events for energy-based devices employed for genitourinary applications. Overall, adverse events were reported for one-quarter of the products currently available for genitourinary applications, with about 80% of reporting to document injury, which is consistent with other published literature. The MAUDE database information relies on voluntary reports by providers and patients but serves as the most comprehensive resource of these events. As evident by the FDA warnings and echoed by the American College of Obstetricians and Gynecologist and the American Urogynecologic Society, these devices need continued assessment to fully evaluate the safety of any individual energy-based device and technology for genitourinary applications utilizing more comprehensive registries.15-17
Disclosures
Dr Ackenbom has received research grant funding from the NIH NICHD Women’s Reproductive Health Research Career Development Program (5K12HD063087) (National Institutes of Health, Bethesda, MD). The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. US Food and Drug Administration (FDA). Product classification.2013. Accessed March 11, 2019https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=5666.
- 2. Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429-436. [DOI] [PubMed] [Google Scholar]
- 3. DeLeon F, Baggish M. Lasers in gynecology. Glob Libr Women’s Med. 2008. doi: 10.3843/GLOWM.10023. [Google Scholar]
- 4. Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(Suppl):S101-S113. [PMC free article] [PubMed] [Google Scholar]
- 5. Welch AJ, Torres JH, Cheong WF. Laser physics and lasertissue interaction. Tex Heart Inst J. 1989;16(3):141-149. [PMC free article] [PubMed] [Google Scholar]
- 6. Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22(8):845-849. [DOI] [PubMed] [Google Scholar]
- 7. Administration US FDA. Statement from the FDA Commissioner, Scott Gottlieb, MD, on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.”2018. Accessed March 11, 2019https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-efforts-safeguard-womens-health-deceptive-health-claims.
- 8. Bhide AA, Khullar V, Swift S, Digesu GA. The use of laser in urogynaecology. Int Urogynecol J. 2019;30(5):683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahluwalia J, Avram MM, Ortiz AE. Lasers and energy-based devices marketed for vaginal rejuvenation: a cross-sectional analysis of the MAUDE database. Lasers Surg Med. 2019;51(8):671-677. [DOI] [PubMed] [Google Scholar]
- 10. Guo JZ, Souders C, McClelland L, et al. Vaginal laser treatment of genitourinary syndrome of menopause: does the evidence support the FDA safety communication? Menopause. 2020;27(10):1177-1184. [DOI] [PubMed] [Google Scholar]
- 11. Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149(2):188-193. [DOI] [PubMed] [Google Scholar]
- 12. Pierce RR, Martell DW. Ablative lasers: 24 years of medical malpractice cases in the United States. Dermatol Surg. 2018;44(5):730-731. [DOI] [PubMed] [Google Scholar]
- 13. Yildiz H, Abuaf OK, Goker K, Bulur I. Why some of the dermatologists choose to avoid carrying out minimally invasive cosmetic procedures? J Cosmet Laser Ther. 2016;18(8):467-471. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg DJ. Cosmetic dermatology: legal issues. Dermatol Clin. 2009;27(4):501-505, vii. [DOI] [PubMed] [Google Scholar]
- 15. Committee on Gynecologic Practice. ACOG committee opinion no. 378: vaginal “rejuvenation” and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738. [DOI] [PubMed] [Google Scholar]
- 16. American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG clinical document (published January 2020 through present): elective female genital cosmetic surgery ACOG committee opinion No. 795. Obstet Gynecol. 2020;135(1):e36e42.31856125 [Google Scholar]
- 17. American Urogynecologic Society. Energy-based devices and vaginal rejuvenation.2018. Accessed March 11, 2019https://www.augs.org/energy-based-devices-and-vaginal-rejuvenation/.