Abstract
Proteasomal degradation of ubiquitylated proteins is tightly regulated at multiple levels1–3. A primary regulatory checkpoint is the removal of ubiquitin chains from substrates by the deubiquitylating enzyme ubiquitin-specific protease 14 (USP14), which reversibly binds the proteasome and confers the ability to edit and reject substrates. How USP14 is activated and regulates proteasome function remain unknown4–7. Here we present high-resolution cryo-electron microscopy structures of human USP14 in complex with the 26S proteasome in 13 distinct conformational states captured during degradation of polyubiquitylated proteins. Time-resolved cryo-electron microscopy analysis of the conformational continuum revealed two parallel pathways of proteasome state transitions induced by USP14, and captured transient conversion of substrate-engaged intermediates into substrate-inhibited intermediates. On the substrate-engaged pathway, ubiquitin-dependent activation of USP14 allosterically reprograms the conformational landscape of the AAA-ATPase motor and stimulates opening of the core particle gate8–10, enabling observation of a near-complete cycle of asymmetric ATP hydrolysis around the ATPase ring during processive substrate unfolding. Dynamic USP14–ATPase interactions decouple the ATPase activity from RPN11-catalysed deubiquitylation11–13 and kinetically introduce three regulatory checkpoints on the proteasome, at the steps of ubiquitin recognition, substrate translocation initiation and ubiquitin chain recycling. These findings provide insights into the complete functional cycle of the USP14-regulated proteasome and establish mechanistic foundations for the discovery of USP14-targeted therapies.
Subject terms: Proteasome, Deubiquitylating enzymes, Cryoelectron microscopy
Structures of the human ubiquitin-specific protease 14 in complex with the 26S proteasome captured in the act of protein degradation provide a detailed view of the functional cycle of the USP14-regulated proteasome.
Main
The majority of cellular proteins in eukaryotes are targeted to the 26S proteasome for degradation by ubiquitylation pathways, which regulate major aspects of cellular processes1,10. The proteasome holoenzyme is assembled from a cylindrical 20S core particle (CP) capped with one or two 19S regulatory particles (RPs), each consisting of the lid and base subcomplexes1,10. The ring-like heterohexameric motor of the AAA (ATPase associated with diverse cellular activities) family of adenosine triphosphatase (ATPase) in the base regulates substrate processing in the proteasome via multiple modes of coordinated ATP hydrolysis10. The proteasome is dynamically regulated by numerous proteins that reversibly associate with it via mechanisms that remain unknown3.
USP14 is one of the three proteasome-associated deubiquitinating enzymes2 (DUBs) and is crucially involved in the regulation of proteostasis, inflammation, neurodegeneration, tumorigenesis and viral infection2,14,15. USP14 is a potential therapeutic target for treating cancer, inflammatory and neurodegenerative diseases6,14,16,17. In common with its yeast orthologue Ubp6, USP14 has major roles in proteasome regulation and is prominently activated upon reversible association with the proteasome4–7. USP14 and Ubp6 stabilize many cellular proteins against proteasomal degradation by ubiquitin chain disassembly as well as noncatalytically18–20. Unlike the stoichiometric subunit RPN11 (also known as PSMD14), the DUB activity of which is coupled to ATP-driven substrate translocation, USP14 catalyses removal of supernumerary ubiquitin chains on a substrate en bloc, independently of ATPase activity, until a single chain remains7. Paradoxically, binding of ubiquitylated substrates to USP14 stimulates proteasomal ATPase activity and CP gate opening20–23. The molecular mechanisms underlying USP14 activation and its regulation of the proteasome remain unknown.
Previous cryo-electron microscopy (cryo-EM) studies have determined the atomic structures of the USP14-free, substrate-engaged proteasomes at key functional steps, including ubiquitin recognition (states EA1 and EA2), deubiquitylation (state EB), translocation initiation (states EC1 and EC2) and processive degradation (states ED1 and ED2)10. Early cryo-EM reconstructions have revealed the approximate locations of USP14 and Ubp6 in the proteasome23–26. However, the insufficient resolution and the absence of polyubiquitylated substrates in these studies preclude understanding of USP14-mediated proteasome regulation at the atomic level. In addition, the highly dynamic nature of USP14–proteasome association has hampered structural determination of full-length USP14. Here we report time-resolved cryo-EM studies of human USP14 in complex with functional proteasome in the act of substrate degradation. Our structural and functional analyses portray a dynamic picture of USP14–proteasome interactions and reveal the mechanism of allosteric ‘tug-of-war’ between USP14 and the proteasome for deciding substrate fate.
Visualizing intermediates of USP14–proteasome
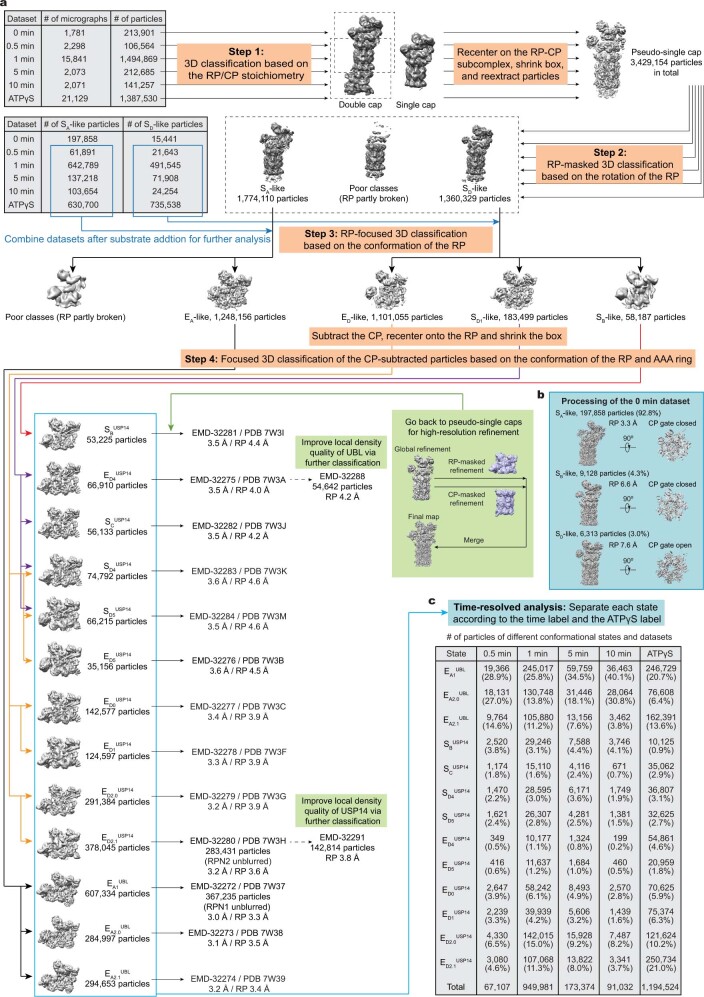
To prepare a substrate-engaged USP14–proteasome complex, we separately purified human USP14, RPN13 (also known as ADRM1) and USP14-free 26S proteasome (Extended Data Fig. 1). Although the ubiquitin receptor RPN13 appears to be present in the purified USP14-free proteasome (Extended Data Fig. 1e), it was absent from previous cryo-EM reconstructions of human proteasomes8–10,25,27–29, probably due to its sub-stoichiometric levels. In an attempt to saturate the proteasome with RPN13, and thereby enhance substrate recruitment by the USP14-bound proteasome, we mixed stoichiometric excesses of both purified USP14 and RPN13 with the USP14-free proteasome before substrate addition. We used Sic1PY conjugated with Lys63-linked polyubiquitin chains (Ubn–Sic1PY) as a model substrate7. Lys63-linked chains are the second most abundant ubiquitin linkages in mammals and regulate essential intracellular functions such as endocytosis, DNA repair and immune responses1. We collected cryo-EM datasets for samples prepared by cryo-plunging at 0.5, 1, 5 and 10 min after mixing Ubn–Sic1PY with the USP14-bound proteasome in the presence of 1 mM ATP at 10 °C. Because 3D classification of cryo-EM data indicated that intermediate states were maximized approximately 1 min after substrate addition, we collected considerably more data at this time point (Fig. 1e, f). To facilitate structural determination at high resolution, we collected another large dataset by exchanging ATP with the slowly hydrolysed ATPγS 1 min after substrate addition at 10 °C, which is expected to stall all coexisting intermediate conformations10. Deep learning-enhanced 3D classification27 enabled us to determine 13 distinct conformational states of the USP14-bound proteasome, including 3 EA-like, 4 SD-like and 6 ED-like conformers, at nominal resolutions of 3.0–3.6 Å (Fig. 1a–d, Extended Data Figs. 2–4, Extended Data Table 1). As expected, each of the 13 states was consistently observed under both the ATP-only and ATP-to-ATPγS exchange conditions despite the differences in state populations (Extended Data Figs. 1r, 2, 5a).
Extended Data Fig. 1. Protein purification and cryo-EM imaging.
a, The human 26S proteasome was purified through gel-filtration column (Superose 6 10/300 GL). b, Native gel analysis of the human 26S proteasome from (a). c, FPLC purification of human USP14 on Superdex 75 10/300 GL column. d–f, SDS-PAGE and Coomassie blue stain analysis of purified USP14 (d), Sic1PY, UBE1, UBCH5A, WW-HECT, human RPN13 (e), USP14 UBL and USP domains (f). g and h, Western blot was used to evaluate the content of RPN13 (g) and USP14 (h) in the purified human proteasomes. The results indicate the presence of RPN13 and the absence of USP14 in the purified human 26S proteasome. i, Western blot was used to verify polyubiquitylation of Sic1PY (Ubn-Sic1PY) using anti-T7 antibody, indicating that most Sic1PY was ubiquitylated. j, In vitro degradation of Ubn-Sic1PY by the purified 26S proteasome at 10 °C in the absence and presence of USP14. The concentration and ratio of each component was same as cryo-EM sample preparation. The experiments were repeated three times. Samples in (i) and (j) were analyzed by SDS–PAGE/Western blot using anti-T7 antibody. W/O, the proteasome without binding to USP14. WT, the wildtype USP14-bound proteasome. k, Kinetics of Ubn-Sic1PY degradation was plotted by measuring Ubn-Sic1PY density in (j) using ImageJ software. Each point is representative of three independent experiments. Data are presented as mean ± s.d. l–o, MST analysis of USP14 binding to the human proteasome. A dissociation constant of 94.6 ± 27.1 nM (l, full-length USP14 in the absence of Ubn-Sic1PY), 137 ± 33.4 nM (m, USP14 UBL domain only), 135 ± 22.9 nM (n, USP14 USP domain only) and 43.9 ± 24.7 nM (o, full-length USP14 in the presence of Ubn-Sic1PY) were calculated from three independent experiments (shown as mean ± s.d.). p and q, Typical motion-corrected cryo-EM micrographs (left) of the substrate-engaged human USP14–proteasome complex in the presence of 1 mM ATP (p) or after ATP-to-ATPγS exchange (q). Power spectrum evaluation of the corresponding micrographs are shown on the right. The exact numbers of micrographs collected under different experimental conditions are provided in Extended Data Fig. 2a. Each experiment was repeated independently at least five times with similar results. r, Comparison of percent population of each conformational state in the presence of 1 mM ATP or ATP-to-ATPγS exchange in 1 min after mixing the substrate with the USP14-bound proteasome. Our cryo-EM analysis suggests that ATP-to-ATPγS exchange enriches states , , , , and , but reduces states , , and . The particle numbers used to derive this plot are provided in Extended Data Fig. 2c. For gel source data, see Supplementary Fig. 1
Fig. 1. Time-resolved cryo-EM analysis of the conformational landscape of USP14–proteasome complexes in the act of substrate degradation.
a, b, Cryo-EM density map of the substrate-engaged USP14–proteasome complex in state , viewed from the top (a) and side (b). c, Side view of the cryo-EM density map of the substrate-engaged USP14–proteasome complex in state . Compared to the view of in b, USP14 is rotated about 30° to dock onto the AAA domain of RPT1. To visualize the substrate density inside the AAA-ATPase motor, the density of RPT5 is omitted in both b and c. d, Atomic model of state viewed from the same perspective as in a. e, Kinetic changes of overall particle populations of SD-like and ED-like states versus EA-like states obtained from time-resolved cryo-EM analysis. EA-like states include , and . SD-like states include , and . ED-like states include , , , , and . The control consists of previously reported data for substrate-free, USP14-free proteasome8. f, Kinetic changes of the particle populations of 13 coexisting conformational states of USP14-bound proteasome from the cryo-EM samples made at different time points after mixing the substrate with the USP14–proteasome complex in the presence of 1 mM ATP at 10 °C. Three substrate-inhibited intermediates (, and ) reach their maximal populations at around 5 min, in contrast to state and six substrate-engaged states, which all reach their maximal populations at approximately 1 min. The number of particles used in e and f are provided in Extended Data Fig. 2b, c.
Extended Data Fig. 2. Cryo-EM data processing workflow and time-resolved analysis.
a, The workflow diagram illustrates the major steps of our focused 3D classification strategy for cryo-EM data analysis. Particle numbers after 3D classification and final reconstruction and the resolutions of the complete RP-CP and RP-masked reconstructions of each state are labelled. b, 3D classification of the dataset corresponding to 0 minute before substrate addition as an overall control. c, Time-resolved analysis of all states by restoring the time label (for the buffer condition with 1 mM ATP) or ATPγS label (for the condition with ATP-to-ATPγS exchange). The percentages were computed using the total particle number corresponding to a given time point.
Extended Data Fig. 4. Cryo-EM maps and quality assessment.
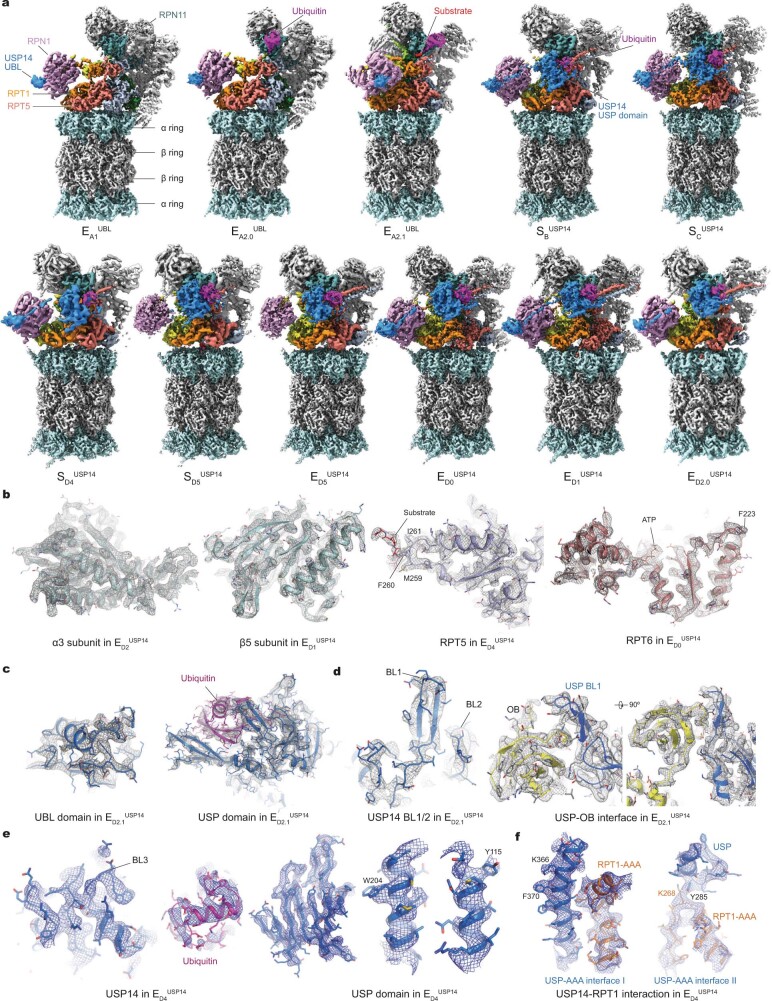
a, Gallery of all refined cryo-EM maps not shown in the main figures. b–f, Typical high-resolution cryo-EM densities (mesh) of secondary structures superimposed with their atomic models. Different subunits of the proteasome are shown in (b), where the substrates shown in the middle panel are modelled using polypeptide chains without assignment of amino acid sequence. c, High-resolution cryo-EM densities of the two domains of USP14 in state . d, High-resolution cryo-EM densities of USP14 BL1 and BL2 loops and USP-OB interface in state . e, High-resolution cryo-EM densities of the BL3 loop, ubiquitin and representative secondary structure elements in the USP domain of USP14 in state . f, High-resolution cryo-EM densities of USP-AAA interfaces in state .
Extended Data Table 1.
Cryo-EM data collection, refinement and validation statistics
Extended Data Fig. 5. Structural comparison of the proteasome under distinct conditions.
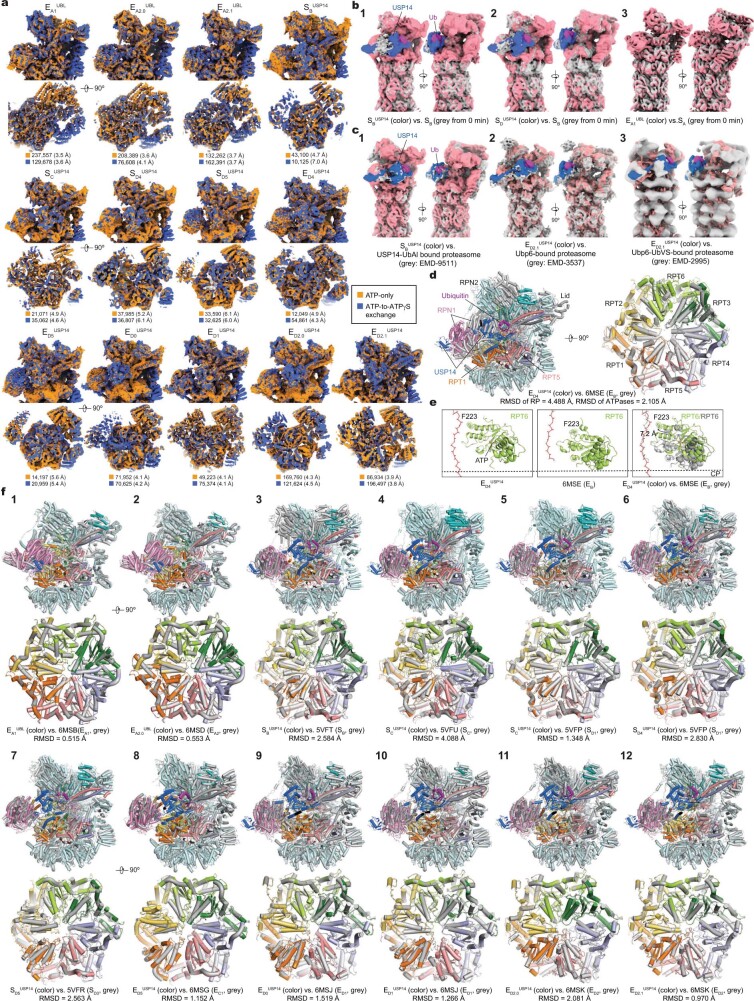
a, Comparison of cryo-EM reconstructions of the thirteen states of USP14-proteasome complex from the conditions of including 1 mM ATP (ATP-only, no ATPγS in the degradation buffer) (orange) and with ATP-to-ATPγS exchange at 1 min after substrate addition (blue). Particle number and the RP resolution for each class are labelled below each panel of structural comparison. The results show that consistent features of the same states were obtained from the two conditions, although the N-terminal densities of RPN5 are slightly stronger under the condition with ATP-to-ATPγS exchange. b, Comparison of the three major states at 0 min (before substrate addition and after USP14 was bound to the proteasome) with those after the substrate was mixed with the USP14-proteasome complex. For visual clarity, all maps were low-pass filtered to 8 Å. c, Comparison of state with the USP14-UbAl-bound proteasome map EMD-9511 (panel 1)25 and of state with the Ubp6-bound proteasome map EMD-3537 (panel 2)26 and Ubp6-UbVS-bound proteasome map EMD-2995 (panel 3)23. Due to the low-resolution nature of the previously published maps, the cryo-EM maps of states and were low-pass filtered to 8 Å for the visual clarity of comparison. d, Structural comparison of state with state EB (6MSE) suggests that they have comparable ATPase conformations with defined differences in RPT6 (shown in panel e), whereas the lid subcomplexes are in very different conformations with large rotations. e, Structural comparison of RPT6 in states and EB shows that the RPT6 pore-1 loop, highlighted by transparent sphere representation of Phe223, is moved about 7.2 Å toward the substrate in state relative to state EB. The right panel show the two RPT6 structures superimposed after aligning the entire ATPase motor subcomplex structures together (as shown in the right of panel d). f, Comparison of the RP and ATPase structures in different states and previously published cryo-EM structures8–10. These structures are aligned together against their CPs. Each pair of compared structures are shown in two orthogonal orientations, with the root-mean-squared-deviation (RMSD) values for the ATPase components shown below each panel of structural comparison. Previous USP14-free structures (PDB ID) used for the comparison include substrate-free states SB (5VFT), SC (5VFU), SD1 (5VFP), SD3 (5VFR) and substrate-bound states EA1 (6MSB), EA2 (6MSD), EB (6MSE), EC1 (6MSG), ED1 (6MSJ), and ED2 (6MSK).
The six ED-like substrate-engaged states—designated , , , , and —captured sequential intermediates during processive substrate unfolding and translocation, which are compatible with the hand-over-hand translocation model10,30 (Extended Data Figs. 6, 7, Extended Data Table 2). The cryo-EM density of USP14 in state is of sufficient quality to allow atomic modelling of full-length USP14 (Extended Data Fig. 4c, d). An unfolded polypeptide substrate is observed in the AAA-ATPase channel in all of the ED-like states. These states also exhibit an open CP gate, with five C-terminal tails (C-tails) of ATPase subunits (RPT1–RPT6, excluding RPT4) inserted into the inter-subunit surface pockets on the α-ring (α-pockets) of the CP8–10,30,31 (Fig. 3a, Extended Data Fig. 6h).
Extended Data Fig. 6. Substrate interactions with the pore loops in the AAA-ATPase motor, the RP-CP interfaces and the nucleotide densities in different USP14-bound states.
a–f, Cryo-EM densities of the ATPase motor bound to the substrate. Substrate densities are colored in red. Right insets show the zoomed-in side views of the substrate interacting with the pore loops of the ATPases. The substrate in each state is modelled as a polypeptide backbone structure and is represented with red sticks. The ATPases and substrates are rendered as surface (left) and mesh (right) representations. g, Top views of the ATPase motor structures of all states not shown in the main figure are shown in cartoon representations. Nucleotides are shown in sphere representations. The sphere representations of ADP and ATP are in blue and in red, respectively. The structures are aligned together against their CP components. Top right, color codes of subunits used in all panels. h, Comparison of the RP–CP interface and RPT C-terminal tail insertions into the α-pockets of the CP in different states. The cryo-EM densities of the CP subcomplexes are shown as grey surface representations, while the RPT C-tails are colored in teal blue. i, Comparison of the nucleotide densities in the cryo-EM reconstructions of USP14-bound proteasome under two different nucleotide conditions. The side-by-side comparison of the nucleotide densities in two best resolved states show that the ATP-to-ATPγS exchange did not change the observed nucleotide states under our experimental conditions relative to that only used 1 mM ATP in the degradation buffer. j, Comparison of the nucleotide densities in thirteen distinct states of UBP14-bound proteasome. The nucleotide densities fitted with atomic models are shown in blue mesh representation. All close-up views were directly screen-copied from Coot after atomic modelling into the density maps without modification. At the contour level commonly used for atomic modelling, the potential nucleotide densities in the apo-like subunits mostly disappear, although they can occasionally appear as partial nucleotide shapes at a much lower contour level. The states with limited local resolutions are hypothetically assigned for nucleotide types based on the densities, the openness of corresponding nucleotide-binding pockets as well as their homologous structural models of higher resolution from states with similar conformations.
Extended Data Fig. 7. Structural comparison of the USP14-bound proteasome of different states.
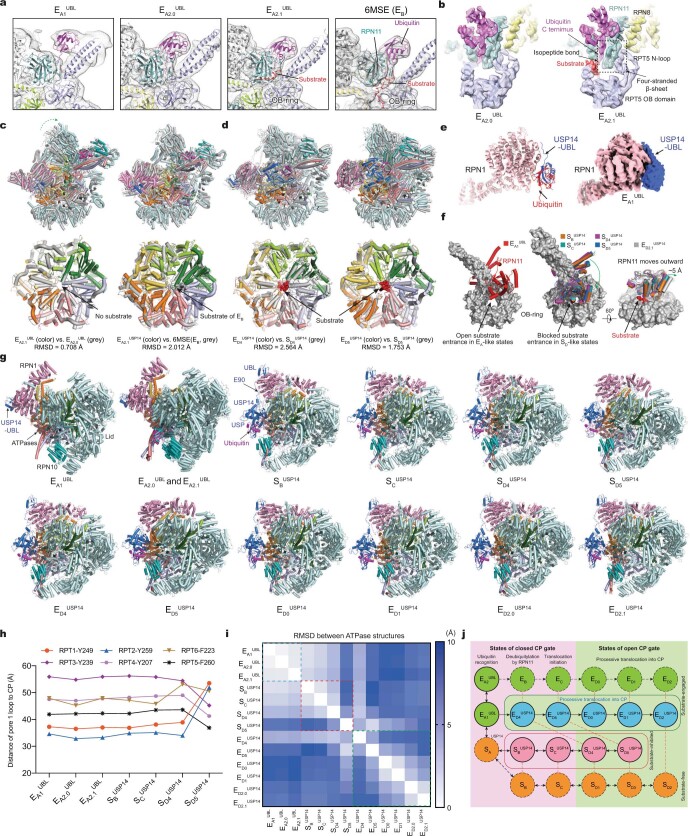
a, Structural comparison of states , and shows the ubiquitin transfer from the RPT4-RPT5 coiled-coil (CC) domain to RPN11. The cryo-EM densities rendered as grey mesh representations are low-pass-filtered to 8 Å for visual clarity of comparison. b, Structural comparison of ubiquitin-RPN11-RPT5 interaction in state and . Cryo-EM densities rendered transparent surfaces are superimposed with the atomic models. The overall conformation of resides between and . Both states and exhibited RPN11-bound ubiquitin and no substrate densities in the AAA-ATPase motor. A short stretch of ubiquitin-linked substrate density is bound to the cleft between RPN11 and the OB ring in but not . In both states, the RPT5 N-loop (residues 99-119) pairs with one side of the Insert-1 β-hairpin of RPN11, the other side of which is paired with the C terminus of ubiquitin. They together form a four-stranded β-sheet, a feature that was previously visualized at atomic level only in state EB of the USP14-free proteasome10. c, Structural comparison of the RP and ATPase between and and between and EB (PDB ID 6MSE). d, Structural comparison of the RP and ATPase between and and between and . The root-mean-squared-deviation (RMSD) values for the ATPase components are shown below each panel of structural comparison in (c) and (d). e, Binding of UBL of USP14 to the T2 site of RPN1 in state as compared to the binding of ubiquitin to RPN1 in USP14-free state EA1 from previous studies10. Right inset shows the cryo-EM density of UBL-bound RPN1 in . f, Comparison of the RPN11-OB ring interface in different states. Left panel, the interface in the EA-like states shows an open OB ring for substrate entrance. Middle panel, the substrate entrance of the OB ring is blocked by RPN11 in the substrate-inhibited states. Right panel, RPN11 is rotated outward slightly by ~5 Å to make way for substrate translocation through the OB ring in state (grey cartoon) as compared to those in the substrate-inhibited states (colorful cartoon). g, Side-by-side structural comparison of the USP14-bound RP in all states from the top view showing differential rotation of the lid and RPN1. h, Plots of the distance of pore-1 loop to the CP for those states not shown in Fig. 3c. The comparison shows that the pore-loop staircase architecture in state , or is similar to that of EA-like state. i, Root-mean-squared-deviation (RMSD) values of the ATPase structures are mapped between any pairs of the thirteen states. j, An integrated schematic diagram of proteasome state transitions illustrates the full functional cycles of the proteasome in the presence and absence of USP14. The solid circles are the states observed in the current study, whereas the dashed circles are the states observed in previous studies of substrate-free8 (orange) or substrate-engaged human proteasome10 (green) in the absence of USP14. Color blue and salmon label the substrate-engaged and substrate-inhibited USP14-proteasome states, respectively. The states with closed and open CP gate are placed in pink and limon backgrounds, respectively. Vertical orange dashed lines link the state pairs with comparable AAA-ATPase structures. Black solid arrows and dashed arrows represent the possible structural transitions connecting the states observed in the current study and pervious USP14-free studies8,10, respectively
Extended Data Table 2.
Summary of key structural features of USP14-bound proteasome states
Comparison is meant for the human proteasome under different conditions. The nucleotide states in the substrate-engaged states share certain comparable features consistent with the previously reported results10. ATP is generally bound to the substrate-engaged ATPases above the penultimate contact along the substate. ADP is bound to the ATPase subunit at the lowest position in contact with substrate, which is poised for dissociation from substrate, whereas an apo-like or ADP-bound state is generally found in the substrate-disengaged ATPase subunits that are in the seam of the lock-washer-like ATPase ring. CC, coiled coil. CP, core particle.
Fig. 3. Structural dynamics and mechanism of allosteric regulation of the AAA-ATPase motor by USP14.
a, b, Side-by-side comparison of the USP14–ATPase subcomplex structures aligned against the CP in six substrate-engaged states (a) and four substrate-inhibited states (b). c, d, Plots of distance from the pore-1 loop of each ATPase to the CP (c) and to the substrate (d) in distinct states. e, The AAA domain structures of the ATPase motor in six substrate-engaged states. f, Varying architecture of the pore-1 loop staircase interacting with the substrate in distinct states. The distances from disengaged pore-1 loops to the substrate are marked. The side chains of the pore-1 loop residues, featuring a consensus sequence of K/M-Y/F-V/L/I, are shown in stick representation, with the aromatic residues highlighted in transparent sphere representation. g, Electrostatic surface representation of the full-length USP14, coloured according to electrostatic potential from red (−5.0 kT e−1, negatively charged) to blue (5.0 kT e−1, positively charged). h, Atomic model of the USP14–RPT1 subcomplex in state in cartoon representation. i, j, Magnified views of the USP-AAA interface I (i) and II (j) in state , with the interacting pairs of residues in stick representation. k, Changes of the USP-AAA interface in distinct states, characterized by measuring the shortest distance between four USP14 residues (Y285, R371, K375 and N383) and the main chains of RPT1 AAA domain. l, ATPase activity was quantified by measuring the release of phosphate from ATP hydrolysis of the proteasome. All labelled P values were computed by comparison with wild-type USP14 using a two-tailed unpaired t-test. Data are mean ± s.d. from three independent experiments, each with three replicates. The quantification of USP14-free proteasome was used as a denominator to normalize all measurements in each experiment.
There are no substrate densities observed in the AAA-ATPase channel of the four SD-like states, designated , , and . Substrate insertion into the AAA-ATPase channel is sterically inhibited in these states, because RPN11 blocks the substrate entrance at the oligonucleotide- or oligosaccharide-binding (OB) ring of the AAA-ATPase motor (Extended Data Fig. 7f). The AAA-ATPase conformations of , , and resemble those of SB, SD1, and , respectively8,9 (Extended Data Figs. 5f, 7d). However, the ATPase–CP interfaces of states , and resemble those of states EA, EC and ED, where two, four and five RPT C-tails are inserted into the α-pockets, respectively10 (Extended Data Fig. 6h). Thus, the CP gate remains closed in and , but is open in and .
Human USP14 comprises 494 amino acids and features a 9-kDa ubiquitin-like (UBL) domain at its N terminus, followed by a 43-kDa USP domain joined via a flexible linker region of 23 amino acids. The overall USP14 structure bridges the RPN1 and RPT1 subunits in all the SD-like and ED-like states, in agreement with previous low-resolution studies23–26 (Extended Data Fig. 5c). By contrast, the three EA-like states, designated , and , show no visible density for the catalytic USP domain of USP14, although a density consistent with the UBL domain is observed at the T2 site of RPN127,32 (also known as PSMD2) (Extended Data Figs. 4a, 7e).
Time-resolved conformational continuum
Time-dependent quantification of the degraded Ubn–Sic1PY substrate indicates that USP14 reduced the degradation rate by approximately two-fold compared with that of the USP14-free proteasome7 (Extended Data Fig. 1k). The majority (about 80%) of substrates were eventually degraded by the USP14–proteasome mixture after 30 min of reaction under the same conditions used for our time-resolved cryo-EM analysis. Notably, the substrate-engaged and substrate-inhibited intermediates coexisted at all measured time points and reached their maximal per cent levels at approximately 1 and 5 min, respectively, after substrate addition (Fig. 1e). The population of became the largest among the substrate-inhibited states in 5–10 min (Fig. 1f). The overall population of substrate-inhibited intermediates varied within a small range of 7.7–10.3% from 0.5 to 5 min and was comparable to the 7.3 % of the SD-like states before substrate addition (Fig. 1e, f, Extended Data Fig. 2b, c), suggesting that the substrate-inhibited pathway was induced in parallel to the substrate-engaged pathway and was promoted by USP14 despite the stoichiometric excess of ubiquitylated substrates. Consistent with previous studies23, these results indicate that the substrate-engaged intermediates were converted to substrate-inhibited states upon termination of substrate translocation.
Despite exhaustive 3D classification, we did not observe in any USP14-loaded experimental conditions the proteasome states EB and EC, which represent RPN11-mediated deubiquitylation and translocation initiation prior to the CP gate opening, respectively10. This implies that USP14 prevents the proteasome from assuming the conformation of RPN11-catalysed deubiquitylation. In contrast to the RPN11-bound ubiquitin in states and , no ubiquitin was observed on RPN11 in any state in which USP14 is engaged with ubiquitin (Fig. 1a–d, Extended Data Figs. 4a, 7a–c), suggesting that activated USP14 and RPN11 do not bind ubiquitin simultaneously.
Dynamic USP14–proteasome interactions
The UBL domain of USP14 binds the RPN1 T2 site via a hydrophobic patch centred on residue Leu70, which is structurally homologous to the Ile44 patch of ubiquitin (Fig. 2a). The RPN1 T2 site is composed of residues Asp423, Leu426, Asp430, Tyr434, Glu458, Asp460 and Leu465 on two adjacent helix-loop regions, in agreement with previous findings27,32. The N-terminal stretch of the linker (residues Ala77 to Phe88) in USP14 appears to bind the ridge of the toroid domain of RPN1 (Fig. 2a).
Fig. 2. Structural basis of proteasome-mediated activation of USP14.
a, Side-chain interactions between the USP14 UBL domain and the RPN1 T2 site in the proteasome state . b, Structural comparison of the blocking loops by superimposing the USP14 structure in state with two crystal structures of USP14 in its isolated form (PDB ID: 2AYN) and in complex with ubiquitin aldehyde5 (UbAl) (PDB ID: 2AYO). c, Magnified view of the ubiquitin–USP–OB sandwich architecture in state . d, Local cryo-EM density of the BL1 motif in state in mesh representation superimposed with its atomic model in cartoon representation from two opposite orientations, showing its β-hairpin conformation. e–g, Magnified views of the interfaces between the catalytic Cys114 of USP14 and the C-terminal Gly76 of ubiquitin (e), between the USP14 BL1 motif and the RPT1 OB domain (f), and between the USP14 BL3 motif and the RPT2 OB domain (g). Key residues mediating the inter-molecular interactions are shown in stick representation in a–g. h, In vitro degradation of Ubn–Sic1PY by the human proteasome assembled with USP14 variants at 37 °C, analysed by SDS–PAGE and western blot using anti-T7 antibody to visualize the fusion protein T7–Sic1PY. See Supplementary Fig. 1 for gel source data. These experiments were repeated independently three times with consistent results. The proteasome without USP14 (no USP14; labelled W/O above the leftmost lanes) is used as a negative control. Lanes labelled WT correspond to the proteasome bound to wild-type USP14. i, Ubiquitin–AMC hydrolysis by the USP14 mutants dictates their DUB activity in the proteasome. RFU, relative fluorescence units. RFU values at 60 min are shown. All labelled P values were computed against the wild-type USP14 using a two-tailed unpaired t-test. Data are mean ± s.d. from three independent experiments. Each experiment includes three replicates. The quantification of wild-type USP14 was used as a denominator to normalize all measurements.
USP14 interacts with both the OB and AAA domains of the ATPase ring. The USP–OB interaction is mediated by the blocking loops 1 (BL1), 2 (BL2) and 3 (BL3) of the USP domain (Fig. 2b), which buries a solvent-accessible area of approximately 527 Å2 on USP14. BL1 makes the most extensive interface with the OB ring around residues Gln128 and Asp133 of RPT1 (also known as PSMC2), whereas Ser430 in BL2 and Trp472 in BL3 interact with Gln128 of RPT1 and Asp118 of RPT2 (also known as PSMC1), respectively (Fig. 2c). The movement of the USP domain between different proteasome states appears to adapt to the rocking motion of the AAA-ATPase motor and maintains its interaction with the OB ring (Fig. 3a–d, Extended Data Fig. 8a).
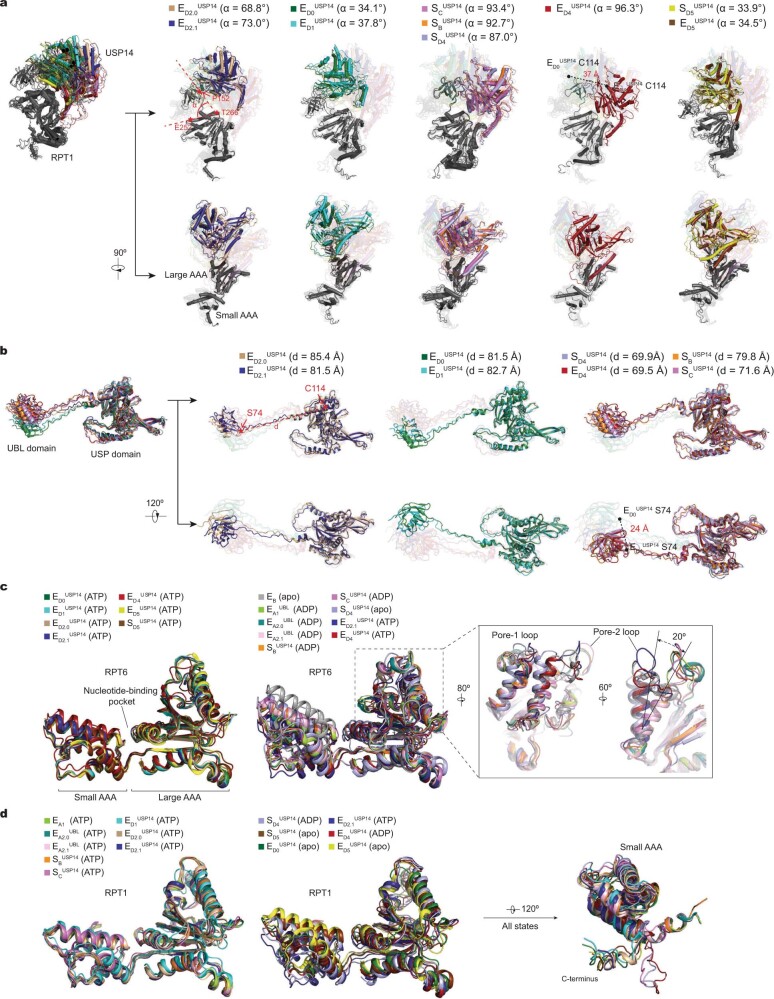
Extended Data Fig. 8. Dynamics of USP14 and key ATPase subunits in the proteasome.
a, Superposition of the USP14-RPT1 subcomplex structures from different states aligned against the RPT1 large AAA subdomain. USP14 rotates together with the RPT1 OB domain and moves up over 37 Å (from to ) relative to the RPT1 AAA domain. The angle between the OB domain and the AAA domain is measured and labelled for each state. b, Superposition of the USP14 structures from different states aligned against their USP domain. The UBL domain moves up over 24 Å (from to ) relative to the USP domain. The distance between Ser74 and Cys114 is measured and labelled for each state. c, Superposition of the RPT6 AAA domain structures from different states aligned against the large AAA subdomain. Left, comparison of the RPT6 AAA structures in the ATP-bound states. Middle, comparison of states , and , the ADP-bound states and state EB (PDB ID: 6MSE) shows conformational changes of the AAA domain driven by the ATP hydrolysis and nucleotide exchange. Right, the open-gate states , , and show different refolding of both the pore-1 and pore-2 loops. d, Superposition of the RPT1 AAA domain structures from different states aligned against the large AAA subdomain. Left, comparison of the structures in the ATP-bound states. Middle, comparison of the structures in different nucleotide-binding states. Right, the C-terminal tails of RPT6 exhibit three major orientations.
In contrast to the USP–OB interface, which is nearly invariant in all SD-like and ED-like states, USP-AAA interactions vary prominently (Fig. 3a, b). In states , , and , the USP domain is flipped up and completely detached from the AAA domain of RPT1. By contrast, the USP domain exhibits differential interactions with the AAA domain of RPT1 in other states (Fig. 3h, Extended Data Fig. 8a), suggesting that USP14 preferentially recognizes these AAA conformations and stabilizes these states by direct interactions. The most extensive USP–AAA interactions are observed in state (Fig. 3h–k). In this conformation, a helix-loop region (residues 371-391) protruding from the USP domain contacts the AAA domain of RPT1, which buries a solvent-accessible area of approximately 850 Å2 on USP14.
USP14 activation by the proteasome
Occlusion of the ubiquitin C terminus-binding groove in USP14 by BL1 and BL2 auto-inhibits the DUB activity in the absence of the proteasome5. Comparison of the USP14 structure in state with the crystal structures of the USP domain in isolated and ubiquitin aldehyde-bound forms5 reveals differential conformational changes in BL1 and BL2 (Fig. 2b). The BL1 region is an open loop in the crystal structures but is folded into a β-hairpin sandwiched between the OB ring and ubiquitin in the proteasome (Fig. 2b, d). By contrast, the BL2 loop is moved approximately 4 Å to make way for docking of the ubiquitin C terminus into the active site groove. Stabilized by the USP–OB interface, BL1 and BL2 together hold the ubiquitin C terminus in a β-strand conformation (Fig. 2c), in which the main chain carbon of ubiquitin C-terminal Gly76 is placed approximately 3.4 Å away from the sulfur atom of the catalytic Cys114 and is fully detached from the substrate, indicating that the structure represents a post-deubiquitylation state of USP14 (Fig. 2e). This ternary architecture of the ubiquitin–USP–OB sandwich indicates that ubiquitin binding stabilizes the BL1 β-hairpin conformation and the USP interaction with the OB ring. Indeed, microscale thermophoresis (MST) measurements show that the dissociation constant (approximately 44 nM) of USP14 with the proteasome in the presence of Ubn–Sic1PY is approximately half of that (approximately 95 nM) in the absence of ubiquitin conjugates and one third of that (approximately 137 or 135 nM, respectively) of either UBL or USP domain alone (Extended Data Fig. 1l–o). Thus, USP14 affinity towards the proteasome reflects interactions of both its UBL and USP domains and is enhanced by Ubn–Sic1PY, consistent with previous studies33.
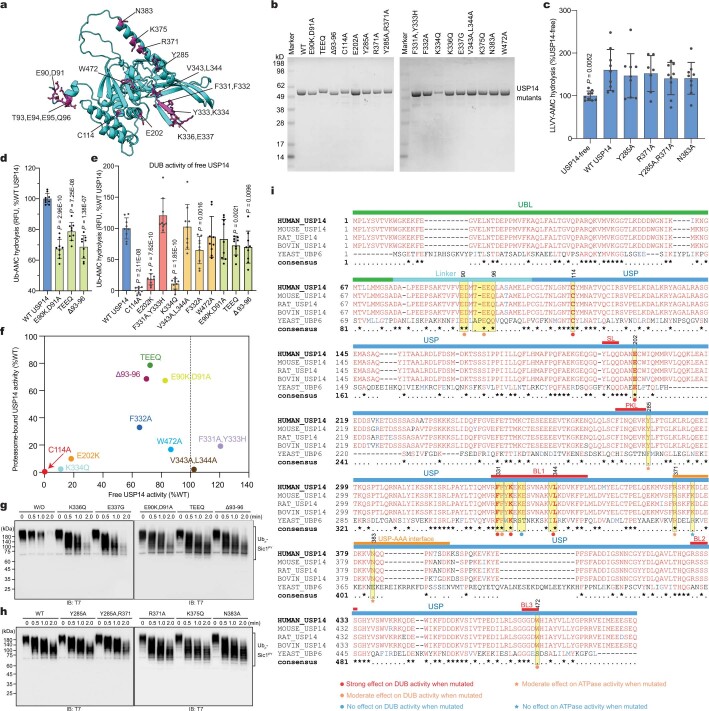
To test the functional importance of the ubiquitin–USP–OB interface in USP14 activation, we performed structure-based site-directed mutagenesis (Extended Data Fig. 9). Indeed, the USP mutations, including the single mutant K334Q and the double mutant V343A/L344A at the BL1–OB interface (Fig. 2f), F331A/Y333A at the ubiquitin–BL1 interface, as well as the single mutant E202K that disrupts the salt bridge between Glu202 of USP and Arg42 of ubiquitin (Fig. 2c), all abrogated the DUB activity of USP14—similar to the effect by C114A mutation that removes the active site of USP14—and showed no obvious inhibition of proteasomal degradation (Fig. 2h, i, Extended Data Fig. 9e, f). By contrast, the single mutants BL1(F332A) and BL3(W472A) retained a reduced DUB activity and suppressed proteasome function to a lesser extent (Fig. 2f–i). These contrasting phenotypes substantiate our structural finding that the ubiquitin–USP–OB interactions are essential for USP14 activation towards efficacious deubiquitylation.
Extended Data Fig. 9. Structure-based site-directed mutagenesis.
a, Mapping of the potential RPT-binding sites and other residues affecting USP14 activation onto the USP14 structure in the model. The UBL domain of USP14 is not shown. b, Purification of USP14 mutants and analyzed by SDS/PAGE and stained with Coomassie blue. c, Peptidase activity assay was used to evaluate the effects of USP14 variants on regulating the CP gate opening. P values were analyzed using a two-tailed unpaired t-test between wild-type USP14-bound and USP14-free proteasomes. The results suggest that the mutants promote the CP gate opening to the same degree as that of wild-type USP14 as compared to that of the USP14-free proteasome. d and e, Ubiquitin-AMC hydrolysis assay to measure the DUB activity of USP14 mutants in the presence (panel d) or absence (panel e) of the human proteasome. Data are presented as mean ± s.d. from three independent experiments. P values were analyzed using a two-tailed unpaired t-test between USP14 mutants and wild-type USP14. P value is not labelled for data with P > 0.05, which is not significant. Data in c–e are presented as mean ± s.d. from three independent experiments, each with three replicates. Dots, individual data points. f, The DUB activity of USP14 mutants in the presence or absence of proteasome. Data points are the average of individual data points shown in panels (d) and (e) and Fig. 2i. g and h, In vitro degradation of Ubn-Sic1PY by the 26S proteasome in the presence of USP14 mutants testing the USP-OB interface or linker region (panel g) and affecting the USP-AAA interfaces (panel h) (repeated three times with similar results). Samples were analyzed by SDS–PAGE/Western blot using anti-T7 antibody. TEEQ, insertion of TEEQ after residue 92 in the linker region. Δ93–96, mutant with deletion of residues 93-96 in the linker region. W/O, the proteasome without binding to USP14. WT, the wildtype USP14-bound proteasome. i, Multiple sequence alignment of USP14 from five species was performed by Chimera. Annotation is based on the structural and mutational data from Figs. 2 and 3. The mutations with the strongest phenotypes (marked by red stars) all correspond to the amino acids (highlighted bold) that are fully conserved from yeast to human. Those mutations with moderate phenotypes correspond to the amino acids that are well conserved in mammals but may vary in yeast. For gel source data, see Supplementary Fig. 1
The overall structure of full-length USP14 exhibits three major states (Extended Data Fig. 8b), far fewer than the number of USP14-bound proteasome states, suggesting that the conformational entropy of the linker region is greatly reduced upon USP14 assembly and activation on the proteasome. In the linker region, the residues Glu90 and Asp91 are potentially involved in transient interactions with the RPN1 toroid (Extended Data Figs. 7g, 9a). Deletion of residues 93–96 and insertion of TEEQ after residue 92 in the linker suppressed proteasomal degradation of Ubn–Sic1PY more potently than the double point mutation E90K/D91A (Extended Data Fig. 9d, g). All three USP14 mutants showed 20–30% reductions in DUB activity relative to the wild-type USP14. These observations suggest that the linker length and composition of USP14 may have been evolutionarily optimized for DUB activity, although the UBL and USP domain architecture appears to be functionally robust against variations of the linker region.
Allosteric regulation of ATPase activity
USP14 interacts with the AAA domain of RPT1 via two discrete interfaces in state (Fig. 3a, Extended Data Fig. 4f). The primary USP–AAA interface is composed of a negatively charged surface on a helix (residues 371–383) and a loop (residues 384–391) protruding out of the USP domain, which buries the majority of the USP–AAA interface (Fig. 3g, h). The helix region appears to pack against a convex surface of the large AAA subdomains of RPT1, on which Arg371, Lys375 and Asn383 of USP14 interact with Glu185, His197 and Glu199 of RPT1, whereas the loop region (384–391) reaches the small AAA subdomain of RPT1 (Fig. 3i). The second USP–AAA interface is centred on Tyr285 in the PKL loop region of USP14 that interacts with Lys267 and Lys268 of RPT1 (Fig. 3j).
In state , ADP is bound to the Walker A motif between the large and small AAA subdomain of RPT1, the dihedral angle of which is directly stabilized by the USP–AAA interaction (Fig. 3h). In other substrate-engaged states, in which the USP domain is detached from the AAA domain, the nucleotide-binding pocket of RPT1 is either empty or bound to ATP (Fig. 3e, Extended Data Fig. 6j). Thus, the USP–AAA interaction energetically stabilizes the ADP-bound conformation of RPT1 and USP–AAA dissociation promotes nucleotide exchange in RPT1. We postulate that the USP–AAA interaction may allosterically promote the ATPase activity during substrate degradation. Indeed, wild-type USP14 enhanced the ATPase activity during proteasomal degradation of Ubn–Sic1PY by approximately 10% relative to that of the USP14-free proteasome (Fig. 3l).
To test the functional roles of the USP–AAA interfaces, we mutated several USP14 residues at the USP–AAA interfaces to alanine. Although none of these USP14 mutants exhibited any observable defects in the DUB activity of USP14 or the peptidase activity of USP14-bound proteasome (Extended Data Fig. 9c, h), they perturbed the ATPase activity in the presence of polyubiquitylated substrate, in line with previous studies21–23,34,35. Two mutants, Y285A and N383A, showed approximately 20–30% reduction of the ATPase activity to a level 10–20% below that of the USP14-free proteasome (Fig. 3l). As Tyr285 is located halfway between the primary USP–AAA and USP–OB interfaces (Fig. 3f, h), it may be mechanically important in transmitting the allosteric effect from USP to AAA. Both Y285A and N383A mutations potentially reduce the overall USP association with the large AAA subdomain of RPT1 (Fig. 3i). By contrast, the single mutant R371A enhanced the ATPase activity by approximately 20% relative to the wild-type USP14, probably by tightening the USP–AAA interactions; whereas K375Q showed no significant effect on the ATPase activity, probably because only the main chain of K375 interacts with RPT1 His197 (Fig. 3j). Consistently, the double mutant Y285A/R371A restored the ATPase rate to the approximate level of the USP14-free proteasome, presumably owing to the cancellation of two counteracting allosteric effects between the two mutated residues (Fig. 3l). Altogether, our structure-guided mutagenesis supports the notion that the USP–AAA interfaces mediate the non-catalytic, allosteric regulation of the ATPase activity in the proteasome.
Asymmetric ATP hydrolysis around ATPase ring
The six substrate-engaged USP14–proteasome structures characterize a detailed intermediate sequence of substrate translocation, which deviates substantially from the pathway of translocation initiation of the USP14-free proteasome10 (Extended Data Figs. 5, 6). Whereas the RP conformations of states and closely resemble those of USP14-free proteasomes in previously reported states ED1 and ED2, respectively, other substrate-engaged states of USP14–proteasome present distinct, hitherto unknown RP conformations10 (Extended Data Figs. 5d–f, 6). Of note, the AAA-ATPase conformations in states and can be compared to those of USP14-free, closed-CP states EB and EC1, respectively, albeit with defined structural differences10,27 (Extended Data Fig. 5d, f). In particular, the pore-1 loop of RPT6 in state has considerably moved up towards the substrate as compared with that in state EB (Extended Data Fig. 5e). Given the absence of the EB- and EC-like states in the presence of USP14, our structural data together prompt the hypothesis that and may replace the USP14-free states EB and EC during initiation of substrate translocation. Thus, we infer that these states present a continuum of USP14-altered conformations following the state-transition sequence → → → → → → (Fig. 3c, Extended Data Fig. 7i, j). In support of this sequence assignment, the RPT3 pore-1 loop is moved sequentially from the top to the bottom of the substrate–pore loop staircase, whereas coordinated ATP hydrolysis navigates around the ATPase ring for a near-complete cycle (Fig. 3c–f, Extended Data Fig. 6a–f).
The newly resolved states fill a major gap in our understanding of substrate translocation by the proteasomal AAA-ATPase motor. It has remained unclear whether a strict sequential hand-over-hand mechanism is used by the AAA-ATPase motor for processive substrate translocation36. Of note, four of the six ED-like states—, , and —exhibit an AAA-ATPase conformation with two adjacent RPT pore-1 loops disengaged from the substrate: one moving away from the substrate while releasing ADP for nucleotide exchange, and the other moving up towards the substrate upon binding ATP for substrate re-engagement (Fig. 3d–f). Recent studies of the proteasome have found that substrate re-engagement of an ATPase subunit can be the rate-limiting step in single-nucleotide exchange dynamics27. Coupling of conformational changes between adjacent AAA domains may vary from tightly coupled kinetics—that is, only one dissociated pore-1 loop at a time—to loosely coupled kinetics exhibiting two adjacent pore-1 loops dissociated from the substrate simultaneously10,27. Thus, two adjacent pore-1 loops dissociated from the substrate represent an inevitable intermediate in the sequential hand-over-hand model of substrate translocation, which would be very short-lived in tightly coupled kinetics37. Under the allosteric influence of USP14, such kinetics can become loosely coupled around RPT1, leading to meta-stabilization of these short-lived intermediate states. The observations of both tightly and loosely coupled kinetics at different locations around the ATPase ring rationalize broken symmetry of coordinated ATP hydrolysis in the AAA-ATPase motor—an effect that has been previously informed by functional studies38,39.
Insights into proteasome regulation by USP14
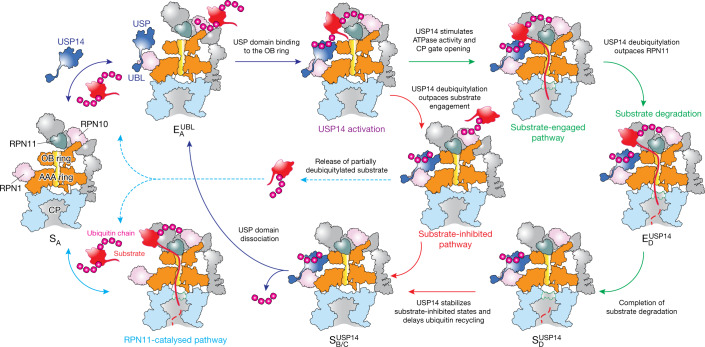
Our structural, kinetic and functional data collectively provide insights into how USP14 regulates proteasome activity at multiple checkpoints by inducing parallel pathways of proteasome state transitions (Fig. 4, Extended Data Fig. 7j). The first checkpoint is at the initial ubiquitin recognition before substrate engagement with the ATPases. USP14 assembly on the proteasome is initiated by its UBL domain binding to the T2 site of the RPN1 toroid27,32 as suggested in state . Binding of the UBL domain alone has been previously suggested to allosterically stimulate proteasome activity34,35. This is followed by a distinct association step of its USP domain with the OB ring of the AAA-ATPase motor in a ubiquitin-dependent manner, which allosterically activates USP14 and reciprocally stabilizes the proteasome in the substrate-inhibited states. It appears that the USP-ATPase association is allosterically precluded at large once substrate-conjugated ubiquitin is recruited to RPN11, as suggested by states and (Extended Data Fig. 7a, b). Such a competition in ubiquitin recruitment forces the proteasome to bypass the conformational transition pathway represented by USP14-free states EB and EC, which are needed to couple RPN11-catalysed deubiquitylation with ATP-dependent substrate engagement and translocation initiation10. Thus, the first checkpoint allows USP14 to allosterically compete against RPN11 in accepting substrate-conjugated ubiquitin.
Fig. 4. Proposed model of USP14-mediated regulation of proteasome function.
USP14 binding to the RPN1 and RPT1 subunits of the proteasome primes USP14 activation, whereas ubiquitin–substrate conjugates recruited to the proteasome’s ubiquitin receptors facilitate ubiquitin recognition by USP14. RPN11-catalysed pathway (turquoise solid arrow) is allosterically excluded once USP14 is recruited to the proteasome (dark blue arrows). USP14 binding creates two parallel state-transition pathways of the proteasome. Along the substrate-inhibited pathway (red arrows), which has RPN11 blocking the substrate entrance of the OB ring before any substrate insertion takes place, USP14 trims ubiquitin chains and releases the substrate from the proteasome, thus preventing the substrate degradation (dashed turquoise arrows). Along the substrate-engaged pathway (green arrows), a substrate has already been inserted into the ATPase ring and RPN11 narrows down on the OB ring but does not block substrate translocation through the OB ring (Extended Data Fig. 7f). Although our data do not intuitively explain why USP14 trims ubiquitin until the last one on a substrate remains, the structures provide geometric constraints for polyubiquitin chain binding to both ubiquitin receptors and USP14 and suggest that ubiquitin recognition by USP14 in the proteasome requires at least one additional helper ubiquitin chain that is already anchored on a nearby ubiquitin receptor. This helper ubiquitin chain may not be available for USP14 binding but can be readily trimmed by RPN11.
However, the stabilization of substrate-inhibited states by USP14 activation does not completely exclude the possibility of substrate engagement with the ATPases. Should an unfolded or loosely folded initiation region of the substrate be available40, substrate insertion into the AAA-ATPase channel can stochastically occur before USP14 activation by the proteasome, which presumably depends on the specific structures of substrate-ubiquitin conjugates. In the presence of ubiquitylated substrates, USP14 stimulates the ATPase rate, tightens the RP–CP interface and induces early CP gate opening21–23, as represented in states and . Thus, USP14 creates a second kinetic checkpoint and drives the proteasome to choose between two alternative pathways of USP14-regulated conformational transitions—one that sterically impedes substrate commitment and the other that kinetically antagonizes RPN11 by outpacing the coupling of RPN11-directed deubiquitylation and substrate translocation, rather than directly inhibiting the DUB activity of RPN11. In support of this model, all SD-like and ED-like states of the USP14-bound proteasome showed no ubiquitin binding to RPN11.
Previous studies have demonstrated that USP14 trims ubiquitin chains from the substrate on a millisecond time scale7. Consistently, all observed USP14-bound SD-like and ED-like states appear to present USP-bound ubiquitin in a post-cleavage state, perhaps owing to the stabilization of trimmed ubiquitin by ternary interactions of the ubiquitin–USP–OB sandwich (Fig. 2c). The lack of observation of ubiquitin-free USP14 in the proteasome suggests that the rate of ubiquitin release from USP14 on the proteasome is slow as compared with that of ubiquitin-free USP14 from the proteasome. Thus, it is conceivable that the cleaved ubiquitin chain is released only upon dissociation of the USP domain from the OB ring21,22,33. This is in contrast to RPN11 in all the USP14-free ED-like states10, which exhibit no RPN11-bound ubiquitin, implying rapid release of cleaved ubiquitin. Therefore, USP14 creates a third checkpoint, where the polyubiquitin-bound USP14 kinetically delays ubiquitin release from the proteasome and suppresses additional substrate recruitment. The third checkpoint may further control the ubiquitin-recycling function of the proteasome that is critical for regulating the free ubiquitin reservoir in cells41.
In summary, USP14 acts as an adaptive regulator of proteasomal DUB and ATPase activities that times intermediate steps of substrate processing, as USP14 bridges the gap between RPN1 and the AAA-ATPase motor in a ubiquitin-dependent, switchable fashion. USP14 interactions create three branching checkpoints on the proteasome, at the steps of initial ubiquitin recognition, substrate translocation initiation by the AAA-ATPase motor, and recycling of trimmed ubiquitin chains. This multi-checkpoint mechanism integrates catalytic and non-catalytic effects of USP14-mediated proteasome regulation into a comprehensively coordinated, elegantly timed process of substrate degradation. As partly supported by another study on the yeast Ubp6-proteasome interactions42, such a mechanism is expected to be conserved from yeast to human and to inform on how reversibly associated DUBs and other proteins regulate proteasome function in general (Extended Data Fig. 9i). Importantly, our time-resolved cryo-EM studies, which had been enhanced by deep learning-improved 3D classification27, present an emerging paradigm that enables atomic-level visualization of hitherto inaccessible functional kinetics and complex dynamics in general.
Methods
Expression and purification of human USP14
Wild-type USP14 and its mutants were cloned into pGEX-4T vector obtained from GenScript (Nanjing, China). For purification of recombinant USP14 and mutants, BL21-CondonPlus (DE3)-RIPL cells (Shanghai Weidi) transformed with plasmids encoding wild-type or mutant USP14 were grown to an OD600 of 0.6–0.7 in LB medium supplemented with 100 mg ml−1 ampicillin. Cultures were cooled to 20 °C and induced with 0.2 mM IPTG overnight. Cells were collected by centrifugation at 3,000g for 15 min and resuspended in lysis buffer (25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.2% NP-40, 1 mM DTT, 10% glycerol and 1× protease inhibitor cocktail). Cells were lysed by sonication and the lysate was cleared through centrifugation at 20,000g for 30 min at 4 °C. The supernatant was incubated with glutathione Sepharose 4B resin (GE Healthcare) for 3 h at 4 °C. For the purification of wild-type USP14, USP14 UBL domain (USP14-UBL) and USP14 USP domain (USP14-USP), the resin was washed with 20 column volumes of washing buffer (25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM DTT, 10% glycerol), then incubated with cleavage buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl) containing thrombin (Sigma) overnight at 4 °C. The eluted samples were further purified on a gel-filtration column (Superdex 75, GE Healthcare) equilibrated with 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM DTT, 10% glycerol. For the purification of USP14 mutants, the resin was washed with 20 column volumes of washing buffer (25 mM Tris-HCl (pH 8.0), 300 mM NaCl, 1 mM DTT), then incubated with cleavage buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl) containing thrombin (Sigma) overnight at 4 °C. To remove thrombin, the GST eluent was incubated with Benzamidine-Sepharose (GE Healthcare) for 30 min at 4 °C.
Expression and purification of human 26S proteasome
Hexahistidine, TEV cleavage site, biotin and hexahistidine (HTBH)-tagged human 26S proteasomes were affinity purified as described8–10 from a stable HEK 293 cell line (a gift from L. Huang). Further authentication of cell lines was not performed for this study. Mycoplasma testing was not performed for this study. In brief, HEK 293 cells were Dounce-homogenized in a lysis buffer (50 mM PBS (77.4% Na2HPO4, 22.6% NaH2PO4, pH 7.4), 5 mM MgCl2, 5 mM ATP, 0.5% NP-40, 1 mM DTT and 10% glycerol) containing 1× protease inhibitor cocktail. The cleared lysates were incubated with Streptavidin Agarose resin (Yeasen) for 3 h at 4 °C. The resin was washed with 20 bed volumes of lysis buffer to remove endogenous USP14 and UCH37 associated with the proteasome7,10. The 26S proteasomes were cleaved from the beads by TEV protease (Invitrogen) and further purified by gel filtration on a Superose 6 10/300 GL column. Western blot was used to detect RPN13 and USP14 in the proteasomes using anti-RPN13 antibody (Abcam, 1:10,000 dilution) and anti-USP14 antibody (Novus, 1:1,000 dilution). For ubiquitin–vinyl-sulfone (Ub–VS)-treated human proteasome, 1 µM Ub–VS (Boston Biochem) was added to the proteasome-binding resin and incubated for 2 h at 30 °C. Residual Ub–VS was removed by washing the beads with 30 bed volumes of wash buffer (50 mM Tris-HCl (pH7.5), 1 mM MgCl2 and 1 mM ATP). The proteasomes were cleaved from the beads using TEV protease (Invitrogen) and used to measure the DUB activity of USP14 using the Ub–AMC hydrolysis assay.
Preparation of polyubiquitylated Sic1PY
Sic1PY and WW-HECT were purified as previously described10. The PY motif (Pro-Pro-Pro-Tyr) is recognized by the WW domains of the Rsp5 family of E3 ligases. In the Sic1PY construct, a PY motif was inserted to the N-terminal segment (MTPSTPPSRGTRYLA) of the Cdk inhibitor Sic1, resulting in a modified N terminus of MTPSTPPPPYSRGTRYLA43,44 (the PY motif is underlined). Human UBE1 (plasmid obtained as a gift from C. Tang) and human UBCH5A (obtained from GenScript) were expressed as GST fusion proteins from pGEX-4T vectors. In brief, UBE1-expressing BL21-CondonPlus (DE3)-RIPL cell cultures were induced with 0.2 mM IPTG for 20 h at 16 °C, whereas UBCH5A expression was induced with 0.2 mM IPTG overnight at 18 °C. Cells were collected in lysis buffer (25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM MgCl2, 0.2% Triton-X-100, 1 mM DTT) containing 1× protease inhibitor cocktail and lysed by sonication. The cleared lysates were incubated with glutathione Sepharose 4B resin for 3 h at 4 °C and subsequently washed with 20 bed volumes of lysis buffer. The GST tag was removed by thrombin protease (Sigma) in cleavage buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1mM DTT) overnight at 4 °C. The eluted samples were further purified by gel-filtration column (Superdex 75, GE Healthcare) equilibrated with 25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, 10% glycerol.
To ubiquitylate Sic1PY, 1.2 μM Sic1PY, 0.5 μM UBE1, 2 μM UBCH5A, 1.4 μM WW-HECT and 1 mg ml−1 ubiquitin (Boston Biochem) were incubated in reaction buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 2 mM ATP, 1 mM DTT and 10% glycerol) for 2 h at room temperature. His-tagged Sic1PY conjugates (polyubiquitylated Sic1PY, Ubn–Sic1PY) were purified by incubating with Ni-NTA resin (Qiagen) at 4 °C for 1 h. Afterwards the resin was washed with 20 column volumes of the wash buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10% glycerol). The Ubn–Sic1PY was eluted with the same buffer containing 150 mM imidazole, and finally exchanged to the storage buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10% glycerol) using an Amicon ultrafiltration device with 30K molecular cut-off (Millipore).
Expression and purification of human RPN13
To purify human RPN13, pGEX-4T-RPN13-transformed BL21-CondonPlus (DE3)-RIPL cells were cultured to an OD600 of 0.6 and then induced by 0.2 mM IPTG for 20 h at 16 °C. Cells were resuspended in lysis buffer (25 mM Tris-HCl (pH 7.5), 300 mM NaCl, 1 mM EDTA, 0.2% Triton-X-100, 1 mM DTT) containing 1× protease inhibitor cocktail and lysed by sonication. A 20,000g supernatant was incubated with glutathione Sepharose 4B resin (GE Healthcare) for 3 h at 4 °C. The resin was washed with 20 column volumes of washing buffer (25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM DTT, 10% glycerol) and 10 column volumes of cleavage buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl). The GST tag was cleaved by incubating with thrombin (Sigma) overnight at 4 °C. The eluted samples were further purified by gel-filtration column (Superdex 75, GE Healthcare) equilibrated with 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM DTT, 10% glycerol.
In vitro degradation assay
Purified human proteasomes (~30 nM) were incubated with RPN13 (~300 nM), Ubn–Sic1PY (~300 nM) in degradation buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 5 mM ATP) at 37 °C. Purified recombinant USP14 variants (~1.2 μM) were incubated with proteasome for 20 min at room temperature before initiating the degradation reaction. The reaction mixtures were incubated at 37 °C for 0, 0.5, 1.0 and 2.0 min, or 10 °C for 0, 0.5, 1.0, 5.0, 10 and 30 min, then terminated by adding SDS loading buffer and subsequently analysed by western blot using anti-T7 antibody (Abcam, 1:1,000 dilution), which was used to examine fusion protein T7–Sic1PY.
Ubiquitin–AMC hydrolysis assay
Ubiquitin–AMC (Ub–AMC; Boston Biochem) hydrolysis assay was used to quantify the deubiquitylating activity of wild-type and mutant USP14 in the human proteasome. The reactions were performed in reaction buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM ATP, 1 mM DTT, 1 mM EDTA and 1 mg ml−1 ovalbumin (Diamond)), containing 1 nM Ub–VS-treated proteasome, 0.2 μM USP14 variants and 10 nM RPN13. The reaction was initiated by adding 1 μM Ub–AMC. Ub–AMC hydrolysis was measured in a Varioskan Flash spectral scanning multimode reader (Thermo Fisher) by monitoring an increase of fluorescence excitation at 345 nm with an emission at 445 nm. For free USP14 activity, the reaction was performed using 1 μM USP14 variants and 1 μM Ub–AMC (BioVision).
ATPase activity assay
ATPase activity was quantified using malachite green phosphate assay kits (Sigma). Human proteasomes (30 nM), RPN13 (300 nM) and USP14 variants (1.2 μM) were incubated in assembly buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 0.5 mM ATP) for 20 min at room temperature. Ubn–Sic1PY (300 nM) was subsequently added, and the sample was incubated for 1 min at 37 °C. The reaction mixtures were mixed with malachite green buffers as described by the manufacturer (Sigma). After 30 min of room temperature incubation, the absorbance at 620 nm was determined using a Varioskan Flash spectral scanning multimode reader (Thermo Fisher).
Peptidase activity assay
Peptide hydrolysis by the human proteasomes was measured using fluorogenic substrate Suc-LLVY-AMC (MCE). Human proteasomes (1 nM) were incubated with USP14 variants (1 μM) in buffer (50 mM Tris-HCl (pH 7.5), 100 mM KCl, 0.5 mM MgCl2, 0.1 mM ATP and 25 ng μl−1 BSA) for 20 min at room temperature. 10 μM Suc-LLVY-AMC was added to the reaction mixture, which was incubated for 30 min at 37 °C in the dark. Peptide activity was measured in a Varioskan Flash spectral scanning multimode reader (Thermo Fisher) by excitation at 380 nm with an emission at 460 nm.
Microscale thermophoresis
The human proteasomes were labelled with red fluorescent dye NT-650-NHS using the Monolith NT Protein Labeling Kit (NanoTemper). After labelling, excess dye was removed by applying the sample on column B (provided in the kit) equilibrated with reaction buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 1 mM ATP). 0.05% Tween-20 was added to the sample before MST measurements. For interaction of NT-650-NHS-labelled proteasomes with USP14, USP14-UBL or USP14-USP, concentration series of USP14, USP14-UBL or USP14-USP were prepared using a 1:1 serial dilution of protein in reaction buffer containing 0.05% Tween-20. The range of USP14, USP14-UBL or USP14-USP concentration used began at 8 μM, with 16 serial dilution in 10-μl aliquots. The interaction was initiated by the addition of 10 μl of 30 nM NT-650-NHS-labelled proteasomes to each reaction mixture and measured by Monolith NT.115 (NanoTemper) at 20% LED excitation power and 40% MST power. To evaluate the effect of Ubn–Sic1PY on the interaction of USP14 with the proteasome, 30 nM Ubn–Sic1PY was added to the reaction mixture. Data were analysed using MO Control software provided by NanoTemper.
Cryo-EM sample preparation
To prepare cryo-EM samples, all purified proteins were exchanged to imaging buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 1 mM ATP). Human proteasomes (1 μM) were incubated with 10 μM RPN13, 10 μM USP14 in imaging buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 1 mM ATP) for 20 min at 30 °C, then cooled to 10 °C. 10 μM Ubn–Sic1PY was added to the mixture and incubated at 10 °C for 0.5, 1, 5 and 10 min. 0.005% NP-40 was added to the reaction mixture immediately before cryo-plunging. Cryo-grids made without the addition of substrate corresponded to the condition of 0 min of reaction time and were used as a baseline control for time-resolved analysis (Fig. 1e). For ATP-to-ATPγS exchange and ATPase quenching, after the reaction mixture was incubated at 10 °C for 1 min, 1 mM ATPγS was added to the reaction mixture at once, and incubated for another 1 min, then NP-40 was added to the mixture to a final concentration of 0.005% before cryo-plunging.
Cryo-EM data collection
The cryo-grids were initially screened in a 200 kV Tecnai Arctica microscope (Thermo Fisher). Good-quality grids were then transferred to a 300 kV Titan Krios G2 microscope (Thermo Fisher) equipped with the post-column BioQuantum energy filter (Gatan) connected to a K2 Summit direct electron detector (Gatan). Coma-free alignment and parallel illumination were manually optimized prior to each data collection session. Cryo-EM data were acquired automatically using SerialEM software45 in a super-resolution counting mode with 20 eV energy slit, with the nominal defocus set in the range of −0.8 to −2.0 μm. A total exposure time of 10 s with 250 ms per frame resulted in a 40-frame movie per exposure with an accumulated dose of ~50 electrons per Å2. The calibrated physical pixel size and the super-resolution pixel size were 1.37 Å and 0.685 Å, respectively. For time-resolved sample conditions, 1,781, 2,298, 15,841, 2,073 and 2,071 movies were collected for cryo-grids made with the reaction time of 0, 0.5, 1, 5, and 10 min, respectively. For the condition of exchanging ATP to ATPγS at 1 min after substrate addition, 21,129 movies were collected.
Reference structures
Comparisons to protein structures from previous publications used the atomic models in the PDB under accession codes: 2AYN (USP domain of USP14 in its isolated form5), 2AYO (USP domain of USP14 bound to ubiquitin aldehyde5), 6MSB (state EA1 of substrate-engaged human proteasome10), 6MSD (state EA2), 6MSE (state EB), 6MSG (state EC1), 6MSJ (state ED1), 6MSK (state ED2), 5VFT (state SB of substrate-free human proteasome8,9), 5VFU (state SC), 5VFP (state SD1) and 5VFR (state SD3). Cryo-EM maps from previous publications used in comparison are available from EMDB under access codes EMD-9511 (USP14–UbAl-bound proteasome25), EMD-3537 (Ubp6-bound proteasome map26) and EMD-2995 (Ubp6–UbVS-bound proteasome23).
Cryo-EM data processing
Drift correction and dose weighting were performed using the MotionCor2 program46 at a super-resolution pixel size of 0.685 Å. Drift-corrected micrographs were used for the determination of the micrograph CTF parameters with the Gctf program47. Particles were automatically picked on micrographs that were fourfold binned to a pixel size of 2.74 Å using an improved version of the DeepEM program48. Micrographs screening and auto-picked particles checking were both preformed in the EMAN2 software49. A total of 213,901, 106,564, 1,494,869, 212,685, 141,257 and 1,387,530 particles were picked for the 0 min, 0.5 min, 1 min, 5 min, 10 min and ATPγS datasets, respectively. Reference-free 2D classification and 3D classification were carried out in software packages RELION50 version 3.1 and ROME51. Focused 3D classification, CTF and aberration refinement, and high-resolution auto-refinement were mainly done with RELION 3.1, whereas the AlphaCryo4D software27 was used to analyse the conformational changes and conduct the in-depth 3D classification for time-resolved analysis. Particle subtraction and re-centering were performed using RELION 3.1 and SPIDER52 software. We applied a hierarchical 3D classification strategy to analyse the data (Extended Data Fig. 2), which were optimized as previously described10. The entire data-processing procedure consisted of five steps. Datasets of different conditions were processed separately at steps 1 and 2 and combined at steps 3 and 4.
Step 1: doubly capped proteasome particles were separated from singly capped ones through several rounds of 2D and 3D classification. These particles were aligned to the consensus models of the doubly and singly capped proteasome to obtain their approximate shift and angular parameters. With these parameters, each doubly capped particle was split into two pseudo-singly capped particles by re-centring the box onto the RP–CP subcomplex. Then the box sizes of pseudo-singly capped particles and true singly capped particles were both shrunk to 640 × 640 pixels with a pixel size of 0.685 Å, and down-sampled by two-fold to a pixel size of 1.37 Å for the following processing. A total of 3,429,154 particles from all datasets were obtained after this step.
Step 2: particles were aligned to the CP subcomplex through auto-refinement, followed by one round of CTF refinement to correct optical aberration (up to the fourth order), magnification anisotropy, and per-particle defocus together with per-particle astigmatism. After another run of the CP-masked auto-refinement, an alignment-skipped RP-masked 3D classification was performed to separate the SA-like states from the SD-like states. Poor 3D classes showing broken 26S proteasome were removed for further analysis at this step. The RP subcomplex of the SD-like states rotated by a large angle compared to the SA-like states, and only in SD-like states was the USP domain of USP14 observed to bind the OB ring of the proteasome. There were 1,774,110 particles in total in SA-like states and 1,360,329 particles in total in SD-like states in all datasets after this step.
Step 3: considering the particle number of some datasets were not enough to ensure high accuracy of independent 3D classification, in the following procedure we pooled particles together from all datasets except for the 0-min condition, in which the substrate was not yet added into the reaction system. For the SD-like state, CP-masked auto-refinement was performed, followed with two rounds of CTF refinement and another run of CP-masked auto-refinement. Alignment-skipped RP-masked 3D classification was then performed, while conformational changes were analysed using AlphaCryo4D27, which yielded three clusters, designated SB-like, SD-like, and ED-like states. These names were correspondingly referred to their similar states in previously published studies9,10. The SA-like particles were processed in the same way, resulting in a cluster named EA-like state; bad classes showed blurred densities in RPN10 and part of the lid. The 0-min dataset was processed independently for the lack of substrate, resulting in three classes, named SA-like (92.8%), SB-like (4.3%) and SD-like (3.0%).
Step 4: particles in different clusters were individually refined with the CP masked. The CP density was then subtracted, and the particle box was recentred to the RP subcomplex and shrunk to 240 × 240 pixels, with a pixel size of 1.37 Å. For each cluster, the CP-subtracted particles were subjected to several rounds of RP-masked auto-refinement and alignment-skipped RP-masked 3D classification followed by AlphaCryo4D analysis27, finally resulting in thirteen major conformational states of the USP14-bound proteasome, named , , , , , , , , , , , and . For state , particles with blurred RPN1 were excluded for final high-resolution reconstruction. For state , particles with blurred RPN2 were excluded for final high-resolution reconstruction. These states exhibit remarkable conformational changes of the AAA ring and the full RP, as well as the interactions of the RP and USP14. Time-resolved analysis of conformational changes and comparison in the presence and absence of ATPγS were both done after this step, by simply separating the particles for each state based on their time labels. Namely, the proportion of particles of each state at a given time point was obtained by summing up the number of particles for each state at the same time point and then calculating the fraction of particles of each state with respect to the total number of particles at this time point53,54. Similarly, final analysis of state percentage for the ATP-to-ATPγS exchange condition was done by counting the particles of each state under this condition, with the particles of each state used for separate refinement, reconstruction and comparison with those under ATP-only conditions (Extended Data Figs. 2c, 5a, 6i).
Final refinement of each state was performed using pseudo-singly capped particles with the pixel size of 1.37 Å. Two types of local mask were applied for the auto-refinement, one focusing on the complete RP and the other focusing on the CP, resulting in two maps for each state, which were merged in Fourier space into one single map. Based on the in-plane shift and Euler angle of each particle from the auto-refinement, we reconstructed the two half-maps of each state using pseudo-singly capped particles with the pixel size of 0.685 Å. The Fourier shell correlation (FSC) curves of thirteen states were calculated from two separately refined half maps in a gold-standard procedure, yielding the nominal resolution ranging from 3.0 to 3.6 Å, and the local RP resolution ranging from 3.3 to 4.6 Å (Extended Data Figs. 2a, 3b–d). Before visualization, all density maps were sharpened by applying a negative B-factor ranging from −10 to −50 Å2.
In order to further improve the local density quality of USP14 and RPN1, which suffered from local conformational dynamics, another round of RP-masked 3D classification was done using CP-subtracted particles for some states to exclude 3D classes with blurred USP14 and RPN1. These locally improved maps were only used for visualization and adjustment of atomic models of USP14 and RPN1. For states , and , 3D classes with unblurred RPN1 and especially visible UBL on the RPN1 T2 site (including 152,802, 66,966 and 61,930 particles, respectively) were selected and refined by applying a mask on the RPN1-UBL component. The resulting RPN1-UBL density in these states were compared with previously reported EA1 state (Extended Data Fig. 7e). For states and , 3D classes with unblurred RPN1 density (including 61,447 and 53,145 particles, respectively) were selected and refined to 4.1 and 4.2 Å for the RP, respectively. For state , and , 3D classes with improved USP14 densities (including 34,659, 54,642, 142,814 particles, respectively) were selected and refined to 4.5, 4.2 and 3.8 Å for the RP component, respectively, for better visualization of the full-length USP14 in the proteasome.
Atomic model building and refinement
Atomic model building was based on the previously published cryo-EM structures of the human proteasome10. For the CP subcomplex, initial models of the closed-gate CP and open-gate CP were respectively derived from the EA1 model (PDB 6MSB) and the ED2 model (PDB 6MSK). For the RP subcomplex, the previous ED2 model was used as an initial model. All subunits of the initial model were individually fitted as a rigid body into each of the reconstructed maps with UCSF Chimera55, followed by further adjustment of the main chain traces using Coot56. Initial model of the full-length USP14 was first derived from a predicted one by AlphaFold57, which was verified by comparing to a crystal structure5 (PDB 2AYO). The USP14 model was then merged with the initial proteasome model by independently fitting models of the USP14 UBL and USP domains as rigid bodies into the cryo-EM maps, and manually fitting the linker between the UBL and USP domains in Coot56. Despite the presence of RPN13 in our purified proteasome (Extended Data Fig. 1g) and the addition of excessive RPN13 to saturate the proteasome, no reliable density was observed for RPN13 in all cryo-EM maps, thus precluding the atomic modelling of RPN13 and likely reflecting its highly dynamic association with the proteasome. The atomic models of subunit SEM1 and the N terminus of subunit RPN5 fitted into their corresponding local densities of lower resolution were rebuilt by considering the prediction of AlphaFold57. The atomic model of USP14 was first rebuilt and refined against the map of state with improved local USP14 density, the resulting model of which was then used as starting USP14 models to fit into the USP14 densities in other states. For some structures with partially blurred or missing subunit densities, the atomic models were revised by removing these regions, for example, the UBL domain of USP14 was removed in the models of and . Given that the substrates were not stalled in a homogeneous location during their degradation and that substrate translocation through the proteasome is not sequence-specific, the substrate densities were modelled using polypeptide chains without assignment of amino acid sequence. For states , , , , , , and , the nucleotide densities are of sufficient quality for differentiating ADP from ATP, which enabled us to build the atomic models of ADP and ATP into their densities (Extended Data Fig. 6j). For other states with the local RP resolution worse than 4.0 Å, the nucleotide types or states were hypothetically inferred from their adjacent states at higher resolution with the closest structural similarity, based on the local densities, the openness of corresponding nucleotide-binding pockets as well as their homologous structural models of higher resolution if available.
After manually rebuilding, atomic models were all subjected to the real-space refinement in Phenix58. Both stimulated annealing and global minimization were applied with non-crystallographic symmetry (NCS), rotamer and Ramachandran constraints. Partial rebuilding, model correction and density-fitting improvement in Coot56 were then iterated after each round of atomic model refinement in Phenix58. The refinement and rebuilding cycle were often repeated for three rounds or until the model quality reached expectation (Extended Data Table 1).
Structural analysis and visualization
All structures were analysed in Coot56, PyMOL59, UCSF Chimera55, and ChimeraX60. Inter-subunit interactions and interfacial areas were computed and analysed using the PISA server61 (https://www.ebi.ac.uk/pdbe/prot_int/pistart.html). Local resolution variations were estimated using ResMap62. Figures of structures were plotted in PyMOL59, ChimeraX60, or Coot56. Structural alignment and comparison were performed in PyMOL59 and ChimeraX60.
Data reporting
No statistical methods were used to predetermine sample size. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Statistical analysis
Statistical analyses of mutagenesis data were performed using two-tailed unpaired t-tests with SPSS v.27.0 unless otherwise indicated. Statistical significance was assessed with a 95% confidence interval and a P value of < 0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-04671-8.
Supplementary information
This file contains the raw (uncropped) gel images for Fig. 2 and for Extended Data Figs. 1, 9.
Source data
Acknowledgements
We thank Y. Saeki for the plasmids expressing Sic1PY and WWHECT; L. Huang for the proteasome-expressing cell lines; C. Tang for the plasmid expressing UBE1; X. Li, Z. Guo, X. Pei, B. Shao, G. Wang, Y. Ma, C. Fan, B. Liu and L. Lai for technical assistance; and A. Goldberg and Q. Ouyang for discussions and comments. This work was funded in part by grants from the Beijing Natural Science Foundation (Z180016/Z18J008) and the National Natural Science Foundation of China (11774012, 12125401) to Y.M., and by a grant from the National Institute of Health (R01 GM043601) to D.F. The cryo-EM data were collected at the Cryo-EM Core Facility Platform and Laboratory of Electron Microscopy at Peking University. The data processing was performed in the High-Performance Computing Platform, the Weiming No. 1 and Life Science No. 1 supercomputing systems, at Peking University.
Extended data figures and tables
Extended Data Fig. 3. Cryo-EM reconstructions and resolution measurement.
a, Local resolution estimation of the RP reconstructions of thirteen states calculated by ResMap62. Each state is shown in two orthogonal orientations, with the second orientation (top view) shown in two different cross-sections at the AAA (middle row) and OB (lower row) domains. All local resolutions are shown with the same color bar in the upper right inset. b and c, Gold-standard Fourier shell correlation (FSC) plots of the complete RP-CP maps of all states calculated without (b) or with (c) masking the separately refined half-maps. d, Gold-standard FSC plots of the RP-masked reconstructions of all states. The RP maps were refined by focusing the mask on the RP subcomplex. e, Model-map FSC plots calculated by Phenix58 between each RP-CP map (masked) and its corresponding atomic model. For each state, separately refined RP and CP maps (using RP and CP masks, respectively) were merged in Fourier space into a single RP-CP map, which was used for the model-map FSC calculation. The same color code is used in (b–e).
Author contributions
Y.M. conceived this study. S. Zou purified the protein complexes and prepared the cryo-EM samples. S. Zou and L.Z. conducted the biochemical experiments and mutagenesis study. S. Zhang, S. Zou, and D.Y. collected cryo-EM data and analysed the experimental cryo-EM datasets. S. Zhang refined the density maps. S. Zhang and Y.M. built and refined the atomic models. D.F. and Z.W. contributed to the analysis of the data. Y.M. supervised this study, analysed the data and wrote the manuscript with input from all authors.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Data availability
Cryo-EM density maps of USP14–proteasome complexes resolved in this study have been deposited in the Electron Microscopy Data Bank (EMDB) (www.emdataresource.org) under accession codes EMD-32272 (), EMD-32273 (), EMD-32274 (), EMD-32275 (), EMD-32276 (), EMD-32277 (), EMD-32278 (), EMD-32279 (), EMD-32280 (), EMD-32281 (), EMD-32282 (), EMD-32283 (), EMD-32284 (), EMD-32285 ( with the local RPN1 density improved), EMD-32286 ( with the local RPN1 density improved), EMD-32287 ( with the local RPN1 density improved), EMD-32288 ( with the USP14 density improved), EMD-32289 ( with the RPN1 density improved), EMD-32290 ( with the RPN1 density improved), EMD-32291 ( with the USP14 density improved) and EMD-32292 ( with the USP14 density improved). The corresponding coordinates have been deposited in the Protein Data Bank (PDB) (https://www.pdb.org) under accession codes 7W37 (), 7W38 (), 7W39 (), 7W3A (), 7W3B (), 7W3C (), 7W3F (), 7W3G (), 7W3H (), 7W3I (), 7W3J (), 7W3K () and 7W3M (). Uncropped versions of all gels and blots are provided in Supplementary Fig. 1. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuwen Zhang, Shitao Zou
Extended data
is available for this paper at 10.1038/s41586-022-04671-8.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-04671-8.
References
- 1.Mao, Y. In Macromolecular Protein Complexes III: Structure and Function (eds Harris J. R. & Marles-Wright, J.) Ch. 1 (Springer, 2021).
- 2.de Poot SAH, Tian G, Finley D. Meddling with fate: the proteasomal deubiquitinating enzymes. J. Mol. Biol. 2017;429:3525–3545. doi: 10.1016/j.jmb.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leggett DS, et al. Multiple associated proteins regulate proteasome structure and function. Mol. Cell. 2002;10:495–507. doi: 10.1016/S1097-2765(02)00638-X. [DOI] [PubMed] [Google Scholar]
- 4.Borodovsky A, et al. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu M, et al. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BH, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BH, et al. USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature. 2016;532:398–401. doi: 10.1038/nature17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, et al. Structural basis for dynamic regulation of the human 26S proteasome. Proc. Natl Acad. Sci. USA. 2016;113:12991–12996. doi: 10.1073/pnas.1614614113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, et al. Structural mechanism for nucleotide-driven remodeling of the AAA-ATPase unfoldase in the activated human 26S proteasome. Nat. Commun. 2018;9:1360. doi: 10.1038/s41467-018-03785-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, et al. Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature. 2019;565:49–55. doi: 10.1038/s41586-018-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 12.Verma R, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 13.Worden EJ, Dong KC, Martin A. An AAA motor-driven mechanical switch in Rpn11 controls deubiquitination at the 26S proteasome. Mol. Cell. 2017;67:799–811.e798. doi: 10.1016/j.molcel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Ma H, Zhao Y, Zhao J. Ubiquitin-specific protease 14 is a new therapeutic target for the treatment of diseases. J. Cell. Physiol. 2021;236:3396–3405. doi: 10.1002/jcp.30124. [DOI] [PubMed] [Google Scholar]
- 15.Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat. Rev. Drug Discov. 2018;17:57–78. doi: 10.1038/nrd.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wertz IE, Wang X. From discovery to bedside: targeting the ubiquitin system. Cell Chem. Biol. 2019;26:156–177. doi: 10.1016/j.chembiol.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci. Rep. 2016;6:26979. doi: 10.1038/srep26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna J, et al. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 19.Crosas B, et al. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. doi: 10.1016/j.cell.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Kim HT, Goldberg AL. The deubiquitinating enzyme Usp14 allosterically inhibits multiple proteasomal activities and ubiquitin-independent proteolysis. J. Biol. Chem. 2017;292:9830–9839. doi: 10.1074/jbc.M116.763128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peth A, Kukushkin N, Bosse M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J. Biol. Chem. 2013;288:7781–7790. doi: 10.1074/jbc.M112.441907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peth A, Besche HC, Goldberg AL. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol. Cell. 2009;36:794–804. doi: 10.1016/j.molcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashore C, et al. Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome. Nat. Struct. Mol. Biol. 2015;22:712–719. doi: 10.1038/nsmb.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aufderheide A, et al. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc. Natl Acad. Sci. USA. 2015;112:8626–8631. doi: 10.1073/pnas.1510449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Luan B, Wu J, Shi Y. An atomic structure of the human 26S proteasome. Nat. Struct. Mol. Biol. 2016;23:778–785. doi: 10.1038/nsmb.3273. [DOI] [PubMed] [Google Scholar]
- 26.Wehmer M, et al. Structural insights into the functional cycle of the ATPase module of the 26S proteasome. Proc. Natl Acad. Sci. USA. 2017;114:1305–1310. doi: 10.1073/pnas.1621129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, Z. et al. Hidden dynamics of human proteasome autoregulation discovered by cryo-EM data-driven deep learning. Preprint at 10.1101/2020.12.22.423932 (2021).
- 28.Schweitzer A, et al. Structure of the human 26S proteasome at a resolution of 3.9 Å. Proc. Natl Acad. Sci. USA. 2016;113:7816–7821. doi: 10.1073/pnas.1608050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haselbach D, et al. Long-range allosteric regulation of the human 26S proteasome by 20S proteasome-targeting cancer drugs. Nat. Commun. 2017;8:15578. doi: 10.1038/ncomms15578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Pena AH, Goodall EA, Gates SN, Lander GC, Martin A. Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science. 2018;362:eaav0725. doi: 10.1126/science.aav0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisele MR, et al. Expanded coverage of the 26S proteasome conformational landscape reveals mechanisms of peptidase gating. Cell Rep. 2018;24:1301–1315.e1305. doi: 10.1016/j.celrep.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science. 2016;351:eaad9421. doi: 10.1126/science.aad9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo CL, Goldberg AL. Ubiquitinated proteins promote the association of proteasomes with the deubiquitinating enzyme Usp14 and the ubiquitin ligase Ube3c. Proc. Natl Acad. Sci. USA. 2017;114:E3404–E3413. doi: 10.1073/pnas.1701734114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HT, Goldberg AL. UBL domain of Usp14 and other proteins stimulates proteasome activities and protein degradation in cells. Proc. Natl Acad. Sci. USA. 2018;115:E11642–E11650. doi: 10.1073/pnas.1808731115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins GA, Goldberg AL. Proteins containing ubiquitin-like (Ubl) domains not only bind to 26S proteasomes but also induce their activation. Proc. Natl Acad. Sci. USA. 2020;117:4664–4674. doi: 10.1073/pnas.1915534117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Mao Y. AAA+ ATPases in protein degradation: structures, functions and mechanisms. Biomolecules. 2020;10:629. doi: 10.3390/biom10040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha A, Warshel A. Simulating the directional translocation of a substrate by the AAA+ motor in the 26S proteasome. Proc. Natl Acad. Sci. USA. 2021;118:e2104245118. doi: 10.1073/pnas.2104245118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erales J, Hoyt MA, Troll F, Coffino P. Functional asymmetries of proteasome translocase pore. J. Biol. Chem. 2012;287:18535–18543. doi: 10.1074/jbc.M112.357327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckwith R, Estrin E, Worden EJ, Martin A. Reconstitution of the 26S proteasome reveals functional asymmetries in its AAA+ unfoldase. Nat. Struct. Mol. Biol. 2013;20:1164–1172. doi: 10.1038/nsmb.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat. Struct. Mol. Biol. 2004;11:830–837. doi: 10.1038/nsmb814. [DOI] [PubMed] [Google Scholar]
- 41.Chen PC, et al. The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J. Neurosci. 2009;29:10909–10919. doi: 10.1523/JNEUROSCI.2635-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung KYS, et al. Allosteric control of Ubp6 and the proteasome via a bidirectional switch. Nat. Commun. 2022;13:838. doi: 10.1038/s41467-022-28186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma R, McDonald H, Yates JR, 3rd, Deshaies RJ. Selective degradation of ubiquitinated Sic1 by purified 26S proteasome yields active S phase cyclin–Cdk. Mol. Cell. 2001;8:439–448. doi: 10.1016/S1097-2765(01)00308-2. [DOI] [PubMed] [Google Scholar]
- 44.Verma R, Oania R, Graumann J, Deshaies RJ. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell. 2004;118:99–110. doi: 10.1016/j.cell.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Ouyang Q, Mao Y. A deep convolutional neural network approach to single-particle recognition in cryo-electron microscopy. BMC Bioinformatics. 2017;18:348. doi: 10.1186/s12859-017-1757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, et al. Massively parallel unsupervised single-particle cryo-EM data clustering via statistical manifold learning. PLoS ONE. 2017;12:e0182130. doi: 10.1371/journal.pone.0182130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaikh TR, et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 2008;3:1941–1974. doi: 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 54.Kaledhonkar S, et al. Late steps in bacterial translation initiation visualized using time-resolved cryo-EM. Nature. 2019;570:400–404. doi: 10.1038/s41586-019-1249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 56.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 57.Tunyasuvunakool K, et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590–596. doi: 10.1038/s41586-021-03828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The PyMOL Molecular Graphics System Version 2.0 (Schrödinger, LLC).
- 60.Goddard TD, et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat. Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the raw (uncropped) gel images for Fig. 2 and for Extended Data Figs. 1, 9.
Data Availability Statement
Cryo-EM density maps of USP14–proteasome complexes resolved in this study have been deposited in the Electron Microscopy Data Bank (EMDB) (www.emdataresource.org) under accession codes EMD-32272 (), EMD-32273 (), EMD-32274 (), EMD-32275 (), EMD-32276 (), EMD-32277 (), EMD-32278 (), EMD-32279 (), EMD-32280 (), EMD-32281 (), EMD-32282 (), EMD-32283 (), EMD-32284 (), EMD-32285 ( with the local RPN1 density improved), EMD-32286 ( with the local RPN1 density improved), EMD-32287 ( with the local RPN1 density improved), EMD-32288 ( with the USP14 density improved), EMD-32289 ( with the RPN1 density improved), EMD-32290 ( with the RPN1 density improved), EMD-32291 ( with the USP14 density improved) and EMD-32292 ( with the USP14 density improved). The corresponding coordinates have been deposited in the Protein Data Bank (PDB) (https://www.pdb.org) under accession codes 7W37 (), 7W38 (), 7W39 (), 7W3A (), 7W3B (), 7W3C (), 7W3F (), 7W3G (), 7W3H (), 7W3I (), 7W3J (), 7W3K () and 7W3M (). Uncropped versions of all gels and blots are provided in Supplementary Fig. 1. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.