Abstract
Patients with severe coronary artery disease with a clinical indication for revascularization but who are at high procedural risk because of patient comorbidities, complexity of coronary anatomy, and/or poor hemodynamics represent an understudied and potentially underserved patient population. Through advances in percutaneous interventional techniques and technologies and improvements in patient selection, current percutaneous coronary intervention may allow appropriate patients to benefit safely from revascularization procedures that might not have been offered in the past. The burgeoning interest in these procedures in some respects reflects an evolutionary step within the field of percutaneous coronary intervention. However, because of the clinical complexity of many of these patients and procedures, it is critical to develop dedicated specialists within interventional cardiology who are trained with the cognitive and technical skills to select these patients appropriately and to perform these procedures safely. Preprocedural issues such as multidisciplinary risk and treatment assessments are highly relevant to the successful treatment of these patients, and knowledge gaps and future directions to improve outcomes in this emerging area are discussed. Ultimately, an evolution of contemporary interventional cardiology is necessary to treat the increasingly higher-risk patients with whom we are confronted.
Keywords: coronary artery disease, coronary occlusion, heart failure, percutaneous coronary intervention
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the developed world, affecting 15.5 million adults in the United States, with 635 000 Americans projected to have a new coronary event (either first hospitalized myocardial infarction or CAD death) this year.1 The profound burden of CAD, coupled with these high event rates, underscores the need to identify and offer treatment to patients with CAD at higher risk for these adverse clinical events. Unfortunately, despite the availability and implementation of disease-modifying guideline-directed medical therapy (GDMT; eg, lifestyle modification, aspirin, statins, and control of risk factors such as blood pressure and diabetes mellitus), a significant proportion of patients still present with prognostically important and anatomically severe CAD as their initial manifestation of CAD.2
For these higher-risk CAD patients, coronary revascularization (in addition to GDMT) can both improve quality of life and reduce adverse clinical events.3–6 A strategy of offering revascularization to patients with high-risk clinical presentations (acute coronary syndromes or stable ischemic heart disease with high-risk anatomy or refractory symptoms) is supported in current clinical practice guidelines and appropriate use documents.7–10 Nonetheless, the rate of revascularization procedures, especially for stable ischemic CAD, has declined considerably over the past decade.11–13 Several factors have been identified as contributing to this decline. More effective implementation of GDMT after the publication of randomized trials examining the role of revascularization strategies for patients with stable CAD,14,15 more judicious CAD screening protocols, and concerns about inappropriate percutaneous coronary intervention (PCI) have likely resulted in more selective use of diagnostic and revascularization procedures.12,16–18 Declines in revascularization because of these factors are entirely appropriate. However, it is possible that the decline in the rate of revascularization may be out of proportion to clinically inappropriate use. On the basis of a comprehensive analysis of the US CathPCI Registry frequently cited as evidence for the overuse of PCI in the midst of this decline, <5% of all urgent and electively performed PCIs were rated as inappropriate using appropriate use criteria.19 Thus, although revascularization may have been overused in lower-risk patients, within the overall decline in volume is the possibility of underuse of invasive testing and revascularization procedures in other subgroups of patients such as those at higher risk for adverse events.12,20–25
A patient population among the least likely to be offered PCI but with a clinical indication for revascularization consists of patients with CAD who also are at higher or extreme (inoperable) surgical risk.26–28 Although complete revascularization through PCI is a less invasive alternative to surgical revascularization and may therefore offer advantages to patients at high risk for surgery, early experiences with PCI conducted in the balloon angioplasty and early stent eras demonstrated lower success rates and higher rates of complications with PCI in this group of patients. Nonetheless, with improved patient selection, in conjunction with advances in interventional techniques and technologies, complete revascularization through PCI may allow appropriate patients to safely benefit from revascularization procedures that otherwise might not have been possible or were unwise to offer in the past. Here, we attempt to characterize these patients, increasingly referred to as the “complex higher-risk (and indicated) patient” population, on the basis of growing interest within the field of interventional cardiology. We also examine the potential unmet need for revascularization in this patient subset and discuss the evolution in treatment paradigms essential for the effective care and treatment of these patients.
CANDIDACY FOR REVASCULARIZATION: AN UNMET NEED FOR PCI
Risk assessment with established and evolving contemporary risk models is an integral component for appropriately identifying and selecting patients for coronary revascularization. Objective risk assessment also can provide patients and referring providers with information to allow them to make shared and informed decisions about treatment. The risk selection algorithm for the advanced CAD patient should be an integrated process aimed at determining the risks of all potential therapies that can be offered to the patient: surgical, percutaneous, or GDMT alone. Large, multicenter, randomized trials have generally demonstrated superior outcomes with coronary artery bypass grafting (CABG) compared with PCI or GDMT in patients with complex multivessel/left main CAD and complex anatomy as identified through an intermediate to high SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) score.29,30 However, patients who are deemed either inoperable or at higher risk (eg, >5% estimated risk of mortality for a surgical revascularization procedure) are potential candidates for a percutaneous approach. An additional group of patients for whom percutaneous revascularization is frequently considered is composed of patients who have already had a CABG procedure, especially those for whom the left internal mammary has already been used as a conduit.
Contemporary data demonstrate that patients with higher-risk CAD (such as those with comorbidities or presentations with heart failure) are among the least likely to undergo or even be offered revascularization via a percutaneous approach.23,24,26–28 There are several possible reasons why this group of patients may not be offered PCI. Some patients may have comorbidities that are too extensive for a (potentially futile) revascularization procedure to make an appreciable difference in outcome. For other patients at high surgical risk, other practical obstacles can lead to the underuse of PCI. For most interventional cardiologists, the percutaneous treatment of these high-risk patients represents a challenge that is often avoided, given a lack of widespread technical expertise, the perception of low procedural success, and confusion about accepted indications for PCI in this population. Physicians may intuitively (and perhaps incorrectly) think that these patients have such far advanced CAD to preclude any meaningful clinical benefit. This is, no doubt, compounded by a relative scarcity of data on the efficacy of revascularization in this population. Public reporting of adverse outcomes can also serve as a deterrent to PCI among higher-risk patients.31
At the present time, it is not known precisely how many of these higher-risk patients who might potentially benefit from revascularization are not ultimately offered it. The difficulty in approximating this number stems from the fact that many of these patients may never come to the attention of interventionalists or cardiac surgeons.24 Additionally, higher-risk patients are almost uniformly excluded from most clinical trials. Many of these patients do not even undergo the diagnostic testing necessary to make the diagnosis of severe CAD.24,32 Furthermore, a misalignment exists in the use of cardiac catheterization in many patients relative to their predicted probability of severe CAD in which the use of catheterization appears to target patients who would derive less benefit from revascularization, consistent with a treatment-risk paradox.21
PCI may be considered a beneficial option for the subgroup of patients with severe CAD in whom the revascularization hypothesis (of incremental benefit compared with GDMT alone) may in fact be demonstrable, assuming that these patients can safely undergo revascularization. Even among patients who may be candidates for surgical revascularization, some patients may be willing to accept a higher rate of repeat revascularization with PCI if it minimizes stroke risk compared with CABG, whereas other patients may be willing to accept a longer perioperative recovery from a CABG in hopes of avoiding the need for repeat revascularization and potentially shorter overall longevity with PCI. If CABG is not an option or not desired by the patient, complete revascularization through PCI in many of these patients would require specialized technical and cognitive skills not readily possessed by most coronary interventionalists. Nevertheless, if complete revascularization can be safely and effectively achieved, these patients are among the most likely to derive a robust clinical benefit. Therefore, effective treatment of these patients falls into a true “higher risk, higher reward” paradigm.
DEFINING THE STABLE ISCHEMIC CAD PATIENT AT HIGHER RISK FOR ADVERSE OUTCOMES WITH REVASCULARIZATION THERAPIES
Accurate risk stratification is critical in the evaluation and management of patients with stable ischemic CAD who are candidates for revascularization therapies. The clinical characteristics and presentation, noninvasive testing including functional testing, and anatomic delineation of CAD all inform the overall risk assessment of CAD patients, and diagnostic and therapeutic strategies usually are tailored by weighing the anticipated benefits of treatment against an individual’s predicted risk for adverse events. Among patients who might benefit from revascularization, a careful assessment of anticipated procedural benefits and estimated procedural risk is critical, and communication of these benefits and risks to the patients, their family, and any physicians comanaging them is essential. Despite the presence of procedural risk calculators for both CABG and PCI, formal consensus on the exact definition of high procedural risk still remains somewhat of an art.33–35 This may be in part due to factors not currently captured in validated risk calculators,36,37 combined with the observation that in higher-risk patients, conventional risk calculators that estimate 30-day mortality across both elective and emergent patients may be wanting, particularly for stable patients undergoing higher-risk interventions.34 In addition, there may be individual variability in levels of accepted or tolerated risk (by both patients and providers/institutions, and depending on the clinical scenario).
All proposed definitions of risk for revascularization procedures incorporate features specific to 3 clinical spheres: patient risk factors and comorbid conditions (including those that preclude surgical or percutaneous revascularization); location and complexity of coronary anatomy (including adequacy of vessels for PCI or for surgical targets); and hemodynamics, ventricular function, and concomitant valvular disease (Figure). It is the composite risk derived from the integration of each of these 3 areas that leads to the cumulative procedural risk profile of any individual CAD patient for whom revascularization is considered.
Figure. The increasingly high-risk patient population with indications for revascularization who may be considered for percutaneous coronary intervention.
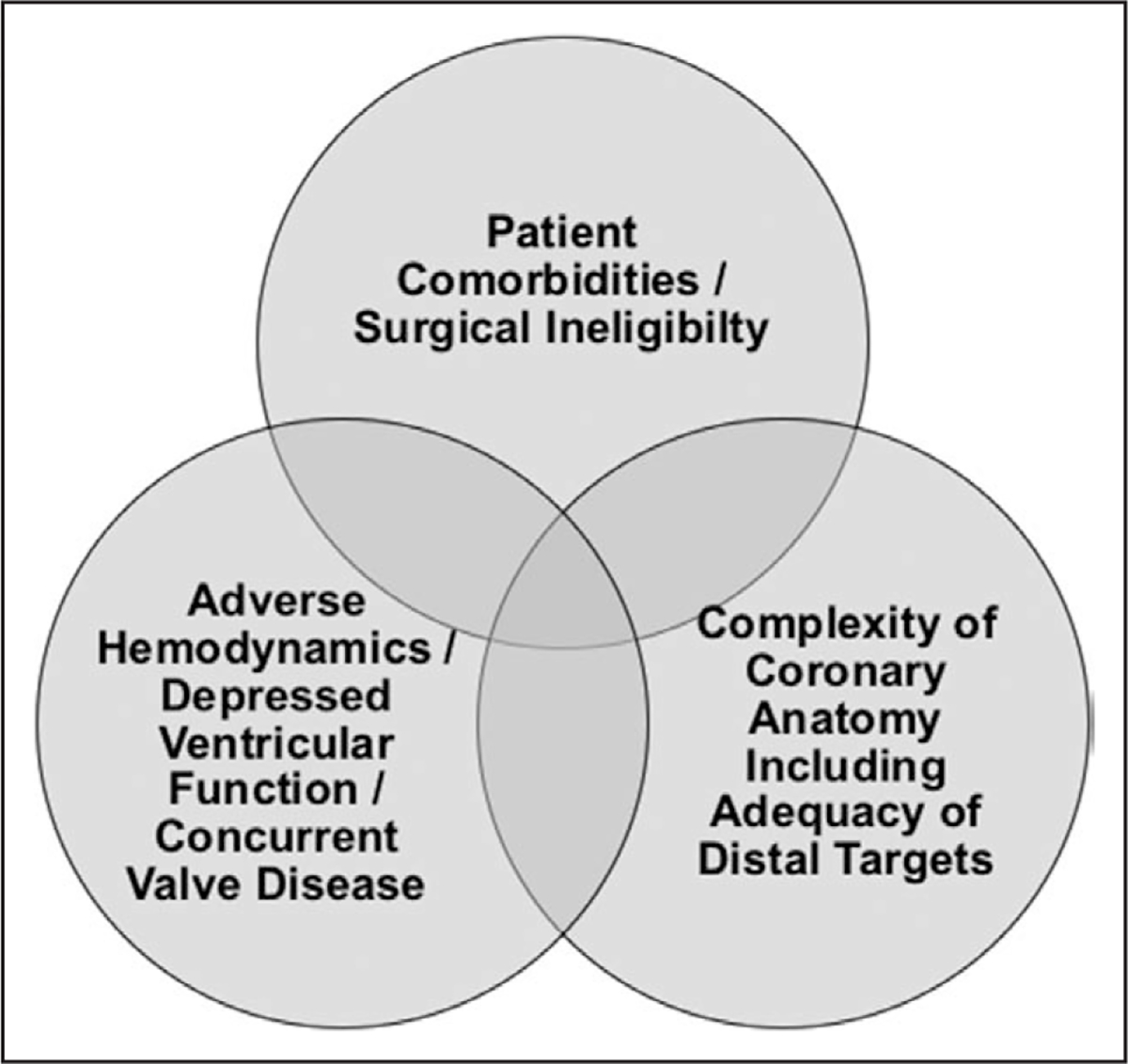
Patient risk is reflected by the 3 separate (but overlapping and potentially additive) areas.
Comorbid characteristics and adverse patient risk factors can result in increased mortality, decreased functional capacity, inferior quality of life, and greater cost and resource use, including rehospitalization. Epidemiologic data demonstrate that the odds of having multiple cardiovascular comorbidities in CAD patients has increased significantly over time.38 In addition, certain comorbidities may disproportionately modify procedural risk for CABG compared with PCI. In particular, patients with oxygen-dependent chronic obstructive pulmonary disease, severe liver disease, carotid artery disease, prior stroke, frailty, or even prior CABG have been shown to have increased risk with CABG compared with PCI.33,39–41 Other factors such as the presence of a hostile chest (eg, resulting from anatomic deformities or prior radiation therapy), severe aortic calcification (porcelain aorta), and other factors specifically increasing surgical risk may also weigh into the decision making for revascularization strategies in CAD.
When a revascularization strategy is being considered for patients with CAD, anatomic considerations such as the presence of unprotected left main CAD, complex bifurcation and trifurcation lesions, chronic total occlusions, and heavily calcified lesions, as well as high SYNTAX score, also can factor heavily into the estimation of risk, particularly for patients for whom PCI is being considered.42 Each of these factors can influence the degree of difficulty of a complex PCI procedure. There also is a disparate set of anatomic considerations that affect the risk and potential success of CABG, including suitability of conduits (arterial and venous) and the adequacy of distal targets within the native coronary arteries, especially because this may affect the suitability of left internal mammary placement to a diseased left anterior descending coronary artery. A fundamental assumption before consideration of higher-risk revascularization procedures is that the territories being revascularized are both ischemic and viable. In patients with severe left ventricular dysfunction or regional wall motion abnormalities, performance of either noninvasive testing to confirm ischemia/viability or fractional flow reserve to determine the physiologic significance of lesions should be considered to ensure that a meaningful degree of myocardium subtended by the vasculature to be intervened on is recoverable.
Poor hemodynamic status, impaired ventricular function, and the presence of concomitant valvular heart disease are the final critical components of the assessment of procedural risk. Patients with abnormalities in this sphere typically have low physiologic reserve and are at high risk for hemodynamic decompensation during either PCI or CABG. Revascularization in these patients also requires careful planning, with preprocedural hemodynamic optimization and consideration of the use of hemodynamic support before intervention in selected cases and continuing through the immediate postprocedural time period. An additional (often unrecognized) subgroup of patients at high risk for adverse outcomes are those with pulmonary hypertension or right ventricular failure; surgical outcomes in this group in particular are among the poorest.43
MULTIDISCIPLINARY TEAM-BASED APPROACH TO HIGHER-RISK CAD PATIENTS
Given the complexity of managing higher-risk patients with CAD, a collaborative team-based model is essential for appropriate patient selection, treatment, and subsequent care of these patients. The coordinated approach of a dedicated heart team has the potential to lead to enhanced decision making, superior outcomes, and ultimately exceptional overall patient care,7–9 although institutional protocols encapsulating heart team decision making algorithms may sometimes be substituted for formal heart team deliberations in an institution with well-established and high-quality practice patterns.44 Patients should be considered potentially for PCI or surgical revascularization if they have ongoing symptoms despite GDMT and are thought by the heart team to derive a likely meaningful clinical benefit from revascularization. A thorough assessment of the extent of CAD burden, hemodynamics, and global cardiac function should be undertaken. The optimal revascularization strategy, timing, and alternative approaches then should be closely mapped out within the constructs of the heart team. Given the nuances of clinical decision making in these patients and scenarios in which there may be clinical equipoise, the collective experience of the multidisciplinary heart team is vital to optimizing patient outcomes.
Beyond the decision making for a revascularization strategy, when patients with higher-risk CAD are treated, input from primary treating physicians, interventional cardiologists, cardiothoracic surgeons, heart failure specialists, multimodality imaging specialists, intensivists, and even electrophysiologists (for patients with depressed ventricular function or concomitant arrhythmias) may become relevant (Table 1). For example, heart failure or critical care specialists with a dedicated interest in the acute care of decompensated congestive heart failure should be engaged when issues related to candidacy for advanced heart failure therapies or cardiac transplantation are being considered. Additionally, imaging experts with specific knowledge of applied imaging as an adjunct to interventional vascular procedures would be best suited to participate in discussions of the cause and management of concurrent valvular heart disease (eg, ischemic mitral regurgitation). Furthermore, it is important to explicitly state that all patients with CAD should have their medical regimen optimized before proceeding with any revascularization procedure. Optimization should include a thorough assessment of implementation and adherence to GDMT, related to both disease modification and symptom relief. A careful and systematic review of both medical and adjunctive lifestyle-modifying therapies can reveal significant opportunities for improvements in overall cardiac care. Finally, careful optimization of hemodynamic status and adjunctive periprocedural therapies (eg, management of kidney dysfunction, including optimization of hydration, in conjunction with low-contrast protocols, prophylaxis for contrast allergy as needed) can be critical to ensuring that a patient undergoes a safe procedure.
Table 1.
Roles and Team Members Who May Be Called on in a Multidisciplinary Approach to Patients With Higher-risk, severe Cad
| Role | Member | |
|---|---|---|
| Patient/family | Shared decision making | Patient and family members |
| Physicians | Defining goals of care and preprocedural optimization | Primary care physician |
| Primary cardiologist | ||
| Advanced heart failure/critical care specialist (experience in advanced therapies, transplantation) | ||
| Formulating revascularization strategy | Specialized coronary interventionalist | |
| Cardiothoracic surgeon | ||
| Managing concomitant structural heart disease | Multimodality imaging specialist | |
| Structural heart interventionalist (for concomitant valvular disease) | ||
| Managing concomitant rhythm therapies | Electrophysiologist | |
| Postprocedural care | Cardiac intensivist, primary cardiologist | |
| Staff | Care facilitation | Nurse or advanced practice provider to assist in preprocedural/postprocedural optimization Social worker/services |
CAD indicates coronary artery disease.
SPECIALIZED COGNITIVE SKILLS AND TECHNICAL SKILLS REQUIREMENTS
To treat patients safely and effectively, there needs to be a cadre of interventional cardiologists who possess the skill sets necessary to perform complete revascularization safely and effectively in the most complex and higher-risk patients. In the current era of PCI with advances in functionally based revascularization, adjunctive pharmacology, and PCI techniques and devices, the success rates for treating the most complex lesion subsets have improved among operators trained in specialized techniques45,46 but have remained significantly lower among everyday interventionalists.47 Armed with the knowledge of how and when to use these techniques, dedicated interventionalists with expertise in treating these patients could be more apt to choose the optimal treatment strategies and, most important, improve overall outcomes in these patients.
For those interventionalists who wish to evolve beyond those performing conventional contemporary PCI, adequate technical training complemented by an adequate procedural volume of complex cases (eg, chronic total occlusions, calcified vessels, complex bifurcation disease, cases requiring hemodynamic support) and specific techniques and devices is a prerequisite (Table 2). The experience and clinical judgment required to perform these procedures in most cases will be beyond that obtained in traditional single-year interventional cardiology fellowships in which the exposure to the most complex patient and lesion subsets may be limited. Moreover, the nuances of case selection and clinical judgment necessary to become an expert interventional cardiologist require time and an accumulated case load.
Table 2.
Technical Skills and Training/Infrastructure Requirements (for Physicians, Staff, and Institutions) for the Care and revascularization of Patients With Higher-risk, severe CAD
| Patient/Lesion subsets | Techniques/devices |
|---|---|
| Chronic total occlusions | Dual access and injections |
| Antegrade and retrograde techniques, including dissection/re-entry devices Specialty wires, microcatheters, devices for increasing guide/catheter support, externalization techniques | |
| Left main stenosis/ bifurcations | Single- and 2-stent strategies (both primary and for provisional/bailout use) Intravascular imaging |
| Calcific disease | Rotational/orbital atherectomy |
| Intravascular imaging | |
| Multivessel disease | Coronary physiological studies (eg, fractional flow reserve) Intravascular imaging |
| Poor hemodynamic status/ventricular function coexisting with complex anatomy | Left/right ventricular percutaneously implanted support devices |
| Intra-aortic balloon counterpulsation | |
| Extracorporeal membrane oxygenation | |
| Large-vessel access/closure management | |
| Transradial expertise (when both femoral arteries are used) | |
| Alternative access considerations (axillary, transcaval) | |
| Stent underexpansion/ restenosis | Intravascular imaging |
| Aggressive noncompliant and plaque-modification balloons | |
| Atherectomy (laser, rotational) | |
| Vascular brachytherapy | |
| Complication management | Echocardiography-guided pericardiocentesis |
| Covered stents, coils, beads | |
| Snares/snaring techniques | |
| Dual guide techniques | |
| Dissection/re-entry to salvage distal flow | |
| Endovascular rescue |
CAD indicates coronary artery disease.
The development and eventual success of this field are, however, predicated on appropriately identifying and treating the correct patient population and ensuring that the desired outcomes can be achieved. Although performing PCI in patients who are either ineligible or too high risk for surgical revascularization makes empirical sense, a movement toward the performance of PCI in these populations is not to be taken lightly. Given the procedural complexity and the patient comorbidities associated with an intrinsically high-risk population, the potential for considerable harm exists if potentially unprepared interventionalists are given free rein to perform the highest-risk procedures for contemporary PCI in a potentially vulnerable population. There is a difference between complex intervention and higher-risk intervention. Whereas complex intervention requires advanced and specialized techniques, not all of these confer increased risk to an individual patient. For example, although a patient with an ejection fraction of 10% and a focal noncomplex LAD lesion might not at first blush appear to be at significantly higher risk for an interventional procedure, if the patient had pulmonary artery pressures of 75 mm Hg with a pulmonary capillary wedge pressure of 35 mm Hg and a pulmonary artery saturation of 30%, that patient might indeed be at higher risk than a patient with a complex distal left main coronary artery lesion and normal ventricular function. Therefore, successful establishment of specialized programs must incorporate training/expertise in both complex techniques (eg, treating the distal left main bifurcation) and the adequate assessment of procedural risk (eg, through knowing when to perform right-sided heart catheterization before undertaking PCI).
In many respects, a focused core curriculum for interventionalists performing procedures in these patients is therefore essential. Such a curriculum could focus not only on how to safely and skillfully treat these patients from a technical standpoint but also on the cognitive development necessary for preprocedural screening and evaluation in conjunction with an understanding of the rationale and goals for revascularization. Having a dedicated and case-based curriculum could additionally help to ensure a shared level of expertise and knowledge among interventional cardiologists performing these procedures. If this curriculum could be robustly developed and broadly applied, it could truly be transformative in defining what it means to be an advanced coronary specialist in an era of continued differentiation within the field of interventional cardiology.
The specialized techniques needed for effective treatment of higher-risk patients with indications for revascularization simply cannot be taught in an abridged course or without some element of hands-on training. Thus, a considerable investment in time and effort likely would be needed for physicians to become truly proficient. The development of formalized training programs, observerships, or even proctorships could help with some of the practical hands-on skills necessary, particularly for practitioners who lack the procedural experience and support to begin to tackle more complex procedures with the goal of complete revascularization. In addition, we could envision specific training and mock scenarios administered within the cardiac catheterization laboratory and intensive care units to ensure adequate training by staff in both areas. It is imperative, however, that designated specialists and programs continue to use their accumulated skills set on a regular basis because outcomes are undoubtedly likely to suffer if appropriate volume thresholds are not maintained. Finally, further work is required to address the ideal reimbursement and cost structures for these complex procedures that can often lead to substantial variances in time, equipment costs, and hospital use.
KNOWLEDGE GAPS AND FUTURE DIRECTIONS
There are still many unanswered questions related to the evolving population of higher-risk PCI patients. The exact size of the patient population that can benefit from higher-risk PCI procedures remains unknown because these patients have historically been underrepresented in clinical trials and registries, and many patients who could be eligible for revascularization never come to the attention of interventional cardiologists or cardiothoracic surgeons. There have also been no trials comparing PCI with GDMT in this patient population. Moreover, in patients with complex coronary anatomy at very high (but not inoperable) surgical risk, it is unknown whether PCI is truly a viable alternative to CABG over the long term. Whereas surgical ineligibility can confer risk independently for patients undergoing high-risk PCI,36,37 no risk models can calculate the differential risk of PCI appropriately compared with optimal GDMT. As a result, providers inappropriately may ascribe too high or too low a risk to PCI and adversely affect the decision about revascularization. Lastly, it is not known how many patients fall into an area of futility where no benefit can be achieved by revascularization.
To begin to answer some of these questions, it is critical to start gathering systematic disease-based data on patients with complex and severe CAD and the current treatments offered to these patients. Various research priorities within this space are listed in Table 3. One of the first steps in investigating this patient population would involve the formation of a large, multicenter registry that could allow the systematic tracking of short- and long-term outcomes for higher-risk patients already undergoing more complex procedures. This has already started within the chronic total occlusion space.45 The data from similarly developed and more broadly based registries would be hypothesis generating but could help shape guidelines for the management of these patients. These registries ultimately could lead to the creation of a preliminary database infrastructure that could be used to construct formalized prospective studies (even randomized trials) within this population. Such registries and any subsequent studies also may be mined to develop finally an accurate risk model to help guide physicians in the decision making process for revascularization in this population. Ultimately, the recognition of the evolution in risk profiles among patients undergoing PCI concept may lead to the collective improvement in the quality of PCI as a whole because patients may be more apt to undergo PCI by well-trained interventionalists possessing the breadth and depth of technical and cognitive skills to treat them safely and effectively.
Table 3.
Research Priorities in the Higher-Risk CAD Population Potentially Eligible for PCi
| Research Priority/Question | Study design/Cohort |
|---|---|
| What is the prevalence of severe (and nonrevascularized) CAD? | Disease-based (as opposed to solely procedure-based) registries |
| What are the outcomes of PCI in higher-risk CAD patients (eg, nonsurgical patients), and are there specific operator/institution volumes that are required to achieve the best procedural outcomes? | Procedural registries |
| What are the costs associated with revascularization in higher-risk CAD patients? | Dedicated cost-effectiveness studies within procedure- and disease-based registries |
| What are the outcomes with PCI, surgical revascularization, and medical therapy among higher-risk patients with an indication for revascularization? | Disease-based registries with embedded procedural data Potential randomized trials |
| What is the variability in care patterns for patients meriting consideration of revascularization? | Disease-based registries with embedded procedural data |
| To what extent are contemporary interventionalists trained and skilled to perform complete revascularization across complex lesion subsets? | Procedure- and disease-based registries |
| To what extent can PCI achieve surgery-like outcomes in higher-risk CAD patients? | Randomized trials, possible comparative-effectiveness assessments |
CAD indicates coronary artery disease; and PCI, percutaneous coronary intervention.
CONCLUSIONS
Patients with severe CAD who are candidates for PCI but at high risk for established coronary revascularization procedures such as CABG because of patient comorbidities, complexity of coronary anatomy, and/or poor hemodynamic status represent an understudied and potentially underserved patient population. The characterization of a new field of coronary interventional procedures aims to fulfill an unmet need to better define this population and to focus the use of PCI in these patients who potentially have the most to gain from coronary revascularization procedures. The most critical requirements at present relate to training adequately a dedicated cadre of coronary interventionalists who possess the cognitive and technical skills to manage these patients. The impact of these procedures on the hospital level and health system must be formally assessed, but it is our belief that this treatment paradigm has the potential to maximally benefit patients judiciously and safely.
ACKNOWLEDGMENT
We thank Dominic P. Francese, MPH, for assistance in formatting and preparing this manuscript.
DISCLOSURES
Dr Kirtane reports institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, and Eli Lilly. Dr Doshi reports an educational grant from Abiomed Inc. Dr Karmpaliotis reports serving on the speakers’ bureau for Abbott Vascular, Boston Scientific, Medtronic, and Asahi. Dr Leon reports serving on the advisory board for Boston Scientific and Medtronic. Dr. Lasala reports speaker fees from Abiomed, St. Jude Medical, Eli Lilly, Boston Scientific, and Daiichi Sankyo; serving on the advisory board for Abiomed and Boston Scientific; and being a stockholder in Abiomed. Dr Ohman has received grant support from Gilead Sciences and Daiichi-Sankyo/Eli Lilly and Co, as well as consultant fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, The Medicines Company, Merck/Schering Plow, Pozen, Roche, Sanofi-Aventis, and WebMD. Dr Shroff has been a consultant for Medtronic, Abiomed, Terumo Medical, and The Medicines Company. Dr Cohen has been on the speakers’ bureau for Medtronic; has been a consultant for AstraZeneca, Abiomed, Daiichi Sankyo, and Terumo Medical; and has received research support from Boston Scientific and Abbott Vascular. Dr Uriel has been a consultant for Thoratec, Heartware, Abiomed, and Medtronic and has received grant support from Thoratec and Heartware. Dr Kapur reports serving on the speakers’ bureau for Abiomed, Maquet, and Heartware; being a consultant for Abiomed, Cardiac Assist, Maquet, and Thoratec; and receiving research support from Abiomed, Cardiac Assist, and Maquet. Dr Dangas reports institutional research grant support from The Medicines Company, Bristol-Myers Squibb/Sanofi, and Eli Lilly and Co/Daiichi-Sankyo, as well as consulting fees from Abbott Vascular, AstraZeneca, Boston Scientific, Covidien, Janssen Pharmaceuticals, Regado Biosciences, Maya Medical, Merck & Co, and The Medicines Company. Dr Lombardi has served as a consultant to Abbott Vascular, Boston Scientific, and Abiomed; has been on the advisory board for Abbott Vascular and Boston Scientific; is an employee (spouse) of Spectranetics; and holds equity in Bridgepoint Medical Systems. Dr Parikh reports serving on the speakers’ bureau for Abbott Vascular, Medtronic, CSI, and Boston Scientific and on the advisory board for Abbott Vascular, Medtronic, and Philips. Dr Moses reports consultant fees from Boston Scientific and Abiomed. The remaining authors report no conflicts.
Contributor Information
Ajay J. Kirtane, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
Darshan Doshi, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
Martin B. Leon, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
John M. Lasala, Washington University in St. Louis, St. Louis, MO.
E. Magnus Ohman, The Program for Advanced Coronary Disease, Duke University Medical Center, Durham, NC.
William W. O’Neill, Henry Ford Hospital, Detroit, MI.
Adhir Shroff, University of Illinois, Chicago.
Mauricio G. Cohen, University of Miami Miller School of Medicine, Miami, FL.
Igor F. Palacios, Massachusetts General Hospital, Harvard Medical School, Boston.
Nirat Beohar, Mount Sinai Medical Center, Miami, FL.
Nir Uriel, University of Chicago, Chicago, IL.
Navin K. Kapur, Tufts Medical Center, Boston, MA.
Dimitri Karmpaliotis, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
William Lombardi, University of Washington Medical Center, Seattle.
George D. Dangas, Mount Sinai Medical Center, New York, NY..
Manish A. Parikh, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
Gregg W. Stone, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
Jeffrey W. Moses, Herbert and Sandi Feinberg Interventional Cardiology and Heart Valve Center, Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; Cardiovascular Research Foundation, New York, NY.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DM, Letter AJ, Howard G, Howard VJ, Safford MM, Prince V, Muntner P. Medication adherence and stroke/TIA risk in treated hypertensives: results from the REGARDS study. J Am Soc Hypertens. 2013;7:363–369. doi: 10.1016/j.jash.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ; FREEDOM Trial Investigators. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310:1581–1590. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen DJ, Van Hout B, Serruys PW, Mohr FW, Macaya C, den Heijer P, Vrakking MM, Wang K, Mahoney EM, Audi S, Leadley K, Dawkins KD, Kappetein AP; Synergy between PCI with Taxus and Cardiac Surgery Investigators. Quality of life after PCI with drugeluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;364:1016–1026. doi: 10.1056/NEJMoa1001508. [DOI] [PubMed] [Google Scholar]
- 5.Norris CM, Saunders LD, Ghali WA, Brant R, Galbraith PD, Graham M, Faris P, Dzavik V, Knudtson ML; APPROACH Investigators. Health-related quality of life outcomes of patients with coronary artery disease treated with cardiac surgery, percutaneous coronary intervention or medical management. Can J Cardiol. 2004;20:1259–1266. [PubMed] [Google Scholar]
- 6.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 8.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, Anderson JL; American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 9.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2012;126:875–910. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Bailey SR, Bonow RO, Chambers CE, Chan PS, Dehmer GJ, Kirtane AJ, Wann LS, Ward RP. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59:1995–2027. doi: 10.1016/j.jacc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh RW, Mauri L, Wolf RE, Romm IK, Lovett A, Shahian D, Normand SL. Population trends in rates of coronary revascularization. JAMA Intern Med. 2015;175:454–456. doi: 10.1001/jamainternmed.2014.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–1776. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, Kostuk W, Chaitman BR, Berman D, Lorin JD, Dada M, Weintraub WS, Boden WE; COURAGE Trial Investigators. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373:1937–1946. doi: 10.1056/NEJMoa1505532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL; STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim LK, Feldman DN, Swaminathan RV, Minutello RM, Chanin J, Yang DC, Lee MK, Charitakis K, Shah A, Kaple RK, Bergman G, Singh H, Wong SC. Rate of percutaneous coronary intervention for the management of acute coronary syndromes and stable coronary artery disease in the United States (2007 to 2011). Am J Cardiol. 2014;114:1003–1010. doi: 10.1016/j.amjcard.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Bradley SM, Bohn CM, Malenka DJ, Graham MM, Bryson CL, McCabe JM, Curtis JP, Lambert-Kerzner A, Maynard C. Temporal trends in percutaneous coronary intervention appropriateness: insights from the Clinical Outcomes Assessment Program. Circulation. 2015;132:20–26. doi: 10.1161/CIRCULATIONAHA.114.015156. [DOI] [PubMed] [Google Scholar]
- 18.Marso SP, Teirstein PS, Kereiakes DJ, Moses J, Lasala J, Grantham JA. Percutaneous coronary intervention use in the United States: defining measures of appropriateness. JACC Cardiovasc Interv. 2012;5:229–235. doi: 10.1016/j.jcin.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MG, Filby SJ, Roe MT, Chen AY, Menon V, Stouffer GA, Gibler WB, Smith SC Jr, Pollack CV Jr, Peterson ED, Ohman EM. The paradoxical use of cardiac catheterization in patients with non-ST-elevation acute coronary syndromes: lessons from the Can Rapid Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) Quality Improvement Initiative. Am Heart J. 2009;158:263–270. [DOI] [PubMed] [Google Scholar]
- 22.Harskamp RE, Wang TY, Bhatt DL, Wiviott SD, Amsterdam EA, Li S, Thomas L, de Winter RJ, Roe MT. Hospital patterns of medical management strategy use for patients with non-ST-elevation myocardial infarction and 3-vessel or left main coronary artery disease. Am Heart J. 2014;167:355–362.e3. [DOI] [PubMed] [Google Scholar]
- 23.Pandey A, McGuire DK, de Lemos JA, Das SR, Berry JD, Brilakis ES, Banerjee S, Marso SP, Barsness GW, Simon DN, Roe M, Goyal A, Kosiborod M, Amsterdam EA, Kumbhani DJ. Revascularization trends in patients with diabetes mellitus and multivessel coronary artery disease presenting with non-ST elevation myocardial infarction: insights from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines (NCDR ACTION Registry-GWTG). Circ Cardiovasc Qual Outcomes. 2016;9:197–205. doi: 10.1161/CIRCOUTCOMES.115.002084. [DOI] [PubMed] [Google Scholar]
- 24.Doshi D, Ben-Yehuda O, Bonafede M, Josephy N, Karmpaliotis D, Parikh MA, Moses JW, Stone GW, Leon MB, Schwartz A, Kirtane AJ. Underutilization of coronary artery disease testing among patients hospitalized with new-onset heart failure. J Am Coll Cardiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehmer GJ, Patel MR. Use of appropriate use criteria is increasing, but what are their effects on medical care? Circulation. 2015;132:4–6. doi: 10.1161/CIRCULATIONAHA.115.017243. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugasundaram M Percutaneous coronary intervention in elderly patients: is it beneficial? Tex Heart Inst J. 2011;38: 398–403. [PMC free article] [PubMed] [Google Scholar]
- 27.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216 doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Bortnick AE, Epps KC, Selzer F, Anwaruddin S, Marroquin OC, Srinivas V, Holper EM, Wilensky RL. Five-year follow-up of patients treated for coronary artery disease in the face of an increasing burden of co-morbidity and disease complexity (from the NHLBI Dynamic Registry). Am J Cardiol. 2014;113:573–579. doi: 10.1016/j.amjcard.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 30.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 31.Waldo SW, McCabe JM, O’Brien C, Kennedy KF, Joynt KE, Yeh RW. Association between public reporting of outcomes with procedural management and mortality for patients with acute myocardial infarction. J Am Coll Cardiol. 2015;65:1119–1126. doi: 10.1016/j.jacc.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farmer SA, Lenzo J, Magid DJ, Gurwitz JH, Smith DH, Hsu G, Sung SH, Go AS. Hospital-level variation in use of cardiovascular testing for adults with incident heart failure: findings from the Cardiovascular Research Network Heart Failure Study. JACC Cardiovasc Imaging. 2014;7:690–700. doi: 10.1016/j.jcmg.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP, Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models, part 1: coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. [DOI] [PubMed] [Google Scholar]
- 34.Henriques JP, Claessen BE, Dangas GD, Kirtane AJ, Popma JJ, Massaro JM, Cohen BM, Ohman EM, Moses JW, O’Neill WW. Performance of currently available risk models in a cohort of mechanically supported high-risk percutaneous coronary intervention: from the PROTECT II randomized trial. Int J Cardiol. 2015;189:272–278. doi: 10.1016/j.ijcard.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 35.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KKL, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA, Participants NR. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNulty EJ, Ng W, Spertus JA, Zaroff JG, Yeh RW, Ren XM, Lundstrom RJ. Surgical candidacy and selection biases in nonemergent left main stenting: implications for observational studies. JACC Cardiovasc Interv. 2011;4:1020–1027. doi: 10.1016/j.jcin.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Waldo SW, Secemsky EA, O’Brien C, Kennedy KF, Pomerantsev E, Sundt TM 3rd, McNulty EJ, Scirica BM, Yeh RW. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014;130:2295–2301. doi: 10.1161/CIRCULATIONAHA.114.011541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990–2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol. 2012;4:115–123 doi: 10.2147/CLEP.S30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 40.Farooq V, Brugaletta S, Serruys PW. Contemporary and evolving risk scoring algorithms for percutaneous coronary intervention. Heart. 2011;97:1902–1913. doi: 10.1136/heartjnl-2011-300718. [DOI] [PubMed] [Google Scholar]
- 41.Caceres M, Cheng W, De Robertis M, Mirocha JM, Czer L, Esmailian F, Khoynezhad A, Ramzy D, Kass R, Trento A. Survival and quality of life for nonagenarians after cardiac surgery. Ann Thorac Surg. 2013;95:1598–1602. doi: 10.1016/j.athoracsur.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 43.Maslow AD, Regan MM, Panzica P, Heindel S, Mashikian J, Comunale ME. Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg. 2002;95:1507–1518. [DOI] [PubMed] [Google Scholar]
- 44.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 45.Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham A, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari D, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. The efficacy and safety of the “hybrid” approach to coronary chronic total occlusions: insights from a contemporary multicenter US registry and comparison with prior studies. J Invasive Cardiol. 2014;26:427–432. [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson CA, Jayne JE, Robb JF, Friedman BJ, Kaplan AV, Hettleman BD, Niles NW, Lombardi WL. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2:834–842. doi: 10.1016/j.jcin.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. doi: 10.1016/j.jcin.2014.08.014. [DOI] [PubMed] [Google Scholar]


